Abstract
Background
Recurrent Clostridioides difficile infection (CDI) in patients with inflammatory bowel disease (IBD) is a clinical challenge. Fecal microbiota transplantation (FMT) has emerged as a recurrent CDI therapy. Anecdotal concerns exist regarding worsening of IBD activity; however, prospective data among IBD patients are limited.
Methods
Secondary analysis from an open-label, prospective, multicenter cohort study among IBD patients with 2 or more CDI episodes was performed. Participants underwent a single FMT by colonoscopy (250 mL, healthy universal donor). Secondary IBD-related outcomes included rate of de novo IBD flares, worsening IBD, and IBD improvement—all based on Mayo or Harvey-Bradshaw index (HBI) scores. Stool samples were collected for microbiome and targeted metabolomic profiling.
Results
Fifty patients enrolled in the study, among which 15 had Crohn’s disease (mean HBI, 5.8 ± 3.4) and 35 had ulcerative colitis (mean partial Mayo score, 4.2 ± 2.1). Overall, 49 patients received treatment. Among the Crohn’s disease cohort, 73.3% (11 of 15) had IBD improvement, and 4 (26.6%) had no disease activity change. Among the ulcerative colitis cohort, 62% (22 of 34) had IBD improvement, 29.4% (11 of 34) had no change, and 4% (1 of 34) experienced a de novo flare. Alpha diversity significantly increased post-FMT, and ulcerative colitis patients became more similar to the donor than Crohn’s disease patients (P = 0.04).
Conclusion
This prospective trial assessing FMT in IBD-CDI patients suggests IBD outcomes are better than reported in retrospective studies.
Keywords: inflammatory bowel disease, ulcerative colitis, Crohn’s disease, fecal microbiota transplantation, microbiome, butyrate, Clostridioides difficile infection
BACKGROUND
There has been an increase in the incidence and severity of Clostridioides difficile infection (CDI) over the decade that has been attributed to more virulent and treatment-refractory strains1, 2; and its impact has been especially pernicious on inflammatory bowel disease (IBD) patients.3–5 The prevalence of CDI in the IBD population was noted to be 2.5 to 8-fold higher, with a 10% lifetime chance of getting the infection.6–8 Since 1998, CDI-related IBD hospitalizations have doubled, and in-patient hospital mortality rose significantly from 5.9% to 7.2%.9 Further, the in-hospital death rate of IBD patients is nearly 5 times greater when complicated by CDI.9 After an initial course of anti-CDI therapy, the CDI recurrence rate is 4.5-fold higher, and the prevalence of toxigenic C. difficile carrier state is 8-fold greater in IBD patients compared with those without IBD.9Clostridioides difficile may lead to an IBD flare, worsen disease severity, and negatively impact the disease course.9 In a retrospective study, C. difficile–positive ulcerative colitis (UC) patients were twice as likely to be hospitalized, 8 times as likely to be seen in the emergency room, and had nearly 2-fold the colectomy rate compared with C. difficile–negative UC patients for up to a year after the index hospitalization.10 Additionally, it has been noted that CDI-IBD patients tend to improve on anti-CDI therapy, suggesting that prompt eradiation of CDI may prevent colectomy, at least in the short term.10 Overall, the data suggest that CDI among IBD patients is an important clinical challenge.
The use of fecal microbiota transplantation (FMT) has changed the CDI treatment paradigm and is a recommended therapy in C. difficile treatment guidelines in both the US and Europe for recurrent CDI after several randomized trials compared it with standard-of-care antibiotics and placebo.11–16 However, these trials commonly exclude IBD patients. In a retrospective cohort study, Kelly and colleagues demonstrated an overall cure rate of 94% in immunosuppressed patients, including patients with IBD.17 This study also reported that 14% of post-FMT patients developed an IBD exacerbation, although this was not defined.17 Patients with IBD and recurrent CDI have been shown to have higher rates of FMT failure,18 leading to further questions surrounding the safety and efficacy of FMT in IBD patients with concurrent CDI. While placebo-controlled clinical trials of FMT to treat UC have shown early promise,19 the unique population of IBD patients with concurrent CDI remains poorly understood. Additionally, retrospective data suggesting IBD worsening after FMT for CDI remain an important clinical concern, and there is a paucity of prospective data. Therefor, we performed a prospective open-label trial examining the impact of FMT on IBD in patients receiving treatment for recurrent CDI. We previously published the CDI outcomes, revealing an 8% FMT failure rate. Here we aim to present the secondary IBD outcomes post-FMT.
METHODS
Study Design
We performed secondary analysis of IBD outcomes from an open-label, prospective, single-arm, multicenter cohort study at 4 tertiary care centers (Brigham and Women’s Hospital [BWH], Indiana University, Brown University, and Mount Sinai Hospital). The study protocol was approved by the institutional review boards at the Brigham and Women’s Hospital, Indiana University, Brown University, and Mount Sinai Hospital, and all patients provided written informed consent before participation (NCT03106844). An independent data and safety monitoring committee evaluated the trial regularly. Additionally, FDA approval via investigational new drug application (IND 17379) was acquired.
Study Population
Patients with a confirmed diagnosis of IBD (ulcerative colitis [UC] or Crohn’s Disease [CD] with any colonic involvement) and 2 or more confirmed episodes of CDI within 12 months, with the most recent episode occurring within 3 months, were enrolled. Polymerase chain reaction (PCR) or glutamate dehydrogenase (GDH) with enzyme immunoassay (EIA) for toxin were permitted for the qualifying CDI episodes, as the standard lab testing at each site differed. Patients with a total or subtotal colectomy, isolated ileal or small bowel CD, those pregnant or breastfeeding, those treated with vancomycin or metronidazole for more than 60 days, or those that had undergone a prior FMT within 12 months were excluded.
Baseline Assessments
Patients were assessed before the FMT, and the following data was collected: IBD disease history, baseline IBD clinical scores (Harvey-Bradshaw Index [HBI] for Crohn’s disease and partial Mayo score for UC), concurrent IBD therapy, CDI history, and baseline fecal calprotectin. Endoscopic scores including the Mayo endoscopic score and the simple endoscopic score (SES)-CD were obtained at the time of FMT.
Interventions and Follow-up
Fecal microbiota transplantation donor material was produced at OpenBiome (Cambridge, MA, USA) as previously described.20 Briefly, healthy candidate donors underwent a rigorous health evaluation to rule out infectious and microbiome-mediated diseases. Subsequently, potential donors underwent a battery of stool and serological tests aimed at screening for infectious diseases.21, 22 Material was homogenized and filtered with a cryoprotectant and formulated into a 250-mL preparation under good manufacturing practices (GMP) principles. Fecal microbiota transplantation was stored frozen at −80ºC until it was shipped to sites. Material was thawed according to best practices before administration.
All patients received a single FMT via colonoscopy after undergoing a standard-of-care bowel prep with polyethylene glycol on the day before the colonoscopy. Anti-CDI antibiotics were held for 48 hours before the FMT. Colonoscopy was performed to the cecum, with fecal material administered in the right colon. Each subject received material (250 mL) sourced from only 1 donor. Four unique donors were used across the study, and 2 of these supplied material for the majority of the patients.
Follow-up assessments at weeks 1, 8, and 12 post-FMT were performed. Clinical laboratory assessments and stool testing for fecal calprotectin were also performed. All stool testing was performed centrally at the BWH clinical lab. A final safety assessment was performed at 6 months post-FMT.
Inflammatory Bowel Disease Outcomes
Inflammatory bowel disease outcomes assessed included changes in IBD activity in which we assessed for 3 outcomes utilizing clinical scores: (1) de novo IBD flare (defined as a Mayo or HBI score >4 at week 12 in the absence of CDI if Mayo or HBI scores were 2 or less at baseline); (2) worsening IBD in those with baseline active disease (Mayo or HBI score >4) who experienced an increase in either HBI or Mayo score by 2 or more at week 12); and (3) IBD improvement (defined as a decrease in Mayo or HBI score by 2 or more at week 12 compared with baseline without the need for rescue prednisone). Inflammatory bowel disease medication changes, if made based on ongoing or worsening disease activity, were made by the referring IBD physician—not the study team—and were documented. Safety was also assessed as the proportions of adverse events through 6 months.
Assessment and Analysis of the Microbiome
Stool samples were collected for sequencing from donors and patients at baseline, 1, 8, and 12 weeks post-FMT. Samples were stored by flash freezing at −80°C. DNA extraction, PCR amplification of the 16S rRNA gene’s V4 region, and Illumina paired-end sequencing was performed at the University of Michigan core facility, as described previously.23
Primers were trimmed, paired-ends were merged, and sequences were mapped to the SILVA database,24 forming operational taxonomic units (OTUs) clustered at 99% sequence identity, with a custom pipeline. Samples were sequenced to a median depth of 38,445 reads per sample, and 13,222 unique OTUs were identified across the study.
Samples were rarefied to the lowest sample read count (2299 reads) for alpha diversity calculations or left unrarefied for other analyses. Paired t tests were used to compare diversity or donor similarity before vs after FMT, and independent t tests were used for all other comparisons. Spearman rank was used for assessing correlation. Because previous reports have consistently shown low diversity in recurrent CDI microbiomes and that healthy-donor FMT increases diversity,25 1-sided tests were used for evaluating diversity changes post-FMT. One patient was discarded from analysis due to high pretreatment donor similarity consistent with a sample labeling error.
Metabolomic Assessment
Ultraperformance liquid chromatography-mass spectrometry (UPLC-MS) profiling and analysis of fecal bile acids were performed. The protocols used for fecal extract preparation26 and analysis27, 28 were performed as previously described. In addition, mass spectrometry data was analyzed using peakPantheR, an automated pipeline for the detection, integration, and reporting of predefined features across a large number of mass spectrometry data files. Secondary bile acids were defined as those produced from primary bile acids via gut microbial 7-alpha-dehydroxylation, whereas tertiary bile acids were those produced from primary bile acids via processes involving other forms of microbial modification (eg, 7alpha-/beta-isomerization of chenodeoxycholic acid [CDCA] to form UDCA).
Gas chromatography-mass spectrometry (GC-MS) for identification and quantification of fecal short chain fatty acids (SCFAs) was also performed. This was undertaken using previously described protocols.29 Samples analysis was performed on an Agilent 7890B GC system coupled to an Agilent 5977A mass selective detector (Agilent, Santa Clara, California). Analysis of data was performed using MassHunter software (Agilent).
Statistical Analysis
Categorical data were described using descriptive statistics (proportions and percentages). Continuous data were described using means and standard deviations (normally distributed data) or using medians and interquartile range (nonparametric data). Appropriate comparative statistical tests were chosen based in the variable types (categorical, dichotomous, continuous) and distribution (parametric, nonparametric) and were used to describe significant differences between intervention and control groups. Where appropriate, point estimates, confidence intervals (CIs), and P value 2-tailed with a significance level of 0.05 were reported. If patients withdrew or were lost to follow-up, they were not replaced, and their data from the last assessment were carried forward.
RESULTS
Fifty participants were enrolled (between August 2017 and October 2019), among which 15 had Crohn’s disease (mean HBI, 5.8 ± 3.4) and 35 had ulcerative colitis (mean artial Mayo score, 4.2 ± 2.1). The mean age was 43 years (range 21–91), and the cohort was primarily female (58%). A total of 49 patients received treatment, as 1 patient with UC withdrew before receiving FMT and was not replaced. Among the 49 treated, 1 patient was lost to follow-up after the week-1 visit, and week-1 data were carried forward. The primary outcome, FMT failure, has been previously reported.30
IBD Outcomes
Patients had varying levels of IBD disease activity entering into the study. The mean baseline HBI score was 5.9 ± 3.5, with 20% between 1 and 3, 46.6% between 4 and 6, and 40% were >6. The mean baseline partial Mayo score was 4.1 ± 2.1, with 48.5% between 1 and 3, 34.2% between 4 and 6, and 17% were >6. The baseline IBD medications are listed in Table 1. Among the CD cohort, 26.7% were on prednisone at baseline, 60% were on a biologic, and 36.7% were on an immunomodulator. Among the UC cohort, 48.6% were on prednisone at baseline, 51.4% were on a biologic, and 48.6% were on mesalamine. Inflammatory bowel disease outcomes were calculated based on the baseline and week 12 clinical scores—or the last recorded score. If patients underwent a second FMT, their scores after their second FMT were used. Based on the prespecified definitions, in the CD cohort, 73.3% (11 of 15) of CD patients had IBD improvement, and 4 (26.6%) had no change in clinical scores. In the UC cohort, 62% (22 of 34) patients had IBD improvement, a 29.4% (11 of 34) had no change in clinical scores, and 4% (1 of 34) experienced a de novo flare. This patient had a baseline partial Mayo score of 2 and was found to be 5 at the week-12 assessment.
TABLE 1.
Inflammatory Bowel Disease Baseline Medications
| Crohn’s Disease | N = 15 |
|---|---|
| Steroids % (n) | |
| Prednisone | 26.7% (4) |
| Budesonide | 6.7% (1) |
| Biologics | |
| Vedolizumab | 20% (3) |
| Certolizumab | 13.3% (2) |
| Adalimumab | 6.7% (1) |
| Infliximab | 6.7% (1) |
| Ustekinumab | 6.7% (1) |
| Cyclosporine | 6.7% (1) |
| Mesalamine | |
| Oral 5-ASA | 13.3% (2) |
| Rectal 5-ASA | 6.7% (1) |
| Immunomodulators | |
| Azathioprine | 13.3% (2) |
| Methotrexate | 13.3% (2) |
| Ulcerative Colitis | N = 35 |
| Steroids | |
| Prednisone | 48.6% (17) |
| Budesonide | 5.7% (2) |
| Rectal Corticosteroids | 8.6% (3) |
| Biologics | |
| Adalimumab | 8.6% (3) |
| Vedolizumab | 22.9% (8) |
| Infliximab | 17.1% (6) |
| Tofacitanib | 2.9% (1) |
| Cyclosporine | 2.9% (1) |
| Mesalamine | |
| Rectal 5-ASA | 11.4% (4) |
| Oral 5-ASA | 57% (20) |
| Immunomodulator | |
| Methotrexate | 2.9% (1) |
In the CD cohort, among those noted to have improvement, 54.5% (6 of 11) were able to taper off steroids by week 12. In addition, 3 (27%) of the improved patients were safely started on a biologic after FMT. All were noted to have active disease at baseline. In the UC cohort, 27.2% (6 of 22) of those who improved were able to taper off steroids, whereas 18% remained on a stable prednisone dose throughout. In addition, 3 patients were safely started on biologics post-FMT; all 3 had active disease at baseline.
Safety Outcomes
Overall the treatment was safe and well tolerated. Two serious adverse events (SAEs) were reported; neither was determined treatment-related by the treating physician. The first was the previously noted IBD flare. The subject was hospitalized for infliximab initiation and then was discharged. Another subject had worsening anemia that required a blood transfusion. The event was determined to be related to an underlying disease, not treatment-related and resolved with a blood transfusion. There were several mild (grade 1 and grade 2) AEs reported, mostly gastrointestinal in a nature. A full list of AEs is reported in Table 2.
TABLE 2.
Adverse Events by Body Site
| Body System | N = 49 |
|---|---|
| % (n) | |
| Gastrointestinal | |
| Diarrhea | 44.9% (22) |
| Rectal Bleeding | 16.3% (8) |
| Abdominal Painc, d | 26.5 (13) |
| Nausea | 10.2% (5) |
| Vomiting | 6.1% (3) |
| Heart Burn | 4.1% (2) |
| Constipation | 2% (1) |
| Rectal Abscess | 2% (1) |
| Right lower Quadrant Pain | 2% (1) |
| Gastritis | 2% (1) |
| Bloating | 2% (1) |
| Discoloured Stool | 2% (1) |
| IBD flarea | 2% (1) |
| Systemic | |
| Fever | 10.2% (5) |
| Fatigue | 6.1% (3) |
| Chills | 4.1% (2) |
| Night sweats | 2% (1) |
| Anemiab | 2% (1) |
| Burning pain on feet | 2% (1) |
| Pedal bilateral edema | 2% (1) |
| Respiratory | |
| Cough | 2% (1) |
| Dermatologic | |
| Shingles | 2% (1) |
| Eczema | 2% (1) |
| Cyst | 2% (1) |
| Swollen lymph nodes | 2% (1) |
| Rash | 2% (1) |
| Polymorphous light eruption | 2% (1) |
| Skin abscess | 2% (1) |
| Neurological | |
| Migraine | 4.1% (2) |
| Genitourinary | |
| Yeast infection | 4.1% (2) |
| Kidney stones | 2% (1) |
| Musculoskeletal | |
| Muscle soreness | 2% (1) |
| Back pain | 2% (1) |
| Infection | |
| Oral infection | 2% (1) |
| Pharyngitis | 2% (1) |
aGrade 3 IBD flare that resulted in a hospitalization in 1 subject reported during the week 8 follow-up. Subject was treated with Remicade and discharged from hospital.
bSubject with anemia required blood transfusions and reported the event during their week 8 follow-up. The event was deemed to be disease-related not treatment-related; it was resolved with transfusion.
cSubject went to ER for general GI pain and reported the event during their week 26 follow-up. Subject was given Percocet to take as needed.
dOne subject visited the ER due to abdominal pain and reported this event during their week-12 visit. Subject was treated with pain medication.
Microbiome Outcomes
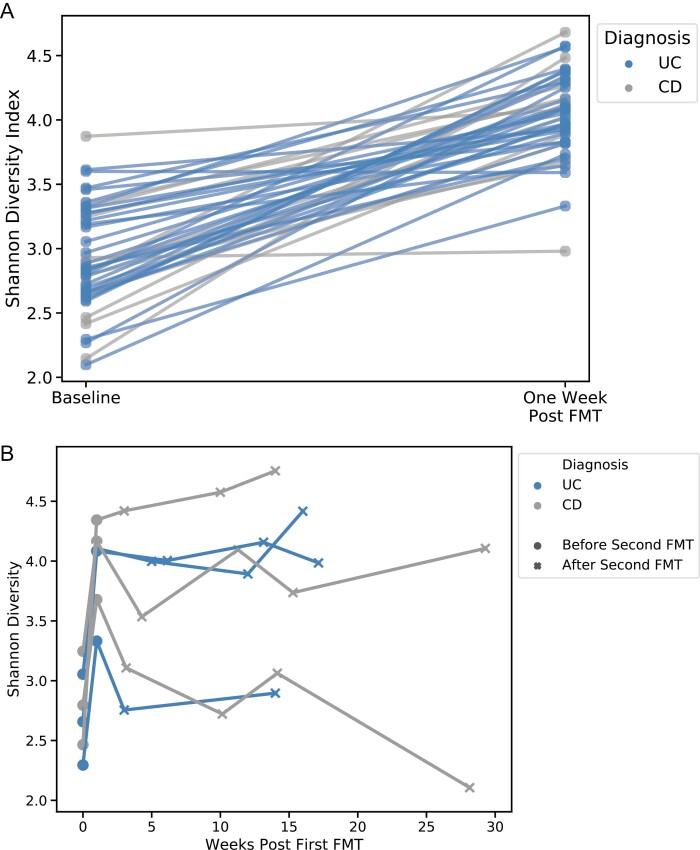
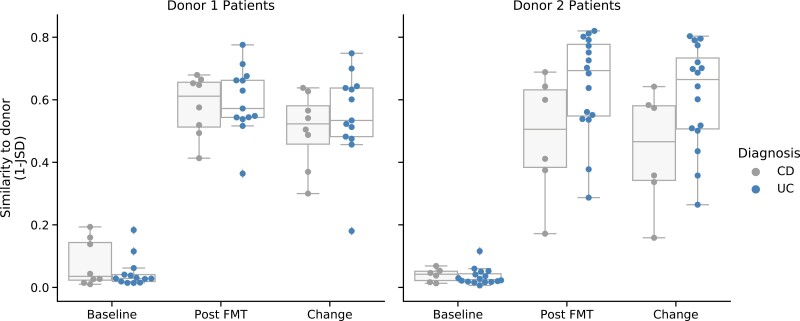
Alpha diversity significantly increased within 1 week of FMT in both patients with UC and CD (Fig. 1A, P < 1e-17), and this was sustained through week 12. Of the 6 patients who underwent a second FMT, alpha diversity did not further increase after the second treatment (Fig. 1B). The microbial composition in UC patients became more similar to the donor than it did in CD patients (P = 0.040), in particular among patients receiving 1 of the 2 major donors (Fig. 2). Similarity to donor did not correlate with IBD clinical improvement.
FIGURE 1.
Alpha diversity after (A) first FMT and (B) in patients receiving second FMT. Increases were significant after first FMT for all patients pooled (P < 1e-17) and when stratified by IBD subtype (UC P < 1e-13; CD P < 1e-5).
FIGURE 2.
Similarity to the donor post-FMT measured by Jensen-Shannon Divergence, stratified by donor and IBD subtype. Data from 2 patients receiving other donors not shown. Change was measured as difference in similarity post-FMT vs baseline and was significantly different between UC and CD patients (all patients, P = 0.040; donor 1 and 2 only, P = 0.038, 2-sided t test). Differences between the subtypes were largest among recipients of donor 2.
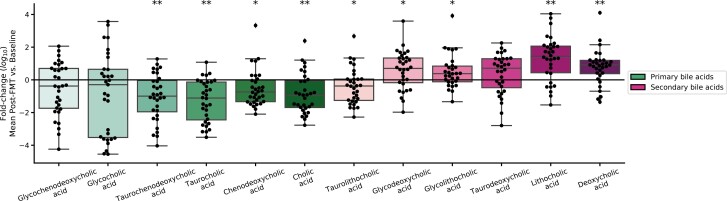
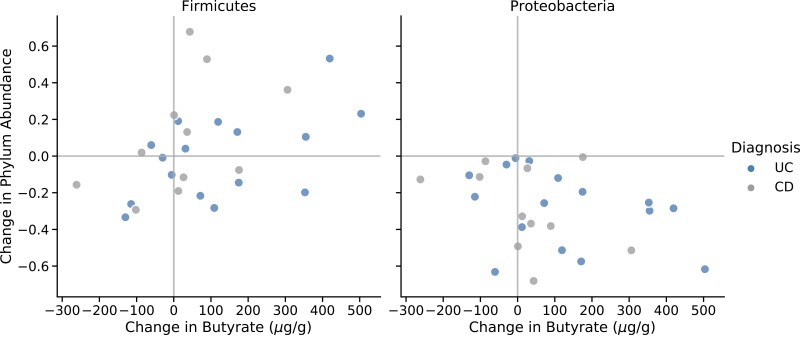
The effect of FMT upon the fecal metabolome was also assessed. Post-FMT, fecal primary bile acids decreased, and secondary bile acids increased. Specifically, at each time point post-FMT, we observed a sustained, significant decrease in conjugated primary bile acids in stool (including taurocholic acid, the major endogenous trigger to C. difficile germination).28 Conversely, the secondary bile acid, deoxycholate (which prevents the growth of C. difficile)28 significantly increased at each time point post-FMT (Fig. 3). Successful FMT was also associated with an overall restoration of short-chain fatty acids (SCFAs) from initial very low levels to levels comparable with those found in the stool of healthy stool donors. Increases in butyrate was positively correlated with Firmicutes and negatively correlated with Proteobacteria in both IBD subtypes (Fig. 4).
FIGURE 3.
Log fold-change in primary and secondary bile acids following FMT. For each patient, all values post-FMT were averaged, then divided by the patient’s baseline. Significant changes are indicated (*indicates P < 0.05, **indicates P < 0.005, 2-sided t test on log abundance).
FIGURE 4.
Associations between changes in fecal butyrate and major phyla abundances. Changes were measured between baseline and week 12 for 2 major phyla: Firmicutes (left) and Proteobacteria (right). Spearman correlations were significant for Firmicutes across all patients (P = 0.021) and when stratified by IBD subtype (UC, P = 0.037; CD, P = 0.039).
DISCUSSION
Here, we report secondary analysis and IBD outcomes from the first prospective trial to assess the effect of FMT in patients with IBD-CDI. We aimed to understand the magnitude of risk for potential IBD flare post-FMT in CDI-IBD patients.17 We found that no patients with CD and only 1 patient with UC (4%) met the definition of de novo IBD flare. Moreover, the vast majority of patients had improved IBD clinical scores after elimination of CDI post-FMT. This finding contradicts previous reports. Our group performed a meta-analysis of studies assessing the rates of IBD flares or of a reported worsening clinical course, in which the pooled rate of IBD worsening was 14.9% (95% CI, 10–21%).31 When separated by patient population, the rate of worsening in IBD activity after FMT for CDI was 22.7% (95% CI, 13–36%) compared with FMT for IBD alone where the IBD worsening rate was 11.1% (95% CI, 7–17%). This trial, in agreement with other randomized controlled trials of FMT for UC found only a marginal risk of worsening in IBD, at 4.6%, (95% CI, 1.8–11%), suggesting reporting bias in small retrospective studies.31 The definition of flare or IBD worsening also varied between previous retrospective studies, and there were often no set definitions. To standardize this, we set 3 definitions: IBD worsening, IBD improvement, and de novo flare to account for baseline IBD activity. We found that many patients had active disease before FMT and continued to have active disease post-FMT. Given the prospective design of this trial, it was clear that active disease post-FMT did not represent FMT treatment-related flare. We did find that with swift eradication of CDI, patients with active disease at the time of FMT were able to safely start appropriate IBD therapy and ultimately led to improvement in their disease activity.
The microbiome analysis revealed significant increase in alpha diversity as early as 1 week post-FMT, and this increase was sustained. Interestingly, patients that received a second FMT did not experience an increase in alpha diversity further but did all achieve cure with a second FMT from the same donor. We had no patients who required more than 2 FMTs or additional anti-CDI therapy beyond that. We did find that the microbiomes of patients with UC became more similar to the donors than patients with CD; they also had higher engraftment efficiency. This convergence may shed light on why investigators are seeing early success with FMT as a therapy for UC. However, we did not observe a correlation between donor similarity and clinical improvement in this trial. We did appreciate patterns in restoration of bile acid profiles post-FMT similar to non-IBD patients, and an increase in butyrate post-FMT correlated with an increase in Firmicutes, including Faecalibacterium, a major contributor to butyrate production in the healthy gut.32 One potential inference of this observation is that restoration of gut microbiome functionality—as well as restoration of composition—is of key importance to the efficacy of FMT. In particular, we noticed early and maintained restoration of both bile acid and SCFA profiles to premorbid patterns after FMT. Though it is already well-established that these metabolites are contributory to the pathogenesis of CDI and efficacy of FMT in its treatment, there is also growing evidence for their importance to IBD, as well. For instance, secondary bile acids have recently been shown to be reduced in UC patients with pouches, and secondary bile acid supplementation is associated with reduced inflammation in animal models of colitis.33, 34
This study had several limitations. We used subjective clinical scores to assess IBD activity, and this may impact interpretation of disease activity in the context of an open-label study, especially with the overlap of symptoms between CDI and active IBD; however, placebo effect in this very ill patient population was likely to be negligible. Additionally, we acknowledge that referring physicians were able to change IBD therapy as they felt necessary; therefore, we were not able to comment on FMT as a treatment for IBD. We do feel that there are several strengths, however. This is the first prospective FMT trial in which patients were systematically followed to assess for changes in IBD clinical activity.
This study was able to demonstrate that FMT was safer and even more well tolerated in patients with IBD requiring treatment for recurrent CDI in comparison with what has been previously reported in retrospective studies. We did not observe IBD worsening, and only 1 patient met the definition of a flare de novo, highlighting the fact that many of these patients have active disease before FMT and will continue to have active disease after FMT. Appropriate treatment with biologics post-FMT after eradication of CDI was safe and led to overall IBD improvement.
Author Contribution: JRA initiated study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript. MF participated in developing study concept, study design, data acquisition, and critical revision of the manuscript. YG and MS analyzed and interpreted microbiome data. BHM, AP, JAKM, JM, GFB, JLA, KIG, and JRM generated, analyzed, and interpreted the metabolomics data. MC, JM, JH, EP, and SN participated in data acquisition. ZK participated in developing study concept, study design, interpretation of data and critical revision of the manuscript. AG, SVS, MB, and CRK participated in data acquisition, interpretation of data, and critical revision of the manuscript. All authors had access to the study data and reviewed and approved the final manuscript.
Supported by: The trial was funded by the Crohn’s and Colitis Foundation. Clinical Research Alliance. In addition, this work was supported in part by the Harvard Digestive Disease Center, National Institute of Health Grant P30 DK034854. Metabonomics studies were performed at the MRC-NIHR National Phenome Centre at Imperial College London; they received financial support from the Medical Research Council (MRC) and National Institute of Health Research (NIHR), grant number MC_PC_12025. BHM is the recipient of a MRC Clinical Research Training Fellowship (grant reference: MR/R000875/1) and NIHR Academic Clinical Lectureship. The Division of Digestive Diseases and MRC-NIHR National Phenome Centre at Imperial College London receive financial and infrastructure support from the NIHR Imperial Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London.
Conflicts of Interest: JRA consults for and has research support from Finch Therapeutics Group. JRA, MF, and CRK have research support from Finch Therapeutics and serves as clinical advisors to OpenBiome. YG, MS, and ZK are employees/shareholders of Finch Therapeutics. BHM consults for Finch Therapeutics.
REFERENCES
- 1. Cohen SH, Gerding DN, Johnson S, et al. ; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–455. [DOI] [PubMed] [Google Scholar]
- 2. Zar FA, Bakkanagari SR, Moorthi KM, et al. . A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007;45:302–307. [DOI] [PubMed] [Google Scholar]
- 3. Ananthakrishnan AN. Clostridium difficile infection: epidemiology, risk factors and management. Nat Rev Gastroenterol Hepatol. 2011;8:17–26. [DOI] [PubMed] [Google Scholar]
- 4. Pépin J, Valiquette L, Alary ME, et al. . Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Cmaj. 2004;171:466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–536. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen GC, Kaplan GG, Harris ML, et al. . A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–1450. [DOI] [PubMed] [Google Scholar]
- 7. Hourigan SK, Oliva-Hemker M, Hutfless S. The prevalence of Clostridium difficile infection in pediatric and adult patients with inflammatory bowel disease. Dig Dis Sci. 2014;59:2222–2227. [DOI] [PubMed] [Google Scholar]
- 8. Binion DG. Clostridium difficile infection in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2012;8:615–617. [PMC free article] [PubMed] [Google Scholar]
- 9. Kelsen JR, Kim J, Latta D, et al. . Recurrence rate of Clostridium difficile infection in hospitalized pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:50–55. [DOI] [PubMed] [Google Scholar]
- 10. Jodorkovsky D, Young Y, Abreu MT. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Dig Dis Sci. 2010;55:415–420. [DOI] [PubMed] [Google Scholar]
- 11. Kassam Z, Lee CH, Yuan Y, et al. . Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–508. [DOI] [PubMed] [Google Scholar]
- 12. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol. 2014;48:693–702. [DOI] [PubMed] [Google Scholar]
- 13. Drekonja D, Reich J, Gezahegn S, et al. . Fecal microbiota transplantation for clostridium difficile infection: a systematic review. Ann Intern Med. 2015;162:630–638. [DOI] [PubMed] [Google Scholar]
- 14. Cammarota G, Masucci L, Ianiro G, et al. . Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther. 2015;41:835–843. [DOI] [PubMed] [Google Scholar]
- 15. Kelly CR, Khoruts A, Staley C, et al. . Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Ann Intern Med. 2016;165:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66:987–994. [DOI] [PubMed] [Google Scholar]
- 17. Kelly CR, Ihunnah C, Fischer M, et al. . Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khoruts A, Rank KM, Newman KM, et al. . Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2016;14:1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paramsothy S, Paramsothy R, Rubin DT, et al. . Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11:1180–1199. [DOI] [PubMed] [Google Scholar]
- 20. Smith MB, Kassam Z, Burgess J, et al. . The International Public Stool Bank: a scalable model for standardized screening and processing of donor stool for fecal microbiota transplantation. Gastroenterology. 2015;148:S–211. [Google Scholar]
- 21. Dubois N, Ling K, Osman M, et al. . Prospective assessment of donor eligibility for fecal microbiota transplantation at a public stool bank: results from the evaluation of 1387 candidate donors. Open Forum Infect Dis. 2016;2(suppl 1):962. [Google Scholar]
- 22. Kassam Z, Dubois N, Ramakrishna B, et al. . Donor screening for fecal microbiota transplantation. N Engl J Med. 2019;381:2070–2072. [DOI] [PubMed] [Google Scholar]
- 23. Kozich JJ, Westcott SL, Baxter NT, et al. . Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Quast C, Pruesse E, Yilmaz P, et al. . The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allegretti JR, Kearney S, Li N, et al. . Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther. 2016;43:1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mullish BH, Pechlivanis A, Barker GF, et al. . Functional microbiomics: evaluation of gut microbiota-bile acid metabolism interactions in health and disease. Methods. 2018;149:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarafian MH, Lewis MR, Pechlivanis A, et al. . Bile acid profiling and quantification in biofluids using ultra-performance liquid chromatography tandem mass spectrometry. Anal Chem. 2015;87:9662–9670. [DOI] [PubMed] [Google Scholar]
- 28. Mullish BH, McDonald JAK, Pechlivanis A, et al. . Microbial bile salt hydrolases mediate the efficacy of faecal microbiota transplant in the treatment of recurrent Clostridioides difficile infection. Gut. 2019;68:1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McDonald JAK, Mullish BH, Pechlivanis A, et al. . Inhibiting growth of Clostridioides difficile by restoring valerate, produced by the intestinal microbiota. Gastroenterology. 2018;155:1495–1507.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allegretti JR, Kelly CR, Grinspan A, et al. . Outcomes of fecal microbiota transplantation in patients with inflammatory bowel diseases and recurrent Clostridioides difficile infection. Gastroenterology. 2020:S0016-5085(20)35008-3. [DOI] [PubMed] [Google Scholar]
- 31. Qazi T, Amaratunga T, Barnes EL, et al. . The risk of inflammatory bowel disease flares after fecal microbiota transplantation: systematic review and meta-analysis. Gut Microbes. 2017;8:574–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martinez-Gili L, McDonald JAK, Liu Z, et al. . Understanding the mechanisms of efficacy of fecal microbiota transplant in treating recurrent Clostridioides difficile infection and beyond: the contribution of gut microbial-derived metabolites. Gut Microbes. 2020;12(1):1810531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sinha SR, Haileselassie Y, Nguyen LP, et al. . Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe. 2020;27:659–670.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quraishi MN, Shaheen W, Oo YH, Iqbal TH. Immunological mechanisms underpinning faecal microbiota transplantation for the treatment of inflammatory bowel disease. Clin Exp Immunol. 2020;199:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]