Abstract
Aim:
The purpose of this in vitro study was to investigate the influence of length and width of implant on primary stability in immediate implants in mandibular first molar.
Materials and Methods:
The study was carried out on 40 cone-beam computed tomography scans selected with defined inclusion and exclusion criteria. According to the diameter and length of implants, they were divided into nine groups (G1 to G9). The virtual implants of different diameters and length were placed in mandibular first molar and measurements were done for peri-implant horizontal and vertical gap defect, peri-implant interradicular bone support and apical bone support for all the groups.
Results:
The study groups Diameter, (D-7 mm) showed least horizontal gap defect (Buccal-1.30 ± 0.56 mm, lingual-1.30 ± 0.56 mm, mesial-1.20 ± 0.51 mm, and distal-1.05 ± 0.59 mm) as compared to regular implant diameter (D-4.7) groups (Buccal-2.35 ± 0.483 mm, lingual-2.10 mm ± 0.44 mm, mesial-2.30 ± 0.64 mm, and distal-2.25 ± 0.43 mm). The unsupported Vertical implant gap defect at the coronal part of the socket was 2.80 mm ± 0.83 mm for all groups in both horizontal and vertical direction. The vertical peri-implant interradicular bone support showed increased bone support with increase in implant length (L). The buccal and lingual inter-radicular bone-support was least for Length (L-8.5 mm), moderate for L-11.5 mm, and highest for L-13.5 mm groups, respectively. The mesial inter-radicular bone support was least for G4G7, moderate for G1G2G5G8, and maximum for G3G6G9 groups. Similarly, the distal inter-radicular bone support was least for G4G7, moderate for G1G5G8, and maximum for G2G3G6G9 groups, respectively. There was no apical bone support in L-8.5 mm group as the tip of implant was 3.5–4 mm within the socket tip. Whereas, L-11.5 mm had decent (0.9–1 mm) and L-13.5 mm had Good (1.35–1.95 mm) apical bone support as the implant tip was beyond the socket tip.
Conclusion:
All the groups showed good interradicular bone support on buccal and lingual surfaces. Regular width implants with longer length showed satisfactory interradicular bone support on mesial and distal surfaces. Longer implants showed good apical bone support in all the four surfaces and hence good apical primary stability expected.
KEYWORDS: Bone support, gap defects, immediate molar implants, implant length and diameter, inter-radicular bone, primary stability
INTRODUCTION
The use of dental implants in restoring missing teeth has been increasingly accepted as a standard dental treatment. Literature provides insertion of implants into freshly extracted sockets with success. Primary implant stability is considered as a fundamental prerequisite for successful osseointegration.[1] In postextraction sites, the anatomical morphology influences primary stability and osseointegration.[2] Factors such as bone quantity, quality, the surgical technique of implant placement, and geometrical factors of the implant including shape, length, and diameter have a definite influence on the primary stability of the implant.[3] Immediate implants placed in the anterior region are more stable than those in the posterior region as, molar teeth being multi-rooted and socket sizes exceed that of most current implant diameters; therefore, it is difficult to estimate the optimal primary stability in the posterior mandible.[4]
Literature reveals cumulative survival rates ranging from 93.9% to 99% for immediate placement of implants in molar areas as compared to those placed in healed sites.[5,6] Martinez et al. reported that the survival rate of implants in posterior mandible is much lower than anterior mandible due to poor quality of bone.[7] In mandibular molar teeth, where interradicular bone is insufficient in providing good primary stability, wider diameter implants may be preferred that can engage the perimeter wall of the molar sockets.[2,8]
Consequently, immediate implants in the posterior region have been somehow a challenge to implantologists. Preservation and skillful engagement of the interseptal/interradicular bone is the cornerstone for the initial stability. A major disadvantage in cases of immediate implants is likely the mismatch, resulting due to the implant size and the socket wall; therefore, a potential gap exists after placement due to the irregularity in the root socket diameter and shape. De Angelis et al. advocated use of bovine inorganic bone substitute if the marginal gap was >2 mm.[9] However, Ferrus et al. concluded that this marginal gap eventually heals with formation of new bony tissue, thereby resolving the defect.[10] Literature does not provide with enough evidence to support or refute favoring augmentation procedures in case implants placed immediately into fresh extracted sockets and also superiority of one augmentation techniques over others.[11]
Increasing the length of implant plays a vital role to reduce the bone stress and enhancing the stability of implant.[3] Increasing implant diameter and also the length ensures a definite reduction in crestal strains and stress, however implant diameter has a significance effect as compared with increase in length in relieving the stress and strain concentration.[12] Some authors[7,13] have reported that implant stability has no correlation with implant length, but diameter was considered as a determinant factor. Increasing the implant diameter will capitalize the buccal and lingual cortical plate engagement and hence better stability.[14] Modifications in surgical techniques such as drilling through the tooth into interradicular bone septa, use of longer and wider implants, and the vertical engagement of the apical threads of the implants, regenerative materials and bone grafts help in success of the Immediate Molar Implant (IMI).[15] The paucity of literature regarding the methods for measuring the primary stability of IMI to validate the effectiveness of variations in implant dimensions for the success rate. Hence, the purpose of this in vitro study was to investigate the influence of length and width of implant on primary stability in bone of posterior multi-rooted teeth (Mandibular 1st molar).
Cone-beam computed tomography (CBCT) provides numerous advantages in clinical decision-making and is considered a well-established virtual simulation tool facilitating extensively as diagnostic and treatment planning particularly in implant dentistry.[16,17]
This study is based on virtual implant placement to quantitatively evaluate stability achieved by different length and diameter of implants with amount of bone engagement by means of CBCT.
MATERIALS AND METHODS
This cross-sectional descriptive study received Institutional ethics committee approval. A total of 40 CBCT Digital Imaging and Communications in Medicine (DICOM) files that had already been made for diagnostic purposes were obtained. The inclusion criteria were: (1) Presence of completely erupted mandibular molar teeth (except third molar), (2) closed root apices with root length of 10 mm and inter root apex distance from 3 to 7 mm, (3) presence of opposing maxillary teeth, (4) each tooth should be normally positioned (the imaginary line connecting the cusp tip of canines, central grooves of premolars, and molars was generally smooth.[18] The exclusion criteria were: (1) Crowding or ectopic eruption of mandibular teeth, (2) presence of periapical radiolucency, periodontal pathology or any other pathology, (3) partial or unclear images.
Imaging protocol
All the scans were taken on Galileo's comfort (Sirona Dental Systems GmbH, Bensheim, Germany) in high definition mode and with metal artifact reduction software. The scan parameters were 85KvP, 21 mAs, and 14 s scan time with FOV of 15 cm × 15 cm × 15 cm and voxel size 0.3 mm3.
Virtual implant placement
The DICOM files were imported in implant planning software (BlueSky bio, blue sky plan. com, IL, United States). The virtual implant was placed in mandibular first molar aligning mesio distally along the long axis of the crown of mandibular first molar and at 2 mm from the adjacent tooth roots. Apico coronally the coronal platform of the implant was at the crestal level. Bucco lingually, the implant was aligned along the imaginary line passing through the central fossae of the adjacent teeth. The mesiodistal and buccolingual angulation of the implant depended on the long axis of the opposing maxillary tooth and the curvature of mandibular occlusal plane. The functional cusp of the opposing teeth was in the center of the implant. Subsequent implants were superimposed on the first one by utilizing the duplicate tool and then changing the dimensions hence maintaining the placement protocol [Figure 1a and b].
Figure 1.
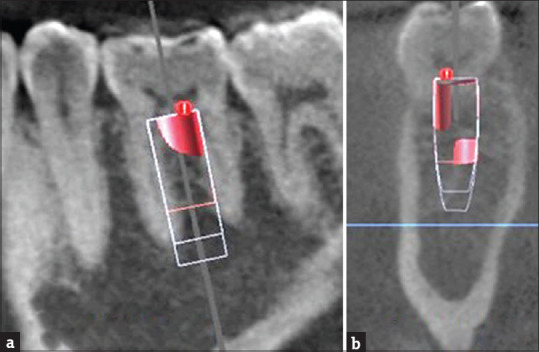
Cone-beam computed tomography images for virtual implant placement protocol in mandibular first molar tooth with various dimensions (G1 to G9) as per surgical guidelines. (a) Sagittal section. (b) Cross section
Different combinations of implant width and height were selected and accordingly groups were made [Table 1].
Table 1.
Different groups with the implant diameter and height
| Groups (G) | Implant diameter coronal (D) | Implant diameter apical | Implant height (L) |
|---|---|---|---|
| 1 | 4.7 | 4.5 | 8.5 |
| 2 | 4.7 | 4.5 | 11.5 |
| 3 | 4.7 | 4.5 | 13.5 |
| 4 | 5.8 | 5.5 | 8.5 |
| 5 | 5.8 | 5.5 | 11.5 |
| 6 | 5.8 | 5.5 | 13.5 |
| 7 | 7 | 6.7 | 8.5 |
| 8 | 7 | 6.7 | 11.5 |
| 9 | 7 | 6.7 | 13.5 |
Measurements
The DICOM file was opened in the software and the image of mandibular first molar aligned in all the three sections. Interradicular and apical bone measurements were done in the sagittal and cross section views for all groups of implants [Table 2 and Figures 2a, b and 3a, b].
Table 2.
Measured parameters of mandibular 1st molar (1 M) sites
| Parameters measured | Acronyms |
|---|---|
| Peri-implant gap defect measurements | |
| Distance from buccal surface of implant to buccal cortical socket bone (buccal peri-implant gap defect at crest) | BGDh |
| Distance from lingual surface of implant to lingual cortical socket bone (lingual peri-implant gap defect at crest) | LGDh |
| Distance from mesial surface of implant to interdental socket bone mesially at crest (mesial peri-implant gap defect at crest) | MGDh |
| Distance from distal surface of implant to interdental socket bone distally at crest (distal peri-implant gap defect at crest) | DGDh |
| Distance between the crest/shoulder of the implant to the interradicular bone (vertical peri-implant gap defect length) | L1 |
| Peri-implant interradicular bone support measurements | |
| Length from the top/shoulder of implant to the point where buccal interradicular bone support starts (buccal interradicular bone support [percentile]) | IRBb (%) |
| Length from the top/shoulder of implant to the point where Lingual interradicular bone support starts (lingual interradicular bone support [percentile]) | IRBl (%) |
| Length from the top/shoulder of implant to the point where mesial interradicular bone support starts (mesial interradicular bone support [percentile]) | IRBm (%) |
| Length from the top/shoulder of implant to the point where distal interradicular bone support starts (distal interradicular bone support [percentile]) | IRBd (%) |
| Apical bone support measurements | |
| Distance from the tip of the implant to the tangent from apex of tooth | AP-TIP |
Figure 2.
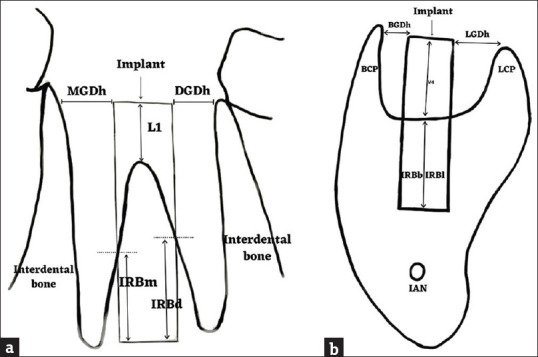
Schematic picture showing gap defects and interradicular bone support around immediate mandibular first molar implants. (a) MGDh: Mesial horizontal gap defect, DGDh: Distal Horizontal, gap defect L1: Vertical gap defect (mesial, distal), IRBm: Mesial interradicular bone support, IRBd: Distal interradicular bone support (b) BGDh: Buccal horizontal gap defect, LGDh: Lingual horizontal, gap defect V4: Vertical gap defect (4 walls – mesial, distal, buccal, and lingual), IRBb: Buccal interradicular bone support, IRBl: Lingual interradicular bone support, BCP: Buccal cortical plate, LCP: Lingual cortical plate
Figure 3.
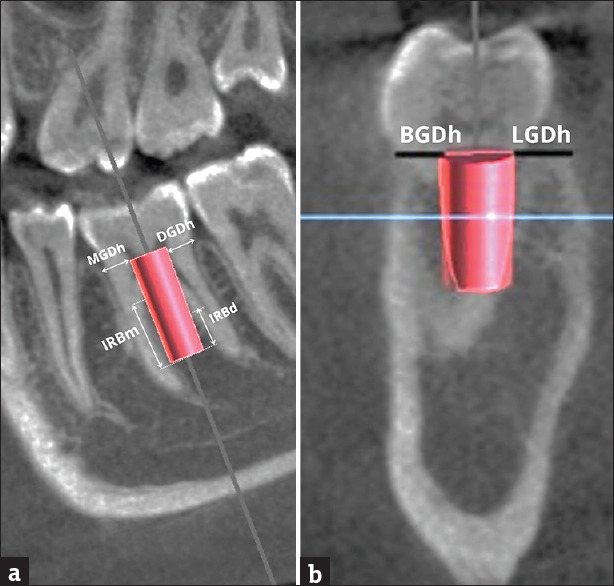
Showing virtual implant with coronal gap defects around implant and Interradicular bone support. (a) Sagittal section. (b) Cross sectio
The measurement of the integrated routing and bridging (IRB) was converted into percentile by using the percentile function in excel.
The qualified observers, for bone measurements, were blinded to the aim of the study. There was a calibration session before the initiation of image evaluation and a training session. In disagreement, the cases were discussed until consensus was reached. For inter- and intra-observer reliability, 25% (10 CBCT images) of the sample were randomly selected and analyzed again by 2 observers using Intraclass Correlation Coefficient.
Statistical analysis
All the parameter data were entered into SPSS v. 11.5 software (SPSS Inc., Chicago, IL, USA) for descriptive statistics for mean and standard deviation analysis. One-way analysis of variance followed by Post hoc Tukey test was done to comparative analysis between the groups and the P < 0.05 were considered statistically significant.
RESULTS
Variation in implant diameter and associated peri-implant bone defect (horizontal and vertical) measurements
The measurements [Table 3] of study groups G1G2G3 (Diameter [D]-4.7 mm) showed buccal peri-implant gap defect of 2.35 mm ± 0.483 mm, G4G5G6 (D-5.8 mm) was 2.00 mm ± 0.453 mm, and G7G8G9 (D-7 mm) was 1.30 mm ± 0.56 mm. This was due to the variation in the implant diameter. Similarly, the peri-implant defects on the lingual side for G1G2G3 (D-4.7 mm) was 2.10 mm ± 0.44 mm, for G4G5G6 (D-5.8 mm) was 1.70 mm ± 0.46 mm, and for G7G8G9 (D-7 mm) was 1.30 mm ± 0.56 mm. Mesial peri-implant defect for 4.7 mm diameter implants (G1G2G3) was 2.30 mm ± 0.64 mm, for 5.8 mm (G4G5G6) was 1.50 mm ± 0.59 mm, and for 7 mm (G7G8G9) was 1.20 mm ± 0.51 mm.
Table 3.
Bone support and gap defect measurements (horizontal and vertical) for the virtual peri-implant parameters, mean with standard deviation values (in mm):
| Groups | n | Mean±SD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| BGDh (buccal gap defect) | LGDh (lingual gap defect) | MGDh (mesial gap defect) | DGDh (distal gap defect) | L1 (vertical gap defect) | ||
| G 1 | 40 | 2.35±0.48 | 2.10±0.44 | 2.30±0.65 | 2.25±0.43 | 2.80±0.82 |
| G 2 | 40 | 2.35±0.48 | 2.10±0.44 | 2.30±0.65 | 2.25±0.44 | 2.80±0.82 |
| G 3 | 40 | 2.35±0.48 | 2.10±0.44 | 2.25±0.63 | 2.25±0.44 | 2.80±0.82 |
| G 4 | 40 | 2.00±0.48 | 1.70±0.46 | 1.45±0.60 | 1.55±0.50 | 2.80±0.82 |
| G 5 | 40 | 2.00±0.45 | 1.70±0.46 | 1.50±0.60 | 1.55±0.50 | 2.80±0.82 |
| G 6 | 40 | 2.00±0.45 | 1.70±0.46 | 1.50±0.59 | 1.55±0.504 | 2.80±0.82 |
| G 7 | 40 | 1.30±0.56 | 1.30±0.56 | 1.20±0.52 | 1.05±0.59 | 2.80±0.82 |
| G 8 | 40 | 1.35±0.58 | 1.30±0.56 | 1.20±0.52 | 1.05±0.59 | 2.80±0.82 |
| G 9 | 40 | 1.30±0.56 | 1.30±0.56 | 1.20±0.51 | 1.05±0.597 | 2.80±0.82 |
N: Number of cases, SD: Standard deviation
Distal peri-implant gap defect for 4.7 mm diameter implants (G1G2G3) was 2.25 mm ± 0.43 mm, for 5.8 mm (G4G5G6) was 1.55 mm ± 0.50 mm, and for 7 mm (G7G8G9) was 1.05 mm ± 0.59 mm. There was statistically significant difference (P ≥ 0.05) between the ultra-wide (G7G8G9) and the other groups for buccal, lingual, mesial, and distal gap defect measurements.
Vertical component of implant gap defect at the coronal part of the socket (L1-shoulder to inter-radicular septa) was 2.80 mm ± 0.83 mm for all groups in vertical direction. There was no statistical significance between these study groups.
Variation in Implant length and associated peri-implant interradicular bone support measurements
The measurements [Table 4] of length variation in implant study groups showed percentage wise increase of buccal bone-support (73.25% ± 11.11%, 80.30% ± 8.34%, and 83.35% ± 7.04%) for L-8.5 mm (G1G4G7), L-11.5 mm (G2G5G8), and L-13.5 mm (G3G6G9) groups respectively.
Table 4.
Measurements of peri-implant vertical interradicular bone support measurements (mean with standard deviation values):
| Mean±SD | ||||
|---|---|---|---|---|
|
| ||||
| IRBb % (buccal bone support) | IRBl % (lingual bone support) | IRBm % (mesial bone support) | IRBd % (distal bone support) | AP-TIP (apical bone support) |
| 73.25±11.11 | 73.35±10.32 | 1.85±6.58 | 7.80±13.86 | −4.25±1.62 |
| 80.30±8.346 | 80.25±7.63 | 6.13±13.96 | 16.53±20.88 | −1.10±1.56 |
| 83.30±7.046 | 83.20±6.32 | 16.35±16.03 | 28.28±26.88 | 1.45±1.37 |
| 73.30±11.08 | 73.55±10.53 | 0.35±0.97 | 3.55±7.61 | −3.90±2.30 |
| 80.30±8.419 | 80.35±7.74 | 2.95±8.37 | 9.55±16.46 | −1.00±1.43 |
| 83.35±6.893 | 83.15±6.51 | 10.05±12.01 | 22.83±24.89 | 1.95±2.23 |
| 73.50±10.83 | 73.50±10.33 | 0.15±0.62 | 0.40±1.29 | −4.00±2.33 |
| 80.45±8.12 | 80.45±7.46 | 3.70±7.89 | 8.80±12.26 | −0.90±1.35 |
| 83.35±6.87 | 83.25±6.24 | 9.30±9.53 | 16.70±13.62 | 1.35±1.67 |
Minus sign in the measurement indicates implant tip short from tip of the socket
Similarly, the lingual bone support was also increased with increase in implant length (73.35% ± 10.32%, 80.25% ± 7.63%, and 83.20% ± 6.32%) for L-8.5 mm (G1G4G7), L-11.5 mm (G2G5G8), and L-13.5 mm (G3G6G9), respectively. Significant Statistical difference (P ≥ 0.05) was seen between L-8.5 mm (G1G4G7) and L-13.5 mm (G3G6G9) for both buccal and lingual bone support.
The mesial inter-radicular bone support varied with change in diameter and length of the implant. The percentage of bone support showed least for G4G7 (0.35% ± 0.97%, 0.15% ± 0.62%), moderate for G1G2G5G8 (1.85% ± 6.58%, 6.13% ± 13.96%, 2.95% ± 8.37%, 3.70% ± 7.89%) and maximum for G3G6G9 (16.35% ± 16.03%, 10.05% ± 12.01%, 9.30 ± 9.53), respectively. Similarly, the distal inter-radicular bone support also varied with change in diameter and length of the implant with percentage of bone support least for G4G7, (3.55% ± 7.61%, 0.40% ± 1.29%), moderate for G1G5G8 (7.80% ± 13.86%, 9.55% ± 16.46%, 8.80% ± 12.26%), and maximum for G2G3G6G9 (16.53% ± 20.88%, 28.28% ± 26.88%, 22.83% ± 24.89%, 16.70% ± 13.62%), respectively.
Significant difference was seen with increase in diameter and length of the implant for mesial and distal bone support.
Measurements' from the tip end of the implant to the socket tip of root for apical bone support
G1G4G7 Short length implant groups, (L-8.5 mm) was 3.5–4 mm within the socket tip indicating no apical bone support. G2G5G8 implant groups (L-11.5 mm) was 0.9–1 mm within socket tip indicating implant tip just engaging the apical bone support. G3G6G9 implant groups (L-13.5 mm) was 1.35–1.95 mm beyond the socket tip indicating good apical bone support.
DISCUSSION
The present study carried out to analyze the alteration in diameter and length of implants for primary stability of IMI with respect to change in peri-implant gap defects and interradicular bone support. Some studies have concluded that length and diameter of implant do not alter the stability of implant if the implants are placed in high quality bone.[19,20]
This concept may be true for implants placed in healed socket or delayed placement. However, in cases of bone with low quality or implant insertion immediately after extraction (IMI) would be a debatable issue since the bone quantity and quality is not optimum.
Peri-implant gap defect
The present study reports showed a significant reduction in peri-implant gap defect in buccal, lingual, mesial and distal surfaces in ultra-wide (7 mm) group as compared with regular diameter (D-4.8 mm) groups. Our results suggest that wider the implants placed in IMI sockets, lesser the peri-implant gap defect (1.05–1.20 mm) and hence, bone augmentation is not always needed in wider or ultra-wide IMI. These findings are similar with other reports, where if the gap defect is <2 mm, natural bone healing can be predicted without the necessity for augmentation techniques.[21,22] However, if the gap defect is more, then Guided bone or soft-tissue augmentation are indicated.[23,24] In conclusion if there is sufficient or larger molar sockets, then larger diameter or wider molar implants are to be preferred to get benefits of better primary stability and faster healing without grafting interventions.
In our study, the vertical component of gap defect (L1) from crest to furcation was 2.80 ± 0.83 mm in all groups on all four peri-implant surfaces. The percentile defect varied depending on the length of the implant and as well the width of the interradicular bone, that is, longer the implants chosen, the vertical gap defect percentile decreased and hence more stability for implants.[25]
Inter-radicular bone support
Our study showed higher inter-radicular bone support for longer (13.5 mm) implant groups (G3, G6, and G9) as compared to shorter (8.5 mm) implant groups (G1, G4, and G7). For both surfaces (buccal, lingual), the inter-radicular bone support was identical (80%–83%) in all groups and did not vary with the alteration in diameter of the implant. Whereas for remaining two surfaces (mesial, lingual), the bone support varied significantly with alteration in diameter and length of the implants [Table 4]. Shorter and wider implant groups (L-8.5 mm and D-5.7 mm) showed least inter-radicular bone support, longer and wider implants (L-13.5 mm and D-7 mm) showed moderate IRB support, whereas, longer and regular width implants (L-13.5 mm and D-4.7 mm) showed maximum IRB support. Reports suggests when the diameter and location are kept constant, the longer implants showed much higher success rate as compared to shorter.[26] If IRB in fresh extraction is intact with <2 mm gap defect in the socket, then the stability of implant will be favorable and the required healing period will be shorter (12–13 weeks) without any need for regenerative interventions.[27] Ostman et al. reported the use of 10–13 mm length implants resulted in satisfactory primary stability as compared to 15–18 mm length. They suggested, higher the length, more heat generated during drilling and hence low primary stability.[28] Whereas, other studies reported increased rate of failure for ultra-wide (D-6-9 mm) implants and suggested a minimum bone thickness of 1.8 mm will provide good success rate.[29,30] As well, Bilhan et al. reported no significance difference in primary stability of implant for variation in implant diameter.[31]
These results suggest implants that are longer (11–13.5) and wider (5–7 mm) are more suitable for IMI. Ultra-wide (7–9 mm) implants may be preferred in selective cases of either very wide inter-radicular septum or in cases of compromised vertical height of bone to get higher primary stability.
Apical bone support
The apical bone support refers to the IRB and Interdental bone around implant, that is normally available if implant in beyond the apex and all four surfaces of implants are in contact with the alveolar bone.
In our study, reports showed tip of shorter (8.5 mm) implants (G1, G4, G7) were within the mesial and distal root socket tip, thus indicating only buccal and lingual wall was providing primary stability for these implants. The 11.5 mm length implants (G2, G5, G8), tip of the implant was either until the socket tip or 0.9–1 mm of apical bone support was available for implants. Whereas the longer (13.5 mm) implants (G3, G6, G9) were 1.35 mm to 1.95 mm apical to socket tip suggesting these implants had good apical bone or, four wall bone support in providing primary stability for IMI as compared with the other length groups. Our results are similar to the findings of other studies where implants with 40% shorter length decreased the implant anchorage by 50%[32] and as well the implant should be placed into a minimum of 3 mm of solid bone apical to the extraction site to gain good apical bone primary stability.[33,34] Many reported short implants failed as compared to longer, as they provide small surface area and decreased crown-to-implant ratio.[35,36] Increasing the implant length will help in increasing the stability due to increase in area of osseointegration and a decrease in stress at the crestal bone with minimum crestal bone resorption. In case of poor bone quality, longer implants impart better stability.[37,38]
CONCLUSION
Variations in length and diameter of implants for placement immediately after extraction plays a crucial role as it varies the gap defects around the coronal part of implants and IRB support at the apical region, which, may influence the primary stability and longevity of the implant. In the present study all the groups showed good interradicular bone support on buccal and lingual surfaces. Regular width implants with longer length showed satisfactory interradicular bone support on mesial and distal surfaces. Wider the implants there was no interradicular bone support in the proximal surfaces and hence less chances for additional primary stability for IMI. Longer the implants better the vertical bone support. Longer implants showed good apical bone support in all the four surfaces and hence good apical primary stability expected. Further long-term randomized control trials should be carried out to accept our findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Zhou Y, Jiang T, Qian M, Zhang X, Wang J, Shi B, et al. Roles of bone scintigraphy and resonance frequency analysis in evaluating osseointegration of endosseous implant. Biomaterials. 2008;29:461–74. doi: 10.1016/j.biomaterials.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Meijer HJ, Raghoebar GM. Immediate implant placement in molar extraction sites: A 1-year prospective case series pilot study. Int J Implant Dent. 2020;6:3. doi: 10.1186/s40729-019-0201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kong L, Sun Y, Hu K, Li D, Hou R, Yang J, et al. Bivariate evaluation of cylinder implant diameter and length: A three-dimensional finite element analysis. J Prosthodont. 2008;17:286–93. doi: 10.1111/j.1532-849X.2007.00286.x. [DOI] [PubMed] [Google Scholar]
- 4.Mesa F, Muñoz R, Noguerol B, de Dios Luna J, Galindo P, O'Valle F. Multivariate study of factors influencing primary dental implant stability. Clin Oral Implants Res. 2008;19:196–200. doi: 10.1111/j.1600-0501.2007.01450.x. [DOI] [PubMed] [Google Scholar]
- 5.Crespi R, Capparè P, Gherlone E. Fresh-socket implants in periapical infected sites in humans. J Periodontol. 2010;81:378–83. doi: 10.1902/jop.2009.090505. [DOI] [PubMed] [Google Scholar]
- 6.Fugazzotto PA. Implant placement at the time of maxillary molar extraction: Treatment protocols and report of results. J Periodontol. 2008;79:216–23. doi: 10.1902/jop.2008.070338. [DOI] [PubMed] [Google Scholar]
- 7.Martinez H, Davarpanah M, Missika P, Celletti R, Lazzara R. Optimal implant stabilization in low density bone. Clin Oral Implants Res. 2001;12:423–32. doi: 10.1034/j.1600-0501.2001.120501.x. [DOI] [PubMed] [Google Scholar]
- 8.Sayed AJ, Shaikh SS, Shaikh SY, Hussain MA. Inter radicular bone dimensions in primary stability of immediate molar implants – A cone beam computed tomography retrospective analysis. Saudi Dent J; 2021;33:71. doi: 10.1016/j.sdentj.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Angelis N, Sorrenti E, Modena C, Benedicenti S. Evaluation of primary stability of single implants placed in fresh extraction sockets: A clinical trial. Biotechnol Biotechnol Equip. 2016;30:354–9. [Google Scholar]
- 10.Ferrus J, Cecchinato D, Pjetursson EB, Lang NP, Sanz M, Lindhe J. Factors influencing ridge alterations following immediate implant placement into extraction sockets. Clin Oral Implants Res. 2010;21:22–9. doi: 10.1111/j.1600-0501.2009.01825.x. [DOI] [PubMed] [Google Scholar]
- 11.Esposito M, Grusovin MG, Polyzos IP, Felice P, Worthington HV. Timing of implant placement after tooth extraction: Immediate, immediate-delayed or delayed implants? A Cochrane systematic review. Eur J Oral Implantol. 2010;3:189–205. [PubMed] [Google Scholar]
- 12.Ding X, Liao SH, Zhu XH, Zhang XH, Zhang L. Effect of diameter and length on stress distribution of the alveolar crest around immediate loading implants. Clin Implant Dent Relat Res. 2009;11:279–87. doi: 10.1111/j.1708-8208.2008.00124.x. [DOI] [PubMed] [Google Scholar]
- 13.Balleri P, Cozzolino A, Ghelli L, Momicchioli G, Varriale A. Stability measurements of osseointegrated implants using Osstell in partially edentulous jaws after 1 year of loading: A pilot study. Clin Implant Dent Relat Res. 2002;4:128–32. doi: 10.1111/j.1708-8208.2002.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Frias V, Lee KW, Wright RF. Effect of implant size and shape on implant success rates: A literature review. J Prosthet Dent. 2005;94:377–81. doi: 10.1016/j.prosdent.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Barone A, Toti P, Marconcini S, Derchi G, Saverio M, Covani U. Esthetic Outcome of Implants Placed in Fresh Extraction Sockets by Clinicians with or without Experience: A Medium-Term Retrospective Evaluation. Int J Oral Maxillofac Implants. 2016;31:1397–406. doi: 10.11607/jomi.4646. [DOI] [PubMed] [Google Scholar]
- 16.Bornstein MM, Horner K, Jacobs R. Use of cone beam computed tomography in implant dentistry: Current concepts, indications and limitations for clinical practice and research. Periodontol. 2000;2017(73):51–72. doi: 10.1111/prd.12161. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh SY, Shaikh SS. Direct linear measurement of root dentin thickness and dentin volume changes with post space preparation: A cone-beam computed tomography study. Contemp Clin Dent. 2018;9:77–82. doi: 10.4103/ccd.ccd_785_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang TY, Kuo PJ, Fu E, Kuo HY, Nie-Shiuh Chang N, Fu MW, et al. Risks of angled implant placement on posterior mandible buccal/lingual plated perforation: A virtual immediate implant placement study using CBCT. J Dent Sci. 2019;14:234–40. doi: 10.1016/j.jds.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barikani H, Rashtak S, Akbari S, Badri S, Daneshparvar N, Rokn A. The effect of implant length and diameter on the primary stability in different bone types. J Dent (Tehran) 2013;10:449–55. [PMC free article] [PubMed] [Google Scholar]
- 20.Bischof M, Nedir R, Szmukler-Moncler S, Bernard JP, Samson J. Implant stability measurement of delayed and immediately loaded implants during healing. Clin Oral Implants Res. 2004;15:529–39. doi: 10.1111/j.1600-0501.2004.01042.x. [DOI] [PubMed] [Google Scholar]
- 21.McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78:377–96. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]
- 22.Chen ST, Darby IB, Reynolds EC, Clement JG. Immediate implant placement postextraction without flap elevation. J Periodontol. 2009;80:163–72. doi: 10.1902/jop.2009.080243. [DOI] [PubMed] [Google Scholar]
- 23.Rominger JW, Triplett RG. The use of guided tissue regeneration to improve implant osseointegration. J Oral Maxillofac Surg. 1994;52:106–12. doi: 10.1016/0278-2391(94)90388-3. [DOI] [PubMed] [Google Scholar]
- 24.Schliephake H, Dard M, Planck H, Hierlemann H, Jakob A. Guided bone regeneration around endosseous implants using a resorbable membrane vs a PTFE membrane. Clin Oral Implants Res. 2000;11:230–41. doi: 10.1034/j.1600-0501.2000.011003230.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbach D. Gap management around immediate implants: A review of the literature and its application in clinical practice. Dentaltown. 2014 Sep;:44–9. [Google Scholar]
- 26.Miyamoto I, Tsuboi Y, Wada E, Suwa H, Iizuka T. Influence of cortical bone thickness and implant length on implant stability at the time of surgery – Clinical, prospective, biomechanical, and imaging study. Bone. 2005;37:776–80. doi: 10.1016/j.bone.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Zamad M, Zamad A, Daga A, Akhare P. Immediate placement of mandibular molar dental implants: Case reports. J Implant Adv Clin Dent. 2015;7:11–9. [Google Scholar]
- 28.Ostman PO, Hellman M, Wendelhag I, Sennerby L. Resonance frequency analysis measurements of implants at placement surgery. Int J Prosthodont. 2006;19:77–83. [PubMed] [Google Scholar]
- 29.Ormianer Z, Palti A, Demiralp B, Heller G, Lewinstein I, Khayat PG. Implant-supported first molar restorations: Correlation of finite element analysis with clinical outcomes. Int J Oral Maxillofac Implants. 2012;27:e1–12. [PubMed] [Google Scholar]
- 30.Mordenfeld MH, Johansson A, Hedin M, Billström C, Fyrberg KA. A retrospective clinical study of wide-diameter implants used in posterior edentulous areas. Int J Oral Maxillofac Implants. 2004;19:387–92. [PubMed] [Google Scholar]
- 31.Bilhan H, Geckili O, Mumcu E, Bozdag E, Sünbüloğlu E, Kutay O. Influence of surgical technique, implant shape and diameter on the primary stability in cancellous bone. J Oral Rehabil. 2010;37:900–7. doi: 10.1111/j.1365-2842.2010.02117.x. [DOI] [PubMed] [Google Scholar]
- 32.Ueno T, Yamada M, Hori N, Suzuki T, Ogawa T. Effect of ultraviolet photoactivation of titanium on osseointegration in a rat model. Int J Oral Maxillofac Implants. 2010;25:287–94. [PubMed] [Google Scholar]
- 33.Esposito M, Grusovin MG, Willings M, Coulthard P, Worthington HV. The effectiveness of immediate, early, and conventional loading of dental implants: A Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2007;22:893–904. [PubMed] [Google Scholar]
- 34.Werbitt MJ, Goldberg PV. The immediate implant: Bone preservation and bone regeneration. Int J Periodontics Restorative Dent. 1992;12:206–17. [PubMed] [Google Scholar]
- 35.Kotsovilis S, Fourmousis I, Karoussis IK, Bamia C. A systematic review and meta-analysis on the effect of implant length on the survival of rough-surface dental implants. J Periodontol. 2009;80:1700–18. doi: 10.1902/jop.2009.090107. [DOI] [PubMed] [Google Scholar]
- 36.Kong L, Gu Z, Li T, Wu J, Hu K, Liu Y, et al. Biomechanical optimization of implant diameter and length for immediate loading: A nonlinear finite element analysis. Int J Prosthodont. 2009;22:607–15. [PubMed] [Google Scholar]
- 37.Bataineh AB, Al-Dakes AM. The influence of length of implant on primary stability: An in vitro study using resonance frequency analysis. J Clin Exp Dent. 2017;9:e1–6. doi: 10.4317/jced.53302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva R, Villalón P, Cáceres F. Effect of macro-design in the primary stability of short and extra-short implants using resonance frequency analysis. An ex vivo study. J Oral Biol Craniofac Res. 2020;10:603–7. doi: 10.1016/j.jobcr.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


