Abstract
Background.
In 2013, New Delhi metallo-β-lactamase (NDM)–producing Escherichia coli, a type of carbapenem-resistant Enterobacteriaceae uncommon in the United States, was identified in a tertiary care hospital (hospital A) in northeastern Illinois. The outbreak was traced to a contaminated duodenoscope. Patient-sharing patterns can be described through social network analysis and ego networks, which could be used to identify hospitals most likely to accept patients from a hospital with an outbreak.
Methods.
Using Illinois’ hospital discharge data and the Illinois extensively drug-resistant organism (XDRO) registry, we constructed an ego network around hospital A. We identified which facilities NDM outbreak patients subsequently visited and whether the facilities reported NDM cases.
Results.
Of the 31 outbreak cases entered into the XDRO registry who visited hospital A, 19 (61%) were subsequently admitted to 13 other hospitals during the following 12 months. Of the 13 hospitals, the majority (n = 9; 69%) were in our defined ego network, and 5 of those 9 hospitals consequently reported at least 1 additional NDM case. Ego network facilities were more likely to identify cases compared to a geographically defined group of facilities (9/22 vs 10/66; P = .01); only 1 reported case fell outside of the ego network.
Conclusions.
The outbreak hospital’s ego network accurately predicted which hospitals the outbreak patients would visit. Many of these hospitals reported additional NDM cases. Prior knowledge of this ego network could have efficiently focused public health resources on these high-risk facilities.
Keywords: social network analysis, ego networks, HAI
Carbapenem-resistant Enterobacteriaceae (CRE) are extensively drug-resistant organisms (XDROs) generally found in patients with a history of healthcare exposure [1, 2]. CRE spread through healthcare networks via patient sharing [2–6], and the prevalence is increasing in many areas, including the greater Chicago region [2, 3, 7–9]. CRE are associated with high mortality rates in infected persons due to the lack of antibacterial options [1, 2, 7–9]. While Klebsiella pneumoniae carbapenemase is the most common CRE resistance mechanism in the United States, other CRE types, including New Delhi metallo-β-lactamase (NDM), present a new challenge to CRE detection and prevention practices [1, 9]. In response to the growing CRE crisis, Illinois initiated mandatory CRE reporting and created the XDRO registry, which serves as a statewide surveillance and interfacility communication tool [8].
In 2013, NDM-producing Escherichia coli (referred to here as “NDM”) was identified in a tertiary care hospital in northeastern Illinois (hospital A) from a urine culture obtained from a hospitalized patient with no international travel history [7]. A regional outbreak of NDM was eventually traced to duodenoscope exposure at hospital A (January 2013–October 2013), precipitating changes to endoscope reprocessing procedures [7].
Social network analysis allowed us to understand complex systems and quantify a healthcare facility’s position within the greater patient-sharing network [10]. We analyzed patterns of patient transfer between the facility that had the original NDM-positive patient (hospital A) and other facilities by constructing hospital A’s ego network. An ego network identifies a group of nodes (ie, healthcare facilities) that are directly connected to a given focal node. In this analysis, we identified the healthcare facilities that were most likely to share patients with hospital A, the index (ie, “ego”) facility [3, 10–14]. We hypothesized that hospital A’s ego network would identify which short- and long-term care hospitals were most likely to encounter NDM-colonized or -infected patients following the outbreak. If successful, during future outbreaks, ego networks could inform an efficient public health response focused on high-risk hospitals [15].
METHODS
We extracted hospital A’s ego network from the statewide patient-sharing network using UCINET software (Analytic Technologies, Harvard, Massachusetts) and Illinois’ inpatient hospital discharge data. We defined a “shared” patient as 2 facilities with a common patient within a 90-day period and defined a “strong connection” as 2 facilities sharing at least 50 patients over the 2-year study period (January 2013–December 2014). CRE epidemiology [16, 17] and network density considerations informed the 90-day period, and we chose a threshold of 50 patients in consultation with public health officials, who specified a reasonable number of facilities feasible for an intervention, after examining the size of the ego network at different patient-sharing thresholds.
We defined outbreak cases as any NDM case reported to the XDRO registry by hospital A in 2013 and then matched individual cases by name and date of birth to their Illinois hospital discharge database records (acute and long-term care hospitals). We identified which facilities outbreak patients visited in the subsequent 12 months. New NDM cases were any NDM cases reported to the XDRO registry in the 12 months after the outbreak that fit the following criteria: the report did not come from hospital A and the colonized individual was not one of the original outbreak patients. We assumed that, due to the rarity of NDMs in Illinois during 2013, such organisms most likely had disseminated from hospital A. We compared the ego network’s identification of high-risk hospitals to those identified in a discrete geographic region by drawing a circle with a radius equidistant to the facility in the ego network that was farthest geographically from hospital A. Then, we compared the number of facilities inside the geographic circle to the number of facilities in the ego network and compared hospital ranks based on number of shared patients with and geographic proximity from hospital A.
RESULTS
The entire patient-sharing network for Illinois consisted of 210 unique hospitals. When the criteria for considering 2 facilities connected was only 1 shared patient, hospital A’s ego network included more than one-half (N = 123) of the facilities in Illinois. Since rare patient transfer events of an uncommon pathogen are unlikely to contribute to spread of the outbreak and because including a large number of facilities in an ego network would diminish the value of an analysis performed to focus resources, we increased the threshold iteratively until we agreed that sharing 50 patients should be considered a strong connection. The 50-patient threshold resulted in a 22-facility ego network. In contrast, varying the patient-sharing threshold would have dramatically impacted the number of facilities as follows: 10 patients shared = 56 ego hospitals, 25 patients shared = 41 ego hospitals, 100 patients shared = 12 ego hospitals. There were 66 possible facilities within the circular geographic limits of our 50 shared-patient social network, the farthest hospital being 19 miles from hospital A.
Of the 31 outbreak cases entered into the XDRO registry who had visited hospital A in the subsequent 12 months, 19 (61%) were eventually admitted to 13 other hospitals following the outbreak. Of the 13 hospitals, the majority (n = 9; 69%) were in the ego network, and 5 of those 9 hospitals subsequently reported at least 1 new NDM case not identified in the original hospital A outbreak (Figure 1). Overall, there were 10 incident NDM cases reported for patients who had not visited hospital A, and 9 of these were reported by facilities in hospital A’s ego network. The 10th case was in the geographically designated region and would have been included in a 25 shared-patient threshold ego network. The facility with the highest number of patients shared with hospital A (n = 256) reported the most incident NDM patients (N = 4; Figure 1).
Figure 1.
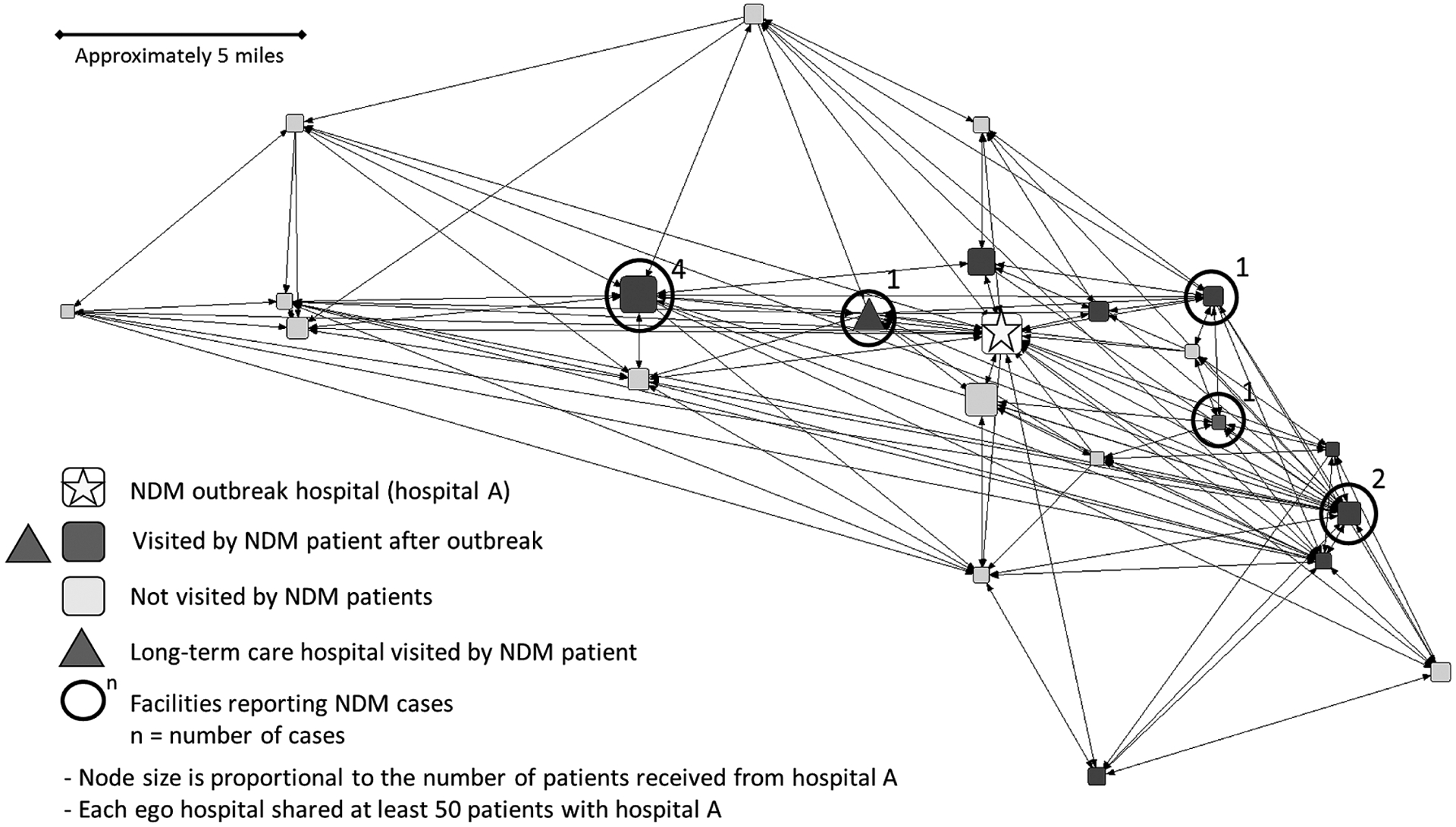
Sociogram representing hospital A’s ego network, arranged geographically. Abbreviation: NDM, New Delhi metallo-β-lactamase–producing Escherichia coli.
When we assigned hospital ranks based on geographic and social network proximity to hospital A, the correlation between the 2 ranks was relatively weak (r = 0.21). A number of facilities that were relatively geographically remote from hospital A shared a high number of patients with hospital A (Figure 2). Overall, the ego network rankings more accurately predicted NDM cases than geographic proximity (Wilcoxon sign-rank test P value = .01). The higher number of cases found in using ego network rankings was supported by our finding that NDM-outbreak patients were more likely to visit hospitals that had a greater degree of patient sharing in the ego network rather than geographically proximate hospitals (Figure 2). Using the proximity rankings by patient sharing, 7 of the incident NDM cases were found in the top 5 ranked hospitals; those same 7 cases were found in the top 11 hospitals when ranked by geographic distance.
Figure 2.
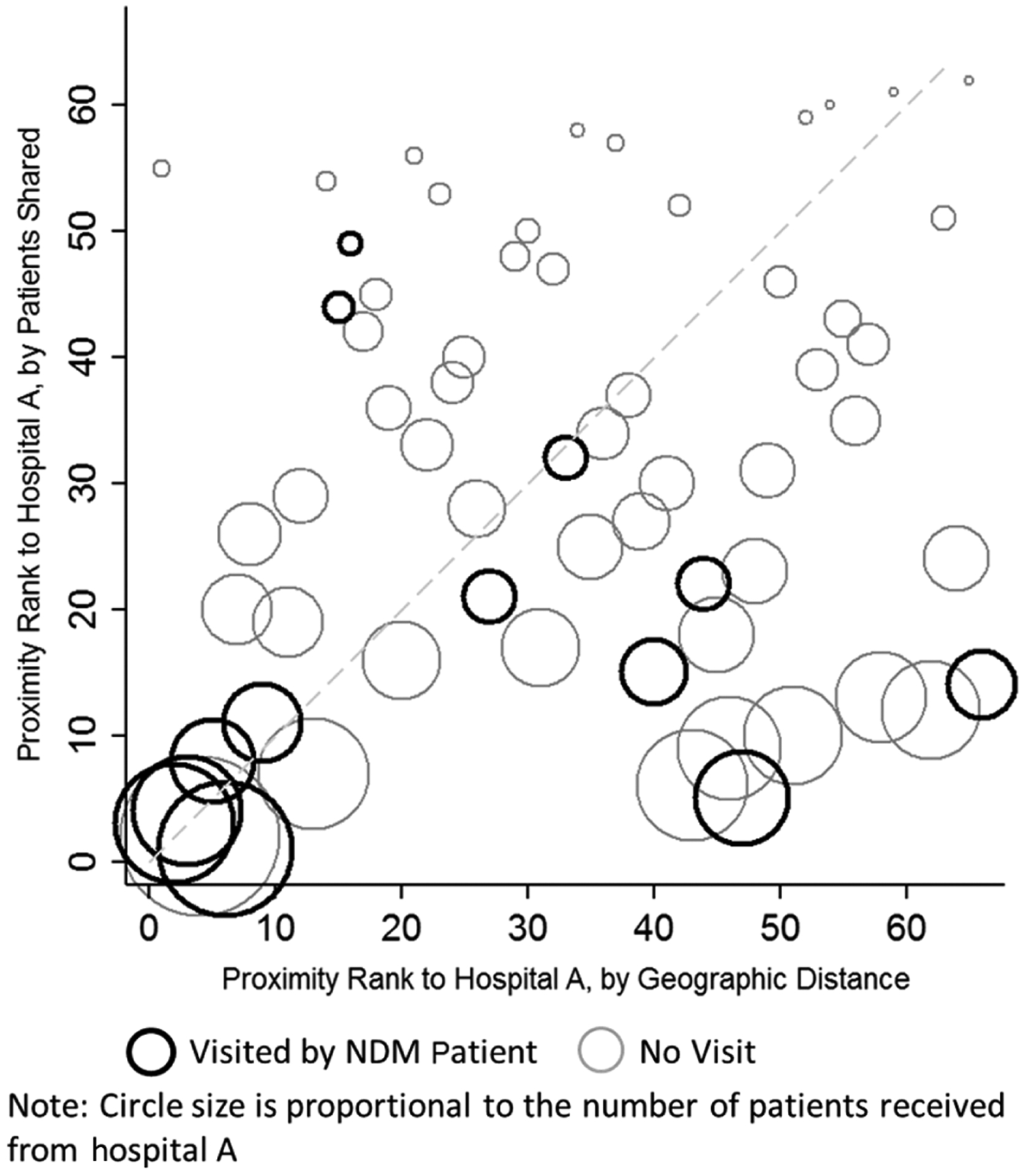
Comparison of patient sharing vs geographic proximity rankings for hospital A. Dashed line represents equality of proximity rank by patients shared vs by geographic distance. More hospitals visited by New Delhi metallo-β-lactamase patients (dark circles) fell below the dashed line, favoring ranking by patient sharing over geographic distance. Abbreviation: NDM, New Delhi metallo-β-lactamase–producing Escherichia coli.
DISCUSSION
Through analysis of an outbreak hospital’s ego network, we identified the majority of hospitals likely to be subsequently visited by an outbreak patient, which was consistent with the distribution of presumed secondary cases. There was rare documented transmission (1 case) outside the ego network, which would have been identified by lowering the patient connection threshold, at the cost, however, of nearly doubling the size of the ego network.
Ego network analysis efficiently identified the highest risk facilities. In Chicago, which has a relatively high density of healthcare facilities, patient sharing between hospitals may be influenced more by business relationships than by geographic proximity. Without development of ego networks, public health interventions would have to rely on global notifications to a large group of hospitals within or across jurisdictions or within a geographically defined region. We identified the hospitals that are closely affiliated through a high volume of patient sharing regardless of geographic proximity.
The correct patient-sharing threshold to define an ego network is unknown and likely contextual, including the following factors: intervention resources, cost or feasibility of the proposed intervention, virulence of the organism, and density of the network. Our proposed threshold was based primarily on what was considered by public health officials to be a reasonable public health response. Because the number of patient-sharing connections likely drives a hospital’s risk of encountering drug-resistant organisms [3, 12], we believe that the “threshold approach” to constructing ego networks that we used is reasonable.
Options for establishing patient-sharing networks include use of a state’s healthcare utilization databases, which may not routinely capture visits to long-term care facilities. One option for capturing long-term care facilities is use of the Centers for Medicare & Medicaid Services (CMS) healthcare utilization dataset; however, this dataset only includes individuals who have Medicare or Medicaid insurance and may not capture those in managed care plans. Although they provide important information, use of CMS-based patient-sharing networks increases costs by the need to purchase data and dedicate personnel time to join datasets and likely results in a less current dataset. However, centralized provision of such patient-sharing networks would be advantageous to state and local public health facilities.
A major strength of this study is that we used Illinois’ hospital discharge dataset, which includes patient identifiers, allowing us to match hospital exposure data to the XDRO registry. The capacity to join 2 public health datasets provides a unique opportunity to explore outbreak propagation through patient-sharing patterns. For future outbreaks, it will be possible to recommend use of the XDRO registry, either through manual searches or an automated alert process, to healthcare facilities in the ego network of a healthcare facility that is experiencing an outbreak. Furthermore, in addition to outbreak response, ego networks could be used for infection prevention. For example, each hospital could receive an individualized risk assessment from public health that is based on reported XDRO rates among facilities in their ego network.
Our study has limitations. We did not have isolates to perform testing of genetic relatedness of all NDM cases, given the retrospective nature of this study. However, because NDM was exceedingly rare in Illinois during the study period, we used an epidemiologic definition that incident NDM cases in the 12 months following the hospital A outbreak were related to the hospital A outbreak. All 39 isolates involved in the 2013 hospital A outbreak were highly genetically related by pulse-field gel electrophoresis, suggesting that NDM was not endemic in our region [7]. Another limitation of our study is that we only had patient-sharing information for short- and long-term care hospitals. Since CRE is often found in certain skilled nursing facilities [2, 18], we were unable to evaluate this important group of facilities.
Had public health professionals known hospital A’s ego network at the time of the outbreak, high-risk hospitals could have been targeted for early intervention such as screening patients at the time of admission, perhaps preventing regional dissemination of NDM. For future outbreaks in Illinois, an ego network now exists to guide public health interventions. While our analysis lacks the sophistication of more complicated network modeling, it demonstrates that knowledge of a region’s patient-sharing patterns through ego networks can be a useful tool in a healthcare-associated infection outbreak situation.
Acknowledgments.
We acknowledge the Strengthening Health Systems through Interprofessional Education (Project SHINE). M. J. R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support.
The study was funded by the Centers for Disease Control and Prevention Epicenter Program (U54CK000161).
Footnotes
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kallen AJ, Ricks P, Edwards JR, et al. Vital signs: carbapenem-resistant Enterobacteriaceae. Morb Mortal Wkly Rep 2013; 62:165–70. [PMC free article] [PubMed] [Google Scholar]
- 2.Won SY, Munoz-Price LS, Lolans K, Hota B, Weinstein RA, Hayden MK; Centers for Disease Control and Prevention Epicenter Program. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2011; 53:532–40. [DOI] [PubMed] [Google Scholar]
- 3.Ray MJ, Lin MY, Weinstein RA, Trick WE. Spread of carbapenem-resistant Enterobacteriaceae among Illinois healthcare facilities: the role of patient sharing. Clin Infect Dis 2016; 63:889–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karkada UH, Adamic LA, Kahn JM, Iwashyna TJ. Limiting the spread of highly resistant hospital-acquired microorganisms via critical care transfers: a simulation study. Intensive Care Med 2011; 37:1633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesosky M, McGeer A, Simor A, Green K, Low DE, Raboud J. Effect of patterns of transferring patients among healthcare institutions on rates of nosocomial methicillin-resistant Staphylococcus aureus transmission: a Monte Carlo simulation. Infect Control Hosp Epidemiol 2011; 32:136–47. [DOI] [PubMed] [Google Scholar]
- 6.Ciccolini M, Donker T, Grundmann H, Bonten MJ, Woolhouse ME. Efficient surveillance for healthcare-associated infections spreading between hospitals. Proc Natl Acad Sci U S A 2014; 111:2271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-β-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA 2014; 312:1447–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trick WE, Lin MY, Cheng-Leidig R, et al. Electronic public health registry of extensively drug-resistant organisms, Illinois, USA. Emerg Infect Dis 2015; 21:1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011; 53:60–7. [DOI] [PubMed] [Google Scholar]
- 10.Borgatti SP, Everett MG, Johnson JC. Analyzing social networks. London: SAGE Publications Limited, 2013:163–9. [Google Scholar]
- 11.Donker T, Wallinga J, Slack R, Grundmann H. Hospital networks and the dispersal of hospital-acquired pathogens by patient transfer. PLoS One 2012; 7:e35002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simmering JE, Polgreen LA, Campbell DR, Cavanaugh JE, Polgreen PM. Hospital transfer network structure as a risk factor for Clostridium difficile infection. Infect Control Hosp Epidemiol 2015; 36:1031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BY, McGlone SM, Song Y, et al. Social network analysis of patient sharing among hospitals in Orange County, California. Am J Public Health 2011; 101:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgatti SP. Identifying sets of key players in a social network. Comput Math Organ Theory 2006; 12:21–34. [Google Scholar]
- 15.Slayton RB, Toth D, Lee BY, et al. Vital signs: estimated effects of a coordinated approach for action to reduce antibiotic-resistant infections in health care facilities—United States. MMWR Morb Mortal Wkly Rep 2015; 64:826–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman N, Adler A, Molshatzki N, et al. Gastrointestinal colonization by KPC-producing Klebsiella pneumoniae following hospital discharge: duration of carriage and risk factors for persistent carriage. Clin Microbiol Infect 2013; 19:E190–6. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman FS, Assous MV, Bdolah-Abram T, Lachish T, Yinnon AM, Wiener-Well Y. Duration of carriage of carbapenem-resistant Enterobacteriaceae following hospital discharge. Am J Infect Control 2013; 41:190–4. [DOI] [PubMed] [Google Scholar]
- 18.Prabaker K, Lin MY, McNally M, et al. ; Centers for Disease Control and Prevention Epicenters Program. Transfer from high-acuity long-term care facilities is associated with carriage of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae: a multihospital study. Infect Control Hosp Epidemiol 2012; 33:1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


