Abstract
Background:
The present study was conducted to compare various methods of disinfection of impression materials such as glutaraldehyde, ultraviolet (UV) radiation, and autoclave.
Materials and Methods:
The present study was conducted on eighty alginate impression materials which were disinfectant with 2.2% glutaraldehyde, UV radiation, and autoclave. The pre- and postbacterial count was assessed.
Results:
The mean premicrobial contamination in Group I was 362.2 106 colony-forming unit (CFU)/ml, in Group II was 306.4 106 CFU/ml, and in Group III was 336.2 106 CFU/ml. The mean postmicrobial contamination in Group I was 65.2 106 CFU/ml, in Group II was 76.7 106 CFU/ml, and in Group III was 28.4 106 CFU/ml.
Conclusion:
The authors found that autoclave proved to be better in terms of reduction in bacterial colonies as compared to glutaraldehyde and UV radiation.
KEYWORDS: Disinfection, glutaraldehyde, ultraviolet radiation
INTRODUCTION
Dental impression is frequently taken in patients requiring removable, partial, or complete dentures. The oral cavity is full of fluids such as saliva and blood.[1] Materials are frequently contaminated with oral fluids such as saliva and blood. Dental professionals dealing with impression material come in contact with saliva, and hence, there are chances to get infected if not handled carefully. Cross infection between dental clinics and dental laboratories are common.[2] It is evident in research that contaminated impressions can cross infect gypsum casts that were poured against them. Various methods for disinfection of impression materials are available. Rinsing with water was the easiest and frequently used method. It is proposed that all dental prostheses and prosthodontic items should be cleaned, disinfected, and rinsed before they are handled in the laboratory using an active hospital disinfectant.[3]
Disinfection is either high-level disinfection which involves bacterial spore inactivity along with other microbial forms, intermediate-level disinfection involves destruction of microorganisms such as tubercle bacilli, and low-level disinfection possesses narrow antimicrobial activity.[4]
Dental impression materials can be disinfected by soaking in chemical disinfectant, autoclave, radiation, etc., The disinfectant solution should show high effectiveness in the reduction of pathogenic microorganisms without interfering with the dimensional stability or ability to reproduce details of the material. Unlike disinfection, sterilization is a procedure that guarantees the elimination of all microorganisms. Sterilization is a preferred method of cross infection control in the dental clinics.[5] The present study was conducted to compare various methods of disinfection of impression materials such as glutaraldehyde, ultraviolet (UV) radiation, and autoclave.
MATERIALS AND METHODS
The present study was conducted in the department of prosthodontic. It consisted of sixty alginate impression materials. The institutional ethical committee approved the study protocol.
We established three groups: Group I in which impression material was disinfected with glutaraldehyde (2.2%) by soaking it in solution for 5 min, Group II in which UV radiation was used for disinfection consisting of an outer body and inner body chamber used for the study and alginate impressions were placed in the chamber for 20 min, and Group III in which sterilization with autoclave for 15 min under 20 lbs pressure was done.
All the impressions were subjected to microbial assessment. Nutrient agar was used as a media to elicit the growth of microorganisms. Pour-plate technique was employed to uniformly dispense the diluted samples on the Petri plates containing the nutrient agar. These Petri plates were then inoculated and incubated at 37°C for 24 h. The Petri plates were examined for recording the total viable count following incubation. The number of colony-forming units (CFUs) of the viable microorganisms on the Petri plates was counted using a digital colony counter. The predisinfectant count was compared with postdisinfectant count. The result thus obtained was evaluated using SPSS version 21 (IBM. Chicago, Illinois, USA). The total viable count was expressed as mean ± standard deviation. The 0.05 value was considered significant.
RESULTS
Table 1 shows that 2.2% glutaraldehyde, UV radiation, and sterilization with autoclave in Groups I, II, III, respectively, were used. Each group had twenty impressions.
Table 1.
Distribution of materials
| Groups | Group I | Group II | Group III |
|---|---|---|---|
| Material | 2.2% glutaraldehyde | UV radiation | Autoclave |
| n | 20 | 20 | 20 |
UV: Ultraviolet
Table 2 and Graph 1 show that the mean premicrobial contamination in Group I was 362.2 × 106 CFU/ml, in Group II was 306.4 × 106 CFU/ml, and in Group III was 336.2 × 106 CFU/ml. The difference was nonsignificant difference (P > 0.05).
Table 2.
Assessment of predisinfection microbial contamination
| Groups | Mean | P |
|---|---|---|
| Group I | 362.1 | 0.075 |
| Group II | 306.4 | |
| Group III | 336.2 |
Graph 1.
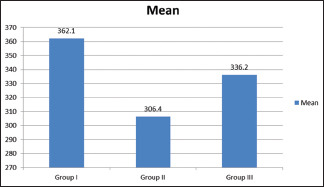
Assessment of predisinfection microbial contamination
Table 3 and Graph 2 show that the mean postmicrobial contamination in Group I was 65.2 × 106 CFU/ml, in Group II was 76.7 × 106 CFU/ml, and in Group III was 28.4 × 106 CFU/ml. The difference was significant difference (P < 0.05).
Table 3.
Assessment of postdisinfection microbial contamination
| Groups | Mean | P |
|---|---|---|
| Group I | 65.2 | 0.001 |
| Group II | 76.7 | |
| Group III | 28.4 |
Graph 2.
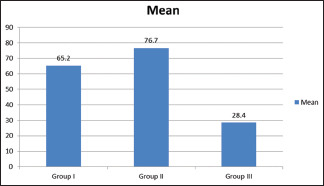
Assessment of postdisinfection microbial contamination
Table 4 shows that there was a significant difference in pre- and postmicrobial CFI in all groups. Maximum reduction was observed in Group III, followed by Groups I and II. A significant difference (P < 0.05) in CFU value in all the groups was found.
Table 4.
Comparison of the pre- and postdisinfection microbial contamination
| Groups | Pre | Post | t | df | P |
|---|---|---|---|---|---|
| Group I | 362.1 | 65.2 | 12.52 | 2 | 0.011 |
| Group II | 306.4 | 76.7 | 12.34 | 2 | 0.017 |
| Group III | 336.2 | 28.4 | 11.96 | 2 | 0.023 |
DISCUSSION
It is evident that microorganisms causing disease are present in human blood and saliva. Contact with blood or saliva mixed with blood may transmit pathogenic microorganisms. Thus, care should be taken while taking impressions. Cross infection control is the most significant and important among dental workers. Cross infection is the transfer of an infectious agent from one individual to another in a clinical environment.[6] New infectious diseases have been found causing cross infection. Impression with different impression materials accelerates the rate of infection transfer. Dental staff including hygienists is at higher risk of getting exposed to infectious agents such as AIDS, hepatitis, herpes simplex, and cytomegalovirus.
Approximately 300–400 million people are chronic hepatitis B carriers all over the world.[7] Hepatitis virus transmission is a major occupational hazard for dental personnel. HIV can be transmitted by transfusions, needle stick injury, or contact of mucous membrane with the blood or body fluids of a carrier. Dentists due to the nature of their work are very prone to such detriments. Thus, it becomes necessary to follow disinfectants and sterilization procedures.[8] The present study was conducted to compare glutaraldehyde, radiation, and autoclave method of disinfection of impression materials.
In the present study, we divided impressions into three groups based on the type of disinfection method used. Jha et al.[9] determined the antimicrobial efficiency of an organic disinfectant Ecosan® with alginate impression material after oral contact. Impression area was dissected into two halves. One half was disinfected with Ecosan® for 10 min and the other was only rinsed with water. Results showed that there was a significant reduction in bacterial count in area sterilized with Ecosan® as compared to water.
We found that the mean premicrobial contamination in Group I was 362.2 × 106 CFU/ml, in Group II was 306.4 × 106 CFU/ml, and in Group III was 336.2 × 106 CFU/ml. The mean postmicrobial contamination in Group I was 65.2 ×106 CFU/ml, in Group II was 76.7 × 106 CFU/ml, and in Group III was 28.4 × 106 CFU/ml. Ganavadiya et al.[10] in their study evaluated total viable count after disinfection with 2% glutaraldehyde, 6% hydrogen peroxide (H2O2), and 99.9% ethyl alcohol with distilled water as a negative control and autoclaving as a positive control. It was found that maximum reduction in microbial load was seen with H2O2 followed by glutaraldehyde, ethyl alcohol, and distilled water. It was also observed that maximum microbial contamination was recorded on locally manufactured mirrors, while standard plain mirrors showed least contamination.
We observed that the mean postmicrobial contamination in Group I was 65.2 106 CFU/ml, in Group II was 76.7 106 CFU/ml, and in Group III was 28.4 106 CFU/ml. Nimonkar et al.[11] compared 1% sodium hypochlorite, 2% glutaraldehyde, and UV disinfectant on the dimensional stability of polyvinyl siloxane impression material. Samples disinfected with 2% glutaraldehyde and 1% sodium hypochlorite showed significant dimensional changes and UV disinfectant unit showed no significant dimensional changes. Impressions disinfected with 1% sodium hypochlorite showed more discrepancy in the dimensions when compared to the 2% glutaraldehyde disinfected group. UV radiation has a powerful bactericidal effect. It reacts with DNA of the cells by the initiation of thymine centenary, which leads to cell death.
Ishida et al.[12] found that UV light was effective against Candida organisms in the silicone impression material within 5 min of exposure without any dimensions or surface roughness change. Lepe et al.[13] determined the disinfection capability of 2% glutaraldehyde on dimensional stability of elastomeric impression materials and found that the accuracy of impressions was extremely affected. It shows that chemical disinfectants induce surface roughness and alters the dimensions of the impression material.
The shortcoming of the study is the small sample size. The inclusion of different disinfecting agents could have provided different and useful results.
CONCLUSION
Numerous methods of disinfection and sterilization of impression materials have been established. There are drawbacks as well as advantages of each method. The authors found that autoclave is the better method of sterilization compared to the use of glutaraldehyde, UV radiation, and herbal disinfectant.
Need of the study
The chances of infection transmission from patients to dental surgeons demand a careful and effective way of disinfecting the material before getting infection. Assessment of different agents may provide useful disinfectant sufficient enough to destroy viable bacterial count.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rbds H, Ganapathy D. Disinfection of dental impression-A current overview. J Pharm Sci Res. 2016;8:661–4. [Google Scholar]
- 2.Elkholy S, Lofty W. Sacred Lotus as an impression disinfectant and its effect on the dimensional stability of an elastomeric impression material. Acta Sci Dent Sci. 2018;2:39–44. [Google Scholar]
- 3.Al Mortadi N, Al-Khatib A, Alzoubi KH, Khabour OF. Disinfection of dental impressions: Knowledge and practice among dental technicians. Clin Cosmet Investig Dent. 2019;11:103–8. doi: 10.2147/CCIDE.S205144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khinnavar PK, Kumar BH, Nandeeshwar DB. An in vitro study to evaluate the effect on dimensional changes of elastomers during cold sterilization. J Indian Prosthodont Soc. 2015;15:131–7. doi: 10.4103/0972-4052.155034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nassar U, Chow AK. Surface detail reproduction and effect of disinfectant and long-term storage on the dimensional stability of a novel vinyl polyether silicone impression material. J Prosthodont. 2015;24:494–8. doi: 10.1111/jopr.12244. [DOI] [PubMed] [Google Scholar]
- 6.Godbole SR, Dahane TM, Patidar NA, Nimonkar SV. “Evaluation of the effect of ultraviolet disinfection on dimensional stability of the polyvinyl silioxane impressions.” An in-vitro study. J Clin Diagn Res. 2014;8:ZC73–6. doi: 10.7860/JCDR/2014/8461.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badrian H, Davoudi A, Molazem M, Zare MH. The effect of spraying different disinfectants on condensational silicone impressions; an in vitro study. J Indian Prosthodont Soc. 2015;15:263–7. doi: 10.4103/0972-4052.161091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aeran H, Sharma S, Kumar V, Gupta N. Use of clinical UV chamber to disinfect dental impressions: A comparative study. J Clin Diagn Res. 2015;9:ZC67–70. doi: 10.7860/JCDR/2015/14025.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jha P, Shetty AK, Anandakrishna L. Efficiency of an organic disinfectant on alginate impressions-A pilot study. JDOR. 2019;17:19–20. [Google Scholar]
- 10.Ganavadiya R, Chandra Shekar BR, Saxena V, Tomar P, Gupta R, Khandelwal G. Disinfecting efficacy of three chemical disinfectants on contaminated diagnostic instruments: A randomized trial. J Basic Clin Pharm. 2014;5:98–104. doi: 10.4103/0976-0105.141946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nimonkar SV, Belkhode VM, Godbole SR, Nimonkar PV, Dahane T, Sathe S. Comparative evaluation of the effect of chemical disinfectants and ultraviolet disinfection on dimensional stability of the polyvinyl siloxane impressions. J Int Soc Prev Community Dent. 2019;9:152–8. doi: 10.4103/jispcd.JISPCD_406_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishida H, Nahara Y, Tamamoto M, Hamada T. The fungicidal effect of ultraviolet light on impression materials. J Prosthet Dent. 1991;65:532–5. doi: 10.1016/0022-3913(91)90295-8. [DOI] [PubMed] [Google Scholar]
- 13.Lepe X, Johnson GH, Berg JC. Surface characteristics of polyether and addition silicone impression materials after long-term disinfection. J Prosthet Dent. 1995;74:181–6. doi: 10.1016/s0022-3913(05)80184-2. [DOI] [PubMed] [Google Scholar]


