Abstract
OBJECTIVE
We examined the magnitude of and trends in the burden of out-of-pocket (OOP) costs among Medicare beneficiaries age 65 years or older with diabetes overall, by income level, by race/ethnicity, and compared with beneficiaries without diabetes.
RESEARCH DESIGN AND METHODS
Using data from the 1999–2017 Medicare Current Beneficiary Survey, we estimated average annual per capita OOP costs and percentage of beneficiaries experiencing high OOP burden, defined as OOP costs >10% or >20% of household income. We used joinpoint regression to examine the trends and generalized linear model and logistic regression for comparisons between beneficiaries with and without diabetes. Cost and income estimates were adjusted to 2017 USD.
RESULTS
Total OOP costs were $3,609–$5,283, with significant increases until 2005 followed by a leveling off. The prevalence of high OOP burden was 57%–72% at the 10% income threshold and 29%–41% at the 20% threshold, with significant increasing trends until 2003 followed by decreases. Total OOP costs were the highest in the ≥75% income quartile, whereas prevalence of high OOP burden was highest in the <25% and 25–50% income quartiles. Non-Hispanic Whites had the highest OOP costs and prevalence of high OOP burden. Beneficiaries with diabetes had significantly higher OOP costs ($498, P < 0.01) and were more likely to have high OOP burden than those without diabetes (odds ratios 1.32 and 1.25 at >10% and >20% thresholds, respectively, P < 0.01).
CONCLUSIONS
Over the past two decades, Medicare beneficiaries age 65 years or older with diabetes have faced substantial OOP burden, with large income-related disparities.
Out-of-pocket (OOP) health care costs are the expenses for services that are paid by patients or their families—as opposed to those paid or reimbursed by insurance. High OOP costs can affect patients in several ways. They can cause financial hardship, such as depleted savings, delinquency on personal bills, increased debts, or higher risk of bankruptcy (1,2). They may also leave people with less money to spend on basic needs, such as food, clothing, heat, or housing (1,2). When the burden of OOP costs is too high, some patients may not be able to follow the treatment plan recommended by their health care provider. For example, they may have to forgo or delay care or stop or skip doses of prescription drugs, which can compromise health outcomes and may lead to increased use of emergency or institutional services (2-4). High OOP cost burden has also been linked to poor clinical outcomes, poor mental health, and poor health-related quality of life (4,5).
Diabetes is the fifth most common disease in older populations, affecting ~27% of adults aged 65 years or older (6). The prevalence of diabetes among older adults has increased over the past two decades and is projected to continue increasing through 2060 (7,8). In addition, older adults with diabetes face a higher risk of OOP cost burden than younger adults and people without diabetes, thus making them economically vulnerable (9,10). They are more likely to be economically insecure while at the same time needing more health care services to treat both diabetes and its complications than younger adults and people without diabetes (9,10). Annual per capita health care costs are twice as high for people with diabetes than for those without diabetes, largely because of diabetes-associated comorbidities (10). This combination of factors may leave some older adults with diabetes unable to pay medical and other bills or save money (9).
Although previous studies have examined OOP costs among younger adults with diabetes and among all Medicare beneficiaries, relatively few studies have estimated OOP health care costs among older adults with diabetes (11-16). Those that have done so provide estimates only for 1 year (11-13). No previous study has examined OOP cost burden for older adults with diabetes over time, by subpopulation group, or in comparison with the burden for those without diabetes.
The objectives of our study were threefold: 1) to estimate the magnitude of and trends in OOP cost burden in Medicare beneficiaries age 65 years or older with diabetes, overall and by type of service; 2) to examine whether there were disparities in OOP cost burden for Medicare beneficiaries with diabetes by income level and race/ethnicity; and 3) to examine differences in OOP cost burden between beneficiaries with and beneficiaries without diabetes.
RESEARCH DESIGN AND METHODS
Data Source and Study Population
Data were obtained from the Medicare Current Beneficiary Survey (MCBS) from 1999 to 2017, with exclusion of 2014 because no data were available for that year (17). MCBS is a continuous, in-person, longitudinal survey of a nationally representative sample of the Medicare population, including beneficiaries age 65 years or older and those age 64 years or younger with disabilities or end-stage renal disease, residing in the U.S. and its territories. MCBS includes comprehensive information on health care costs and use among beneficiaries and links Medicare claims to survey-reported events, if applicable. It also contains beneficiaries’ demographic and socioeconomic characteristics and information on health insurance coverage. For this analysis, we used the Cost and Use files for 1999–2013 and both the Survey File (formerly Access to Care) and Cost Supplement File (formerly Cost and Use) for 2015–2017.
Our study population was made up of Medicare beneficiaries age 65 years or older. We excluded those younger than 65 years of age because younger beneficiaries with disabilities or end-stage renal disease often have different health care needs and spending than the general population of Medicare beneficiaries age 65 years or older (18). We classified people with diabetes as those who answered “yes” to the survey question, “Has a doctor ever told you that you had any type of diabetes?” Characteristics of study population and sample sizes are described in Appendix 5.
Outcome Variables
We examined OOP cost burden using two measures. First, we analyzed average annual total per capita OOP costs for all health care services. We estimated these costs using the patients’ self-reported OOP costs on all health care services, including coinsurance, copayments, deductibles, and medical items; services not covered by insurance; and any portion of health insurance premiums paid OOP. To explore the drivers of changes in OOP costs over time, we estimated OOP spending by service category (prescription drugs, inpatient hospitalizations, outpatient hospital care, medical provider, and other services, including dental, facility, home health, and hospice) and annual insurance premiums paid OOP.
Second, we estimated the percentage of beneficiaries with high OOP cost burden relative to their income. Income included total income before taxes from all sources (e.g., pension, Social Security, and retirement benefits) for the survey participant (and spouse, if applicable) (19). For married persons, we divided the total reported income by a factor of 1.5 to adjust the income to a per capita level. We used two thresholds to define high OOP burden: that OOP spending on health care accounted for >10% of a person’s pretax income or >20% (14,20).
Subgroup Analyses by Income and Race/Ethnicity
We conducted two subgroup analyses to examine disparities in OOP cost burden by income level and by race/ethnicity. All subgroup analyses were controlled for age and the number of chronic conditions including hypertension, hyperlipidemia, cardiovascular disease, arthritis, cancer, Alzheimer disease, depression, and lung diseases such as emphysema, asthma, or chronic obstructive pulmonary disease. Income category was based on income quartile of the survey participant within each survey year: <25%, 25% to <50%, 50% to <75%, and ≥75%. Participants for race/ethnicity were categorized as follows: non-Hispanic Whites, non-Hispanic Blacks, and others. The last category (others) includes Hispanics, non-Hispanic Asians, American Indians, and other defined races.
Statistical Analysis
We estimated the annual total per capita OOP costs and percentage of those with high OOP burden as a weighted mean. We used the Balanced Repeated Replication method (the Fay’s method) of variance estimation to adjust both serial and intracluster correlations in the data, with replicate cross-sectional weights for each year. This adjustment was necessary to obtain nationally representative estimates for Medicare beneficiaries with diabetes and to account for the complex sampling design of MCBS (e.g., the rotating-panel and multistage-sampling design) (19).
For trend analysis, we used a joinpoint regression model to identify changes in the trends of OOP costs and prevalence of high OOP burden over time. The model started with 0 joinpoints and tested whether more joinpoints should be added to the model for a better model fit, with a maximum of three joinpoints (four-line segments). We also estimated the annual percentage change (APC), which characterizes the slope of a single segment, and average annual percentage change (AAPC), which is a summary measure of the trend over the entire study period. We considered the changes in trends to be statistically significant and reported APC or AAPC when the P value was <0.05.
To compare the OOP cost burden between beneficiaries with and without diabetes, we pooled the data from 2012 to 2017 to get a sufficient sample size. As the OOP cost was right skewed with only a few zero values (<0.3%), we used a generalized linear model with γ distribution and log link to estimate the differences in mean total OOP costs. We used logistic regression to estimate the differences in the percentage prevalence of beneficiaries with high OOP burden (>10% and >20% of pretax income) between the two groups. Odds ratios were calculated based on the estimated coefficients from the logistic regression model. All analyses were controlled for age, sex, education, income level, race, and number of other chronic conditions as listed above.
Joinpoint Trend Analysis Software (version 4.7.0.0; National Cancer Institute, Bethesda, MD) was used for trend analysis. All other analyses were conducted with SAS (version 9.4; SAS Institute, Cary, NC) and Stata (version 16.1; StataCorp, College Station, TX). All estimates of costs and income were converted to 2017 USD using the Consumer Price Index (21).
RESULTS
OOP Cost Burden Among Medicare Beneficiaries With Diabetes
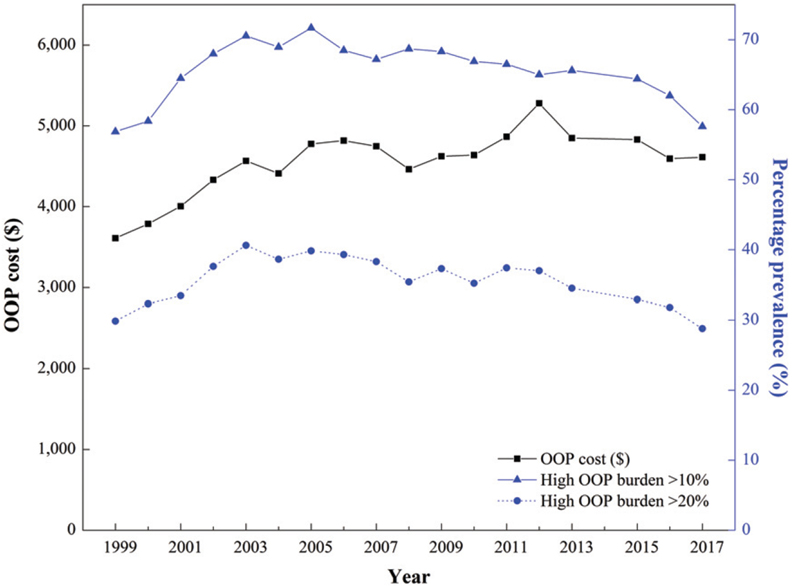
Figure 1 shows overall trends in total OOP costs and the prevalence of high OOP burden for beneficiaries age 65 years or older with diabetes from 1999 to 2017. Overall, mean annual per capita OOP costs increased by 28% from 1999 to 2017 ($3,609–$4,612) (Fig. 1 and Appendix 1), with a significant annual increase of 4.9% from 1999 to 2005 followed by a leveling off (Appendix 2).
Figure 1—
Mean annual total OOP costs (2017 USD) and prevalence of high OOP burden (>10% and >20% of pretax income) among Medicare beneficiaries with diabetes (≥65 years old), 1999 – 2017.
The percentage of beneficiaries with high OOP burden that exceeded 10% of their income ranged from 57 to 72% over the study period, increasing by 5.9% annually from 1999 to 2003, followed by a decrease of 1.0% per year through 2017 (Fig. 1 and Appendix 2). The annual percentage of beneficiaries with high OOP burden that exceeded 20% of their income ranged from 29 to 41%, with a trend similar to that of the 10% threshold. Despite significant changes within the study period, no significant overall trends were found from 1999 to 2017 in OOP costs or the prevalence of high OOP burden over the entire study period (P ≥0.05 for all AAPCs).
Insurance premiums accounted for the largest share of OOP costs, followed by prescription drugs, medical provider services, other services, outpatient hospital care, and inpatient hospitalization ($2,300, $928, $722, $137, and $49 in 2017, respectively) (Appendix 3). OOP spending on insurance premiums increased overall (AAPC 1.5%), with an average increase of 3.2% from 1999 to 2012. OOP spending for prescription drugs increased from 1999 to 2003, declined through 2008, and then increased again until 2015 (APC 8.0%, −5.0%, and 2.0%, respectively). OOP costs for medical provider services increased from 1999 to 2005 and then decreased until 2017 (APC 5.9% and −2.2%, respectively). No joinpoints were identified, but OOP expenditures for other services increased overall from 1999 to 2017 (AAPC 3.6%). OOP costs for outpatient hospital care did not change significantly over the study period, but OOP costs for inpatient hospitalization declined (AAPC −6.7%).
OOP Cost Burden by Income Group Among Beneficiaries With Diabetes
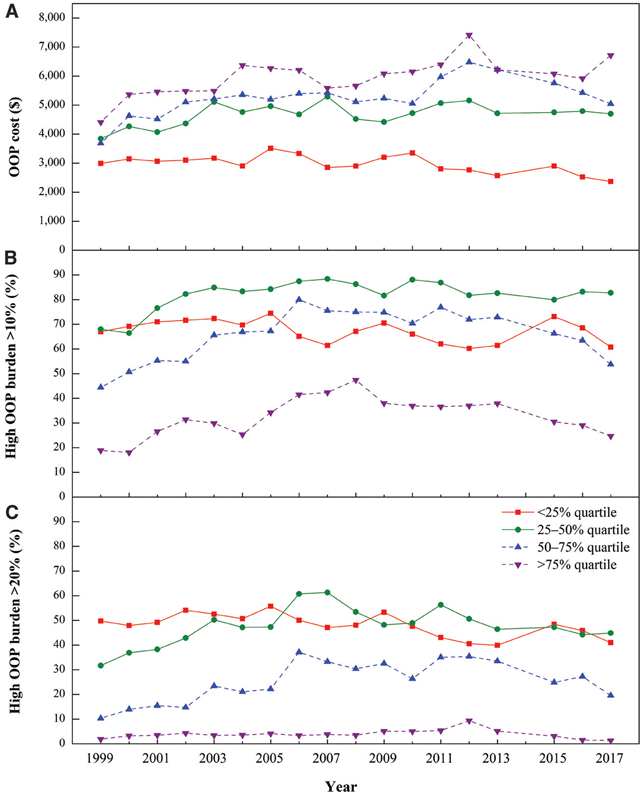
Figure 2 shows that mean annual per capita OOP costs increased with income quartile. For the ≥75% group, OOP costs increased significantly during the study period (AAPC 1.4%) (Appendix 2). The 50–75% group had no significant overall trends in OOP costs, but costs increased significantly from 1999 to 2002 and then decreased from 2013 to 2017 (APC 11.5% and −7.6%, respectively). From 1999 to 2017, OOP costs increased in the 25–50% group (AAPC 1.2%), while they decreased in the <25% group (AAPC −1.3%).
Figure 2—
Mean annual total OOP costs and prevalence of high OOP burden (>10% and >20% of pretax income) by income quartile among Medicare beneficiaries with diabetes (≥65 years old), 1999 – 2017. A: Mean annual total OOP costs (2017 USD) by income level. B: Percentage prevalence of beneficiaries with high OOP burden that exceeded 10% of their pretax income by income level. C: Percentage prevalence of beneficiaries with high OOP burden that exceeded 20% of their pretax income by income level.
Despite their lowest OOP costs, the low-income groups (<25% and 25–50% quartiles) had the highest prevalence of high OOP burden (Fig. 2). At the 10% threshold, >80% of beneficiaries in the 25–50% group had high OOP burden, while the prevalence was <50% in the ≥75% group. At the 20% threshold, the differences between the low-income and middle- and high-income groups became larger. The prevalence of high OOP burden was ~20 times higher on average in the lowest income quartile than it was in the highest quartile. At the 10% threshold, the prevalence of high OOP burden increased until the early or mid-2000s, with significant decreases thereafter (Appendix 2). At the 20% threshold, early increases were followed by significant decreases in the 25–50% and ≥75% groups, with declines starting in the early 2000s for lower-income groups and in 2012 for higher-income groups.
OOP Cost Burden by Race/Ethnicity Among Beneficiaries With Diabetes
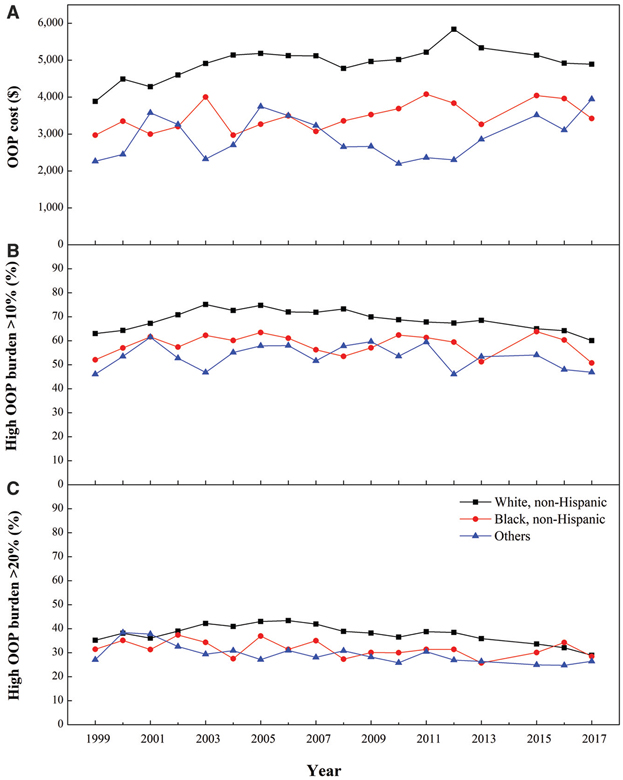
Non-Hispanic Whites had the highest mean annual per capita OOP costs among all groups (Fig. 3 and Appendix 2). Costs increased significantly from 2008 to 2012, followed by decreases since 2012 (APC 3.9% and −2.7%, respectively). An overall increasing trend in OOP costs was observed for non-Hispanic Blacks (AAPC 1.3%). For the others group, costs increased significantly from 2012 to 2017 (APC 9.7%).
Figure 3—
Mean annual total OOP costs and prevalence of high OOP burden (>10% and >20% of pretax income) by race/ethnicity among Medicare beneficiaries with diabetes (≥65 years old), 1999 – 2017. A: Mean annual total OOP costs (2017 USD) by race/ethnicity. B: Percentage prevalence of beneficiaries with high OOP burden that exceeded 10% of their pretax income by race/ethnicity. C: Percentage prevalence of beneficiaries with high OOP burden that exceeded 20% of their pretax income by race/ethnicity.
Similar trends were found in the prevalence of high OOP burden at the 10% threshold (Fig. 3 and Appendix 2). Non-Hispanic Whites had the highest prevalence of high OOP burden; the trend increased from 1999 to 2003 and decreased thereafter (APC 5.1% and −1.2%, respectively). No discernable trends were found for non-Hispanic Blacks and the Others group. At the 20% threshold, the prevalence of high OOP burden was also higher for non-Hispanic Whites, but the differences between groups were less pronounced in comparisons with the 10% threshold. For non-Hispanic Whites, the annual prevalence increased significantly from 1999 to 2006 (APC 2.3%) and decreased from 2012 to 2017 (APC −4.9%). An overall decreasing trend was found for the Others group (AAPC −1.6%).
OOP Cost Burden Between Beneficiaries With and Beneficiaries Without Diabetes
Table 1 shows the differences in OOP cost burden between beneficiaries with and beneficiaries without diabetes. Compared with those without diabetes, beneficiaries with diabetes had a significantly higher per capita total OOP cost (β = $498, P < 0.01) and were more likely to face high OOP burden at both thresholds (odds ratios 1.32 and 1.25 at the >10% and the >20% thresholds, respectively, P < 0.01).
Table 1—
Estimated excess OOP costs and high OOP cost burden rate for Medicare beneficiaries with diabetes (≥65 years old), 2012 – 2017
| Total OOP costs |
||||
|---|---|---|---|---|
| β (SE) | AME (SE) | High OOP burden, >10% of pretax income | High OOP burden, >20% of pretax income | |
| Diabetes | 0.10 (0.02)* | $498 ($82)* | 1.32 (1.21, 1.44)* | 1.25 (1.15, 1.35)* |
Data are odds ratio (95% CI) (with no diabetes as the referent) unless otherwise indicated. Adjustments include age, sex, education, income level, race, and number of chronic conditions. All costs are expressed in 2017 USD. AME, average marginal effects.
Significant at P < 0.01.
CONCLUSIONS
In this study, we analyzed 1999–2017 MCBS data to examine the magnitude of and trends in OOP health care cost burden among Medicare beneficiaries age 65 years or older with diabetes. We found that those with diabetes, on average, paid $4,612 OOP in 2017 for medical care: an increase of 28% from 1999. Additionally, more than one-half of the beneficiaries had OOP costs that exceeded 10% of their income, although this prevalence declined slightly after 2003. Beneficiaries with diabetes had significantly higher OOP cost burden than those without diabetes. We also found large disparities in OOP cost burden, with higher OOP costs among those with higher incomes and greater prevalence of high OOP burden among those with lower incomes. Non-Hispanic Whites had both higher OOP costs and prevalence of high OOP burden compared with non-Hispanic Blacks and other racial/ethnic minority groups. Our study is one of the most comprehensive to date describing the OOP cost burden among Medicare beneficiaries with diabetes.
The findings of high OOP spending amount and a high prevalence of the population spending >10% or >20% of their income on health care provide evidence that Medicare beneficiaries with diabetes experience a large financial burden in managing health care needs—significantly more so than beneficiaries without diabetes (15). This finding is not surprising, as people with diabetes need health care services and medication to treat not only diabetes but also the resulting complications and/or comorbidities (22). Older adults are also likely to have a higher risk and prevalence of chronic conditions, physical disabilities, mental illness, and other comorbidities, which may lead to higher OOP health care costs compared with those of younger adults (23). The higher OOP costs, coupled with lower average earnings for older adults (24), may account for a higher prevalence of high OOP burden compared with the prevalence for younger adults and people without diabetes. However, the difference in OOP costs between those with and without diabetes was much less among Medicare beneficiaries than among the younger population, which may be due to relatively low OOP costs in the younger adults without diabetes (25).
Per capita OOP health care costs in 2017 were significantly higher than in 1999, with increases mostly occurring between 1999 and 2005. These increases appear to be largely attributable to insurance premiums and prescription drugs, which are the largest share of total OOP costs for beneficiaries, consistent with previous literature (26-30). It has been reported that along with a considerable increase in average monthly premiums for private insurance over the past two decades, Medicare Advantage and Part D premiums have also increased, though modestly (26,27,30). The increased OOP spending on prescription drugs reported in this study may be due, in part, to inflation in the price of prescription drugs, with higher-priced specialty drugs more widely available (28). From 2013 to 2015, prescription drug prices increased annually by 10%, six times the rate of general inflation (29). An earlier study found that increased insulin prices led to an OOP cost burden for Medicare beneficiaries with diabetes, even after closure of the coverage gap in Part D benefits under the Affordable Care Act (31).
Along with significant increases in OOP costs from 1999 to 2005, similar trends were observed in the percentage prevalence of older adults with diabetes who had high OOP burden from 1999 to 2003. Based on our findings, since 2003 the prevalence of high OOP burden decreased significantly. Reductions in the prevalence of high OOP burden after 2003 may be partially explained by increases in average income over time (32). Several policies may also have contributed to these decreasing trends. For example, the implementation of Medicare Part D in 2006, which provided Medicare beneficiaries with access to outpatient prescription drug coverage, could decrease OOP costs for prescription drugs (33). Under the Affordable Care Act passed in 2010, the Medicare Part D coverage gap has been gradually closing (34), which has been shown to reduce OOP costs for beneficiaries (35). Analyses by income quartile suggest income-related disparities in OOP cost burden among Medicare beneficiaries with diabetes over the past two decades. Compared with middle- and high-income beneficiaries, poor- and low-income beneficiaries (i.e., those in the <25% and 25–50% quartiles) were more likely to face high OOP burden as measured by the 20% of income threshold. Meanwhile, high-income beneficiaries (≥75% income quartile) had the highest mean per capita OOP costs but the lowest prevalence of high OOP burden. The disproportionate prevalence of high OOP burden between low- and high-income groups was more apparent at the 20% threshold of high OOP burden than the 10% threshold, indicating that poor and low-income beneficiaries are more likely to be burdened with extremely high OOP costs relative to income. Another notable finding is that, from 1999 to 2017, an overall decreasing trend was found in OOP costs or the prevalence of high OOP burden exceeding 20% of income in the <25% income group. This finding could be partially explained by the existence of programs for people with limited incomes, such as Medicaid, Medicare Savings Program, and Extra Help (i.e., Part D Low-Income Subsidy), that provide subsidies to help pay medical costs, premiums, deductibles, coinsurance, copayments, or prescription drug costs (36). Therefore, the <25% income group may include a substantial proportion of beneficiaries enrolled in Medicare subsidy programs, causing their OOP cost burden to continue to decrease over time. We also found that total OOP costs decreased in the 50–75% income group from 2013 to 2017. The Medicare Part D coverage gap reform lowers beneficiaries’ coinsurance rate in the coverage gap in the Part D plan, which may lower the OOP cost associated with prescription drugs. The decrease in OOP prescription drug costs may partially explain the decreases in total OOP costs during this period.
Compared with non-Hispanic Whites, racial/ethnic minority groups consistently had significantly lower OOP costs and a lower prevalence of high OOP burden. In observing similar results, other studies have suggested that the difference is driven by lower total health care use among minority groups (22,37,38), which may indicate problems in accessing health care services. Several socioeconomic factors among racial/ethnic minority groups, such as having less education, having low health literacy, or having low income (or a combination of these factors), may contribute to disparities in health care use or access (37,38). Other possible contributing factors might be cultural differences that influence health-related behaviors or acceptance and adoption of health education messages (39). Future research to identify the reasons for disparities in OOP cost burden could help inform efforts to better understand and increase use of health care for non-Hispanic Blacks and other racial/ethnic minority groups (37-39), given the higher prevalence of diabetes among this group (6).
This study is subject to several limitations. First, we used pretax income to estimate the prevalence of high OOP cost burden, as posttax income information is not available in the MCBS data. Using pretax income is likely to result in underestimation of the proportion of beneficiaries experiencing the high OOP cost burden. The extent of the underestimation would depend on the amount of taxes paid by the beneficiary. The ranking of beneficiaries by income quartile would be unaffected by completely proportionate taxes. Progressive and regressive taxes may also affect the ranking of the proportion of individuals spending >10% or >20% of their income on OOP health care costs. Second, in the study sample all beneficiaries were included except those younger than age <65 years with end-stage renal disease or disabilities and whether beneficiaries participated in Medicare subsidy programs such as the Part D Low-Income Subsidy was not examined. As such, from this study we were not able to provide data on policy changes that may have affected the target population. Third, as we aimed to estimate overall average OOP cost burden, we did not examine confounders, such as socioeconomic factors, to determine their effect on OOP costs and prevalence of high OOP burden. However, we controlled for age and chronic conditions to better estimate the gaps in OOP cost burden by income group and race/ethnicity. Fourth, as with other studies using survey data, in our study we relied in part on self-reported responses and our results are potentially subject to recall or social desirability biases. However, such biases should be small for survey-reported OOP costs with the MCBS, as they were validated by the available administrative records and billing and claims-level data (19). Lastly, this study assessed all-cause OOP health care costs for beneficiaries with diabetes and did not estimate diabetes-attributable costs.
In conclusion, Medicare beneficiaries age 65 years or older with diabetes have faced substantial OOP cost burden over the past two decades. Additionally, income- and race/ethnicity-related disparities were apparent in this burden. Although the burden leveled off and decreased slightly in the later years of the study period, older adults with diabetes may benefit from additional support because of their high risk of comorbidities, complications, and financial constraints (6,23,40). Our findings provide evidence that low-income Medicare beneficiaries with diabetes have higher OOP burden than other income groups, while racial and ethnic minorities do not. These findings may be useful to inform future research addressing the contributing factors of high OOP cost burden, as well as disparities related to income level and race/ethnicity.
Supplementary Material
Acknowledgments
The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
This article contains supplementary material online at https://doi.org/10.2337/figshare.14611329.
References
- 1.Jacoby MB, Holman M. Managing medical bills on the brink of bankruptcy. Yale J Health Policy Law Ethics 2010;10:239–289, 291–297 [PubMed] [Google Scholar]
- 2.Caraballo C, Valero-Elizondo J, Khera R, et al. Burden and consequences of financial hardship from medical bills among nonelderly adults with diabetes mellitus in the united states. Circ Cardiovasc Qual Outcomes 2020;13:e006139. [DOI] [PubMed] [Google Scholar]
- 3.Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care 2004;27:384–391 [DOI] [PubMed] [Google Scholar]
- 4.Heisler M, Choi H, Rosen AB, et al. Hospitalizations and deaths among adults with cardiovascular disease who underuse medications because of cost: a longitudinal analysis. Med Care 2010;48:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morris JL, Chasens ER. Financial difficulty: a barrier to self-care in patients with diabetes. Diabetes Educ 2017;43:247–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Diabetes Statistics Report. Atlanta, GA, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services, 2020 [Google Scholar]
- 7.Andes LJ, Li Y, Srinivasan M, Benoit SR, Gregg E, Rolka DB. Diabetes prevalence and incidence among Medicare beneficiaries – United states, 2001–2015. MMWR Mortal Wkly Rep 2019;68:961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J, Thompson TJ, Cheng YJ, et al. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr 2018;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Council on Aging. Economic Security, 2016. Accessed 18 June 2020. Available from https://oldprod.ncoa.org/wp-content/uploads/NCOA-Economic-Security.pdf
- 10.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong JH. Out-of-pocket health spending among Medicare beneficiaries: which chronic diseases are most costly? PLoS One 2019;14:e0222539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noel-Miller C. Medicare Beneficiaries’ Out-of-pocket Spending for Health Care. Washington, DC, AARP Public Policy Institute, 2017 [Google Scholar]
- 13.Hasche J, Ward C, Schluterman N. Diabetes Occurrence, Costs, and Access to Care Among Medicare Beneficiaries Aged 65 Years and Over. Baltimore, MD, Centers for Medicare & Medicaid Services, 2017 [Google Scholar]
- 14.Li R, Barker LE, Shrestha S, et al. Changes over time in high out-of-pocket health care burden in U.S. adults with diabetes, 2001–2011. Diabetes Care 2014;37:1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley GF. Trends in out-of-pocket healthcare costs among older community-dwelling Medicare beneficiaries. Am J Manag Care 2008;14:692–696 [PubMed] [Google Scholar]
- 16.Cunningham P, Carrier E. Trends in the financial burden of medical care for nonelderly adults with diabetes, 2001 to 2009. Am J Manag Care 2014;20:135–142 [PubMed] [Google Scholar]
- 17.Centers for Medicare & Medicaid Services. Limited Data Set (LDS) Files: Medicare Current Beneficiary Survey (MCBS), 2019. Accessed 27 December 2019. Available from https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/MCBS
- 18.Haile E, Bowen S, Lindenfelser K, Haffer C. Medicare Fee-For-Service Beneficiaries With Disabilities, by End Stage Renal Disease Status, 2014. Baltimore, MD, Centers for Medicare & Medicaid Services Office of Minority Health, 2017 [Google Scholar]
- 19.Centers for Medicare & Medicaid Services. 2015 MCBS Survey File, 2015. Accessed 29 June 2020. Available from https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/MCBS/Codebooks-Items/2015SurveyFile
- 20.Davidoff AJ, Erten M, Shaffer T, et al. Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer 2013;119:1257–1265 [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. Using Appropriate Price Indices for Analyses of Health Care Expenditures or Income Across Multiple Years, 2019. Accessed 12 February 2020. Available from https://meps.ahrq.gov/about_meps/Price_Index.shtml
- 22.Li R, Bilik D, Brown MB, et al. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care 2013;19:421–430 [PMC free article] [PubMed] [Google Scholar]
- 23.Shrivastava SR, Shrivastava PS, Ramasamy J. Health-care of elderly: determinants, needs and services. Int J Prev Med 2013;4:1224–1225 [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Department of Labor. Bureau of Labor Statistics. Usual Weekly Earnings of Wage and Salary Workers Fourth Quarter 2019, 2019. Accessed 18 June 2020. Available from https://www.bls.gov/news.release/archives/wkyeng_01172020.pdf
- 25.Health Care Cost Institute. Per capita health care spending on Diabetes: 2009-2013, 2015. Accessed 14 December 2020. Available from https://healthcostinstitute.org/images/easyblog_articles/112/HCCI-Diabetes-Issue-Brief-5-7-1_20200110-165647_1.pdf
- 26.Rae M, Copeland R, Cox C. Tracking the rise in premium contributions and cost-sharing for families with large employer coverage, 2019. Accessed 6 March 2020. Available from https://www.healthsystemtracker.org/brief/tracking-the-rise-in-premium-contributions-and-cost-sharing-for-families-with-large-employer-coverage/ [Google Scholar]
- 27.Cubanski J, Damico A, Neuman T. Medicare Part D in 2018: the latest on enrollment, premiums, and cost sharing, 2018. Accessed 6 March 2020. Available from https://www.kff.org/medicare/issue-brief/medicare-part-d-in-2018-the-latest-on-enrollment-premiums-and-cost-sharing/ [Google Scholar]
- 28.Medicare Payment Advisory Commission. Report to the Congress: Medicare Payment Policy, 2018. Accessed 25 February 2020. Available from https://www.medpac.gov/docs/default-source/reports/mar18_medpac_entirereport_sec.pdf
- 29.Hernandez I, Good CB, Cutler DM, Gellad WF, Parekh N, Shrank WH. The contribution of new product entry versus existing product inflation in the rising costs of drugs. Health Aff (Millwood) 2019;38:76–83 [DOI] [PubMed] [Google Scholar]
- 30.Kaiser Family Foundation. Medicare Advantage, 2019. Accessed 6 March 2020. Available from https://www.kff.org/medicare/fact-sheet/medicare-advantage/
- 31.Tseng CW, Masuda C, Chen R, Hartung DM. Impact of higher insulin prices on out-of-pocket costs in Medicare Part D. Diabetes Care 2020;43:e50–e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semega J, Kollar M, Shrider EA, Creamer JF. Income and Poverty in the United States. Washington, DC, U.S. Census Bureau, 2019 [Google Scholar]
- 33.Park YJ, Martin EG. Medicare Part D’s effects on drug utilization and out-of-pocket costs: a systematic review. Health Serv Res 2017;52:1685–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser Family Foundation. Summary of the affordable care act, 2013. Accessed 23 June 2020. Available from https://www.kff.org/health-reform/fact-sheet/summary-of-the-affordable-care-act/
- 35.Bonakdar Tehrani A, Cunningham PJ. Closing the Medicare doughnut hole: changes in prescription drug utilization and out-of-pocket spending among Medicare beneficiaries with Part D coverage after the Affordable Care Act. Med Care 2017;55:43–49 [DOI] [PubMed] [Google Scholar]
- 36.Get help paying costs. Accessed 26 February 2020. Available from https://www.medicare.gov/your-medicare-costs/get-help-paying-costs
- 37.Simmons M, Bishu KG, Williams JS, Walker RJ, Dawson AZ, Egede LE. Racial and ethnic differences in out-of-pocket expenses among adults with diabetes. J Natl Med Assoc 2019;111:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canedo JR, Miller ST, Schlundt D, Fadden MK, Sanderson M. Racial/ethnic disparities in diabetes quality of care: the role of healthcare access and socioeconomic status. J Racial Ethn Health Disparities 2018;5:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med 2006;21:667–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iglay K, Hannachi H, Joseph Howie P, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin 2016;32:1243–1252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.