Abstract
Bone is an amazing nanocomposite tissue made of both organic (primarily collagen) and inorganic (primarily nano-hydroxyapatite [n-HA]) elements. Bone grafting is a widely used surgical technique in dental and orthopedic surgeries to enhance bone regeneration. In view of the significant drawbacks of traditional treatments, nanomaterials offer new strategies for bone regeneration. The HA with the chemical formula of Ca10(OH) 2(PO4) 6 is very identical to the inorganic portion of bone. Due to its high stability and minimal solubility, it is often used in orthopedic and dental procedures. Currently, n-HA, which facilitates the growth of new bone, has garnered considerable attention because of better bioactivity and bone integration ability when compared to porous HA. This review gives comprehensive insights related to n-HA structure, chemical composition, surface modification techniques, and their application in bone tissue engineering.
KEYWORDS: Bio-ceramics, biomaterials, bone grafts, materials, nano-hydroxyapatite, tissue engineering
INTRODUCTION
Bone is an interesting nanocomposite tissue, comprising organic (primarily collagen) as well as inorganic (primarily nano-hydroxyapatite [n-HA]) constituents. The hierarchical structure of the bone, at a nanoscale, is presented with a highly organized array of HA nanocrystals within collagen fibrils [Figure 1].[1] Bone regeneration per se might be a complex process and requires an orchestrated sequence of biological events. Bone grafting is a widely used technique in dental and orthopedic surgeries to accelerate bone regeneration.[2] More than two million bone graft operations are carried out each year globally, making it the second most common tissue transplant after blood transfusion.[3]
Figure 1.
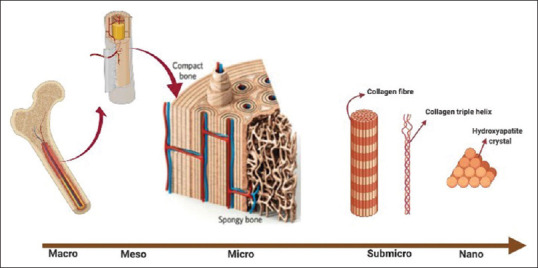
Hierarchical structure of bone
The most widely used technique for bone defect reconstruction is done by using an autogenous bone graft, regarded as a “gold standard.”[4] However, its use is restricted by clinical drawbacks such as limited tissue availability, donor site morbidity, and the poor quality associated with the modification of matrix proteins critical for bone formation and mineralization.[5,6] These drawbacks have led to the development of allografts and xenografts. However, these graft materials carry an inherent risk of infection, immune rejection, and disease transmission.[7,8] To address these shortcomings, enormous alternatives in the form of artificial bone substitutes were developed. These materials only exhibit osteoconductive properties, thus limiting their capacity for bone regeneration.[2]
The hydroxyapatite (HA) with the chemical formula of Ca10(OH) 2(PO4) 6 is almost identical to the inorganic portion of the bone matrix.[9] As a result of this close resemblance, pervasive research is underway to use HA as a bone graft. Due to its excellent osteoconductive and osseo-integrative properties, it is commonly used as a bone graft in orthopedic and dental surgeries.[10] On the other hand, the key mechanical issues related to the use of HA in bone tissue engineering are low resistance to fracture, fatigue failure, and brittleness. In addition, the high Ca/P ratio and crystallinity further hinder the resorption rate of HA by years or even decades.[11]
Recent advances in nanoscience and nanotechnology have opened up new avenues for manufacturing nano bone grafts. Currently, n-HA that facilitates new bone growth has garnered significant attention. n-HA crystals have good biocompatibility, superior bioactivity, and osseointegration ability and show little inflammatory reaction compared to porous HA.[10] Since n-HA has a tiny particle size and a huge surface area, it resorbs quickly within weeks and is substituted by vital bone. Therefore, functionalized n-HA–based nanocomposites would be a promising approach to address the aforementioned concerns in the near future. This review provides a detailed insight related to n-HA structure, chemical composition, surface modification techniques, and their application to bone tissue engineering.
BASIC INFORMATION ABOUT NANOMATERIALS
Nanomaterials are synthetic or natural materials with a scale of < 100 nm in both directions.[12] The two key characteristics of crucial importance which confer unique properties to the nanomaterials are the quantum effects and the huge surface-to-volume ratio. A tremendous driving force for diffusion is provided by the huge surface-to-volume ratio, particularly at elevated temperatures.[13] The size of the nanomaterials should be between 10 and 100 nm to possess excellent biomedical application. Materials smaller than 10 nm in size are cleared easily by the kidneys and the reticulo-endothelial system, whereas sizes greater than 100 nm can cause embolism and can be eliminated through the spleen by phagocytosis.[14] Furthermore, the sizes under 10 nm are extremely lethal and reactive because of their high surface density and increased surface reactive electrons. Nano-biomaterials have the structural identity to numerous body proteins and ligands, allowing them to interact easily with multiple receptors and cross cell membranes.[15] In addition, the solubility of certain substances increases with decreasing particle size.[13,16] Furthermore, the surface properties of nanomaterials determine the type and amount of adsorption of selective proteins for an enhanced osteoblast adhesion.[17]
NANO-HYDROXYAPATITE STRUCTURE AND CHEMICAL COMPOSITION
As described above, n-HA is the hydroxyl end member of the apatite group made up of calcium and phosphates. The structure of HA crystals belongs to the hexagonal symmetry group with ten Ca2+, six PO43−, and two OH − ions contained in a single unit cell [Figure 2].[18] The calcium and phosphates in the HA unit cell are organized in such a fashion that, at M1 position, the four Ca atoms are encircled by nine O atoms and the rest of the six Ca atoms are encircled by the six O atoms at M2 crystallographic position of the Ca atoms.[18,19] HA also contains traces of magnesium (Mg), sodium (Na), chloride ions (Cl−), fluoride ions (F−), phosphite ions (PO33−), and hydroxyl ions (OH-). Of these, PO33 − and Cl − ions tire HA assemble, whereas F − and OH- strengthen it.
Figure 2.
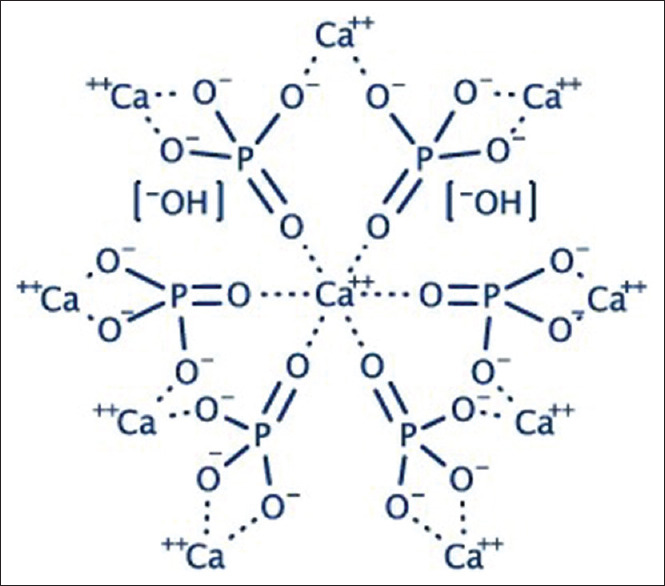
Structure of hydroxyapatite
SURFACE MODIFICATION OF HYDROXYAPATITE NANOPARTICLES
The agglomeration of nanomaterials is a prevalent snag faced by the researchers. Various emulsifying agents and bridging agents have been used for surface modification to overcome this hardship in HA nanoparticles. Tanaka et al. used decanoic and hexanoic acids to modify n-HA and found that the surface of modified HA particles has become hydrophobic.[20] Shimabayashi et al. functionalized the surface of HA with the amphiphilic surfactant sodium dodecyl sulfate.[21]
Inorganic materials can also be used as suitable surface modifiers. For example, silanes serve as the bridging agents for both organic and inorganic materials since they have a bonding capacity to promote adhesion. However, free silanes are found to be cytotoxic when compared to silanes immobilized on n-HA.[22]
ION-DOPING OF HYDROXYAPATITE NANOPARTICLES
In recent years, researchers have focused on alternative methodology for surface modification by doping n-HA with foreign ions. Several ions have been doped into the n-HA framework such as iron (Fe3+), zinc (Zn2+), magnesium (Mg2+), strontium (Sr2+), manganese (Mn2+), carbonate (CO32+), and silicate (SiO44+). The doping of n-HA with these ions did not disrupt the configuration of the n-HA framework but altered the crystallinity, the morphology, and the solubility of n-HA. Although the biocompatible properties of cells were changed in some situations, the ion doping has not altered the behavior of the cells. Zhao et al. demonstrated that cancer cells could be destroyed by Mg2+-doped HA nanoparticles.[23]
Lin et al. analyzed the impact of Sr2 + and Si2 + dopants on osteoblast proliferation, osteogenic differentiation, and angiogenic factor. They found that Si substitution could improve the osteoblast growth and differentiation.[24] Li et al. using a wet chemical process doped HA with iron (Fe3+) and manganese (Mn2+) ions and found that n-HA-doped Fe3 + has improved adhesion of osteoblasts when compared to Mn2+-doped n-HA and pure n-HA.[25] Evidence from these studies clearly indicates that metal cation dopants when incorporated into a tissue-engineering construct can elicit more physio-mechanical and biological benefits.
HYDROXYAPATITE-BASED COMPOSITE MATERIALS IN BONE REGENERATION
Currently, numerous artificial polymers have been explored explicitly or along with n-HA to resemble the bone extracellular matrix. Among the most frequently used polymers are the polylactic acid, polyglycolic acid, and poly (caprolactone). Haider et al. functionalized n-HA surface with BMP-2 along with insulin and then incorporated into poly (lactic-co-glycolic acid) (PLGA) polymer to create a HA/PLGA composite scaffold. These composite scaffolds have enhanced osteoblastic cell growth and increased osteogenesis.[26] In a study performed by Wang et al., it was observed that the n-HA/polyethylene composite has adequate mechanical strength and can be used as scaffolds in bone regeneration.[27] Dalby et al. have demonstrated that increasing n-HA incorporation into poly (methyl methacrylate) composite scaffold improves the human osteoblastic cell adhesion.[28]
Chitosan has a proven ability to form pores, binding capacity to ionic molecules, antibacterial activity, and biodegradation.[29] When n-HA was added to chitosan, it resulted in the formation of bone mimicking natural bone with improved mechanical properties. Kim et al. noted that the osteoblasts exhibited superior binding to nanosized HA/gelatin biocomposites compared to microsized particles.[30] Nanocrystalline HA coatings on the titanium resulted in increased osteoblast and decreased fibroblast adhesion when compared to conventional plasma-sprayed HA coatings.[31]
CONCLUSION
Advances in nanotechnology and materials science are routinely fabricating new biomaterials to improve bone regeneration. n-HA is employed as an orthopedic and dental implants, bone grafts, and vehicle for drug delivery and as a means of coating on metallic materials. Because of its augmented osteoconductive, osteoinductive, and osseointegrative properties, n-HA may represent a promising class of bone graft materials. The biological properties of n-HA can be further intensified by the addition of growth factors and other osteoinductive molecules. However, cell–material interaction, mechanical strength, and biological response of n-HA–based nanocomposites need to be analyzed and investigated for their biomedical significance through clinical studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Abd Razak SI, Ahmad Sharif NF, Abdul Rahman WA. Biodegradable polymers and their bone applications: A review. Int J Basic Appl Sci. 2012;12:31–49. [Google Scholar]
- 2.Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: Current concepts and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, et al. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J Mater Sci Mater Med. 2014;25:2445–61. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer TW, Muschler GF. Bone graft materials An overview of the basic science. Clin Orthop Relat Res. 2000;371:10–27. [PubMed] [Google Scholar]
- 5.Goldberg VM, Stevenson S. Natural history of autografts and allografts. Clin Orthop Relat Res. 1987;225:7–16. [PubMed] [Google Scholar]
- 6.Khan SN, Cammisa FP, Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13:77–86. [PubMed] [Google Scholar]
- 7.Centers for Disease Control (CDC) Transmission of HIV through bone transplantation: case report and public health recommendations. Morbidity Mortality Weekly Rep. 1988;37:597. [PubMed] [Google Scholar]
- 8.Stevenson S. Current concepts review. The response to bone allografts. J Bone Joint Surg Am. 1992;74:939–50. [PubMed] [Google Scholar]
- 9.Dorozhkin SV. Calcium orthophosphates: applications in nature, biology, and medicine. Florida , USA: CRC Press; 2012. [Google Scholar]
- 10.Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7:2769–81. doi: 10.1016/j.actbio.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Goto T, Kojima T, Iijima T, Yokokura S, Kawano H, Yamamoto A, et al. Resorption of synthetic porous hydroxyapatite and replacement by newly formed bone. J Orthop Sci. 2001;6:444–7. doi: 10.1007/s007760170013. [DOI] [PubMed] [Google Scholar]
- 12.Wahajuddin, Arora S. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–71. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson KG. The Kelvin equation and solubility of small particles. J Pharmac Sci. 1972;61:479–80. doi: 10.1002/jps.2600610340. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Urrusuno R, Fattal E, Rodrigues JM, Jr, Féger J, Bedossa P, Couvreur P. Effect of polymeric nanoparticle administration on the clearance activity of the mononuclear phagocyte system in mice. J Biomed Mater Res. 1996;31:401–8. doi: 10.1002/(SICI)1097-4636(199607)31:3<401::AID-JBM15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Katz E, Willner I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew Chem Int Ed Engl. 2004;43:6042–108. doi: 10.1002/anie.200400651. [DOI] [PubMed] [Google Scholar]
- 16.Fan C, Chen J, Chen Y, Ji J, Teng HH. Relationship between solubility and solubility product: The roles of crystal sizes and crystallographic directions. Geochem Cosmochim Acta. 2006;70:3820–9. [Google Scholar]
- 17.Sato M, Webster TJ. Nanobiotechnology: Implications for the future of nanotechnology in orthopedic applications. Expert Rev Med Dev. 2004;1:105–14. doi: 10.1586/17434440.1.1.105. [DOI] [PubMed] [Google Scholar]
- 18.White TJ, ZhiLi D. Structural derivation and crystal chemistry of apatites. Acta Crystallogr B. 2003;59:1–6. doi: 10.1107/s0108768102019894. [DOI] [PubMed] [Google Scholar]
- 19.Uskoković V, Uskoković DP. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J Biomed Mater Res B Appl Biomater. 2011;96:152–91. doi: 10.1002/jbm.b.31746. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Watanabe T, Chikazawa M, Kandori K, Ishikawa T. TPD, FTIR, and molecular adsorption studies of calcium hydroxyapatite surface modified with hexanoic and decanoic acids. J Colloid Interface Sci. 1999;214:31–7. doi: 10.1006/jcis.1999.6154. [DOI] [PubMed] [Google Scholar]
- 21.Shimabayashi S, Tanaka H, Nakagaki M. Adsorption of dodecyl sulfate ion on hydroxyapatite and concurrent release of phosphate and calcium ions from the surface of hydroxyapatite. Chem Pharm Bull. 1986;34:4474–8. [Google Scholar]
- 22.Dupraz AM, De Wijn JR, vd Meer SA, De Groot K. Characterization of silane-treated hydroxyapatite powders for use as filler in biodegradable composites. J Biomed Mater Res. 1996;30:231–8. doi: 10.1002/(SICI)1097-4636(199602)30:2<231::AID-JBM13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Z, Espanol M, Guillem-Marti J, Kempf D, Diez-Escudero A, Ginebra MP. Ion-doping as a strategy to modulate hydroxyapatite nanoparticle internalization. Nanoscale. 2016;8:1595–607. doi: 10.1039/c5nr05262a. [DOI] [PubMed] [Google Scholar]
- 24.Lin K, Wang X, Zhang N, Shen Y. Strontium (Sr) strengthens the silicon (Si) upon osteoblast proliferation, osteogenic differentiation and angiogenic factor expression. J Mater Chem B. 2016;4:3632–8. doi: 10.1039/c6tb00735j. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Widodo J, Lim S, Ooi CP. Synthesis and cytocompatibility of manganese (II) and iron (III) substituted hydroxyapatite nanoparticles. J Mater Sci. 2012;47:754–63. [Google Scholar]
- 26.Haider A, Gupta KC, Kang IK. Morphological effects of HA on the cell compatibility of electrospun HA/PLGA composite nanofiber scaffolds. Biomed Res Int. 2014;2014:308306. doi: 10.1155/2014/308306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Joseph R, Bonfield W. Hydroxyapatite-polyethylene composites for bone substitution: effects of ceramic particle size and morphology. Biomaterials. 1998;19:2357–66. doi: 10.1016/s0142-9612(98)00154-9. [DOI] [PubMed] [Google Scholar]
- 28.Dalby MJ, Di Silvio L, Harper EJ, Bonfield W. Increasing hydroxyapatite incorporation into poly (methyl methacrylate) cement increases osteoblast adhesion and response. Biomaterials. 2002;23:569–76. doi: 10.1016/s0142-9612(01)00139-9. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Nie J, Zhang Q, Li Y, Wang Z, Hu Q. Preparation and characterization of bionic bone structure chitosan/hydroxyapatite scaffold for bone tissue engineering. J Biomater Sci Polym Ed. 2014;25:61–74. doi: 10.1080/09205063.2013.836950. [DOI] [PubMed] [Google Scholar]
- 30.Kim HW, Kim HE, Salih V. Stimulation of osteoblast responses to biomimetic nanocomposites of gelatin-hydroxyapatite for tissue engineering scaffolds. Biomaterials. 2005;26:5221–30. doi: 10.1016/j.biomaterials.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 31.Sato M, Sambito MA, Aslani A, Kalkhoran NM, Slamovich EB, Webster TJ. Increased osteoblast functions on undoped and yttrium-doped nanocrystalline hydroxyapatite coatings on titanium. Biomaterials. 2006;27:2358–69. doi: 10.1016/j.biomaterials.2005.10.041. [DOI] [PubMed] [Google Scholar]


