Abstract
Background:
Bacteria and their by-products are responsible for various pulpal and periapical infections which can be classified as biofilm-mediated infections. Recently, nanoparticles have been introduced to decrease the bacterial load in endodontic infections.
Aim and Objectives:
The aim of the study was to compare and evaluate the antibacterial effect of silver nanoparticles alone and combination of silver nanoparticles with calcium hydroxide and chlorhexidine against Enterococcus faecalis.
Materials and Methods:
A pure culture of E. faecalis was used as the test microorganism. After 24 h of incubation the bacterial colonies were isolated and suspended in 5 ml of infusion broth followed by incubation at 37°C for 4 h. 0.5 McFarland of the bacterial suspension was prepared and then cultured on Mueller–Hinton agar culture medium with the help of a sterile swab. In each culture plate, five wells were created with a sterile pipette for placement of the samples.
Results:
Statistically significant difference in the antibacterial effect of the combination of silver nanocure gel with various medicaments was observed when compared to the effect of silver nanocure gel alone. Silver nanocure gel in combination with calcium hydroxide (Group B) was more efficacious as compared to silver nanocure gel alone (Group A) (P < 0.001). Antibacterial activity of silver nanocure gel in combination with chlorhexidine (Group C) was also significantly higher than silver nanocure gel alone (P < 0.001).
Conclusion:
The present study is an in vitro study, in which we concluded that the combination of all the intracanal medicaments is the best among for elimination of E. faecalis biofilm from the root canal. The above findings need to be tested in vivo also.
KEYWORDS: Enterococcus faecalis, Mueller–Hinton agar, multidrug-resistant bacteria, silver nanocure gel
INTRODUCTION
Enterococcus faecalis is one of the most dominant biofilm-forming bacteria found in teeth with periradicular pathologies.[1] It has a property of an intrinsic resistance to irrigant solutions, several antibiotics, intracanal medicaments, and highly alkaline pH as well as it can tolerate starvation, harsh environment, and invade deeply into the dentinal tubules.[2,3] Reduction in the number of microorganisms in the root canal can be achieved by cleaning and shaping of the root canal along with irrigation protocol. However, these procedures are not able to completely eliminate bacteria from lateral canals, isthmuses, and apical deltas. The augmentation of the above antibacterial protocol can be achieved by utilizing intracanal medicaments.[4,5,6] Usually, bacteria and their by-products are responsible for various pulpal and periapical infections[7,8] which can be classified as biofilm-mediated infections.
Recently, nanoparticles have been introduced to decrease the bacterial load in endodontic infections which is hypothesized to have an antibacterial effect due to their nano size and structure that provides increased surface area which can absorb other medicaments and exert antimicrobial effect,[9,10] while calcium hydroxide and chlorhexidine have been used since a long time for the elimination of E. faecalis. Because of the strong bactericidal potential against Gram-positive, Gram-negative, and multidrug-resistant bacteria, silver nanoparticles are commonly used nanoparticles.[9,10] It has the ability to interact with bacterial cell wall, which leads to the structural changes and disruption of the tissue protein. Hence, the aim of the present study is to compare and evaluate the antibacterial effect of silver nanoparticles alone and a combination of silver nanoparticles with calcium hydroxide and chlorhexidine against E. faecalis.
MATERIALS AND METHODS
There were four groups made according to the medicament used in the study, and they are:
-
Group A
Silver nanocure gel
-
Group B
Silver nanocure gel + Cavisept gel (1:1)
-
Group C
Silver nanocure gel + Aveu-Cal gel (1:1)
Group D
Silver nanocure gel + Cavisept gel + Aveu-Cal gel (1:1:1).
Culture preparation
A pure culture of E. faecalis was used as the test microorganism. The bacterial colonies were isolated after 24 h of incubation and then suspended in 5 ml of infusion broth and then were incubated at 37°C for 4 h. After culturing E. faecalis in the culture media, 0.5 McFarland of the bacterial suspension was prepared and then cultured on Mueller–Hinton agar culture medium with the help of a sterile swab in all directions, and in each culture plate, five wells were created with a sterile pipette for placement of the samples.
Testing procedure
From each group, the wells were then filled with respective medicaments and were repeated five times under aseptic conditions. Incubation of the culture plate was performed at 37°C for 1 week, and the diameter of the growth inhibition zone was evaluated at 24 h, 48 h, and 1 week.
Statistical analysis
The mean of the three values was evaluated and mentioned as the diameter of zone of inhibition, using student's t-test.
RESULTS
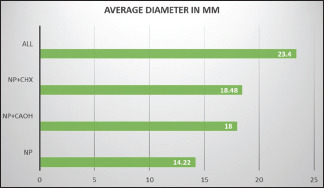
The antibacterial activity of all the three medicaments used in the study with different combinations has been presented in Table 1. There was a statistically significant difference in the antibacterial effect of the combination of silver nanocure gel with various medicaments when compared to silver nanocure gel alone. Silver nanocure gel in combination with calcium hydroxide (Group B) was more efficacious as compared to silver nanocure gel alone (Group A) (P < 0.001). Antibacterial activity of silver nanocure gel in combination with chlorhexidine (Group C) was also significantly higher than silver nanocure gel alone (P < 0.001). On comparison of silver nanocure gel when combined with both calcium hydroxide and chlorhexidine (Group D), the antibacterial activity was found higher in comparison to silver nanocure gel used alone (P < 0.001). The zone of inhibition for a combination of all the three medicaments was maximum [Graph 1].
Table 1.
Diameter of zone of inhibition of different medicaments
| Group | Medicaments | Average diameter (mm) |
|---|---|---|
| A | Silver nanocure gel | 14.22 |
| B | Silver nanocure gel + cavisept gel | (1:1) 18.00 |
| C | Silver nanocure gel + Aveu-cal gel | (1:1) 18.48 |
| D | Silver nanocure gel + Cavisept gel + Aveu cal gel | (1:1:1) 23.4 |
1 to 4 - scores according by Hulsmann et al.[14] *Number of samples showing with a given score, **Percent samples showing with a given grouped score. PUI: Passive ultrasonic irrigation, SAF: Self-adjusting file
Graph 1.
Average diameter
DISCUSSION
The nanoparticles and their different combinations have been used as intracanal medicaments, and their effect on E. faecalis was measured by evaluating the diameter of zone of inhibition.
E. faecalis was used as a test organism in the present study due to its major contribution in the etiology of persistent endodontic infections and its role in the root canal failures because of the ability to thrive in the root canals in adverse situations and form biofilm which has a high resistance against commonly used intracanal medicaments.[11,12,13] When silver nanocure gel was used in combination with Cavisept gel (Group B) showed a more significant antibacterial activity when compared to silver nanocure gel alone, but it was significantly lesser than the other combinations used (P < 0.001). Recent studies have mentioned that biofilm formation by E. faecalis produces a major complication in the working of chlorhexidine.[14] Silver nanoparticles have been added to increase the efficacy of Cavisept gel because silver gets ionized and destroys the cell membrane allows the entry of chlorhexidine which leads to the cell death[15] and helps in increasing the removal of E. faecalis and causes efficient cleaning of the root canal. When silver nanocure gel (Group A) was used alone, we found that there was slight antibacterial activity against E. faecalis because silver ions released due to the oxidation of nanoparticles bind to the cell membrane and penetrate inside the bacteria and react with specific bacterial proteins which alters the bacterial metabolism and inhibits the vital enzymatic systems that result in cell death. The results of our study were in accordance with the study done by Kim et al.[15]
In Group D where silver nanocure gel was used in combination with Cavisept gel and Aveu cal gel, demonstrated most significant reduction in level of E. faecalis activity. In our study, it was shown that, when all the three medicaments combined, there is a synergistic effect and hence it is able to cause maximum eradication of E. faecalis from the microbial flora of root canals. In Group C, where silver nanocure gel is used in combination with Aveu cal gel, this combination also proved significantly more effective against E. faecalis, our findings were in accordance with the other studies,[16,17,18,19] which suggested a combination of silver nanoparticles and calcium hydroxide to be better when compared to the silver nanoparticle gel and calcium hydroxide gel used alone.[20] It has been mentioned that the silver nanoparticles from silver nanocure gel forms pit in the cell wall of microorganism that leads to the disruption of the biofilm.[21,22] The release of hydroxyl ions in the root canal kills bacteria by destroying the cytoplasmic membrane, denaturation of proteins, and damaging DNA.[23,24]
CONCLUSION
The present study is an in vitro study, in which we concluded that the combination of all the intracanal medicaments is the best among for elimination of E. faecalis biofilm from the root canal. The above findings need to be tested in vivo also.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kakehashi S, Stanley HR, Fitzgerald RJ. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol. 1965;20:340–9. doi: 10.1016/0030-4220(65)90166-0. [DOI] [PubMed] [Google Scholar]
- 2.Möller AJ, Fabricius L, Dahlén G, Ohman AE, Heyden G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand J Dent Res. 1981;89:475–84. doi: 10.1111/j.1600-0722.1981.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 3.Madhubala MM, Srinivasan N, Ahamed S. Comparative evaluation of propolis and triantibiotic mixture as an intracanal medicament against Enterococcus faecalis. J Endod. 2011;37:1287–9. doi: 10.1016/j.joen.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Sabrah AH, Yassen GH, Gregory RL. Effectiveness of antibiotic medicaments against biofilm formation of Enterococcus faecalis and Porphyromonas gingivalis. J Endod. 2013;39:1385–9. doi: 10.1016/j.joen.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Wu D, Fan W, Kishen A, Gutmann JL, Fan W. Evaluation of antibacterial efficacy of Silver nanoparticles against Enteroccocus faecalis biofilms. J Endod. 2014:40:285–90. doi: 10.1016/j.joen.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Delgado RH, Gasparoto TH, Sipert CR, Pinheiro CR, Moraes IG, Garcia RB. Antimicrobial effects of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod. 2010:36:1389–93. doi: 10.1016/j.joen.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Z, Al Zaki A, Hui JZ, Muzykantov VR, Tsourkas A. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338:903–10. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: The powerful neoweapon against multi-drug resistant bacteria. J Appl Microbiol. 2012;112:841–52. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- 9.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 10.Ayala-Nunez NV, Villegas HH, Turrent LC, Padilla CR. Silver nanoparticles toxicity and bactericidal effect against methicillin resistant Staphylococcus aureus: Nanoscale does matter. J Nanobiotechnology. 2009;5:2–9. [Google Scholar]
- 11.Siqueira JF, Jr, Araújo MC, Garcia PF, Fraga RC, Dantas CJ. Histological evaluation of the effectiveness of five instrumentation techniques for cleaning the apical third of root canals. J Endod. 1997;23:499–502. doi: 10.1016/S0099-2399(97)80309-3. [DOI] [PubMed] [Google Scholar]
- 12.Kishen A. Advanced therapeutic options for endodontic biofilms. Endod Topics. 2010:22:99–123. [Google Scholar]
- 13.Siqueira JF, Jr, Rocas IN, Ricucci D. Biofilm in endodontic infection. Endod Topics. 2010;22:33–49. [Google Scholar]
- 14.Stuart CH, Schwartz SA, Beeson TJ, Owatz CB. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod. 2006;32:93–8. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 15.Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009;42:288–302. doi: 10.1111/j.1365-2591.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 17.Basrani B, Tjäderhane L, Santos JM, Pascon E, Grad H, Lawrence HP, et al. Efficacy of chlorhexidine-and calcium hydroxidecontaining medicaments against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:618–24. doi: 10.1016/s1079-2104(03)00166-5. [DOI] [PubMed] [Google Scholar]
- 18.Gomes BP, Souza SF, Ferraz CC, Teixeira FB, Zaia AA, Valdrighi L, et al. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro. Int Endod J. 2003;36:267–75. doi: 10.1046/j.1365-2591.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 19.Shen Y, Qian W, Chung C, Olsen I, Haapasalo M. Evaluation of the effect of two chlorhexidine preparations on biofilm bacteria in vitro: A three-dimensional quantitative analysis. J Endod. 2009;35:981–5. doi: 10.1016/j.joen.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 20.Shen Y, Stojicic S, Haapasalo M. Antibacterial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J Endod. 2011;37:657–61. doi: 10.1016/j.joen.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Castellano JJ, Shafii SM, Ko F, Donate G, Wright TE, Mannari RJ, et al. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int Wound J. 2007;4:114–22. doi: 10.1111/j.1742-481X.2007.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza-Filho FJ, Soares Ade J, Vianna ME, Zaia AA, Ferraz CC, Gomes BP. Antimicrobial effect and pH of chlorhexidine gel and calcium hydroxide alone and associated with other materials. Braz Dent J. 2008;19:28–33. doi: 10.1590/s0103-64402008000100005. [DOI] [PubMed] [Google Scholar]
- 23.Javidi M, Afkhami F, Zarei M, Ghazvini K, Rajabi O. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust Endod J. 2014;40:61–5. doi: 10.1111/aej.12028. [DOI] [PubMed] [Google Scholar]
- 24.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]


