Abstract
There is a risk of exposure to drugs in neonates during the lactation period due to maternal drug intake. The ability to predict drugs of potential hazards to the neonates would be useful in a clinical setting. This work aimed to evaluate the possibility of integrating milk‐to‐plasma (M/P) ratio predictive algorithms within the physiologically‐based pharmacokinetic (PBPK) approach and to predict milk exposure for compounds with different physicochemical properties. Drug and physiological milk properties were integrated to develop a lactation PBPK model that takes into account the drug ionization, partitioning between the maternal plasma and milk matrices, and drug partitioning between the milk constituents. Infant dose calculations that take into account maternal and milk physiological variability were incorporated in the model. Predicted M/P ratio for acetaminophen, alprazolam, caffeine, and digoxin were 0.83 ± 0.01, 0.45 ± 0.05, 0.70 ± 0.04, and 0.76 ± 0.02, respectively. These ratios were within 1.26‐fold of the observed ratios. Assuming a daily milk intake of 150 ml, the predicted relative infant dose (%) for these compounds were 4.0, 6.7, 9.9, and 86, respectively, which correspond to a daily ingestion of 2.0 ± 0.5 mg, 3.7 ± 1.2 µg, 2.1 ± 1.0 mg, and 32 ± 4.0 µg by an infant of 5 kg bodyweight. Integration of the lactation model within the PBPK approach will facilitate and extend the application of PBPK models during drug development in high‐throughput screening and in different clinical settings. The model can also be used in designing lactation trials and in the risk assessment of both environmental chemicals and maternally administered drugs.
Study Highlights .
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
The amount of drug excreted into the breast milk depends upon the physicochemical properties of the drug, the mechanism of transport, and the composition of the milk.
WHAT QUESTION DID THIS STUDY ADDRESS?
Integrating different predictive mathematical models within the physiologically‐based pharmacokinetic (PBPK) approach to predict the drug concentration in the milk and to calculate the expected infant dose received during breastfeeding.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Lactation PBPK models can further increase our understanding with respect to gaining insights into drug distribution into the milk during breastfeeding and accounting for drug exposure variability in the milk due to variability in physiological parameters and/or differences in drug properties.
HOW MIGHT THIS CHANGE DRUG DISCOVERY, DEVELOPMENT, AND/OR THERAPEUTICS?
Lactation PBPK models can be implemented during the high throughput screening drug discovery process, and for designing lactation studies, as well as to guide neonatal/infant risk assessments, such as in the case of maternal comedications.
INTRODUCTION
Lactating mothers are either required to use drugs, or are exposed to environmental hazards, during breastfeeding and are therefore confronted with a difficult choice to either discontinue nursing or terminate their maternal medication to avoid potentially harming their infants. Lack, or even poor quality, of information about drug safety during lactation can cause confusion in giving recommendations, which can result in the early cessation of breastfeeding.1
Recently, there has been an increased interest in understanding the pharmacokinetics (PKs) in lactating women and the rate, and extent, of drug distribution into the milk.2, 3 However, published research use different methodology and incomplete information on data variability, calling for a unified scientific framework.4 The US Food and Drug Administration (FDA) has released regulatory guidelines and drafts requesting pharmaceutical companies to address the potential impact of maternal drug exposure, levels of the drug (and metabolite) appearing in breast milk, the potential effects on the feeding infants, and effects of the drug on milk production.5, 6 Unless breastfeeding is contraindicated during drug therapy, a risk and benefit statement must be provided in order to help healthcare providers to make the appropriate prescribing decisions for a lactating patient.
Most drugs carry different levels of risks to the breastfed infant.7 These risks should not be exaggerated, because neonates and infants in most cases receive a much lower dose in breast milk, compared to the known safe dose of the same drug administered to them in clinical pediatrics wards,8 however, some drugs could be contraindicated or may have not yet adequately been studied so precautions should be taken. Quantification of drugs in breast milk is important to raise our awareness of the potential adverse effects, if any, on the infant. In addition, the quantification of drugs will reduce the confusion and anxiety that lactating women may experience during breastfeeding, especially those on chronic medications.
The amount of drugs excreted into the breast milk depends upon the composition of the milk, the physicochemical properties of the drug, and the mechanism of transport. The milk composition and affinity of its components together with the pH are important factors that determine the extent to which drugs are excreted and accumulated in the milk.9 The physicochemical properties of drugs include the size of the molecules, its lipid solubility, and its charge type. The higher the lipid solubility, the greater the concentration in the human milk. Although the majority of drugs are transported into mammary blood capillaries via passive diffusion, a few drugs, such as cimetidine,10 are believed to reach and accumulate in the milk via active transporter mechanisms.
Modeling and simulation have contributed to our understanding of the passage of drugs into human milk. One historical approach still being used is the prediction of the drug level in milk based on plasma concentration and using what is called the milk‐to‐plasma (M/P) ratio. The M/P ratio itself can be predicted using both drug and milk characteristics, as has been shown for different drugs using average population parameters (i.e., deterministic models).9, 11
The objectives of this work are: (1) to collect and integrate physiological data regarding milk parameters that are required for the Fleishaker12 and Atkinson13 models within the PBPK model framework taking into account the interindividual variability in maternal and milk physiological parameters; (2) to use these built‐in models available within the Simcyp Simulator V20 to predict the M/P ratio and milk concentration of different compounds with different physicochemical properties, namely acetaminophen, alprazolam, caffeine, and digoxin. These compounds have been selected because their relevant plasma and milk data have been published, their PBPK models have been developed, and their mechanism of excretion into the milk are mainly via passive diffusion; and (3) to calculate the infant daily dose indices from the predicted milk concentration.
METHODS
Data collection on milk properties
Data on milk volume, pH, and creamatocrit (Crt) were collected from published clinical studies. PubMed was used as the primary search engine for the literature review. A separate search was conducted for each parameter and the following keywords were used in different combinations: breast milk, human milk, pH, composition, lipid, fat, Crt, volume, and production. Only the data from healthy White lactating mothers recorded after the first postpartum week up to 1 year of lactation were included. Data extracted from these studies were converted to standard units. The reported fat content in the milk was converted to Crt.14 Because the goal of this data collection process was not to collect all data available, but rather to get sufficient data to parametrize the integrated model, collected data were pooled together without accounting for the stage of lactation, analytical techniques, or feeding frequency. If the lactation stage was specified as a range, the midpoint of the lactation stage was used. The weighted mean and overall variability coefficient of variation were calculated as described in the Supplementary Material Section 1.
Lactation model building
The built‐in lactation model within the Simcyp Simulator V20 was used to predict the milk concentration for different compounds using the available information on their systemic and milk exposure. The built‐in perfusion lactation model within the Simulator was selected for all investigated compounds. A schematic description of the lactation model is presented in Figure 1.
FIGURE 1.
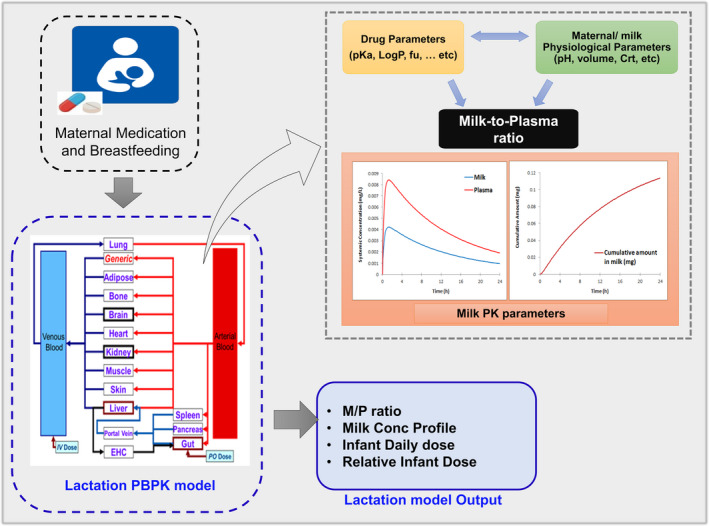
A schematic representation of inputs and outputs of the lactation model used in this study. M/P, milk‐to‐plasma; PBPK, physiologically‐based pharmacokinetic; PK, pharmacokinetic
The assumed lactation model takes the general form of the Fleishaker’s model12 (Equation 1). The model provides an empirical approach and aimed at predicting the M/P ratio using milk composition and physicochemical properties of the drug. It does not account for mammary gland size, perfusion, or composition.
| (1) |
where, is the individualized unbound fraction of the drug in the plasma predicted within the Simulator. The is the individualised unbound fraction of the drug in the milk. In the absence of measurement, it can be calculated according to Equation 215, 16:
| (2) |
The fw and f fat are the fractional volumes of aqueous and fat components, respectively, in the milk sampled from a population mean and distribution using a lognormal distribution. The is the unbound fraction of the drug in skimmed milk. In the absence of required data, this value can be calculated from the unbound fraction in plasma according to Equation (3)17:
| (3) |
Because the value is individualized, the calculated and hence the predicted is individualized. is the milk fat‐to‐aqueous milk partition and for simplicity, the compound octanol‐to‐water partitioning constant (LogPo : w) is used in the current work. The unionized fraction of the drug in plasma, , and the unionized fraction of the drug in milk, , were calculated for a given compound type (monoprotic base, monoprotic acid, and ampholyte) using the compound pKa(s), plasma pH, and milk pH. For a monoprotic base, it is calculated using the following equations:
| (4) |
| (5) |
Where the pH of the milk derived from the population library is based on the data collected from literature (for other compound types, see Supplementary Material Section 2). The S/W ratio is the drug partitioning between skimmed and whole milk and can be predicted using the following equation16:
| (6) |
Where Crt is the individual creamatocrit generated from the Simulator based on collected physiological data. Finally, the drug concentration in milk for each subject (CMilk) is calculated by multiplying the maternal plasma systemic concentration (CPlasma) by the M/P ratio for each individual as follows:
| (7) |
The predicted milk concentration time profiles were used to calculate the infant daily dose (IDD) and relative infant daily dose (RIDD) assuming an infant body weight of 5 kg and a daily milk intake of 0.150 L as a fixed value18:
IDD (mg/kg/day) = average concentration (Caverage; mg/L) * Daily milk intake (L/day) / infant body weight (kg).
IDD (mg/kg/day) = maximum concentration (Cmax; mg/L) * Daily milk intake (L/day) / infant body weight (kg).
where the mother daily dose is the maternal weight‐adjusted dose.
Prediction of the drug concentration profile in milk ‐ Case studies
Acetaminophen
Acetaminophen is a weak acid (pKa 9.38) with a relatively low lipophilicity (LogPo:w = 0.46).19 Its excretion into human milk has been assessed in three lactating women after ingestion of a single 500 mg dose of paracetamol.20 To replicate this study, a previously developed PBPK model for acetaminophen19 was initially used to predict the maternal plasma concentration profile after a single 500 mg oral dose of acetaminophen was administered to 200 virtual female subjects aged 24–31 years and randomly and equally divided into 20 virtual trials (10 women in each trial). The model was then extended to predict the level of acetaminophen in the milk according to the equations described in the method section without any fittings.
Alprazolam
Alprazolam excretion into human milk has been evaluated in eight healthy lactating women 6–28 weeks postpartum after a single oral administration of 0.5 mg alprazolam.21
The compound file for alprazolam is a default compound within the Simulator library, defined as a weak base (pKa of 2.4) and a lipophilic (LogPO:W = 2.12) compound. Default input parameters for alprazolam were used, with the absorption being predicted using the compound polar surface area and hydrogen bond donor available within the Simulator. In vitro data on alprazolam fu in milk 0.74 (7% coefficient of variation [CV]) and S/W ratio 0.86 were measured in eight lactating women21 were incorporated in the model, whereas the rest of the model parameters were predicted as described in the Method section. The model was then used to replicate a clinical trial design that evaluated the disposition kinetics of alprazolam in eight healthy women 6–28 weeks postpartum after oral administration of a single dose of 0.5 mg alprazolam.21 The PBPK trial designs were set to match the clinical settings using 20 trials with eight lactating women aged 20–40 years in each trial.
Caffeine
Caffeine PKs during lactation were assessed in different studies in healthy lactating women at least 2 weeks postpartum.22, 23, 24, 25 The caffeine PBPK compound file within the Simcyp Simulator compound library is defined as a basic (pKa = 1.05) and hydrophilic (LogPo:w = −0.07) compound. The compound file was used as per default settings. The virtual trial designs were set to match the design of three clinical studies as a single oral dose of 200 mg caffeine23 (20 trials with 5 lactating women aged 20–40 years in each trial), a single oral dose of 100 mg caffeine22 (20 trials with 6 lactating women aged 23–35 years in each trial), and a single oral dose of 80 mg caffeine25 (20 trials with 10 lactating women aged 30–35 years in each trial).
Digoxin
Digoxin excretion into human milk has been evaluated in 11 lactating mothers after administration of 0.5 mg of the drug as an intravenous bolus injection.26 To predict digoxin concentration in the milk, the default settings of the digoxin compound file within the Simulator were retained and coupled to the lactation model. The digoxin compound model is defined as a neutral compound with a LogPo:w of 1.26.
The virtual trial design was set up as a single intravenous bolus dose of 0.5 mg of digoxin administered to 220 lactating mothers aged 20–40 years, who were divided randomly into 20 trials. The simulation was then executed for 24 h.
RESULTS
Milk physiological parameters
Collected data for milk volume, pH, and Crt are presented in Table S2 (Supplementary Material Section 3). The weighted means were 0.77 ± 0.26 L (N = 565), 7.02 ± 0.59 (N = 536), and 6.6 ± 2.7% (N = 1231) for the daily milk volume production (both breasts), pH, and Crt (%), respectively. These values were used in the lactation model to generate individual values for the milk volume and Crt, whereas the milk pH was assumed to be fixed.
Acetaminophen
PBPK predictions for acetaminophen plasma and milk levels in lactating mothers were in agreement with the observed profiles (Figure 2). The predicted mean concentration–time profiles closely followed the observed mean profiles. Comparison of the PK parameters, obtained for the simulated profiles, also showed good agreement with those reported in a clinical study20 (Table 1). All PK parameters were within 1.1‐fold of the observed values. Assuming a daily milk intake of 150 ml, the predicted RIDD for acetaminophen, calculated using the Caverage method, was 4.0 ± 0.8%, which corresponds to an average intake of 0.30 (range 0.13–0.51) mg/kg/day. The Cmax method gave a relatively higher RIDD of 8.0 ± 1.6% (range 4.7–12.1%), which corresponds to an average acetaminophen intake of 0.61 (range 0.25–1.03) mg/kg/day.
FIGURE 2.
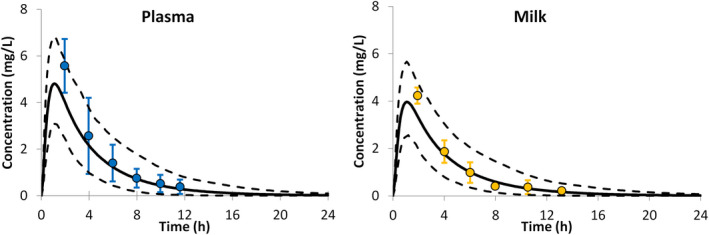
Acetaminophen concentration in plasma and in milk after a single oral administration of 500 mg. Circles with error bars represent the observed mean concentration and associated SD,20 the solid lines represent the predicted mean with the predictive 5th and 95th percentiles given as broken lines
TABLE 1.
Predicted versus observed PK parameters of acetaminophen, alprazolam, and caffeine in plasma and milk during lactation
| Model parameter | Plasma | Milk | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Predicted | Ratio | Observed | Predicted | Ratio | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Acetaminophen20 | ||||||||||
| Half‐life (h) | 2.74 | 0.28 | 2.49 | 0.74 | 0.91 | 2.64 | 0.63 | 2.48 | 0.74 | 0.94 |
|
AUCINF (mg/L/h) |
23.02 | 6.67 | 21.68 | 8.7 | 0.94 | 17.38 | 4.43 | 17.90 | 7.19 | 1.03 |
| fu | 0.802 | 0.0056 | 0.86 | 0.01 | 1.07 | 0.9745 | 0.0035 | 0.99 | 0.0 | 1.02 |
| M/P ratio | 0.76 | 0.04 | 0.83 | 0.01 | 1.09 | |||||
| Alprazolam21 | ||||||||||
| Cmax (ng/ml) | 8.88 | 2.69 | 10.33 | 3.15 | 1.16 | 3.7 | 1.59 | 4.68 | 1.48 | 1.26 |
| Tmax (h)* | 0.6 | (0.45–2.65) | 1.00 | (0.75–1.30) | 1.67 | 1.1 | (0.45 – 3.83) | 1.00 | (0.75–1.30) | 0.91 |
| Half‐life (h) | 12.52 | 3.53 | 11.27 | 5.27 | 0.90 | 14.46 | 6.27 | 11.27 | 5.27 | 0.78 |
| CLpo (ml/min/kg) | 1.15 | 0.32 | 1.04 | 0.36 | 0.90 | |||||
| M/P ratio | 0.36 | 0.11 | 0.45 | 0.05 | 1.26 | |||||
| Caffeine | ||||||||||
| Oo et al., 199523 | ||||||||||
| Half‐life (h) | 5.6 | 1.8 | 5.81 | 5.29 | 1.0 | |||||
| CLpo (ml/min/Kg) | 1.0 | 0.2 | 1.7 | 1.3 | 1.7 | |||||
| M/P ratio | 0.70 | 0.06 | 0.69 | 0.03 | 1.0 | |||||
| Stavchansky et al., 198822 | ||||||||||
| Cmax (mg/L) | 4.4 | 1.0 | 2.5 | 0.7 | 0.6 | 2.8 | 0.6 | 1.7 | 0.5 | 0.6 |
| Tmax (h) | 0.8 | 0.2 | 1.4 | 0.5 | 1.8 | 1.1 | 0.5 | 1.5 | 0.5 | 1.3 |
| Half‐life (h) | 7.3 | 3.6 | 5.8 | 5.3 | 0.8 | 7.2 | 3.4 | 5.8 | 5.3 | 0.8 |
| AUCt (mg/L.hr) | 33.8 | 18.6 | 25.3 | 20.1 | 0.7 | 26.6 | 11.3 | 17.4 | 13.4 | 0.7 |
| M/P ratio | 0.82 | 0.05 | 0.70 | 0.04 | 0.9 | |||||
| Calvaresi et al., 201625, a | ||||||||||
| AUCt (mg/L.hr) | 10.28 | 13.93 | 3.08 | 1.4 | ||||||
| Cmax (mg/L) | 2.06 | 1.40 | 0.43 | 0.7 | ||||||
| Half‐life (h) | 4 | 5.84 | 5.27 | 1.5 | ||||||
Half‐life = elimination half‐life; AUCINF, extrapolated area under the curve to infinity; AUCt, AUC to last time of measurement; fu = fraction unbound; Cmax = maximum concentration; Tmax = time to reach Cmax; CLpo = apparent total body clearance; M/P ratio = milk‐to‐plasma ratio; PK, pharmacokinetic; *median (range)
Individual value.
Alprazolam
The PBPK predictions for alprazolam showed good agreement with the observed drug level in the systemic circulation as well as in the milk of breastfeeding mothers (Figure 3). Comparison of the PK parameters obtained for the simulated profiles with those from clinical studies (Table 1) also showed good agreement and all PK parameters were within twofold of the observed values. The predicted mean (± SD) AUC36hr and AUCINF for plasma were 145 ± 50 and 168 ± 69 ng/ml/h, whereas the corresponding values for milk were 65 ± 23 and 76 ± 31 ng/ml/h, respectively. Assuming a daily milk intake of 150 ml, the predicted RIDD calculated using the Caverage method was 6.7 ± 2.1%, which corresponds to an average alprazolam intake of 554 (range 236–1158) ng/kg/day. The Cmax method predicted RIDD of 11.1±3.0 (range 5.2–18.8%), which corresponds to an average intake of 900 (range 391–1706) ng/kg/day.
FIGURE 3.
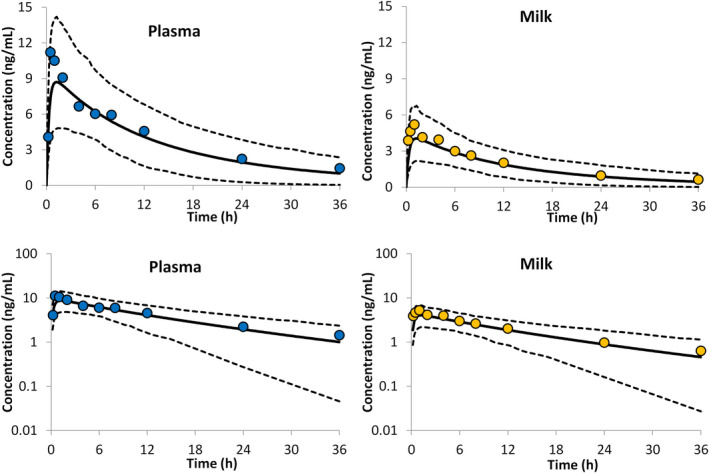
Concentration time profiles of alprazolam in plasma (left) and in milk (right). Circles represent the reported mean values from eight lactating women,21 the lines represent the physiologically‐based pharmacokinetic (PBPK) predicted mean (solid lines) together with the predicted 5th and 95th percentiles (dashed lines). The lower panels show the concentration time profiles on log scales
Caffeine
PBPK predictions for caffeine plasma and milk levels in lactating mothers are presented in Figure 4. A comparison of the observed and predicted PK parameters for caffeine are presented in Table 1. Comparison of the PK parameters obtained for the simulated profiles with those from clinical studies (Table 1) were in good agreement and all PK parameters were within twofold of the observed values.
FIGURE 4.
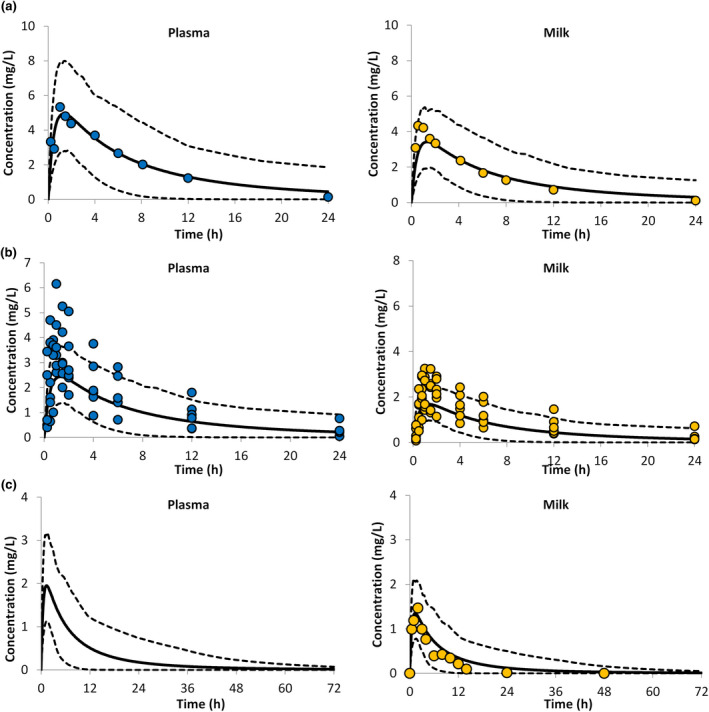
Concentration time profiles of caffeine in plasma (left) and in milk (right). (a) Circles represent the reported mean values from five lactating women (Oo et al., 199523). (b) Circles represent the reported mean values from six lactating women (Stavchansky et al., 198822). (c) Circles represent the reported values for caffeine in the milk of one lactating woman (Calvaresi et al., 201625), the lines represent the PBPK predicted mean (solid lines) together with the predicted 5th and 95th percentiles (dashed lines)
The predicted mean (± SD) AUC24hr for plasma and milk caffeine levels were 49 ± 37 and 34 ± 26 mg/L/h, respectively. Assuming a daily milk intake of 150 ml, the predicted RIDD calculated using the Caverage method was 9.9 ± 4.3%, which corresponds to an average caffeine intake of 0.32 (range 0.10–1.23) mg/kg/day. The Cmax method gave an RIDD of 17.9 ± 6.2 (range 5.6–49.0%), which corresponds to an average intake of 0.58 (range 0.19–1.59) mg/kg/day.
Digoxin
PBPK predictions for digoxin plasma and milk levels in lactating mothers are presented in Figure 5. These predictions were in good agreement with the observed profiles. The predicted unbound fraction of the drug in the milk was 0.95 ± 0.001, which is also close to the measured value of 0.947 (digoxin binding to milk protein was reported to be 5.3%17). The model predicted a M/P ratio of 0.76 ± 0.02 (range 0.70–0.82). Based on a daily milk intake of 150 ml, the predicted RIDD for digoxin, calculated using the Caverage method, was 86 ± 13%, which corresponds to an average intake of 0.0064 (range 0.0045–0.0089) mg/kg/day. The Cmax method gave a RIDD of 171 ± 26 (range 110–231%), which corresponds to an average intake of 0.0127 (range 0.0090–0.0177) mg/kg/day.
FIGURE 5.
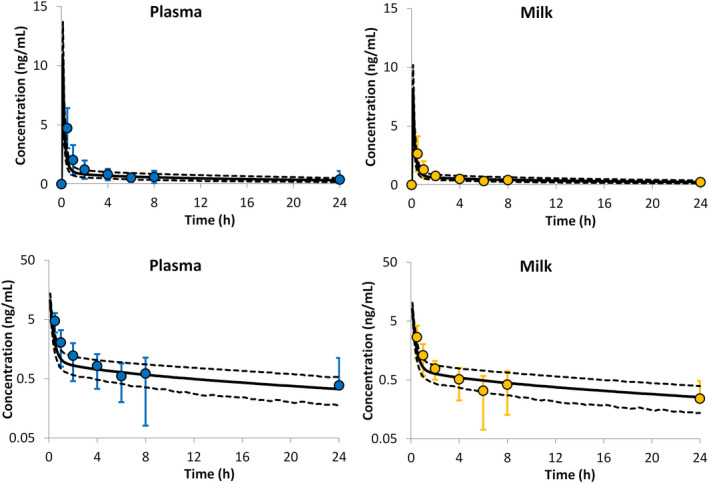
Concentration time profiles of digoxin in plasma and in milk. Circles represent the reported mean values from 11 lactating women26 with error bars representing the SDs. The solid continuous lines represent the PBPK predicted means together with the predicted 5th and 95th percentiles shown as dashed lines. The lower panels show the concentration time profiles on log scales
DISCUSSION
The current study assesses the potential of integrating a lactation model within existing PBPK models. The lactation model uses physicochemical properties of the drug together with physiological parameters to describe the drug’s disposition during lactation using substrates with different properties.
Collected data indicated that the mean milk volume produced per day, the milk pH, and Crt were about 770 ml (33% CV), 7.02 (8% CV), and 6.6% (41%CV), respectively. Utilizing these data in the integrated milk model facilitates the prediction of the M/P ratio and its variability in a virtual population of lactating mothers. Limited published data suggests that full milk production occurs by the second week of lactation and remains relatively constant up to 6 months from the start of lactation when an infant is exclusively breastfed.27, 28 The milk pH is reported to drop from a value of 7.45 after birth to a nadir value of 7.05 and then slowly increase reaching a value of 7.2 during the third month postpartum.29 Other investigations did not observe this trend.30, 31 Crt variability reflects the variations in human milk fat content. It tends to be higher in hind milk samples compared to fore milk samples.14, 32 In a published meta‐analysis, the fat content was reported to be stable between 2 and 12 weeks postpartum (range 3–8.5 g/100 ml).33
Acetaminophen is a weak acid with a pKa of 9.38 (see reference in Ref. 19) and hence more ionized in the plasma pH (~7.4) than in the milk (milk pH ~6.7–7.2) resulting in less exposure in the milk. In addition, the fact that the milk albumin level is lower than the plasma protein level further contributes to this distribution difference. The predicted agreed with the observed value (Table 1). Likewise, the predicted M/P ratio of 0.83 ± 0.01 was also in good agreement with the in vivo value of 0.76 ± 0.0420 and a measured in vitro value of 0.81.20 The PBPK predicted RIDD of 4.0 ± 0.8% (Caverage method) and 8.0 ± 1.6% (Cmax method) suggested that acetaminophen may be safe during breastfeeding.
For alprazolam, the model predictions were within twofold of the observed values (Table 1), with the exemption of the time to Cmax (Tmax) value, however, the predicted parameter range was in a good overlap with the observed range for this parameter. The predicted M/P ratio of 0.45 ± 0.05 (range 0.34–0.60) is close to the reported M/P (area under the curve [AUC]) value of 0.36 ± 0.11 (range 0.19–0.49) from eight lactating women21 and an M/P value of 0.52 (trough conc), 0.49 (at 2 h postdose) and 0.41 (at 2 h postdose) on day 3, day 4, and 1 month postpartum in a breastfeeding woman on a chronic administration of variable alprazolam doses.34 Assuming a daily milk intake of 150 ml, the mean predicted RIDD was 6.7 ± 2.1 (range 3.14–12.88%) (Caverage method). An RIDD value of 3% has been reported assuming a steady‐state alprazolam serum concentration of 0.20–0.40 µg/ml.21 Another study reported a range of 3.11–4.61% for alprazolam RIDD based on limited observations.34 Although the predicted mean RIDD may support the propriety of breastfeeding alprazolam therapy, the predicted wide range for RIDD indicates that each individual situation should be taken into account and good clinical judgment should be exercised in weighing any potential risk to the infant versus the benefits of breastfeeding. Many studies reported mild adverse effects of alprazolam on breastfed infants, such as drowsiness and other infant withdrawal symptoms,35 which probably indicates that sufficient amounts of alprazolam reached the effect site to provoke these symptoms in the infants. High infant drug ingestion can occur in case of maternal comedications with CYP3A4 inducers that can reduce the drug elimination resulting in an elevation in the level of alprazolam in the milk.
The predicted caffeine M/P ratio of 0.70 ± 0.04 (range 0.62–0.77) is in agreement with the observed ratio of 0.70 ± 0.0623 and 0.82 ± 0.05,22 but slightly above a reported value of 0.52 ± 0.10.36 A wider M/P ratio range of 0.44–11.8 has been reported from in vivo data (quoted from Ref. 37). The predicted RIDD of 9.9 ± 4.3% (Caverage method), is in line with the reported value of 7%.23 The predicted RIDD of 17.9 ± 6.2% (Cmax method) is greater than 10% of maternal dose/bodyweight. The predicted infant daily caffeine intake from milk was about 0.32 (range 0.10–1.23) mg/kg/day (Caverage method) and 0.58 (range 0.19–1.59) mg/kg/day (Cmax method) are in line with the reported range of 0.6–0.8 mg/kg/day38 and 0.44–0.55 mg/kg/day.22 The predicted amounts are still lower than the initial 5 mg/kg/day recommended maintenance dose and therefore avoids the risk of apnea in premature infants (caffeine loading dose is about 3–4 higher than the maintenance dose).39 After a maternal intake of 200 mg/day caffeine (roughly 2 cups of coffee), the total amount of caffeine ingested by a 5 kg infant per day is on average 1.6 mg (Caverage method) or 2.9 mg (Cmax method). These levels of caffeine are in agreement with previously reported expected daily infant ingested doses of 1.8–3.1 mg for infants weighing 4–7 kg.22 Although maternal ingestion of 100 mg of caffeine appears to be safe for the breastfed infant, accumulation of caffeine can occur, especially in a premature infant due to the lower capacity of their CYP1A2 enzyme, which can lead to adverse effects.
Digoxin binding to milk protein is predicted to be minimal with of 0.95 ± 0.001, which is in a good agreement with the reported value of 0.947.17 The model predicted an M/P ratio of 0.76 ± 0.02 (range 0.70–0.82), which is also in line with the reported range of measured M/P ratios of 0.59 to 0.85.26, 40, 41 The model predicted RIDD for digoxin seems high, 86 ± 13% (Caverage method) and 171 ± 26% (Cmax method) because of the maternal dose being normalized to kg of maternal bodyweight. However, when translated to absolute daily dose, they correspond to an average of 6.3% (Caverage method) and 12.7% (Cmax method) of a 0.5 mg maternal dose. In fact, the RIDD without body weight adjustment, calculated based on the whole concentration profile, is only about 3.7%. In terms of absolute infant daily dose, the predicted amount of digoxin ingested per day ranged from 0.0009 to 0.0177 mg/kg/day. These amounts are still less than the 35% of the digitalizing dose (0.05 mg/kg) given orally to four newborn infants.42 In addition, digoxin oral bioavailability in newborn infants was found to be around 72%,42 which further support the statement that digoxin can be safely administered routinely in nursing women.26
Although the integrated lactation model describes the observed milk disposition kinetics adequately, it is important to note that the model assumes a passive diffusion process to describe the transfer of drugs across the mammary epithelial membrane. The model also assumes a rapid equilibrium between plasma and milk compartments. Although this is the case for many drugs, like digoxin and acetaminophen, it is intuitive that a more mechanistic model is required to describe the mechanisms by which slowly equilibrated drugs reach their maximum concentrations. Knowing the time at which a maximum drug concentration in the milk is achieved is clinically relevant for drugs of potential risk to the infant. With this information, clinicians can advise lactating mothers to delay the time of a feeding session with respect to the time of drug administration.
Another limitation of the presented model is that the dynamic nature of the milk intake throughout the day and the longitudinal changes in the milk pH were not considered. Although one can update these two parameters in the model to replicate the scenario of interest, built‐in dynamic features, including colostrum, are considered for further model enhancement. Another point to consider is that milk volume and its components are affected by a collection of covariates, including the frequency and stage of lactation, method of measurements, the degree of breast fullness and emptying, and pre‐ and post‐feed samplings.32, 33, 43, 44 Longitudinal quantification of the impact of these covariates on milk parameters is required to increase the confidence in the PBPK model predictions. The change in the milk pH and its composition during the first postpartum week can have significant effect on the amount of the drug retained in the milk, especially for basic compounds, such as B‐blockers or compounds with high LogP. The limited amount of milk that can be produced at this time and ingested by the infant also affected the infant daily dose. Therefore, caution is warranted when using such an empirical model outside the model assumptions for drugs with narrow therapeutic index. Because the described model is empirical and does not include mammary gland, there are no passive or active transfer kinetics from or into the mammary cells.
A previous study described integration of the milk composition and linked it to the plasma concentration using a two‐compartment model.15 The current work integrates the milk composition within the PBPK approach considering the physiological parameter distributions and complement the model with infant dose calculation algorithms. Currently available human lactation PBPK models are limited to those developed for chemical compounds; examples of these models have been published.45, 46, 47 Other human PBPK models developed for therapeutic compounds do not use milk compositions to predict the milk exposure; instead, they use a predefined M/P ratio as a prior parameter.48, 49 Integrating a lactation model within a PBPK platform is a step forward in gaining insights into drug distribution into the milk during breastfeeding.3 Lactation PBPK models have many potential practical applications and clinical settings to improve maternal adherence to the treatment and increase infants’ access to the breast milk by informing drug labels at least for those compounds with low relative infant dose. Scenarios where maternal systemic drug exposure change due to comorbidity and/or comedication can also be evaluated in silico. Because prediction of the relative infant dose can be performed preclinically within the PBPK platform, the lactation model can be used as a guidance during the high throughput screening drug discovery process, where the generated IDD or RIDD are above an accepted value. Identifying such risk signals can inform clinical trial designs of potential drugs that may be administered during breastfeeding. An average milk intake value of 0.15 L/kg/day was used in the model for the calculation of infant dose. Although this value is just representative of the milk intake for a neonate and may not reflect the actual daily milk intake, especially in very young or older infants.
CONCLUSION
This study shows the usefulness of an integrated lactation model within a PBPK platform to predict the drug level in breast milk after maternal administration. The described model could be used to regulate maternal dosing during breastfeeding, predict the expected infant daily dose ingested from the milk, and guide neonatal/infant risk assessments for drugs where such information is not available. Breastfeeding style, milk composition, and volume of milk ingested can affect the amount of the drug ingested by the infant. From an infant safety perspective, the predicted infant daily dose should not be interpreted without considering the infant physiology and the drug kinetics at different infant ages. Infants can eliminate the drug to different magnitudes due to the stage of maturation of the metabolizing enzymes, their genotypes, and renal function. The maturation of the absorption process can affect the amount and extent of the drug that reaches the infant blood circulation. Differences in infant body composition can also affect the distribution of the drugs. Finally, drug receptors also undergo maturation and can therefore affect the manifestation of the response in the breastfed infant.
CONFLICT OF INTEREST
All authors are full‐time employees of Certara UK Limited (Simcyp Division). The activities of Certara are supported by a consortium of pharmaceutical companies. The Simcyp Simulator is currently available at no cost, following completion of the training workshop, to approved members of academic institutions and other not‐for‐profit organizations for research and teaching purposes.
AUTHOR CONTRIBUTIONS
K.A., A.P., and M.J. wrote the manuscript. K.A. and M.J designed the research. K.A., A.P., and J.N. performed the research. K.A., A.P., and J.N. analyzed the data.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Eleanor Savill and Anna Kenworthy for their assistance with technical preparation of the manuscript and submission. The authors also thank Dr. Ruth Clayton for helpful comments and proofreading and the reviewers of this manuscript for constructive suggestions and comments.
Abduljalil K, Pansari A, Ning J, Jamei M. Prediction of drug concentrations in milk during breastfeeding, integrating predictive algorithms within a physiologically‐based pharmacokinetic model. CPT Pharmacometrics Syst Pharmacol. 2021;10:878–889. 10.1002/psp4.12662
Funding information
No funding was received for this work.
REFERENCES
- 1.McClatchey AK, Shield A, Cheong LH, et al. Why does the need for medication become a barrier to breastfeeding? A narrative review. Women Birth. 2018;31:362‐366. [DOI] [PubMed] [Google Scholar]
- 2.Anderson PO, Momper JD. Clinical lactation studies and the role of pharmacokinetic modeling and simulation in predicting drug exposures in breastfed infants. J Pharmacokinet Pharmacodyn. 2020;47:295‐304. [DOI] [PubMed] [Google Scholar]
- 3.Nauwelaerts N, Deferm N, Smits A, et al. A comprehensive review on non‐clinical methods to study transfer of medication into breast milk ‐ a contribution from the ConcePTION project. Biomed Pharmacother. 2021;136:111038. [DOI] [PubMed] [Google Scholar]
- 4.Begg EJ, Duffull SB, Hackett LP, Ilett KF. Studying drugs in human milk: time to unify the approach. J Hum Lact. 2002;18:323‐332. [DOI] [PubMed] [Google Scholar]
- 5.Food and Drug Administration (FDA) . Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling (December 4, 2014). 2014. https://www.govinfo.gov/content/pkg/FR‐2014‐12‐04/pdf/2014‐28241.pdf. Accessed September 29, 2020. [PubMed]
- 6.Food and Drug Administration (FDA) . Clinical lactation studies: considerations for study design. Guidance for Industry (May 2019). 2019. https://www.fda.gov/media/124749/download. Accessed September 15, 2020.
- 7.Beauchamp GA, Hendrickson RG, Horowitz BZ, Spyker DA. Exposures through breast milk: an analysis of exposure and information calls to U.S. poison centers, 2001–2017. Breastfeed Med. 2019;14:508‐512. [DOI] [PubMed] [Google Scholar]
- 8.Anderson PO, Manoguerra AS, Valdes V. A review of adverse reactions in infants from medications in breastmilk. Clin Pediatr (Phila). 2016;55:236‐244. [DOI] [PubMed] [Google Scholar]
- 9.Atkinson HC, Begg EJ, Darlow BA. Drugs in human milk. Clinical pharmacokinetic considerations. Clin Pharmacokinet. 1988;14:217‐240. [DOI] [PubMed] [Google Scholar]
- 10.Oo CY, Kuhn RJ, Desai N, McNamara PJ. Active transport of cimetidine into human milk. Clin Pharmacol Ther. 1995;58:548‐555. [DOI] [PubMed] [Google Scholar]
- 11.Larsen LA, Ito S, Koren G. Prediction of milk/plasma concentration ratio of drugs. Ann Pharmacother. 2003;37:1299‐1306. [DOI] [PubMed] [Google Scholar]
- 12.Fleishaker JC, Desai N, McNamara PJ. Factors affecting the milk‐to‐plasma drug concentration ratio in lactating women: physical interactions with protein and fat. J Pharm Sci. 1987;76:189‐193. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson HC, Begg EJ. Prediction of drug distribution into human milk from physicochemical characteristics. Clin Pharmacokinet. 1990;18:151‐167. [DOI] [PubMed] [Google Scholar]
- 14.Meier PP, Engstrom JL, Zuleger JL, et al. Accuracy of a user‐friendly centrifuge for measuring creamatocrits on mothers’ milk in the clinical setting. Breastfeed Med. 2006;1:79‐87. [DOI] [PubMed] [Google Scholar]
- 15.Koshimichi H, Ito K, Hisaka A, Honma M, Suzuki H. Analysis and prediction of drug transfer into human milk taking into consideration secretion and reuptake clearances across the mammary epithelia. Drug Metab Dispos. 2011;39:2370‐2380. [DOI] [PubMed] [Google Scholar]
- 16.Begg EJ, Atkinson HC. Modelling of the passage of drugs into milk. Pharmacol Ther. 1993;59:301‐310. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson HC, Begg EJ. Prediction of drug concentrations in human skim milk from plasma protein binding and acid‐base characteristics. Br J Clin Pharmacol. 1988;25:495‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neville MC, Keller R, Seacat J, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. 1988;48:1375‐1386. [DOI] [PubMed] [Google Scholar]
- 19.Gaohua L, Neuhoff S, Johnson TN, Rostami‐Hodjegan A, Jamei M. Development of a permeability‐limited model of the human brain and cerebrospinal fluid (CSF) to integrate known physiological and biological knowledge: estimating time varying CSF drug concentrations and their variability using in vitro data. Drug Metab Pharmacokinet. 2016;31:224‐233. [DOI] [PubMed] [Google Scholar]
- 20.Bitzen PO, Gustafsson B, Jostell KG, Melander A, Wahlin‐Boll E. Excretion of paracetamol in human breast milk. Eur J Clin Pharmacol. 1981;20:123‐125. [DOI] [PubMed] [Google Scholar]
- 21.Oo CY, Kuhn RJ, Desai N, Wright CE, McNamara PJ. Pharmacokinetics in lactating women: prediction of alprazolam transfer into milk. Br J Clin Pharmacol. 1995;40:231‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stavchansky S, Combs A, Sagraves R, Delgado M, Joshi A. Pharmacokinetics of caffeine in breast milk and plasma after single oral administration of caffeine to lactating mothers. Biopharm Drug Dispos. 1988;9:285‐299. [DOI] [PubMed] [Google Scholar]
- 23.Oo CY, Burgio DE, Kuhn RC, Desai N, McNamara PJ. Pharmacokinetics of caffeine and its demethylated metabolites in lactation: predictions of milk to serum concentration ratios. Pharm Res. 1995;12:313‐316. [DOI] [PubMed] [Google Scholar]
- 24.Berlin CM Jr, Denson HM, Daniel CH, Ward RM. Disposition of dietary caffeine in milk, saliva, and plasma of lactating women. Pediatrics. 1984;73:59‐63. [PubMed] [Google Scholar]
- 25.Calvaresi V, Escuder D, Minutillo A, et al. Transfer of nicotine, cotinine and caffeine into breast milk in a smoker mother consuming caffeinated drinks. J Anal Toxicol. 2016;40:473‐477. [DOI] [PubMed] [Google Scholar]
- 26.Reinhardt D, Richter O, Genz T, Potthoff S. Kinetics of the translactal passage of digoxin from breast feeding mothers to their infants. Eur J Pediatr. 1982;138:49‐52. [DOI] [PubMed] [Google Scholar]
- 27.Kent JC, Gardner H, Geddes DT. Breastmilk production in the first 4 weeks after birth of term infants. Nutrients. 2016;8:756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kent JC, Mitoulas LR, Cregan MD, et al. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117:e387‐e395. [DOI] [PubMed] [Google Scholar]
- 29.Morriss FH Jr, Brewer ED, Spedale SB, et al. Relationship of human milk pH during course of lactation to concentrations of citrate and fatty acids. Pediatrics. 1986;78:458‐464. [PubMed] [Google Scholar]
- 30.Allen JC, Keller RP, Archer P, Neville MC. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of lactation. Am J Clin Nutr. 1991;54:69‐80. [DOI] [PubMed] [Google Scholar]
- 31.Ansell C, Moore A, Barrie H. Electrolyte pH changes in human milk. Pediatr Res. 1977;11:1177‐1179. [DOI] [PubMed] [Google Scholar]
- 32.Daly SE, Di Rosso A, Owens RA, Hartmann PE. Degree of breast emptying explains changes in the fat content, but not fatty acid composition, of human milk. Exp Physiol. 1993;78:741‐755. [DOI] [PubMed] [Google Scholar]
- 33.Gidrewicz DA, Fenton TR. A systematic review and meta‐analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furugen A, Nishimura A, Kobayashi M, et al. Quantification of eight benzodiazepines in human breastmilk and plasma by liquid‐liquid extraction and liquid‐chromatography tandem mass spectrometry: application to evaluation of alprazolam transfer into breastmilk. J Pharm Biomed Anal. 2019;168:83‐93. [DOI] [PubMed] [Google Scholar]
- 35.Kelly LE, Poon S, Madadi P, Koren G. Neonatal benzodiazepines exposure during breastfeeding. J Pediatr. 2012;161:448‐451. [DOI] [PubMed] [Google Scholar]
- 36.Tyrala EE, Dodson WE. Caffeine secretion into breast milk. Arch Dis Child. 1979;54:787‐789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notarianni LJ, Belk D, Aird SA, Bennett PN. An in vitro technique for the rapid determination of drug entry into breast milk. Br J Clin Pharmacol. 1995;40:333‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu JE. Caffeine in human milk and in serum of breast‐fed infants. Dev Pharmacol Ther. 1985;8:329‐337. [DOI] [PubMed] [Google Scholar]
- 39.WHO) . Pocket Book of Hospital Care for Children: Guidelines for the management of common illnesses with limited resources, 2nd edn. World Health Organisation: Published Online: http://www.ichrc.org/sites/default/files/pocket%20book%20high%20res_0.pdf, 2013. [PubMed] [Google Scholar]
- 40.Chan V, Tse TF, Wong V. Transfer of digoxin across the placenta and into breast milk. Br J Obstet Gynaecol. 1978;85:605‐609. [DOI] [PubMed] [Google Scholar]
- 41.Loughnan PM. Digoxin excretion in human breast milk. J Pediatr. 1978;92:1019‐1020. [DOI] [PubMed] [Google Scholar]
- 42.Wettrell G, Andersson KE. Absorption of digoxin in infants. Eur J Clin Pharmacol. 1975;9:49‐55. [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Jackson RT, Khan SA, Ahuja J, Pehrsson PR. Human milk nutrient composition in the United States: current knowledge, challenges, and research needs. Curr Dev Nutr. 2018;2:nzy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kent JC, Mitoulas L, Cox DB, Owens RA, Hartmann PE. Breast volume and milk production during extended lactation in women. Exp Physiol. 1999;84:435‐447. [PubMed] [Google Scholar]
- 45.Emond C, DeVito M, Warner M, et al. An assessment of dioxin exposure across gestation and lactation using a PBPK model and new data from Seveso. Environ Int. 2016;92–93:23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verner MA, Ayotte P, Muckle G, Charbonneau M, Haddad S. A physiologically based pharmacokinetic model for the assessment of infant exposure to persistent organic pollutants in epidemiologic studies. Environ Health Perspect. 2009;117:481‐487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon M, Schroeter JD, Nong A, et al. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: describing manganese homeostasis during development. Toxicol Sci. 2011;122:297‐316. [DOI] [PubMed] [Google Scholar]
- 48.Garessus EDG, Mielke H, Gundert‐Remy U. Exposure of infants to isoniazid via breast milk after maternal drug intake of recommended doses is clinically insignificant irrespective of metaboliser status. A physiologically‐based pharmacokinetic (PBPK) modelling approach to estimate drug exposure of infants via breast‐feeding. Front Pharmacol. 2019;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willmann S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast‐fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharmacol Ther. 2009;86:634‐643. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


