Abstract
Background.
Since the identification of the first 2 Candida auris cases in Chicago, Illinois, in 2016, ongoing spread has been documented in the Chicago area. We describe C. auris emergence in high-acuity, long-term healthcare facilities and present a case study of public health response to C. auris and carbapenemase-producing organisms (CPOs) at one ventilator-capable skilled nursing facility (vSNF-A).
Methods.
We performed point prevalence surveys (PPSs) to identify patients colonized with C. auris and infection-control (IC) assessments and provided ongoing support for IC improvements in Illinois acute- and long-term care facilities during August 2016–December 2018. During 2018, we initiated a focused effort at vSNF-A and conducted 7 C. auris PPSs; during 4 PPSs, we also performed CPO screening and environmental sampling.
Results.
During August 2016–December 2018 in Illinois, 490 individuals were found to be colonized or infected with C. auris. PPSs identified the highest prevalence of C. auris colonization in vSNF settings (prevalence, 23–71%). IC assessments in multiple vSNFs identified common challenges in core IC practices. Repeat PPSs at vSNF-A in 2018 identified increasing C. auris prevalence from 43% to 71%. Most residents screened during multiple PPSs remained persistently colonized with C. auris. Among 191 environmental samples collected, 39% were positive for C. auris, including samples from bedrails, windowsills, and shared patient-care items.
Conclusions.
High burden in vSNFs along with persistent colonization of residents and environmental contamination point to the need for prioritizing IC interventions to control the spread of C. auris and CPOs.
Keywords: Candida, fungal, communicable diseases, emerging, infection control
Since the identification of the first 2 cases of Candida auris in Chicago in 2016, ongoing spread has been documented in healthcare facilities in the area, particularly in high-acuity, long-term healthcare settings such as ventilator-capable skilled nursing facilities (vSNFs) and long-term acute-care hospitals (LTACHs) [1–3]. Adults residing in vSNFs and LTACHs are at increased risk of acquiring C. auris and other multidrug-resistant organisms (MDROs) due to multiple factors including serious underlying medical conditions; long healthcare facility stays; indwelling medical devices including tracheostomies, feeding tubes, and central venous catheters; frequent healthcare worker contact; and prolonged, broad-spectrum antibiotic exposure [4–6]. Candida auris persistently colonizes patients and contaminates the healthcare environment, allowing for easy transmission within a facility [7]. Additionally, patient movement between facilities contributes to regional spread [8]. Control measures include early identification of cases and adherence to infection-control practices such as hand hygiene (HH), setting-appropriate Transmission-Based Precautions, and cleaning and disinfection of the environment and patient-care equipment with hospital-grade agents with C. auris or sporicidal claim [9–11]. Ventilator-capable skilled nursing facility settings present opportunities to understand transmission dynamics of C. auris, such as drivers of acquisition and duration of colonization, and to improve targeting of prevention and containment strategies [12, 13]. We describe C. auris emergence in Chicago and present a case study of public health response to C. auris and carbapenemase-producing organisms (CPOs) at one Chicago vSNF.
METHODS
Case Definitions and Illinois Surveillance
Case-patients were defined as individuals for whom laboratory testing with culture or polymerase chain reaction (PCR) yielded a C. auris–positive result during May 2016–December 2018 in Illinois. A clinical case was defined as C. auris identified from a specimen obtained to diagnose or treat infection during routine clinical care in a patient or resident of an Illinois healthcare facility [14]. Healthcare exposures, medical histories, and clinical risk factors were collected during clinical case investigations. Colonized cases were defined as laboratory evidence of C. auris from bilateral axillary/groin specimens obtained for surveillance purposes from a patient or resident of an Illinois healthcare facility. Clinical and colonized case-patient information was also queried against Illinois’ Extensively Drug Resistant Organism (XDRO) registry, a statewide Web-based registry of patients infected or colonized with CPO and C. auris, to detect co-colonization [15, 16].
Active Surveillance and Infection-Control Assessments in Chicago
After the initial 2 cases were detected in August 2016, we conducted point prevalence surveys (PPSs) from August 2016 to December 2018 in Chicago short-term acute-care hospitals (STACHs), LTACHs, vSNFs, and skilled nursing facilities (SNFs) to evaluate C. auris burden and transmission. Facilities were selected based on epidemiologic evidence of having housed a case-patient with C. auris. Screening specimens consisted of composite bilateral axillary/inguinal swabs for C. auris and rectal swabs for CPO testing [17, 18]. In addition, we conducted on-site assessments of infection-control practices in healthcare facilities where C. auris transmission was documented or suspected to develop facility-specific recommendations [19].
Ventilator-capable Skilled Nursing Facility “A” Case Study
To assess for ongoing transmission and understand reasons for persistent spread, we conducted intensive follow-up on the 70-bed ventilator-capable unit of one 300-bed vSNF (vSNF-A). An initial PPS was conducted in response to a report of a C. auris case-patient identified in an LTACH and residing at vSNF-A in March 2017. During 2018, we performed serial PPSs on the ventilator-capable unit; surveys included residents with prior C. auris history of colonization or infection. During 4 surveys, residents were also assessed for CPO colonization with a rectal swab, and environmental samples were collected to assess C. auris contamination. Sample collection focused on high-touch surfaces in the patient-care environment, multiuse patient-care items, and mobile equipment.
The Chicago Department of Public Health (CDPH) provided ongoing assistance with identification and remediation of infection-control gaps. Infection-control interventions focused on the following: (1) querying on admission of the XDRO registry, and interfacility communication during admission and discharge, (2) implementation of Transmission-based Precautions, (3) cohorting of colonized individuals with similar organisms, (4) adherence to appropriate environmental cleaning practices, (5) alcohol-based hand rub (ABHR) availability and HH compliance, and (6) routine bathing with chlorhexidine gluconate (CHG) 2% impregnated wipes for patients on the ventilator-capable unit [20]. These infection-control practices, except for enhanced environmental cleaning, had been recommended to vSNF-A in July 2017 as a response to high CPO prevalence under a pre-existing Centers for Disease Control and Prevention (CDC)–funded regional quality improvement project, with varying degrees of implementation and adherence. Identification of C. auris led to our efforts to optimize adherence to these infection-prevention practices and to improve environmental cleaning and disinfection given that C. auris readily contaminates the healthcare environment and equipment. During 2018, healthcare worker adherence to HH and personal protective equipment (PPE) was assessed using standardized assessment tools [21]. Chlorhexidine gluconate bathing was assessed on a random sample of 40 vSNF-A residents (November 2018–April 2019) with swab sampling of 25 cm2 of skin from the chest (sternal or axilla) region using a colorimetric assay for CHG skin-concentration analysis [22].
Laboratory Testing for Candida auris and Carbapenemase-Producing Organism Identification
Bilateral axillary/inguinal composite skin swabs were collected using a single BD Eswab in 1 mL of liquid Amies Medium (#220 245; BD Diagnostics) and sent to the CDC or Antibiotic Resistance Laboratory Network laboratories for C. auris testing. All samples were processed within 4 days of collection. For surveys conducted January 2018–October 2018, 100 μL of swab buffer was processed with a high-salt (10% NaCl) and high-temperature (40°C) culture broth for select C. auris enrichment [23]. After overnight incubation, enriched cultures were plated onto CHROMagar Candida (CHROMagar; Becton Dickinson) and incubated for 7 days. Growth observed with morphology consistent with C. auris was confirmed with matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF; microflex LT; Bruker). For the survey conducted in October 2018, swabs were also processed with a Taqman quantitative PCR (qPCR) culture-independent test [24, 25]. DNA was extracted from 100 μL of swab buffer using the Magnapure 96 before performing the qPCR on an ABI 7500 Fast Dx instrument. Rectal swabs were tested using Cepheid Xpert Carba-R assay (Cepheid) for rapid detection and differentiation of 5 carbapenemase genes (blaKPC, blaVIM, blaOXA-48, blaIMP-1, blaNDM).
Environmental samples were collected using 3M Sponge-Sticks with 10 mL neutralizing buffer (#SSL10NB; 3M Healthcare) and sent to CDC laboratories for processing. Sponges were homogenized in 40 mL of phosphate-buffered saline with 0.02% Tween 80 at 260 revolutions per minute (RPM) (Stomacher 400C) [26]. Homogenates were concentrated by centrifugation at 2700 RPM for 20 minutes and plated onto CHROMagar Candida plates. Growth observed within 7 days with morphology consistent with C. auris was confirmed with MALDI-TOF.
RESULTS
Illinois Surveillance
During 1 May 2016–31 December 2018, 128 clinical and 362 colonized case-patients were identified in Illinois. Among 128 clinical case-patients, 46 (36%) were identified from blood specimens, 43 (34%) from urine, 15 (12%) from respiratory specimens, 11 (9%) from wound, and 13 (10%) from tissue and unknown sources. Thirty (23%) clinical cases were known to be colonized with C. auris prior to being identified as a clinical case. For these cases, the median time from first detected colonization to clinical infection was 89 days (range, 4–301 days). The median age of individuals colonized or infected with C. auris was 63 years (range, 18–94 years), and 62% were male. Among 490 individuals identified as colonized or infected with C. auris, 261 (53%) were also known to be colonized with a CPO.
Among 106 individuals with C. auris clinical infection for whom risk factor data were available, all had serious concurrent medical conditions requiring hands-on care and medical devices: 80% had an intravenous device, 72% had a wound, 66% were bed-bound, 67% had a feeding tube, 60% had a urinary catheter, 49% had a tracheostomy and were mechanically ventilated, 6% had a tracheostomy and were not mechanically ventilated, and 26% were on hemodialysis. Additionally, 93% of individuals with C. auris clinical infection had previously resided in an LTACH or vSNF within 3 months prior to identification.
Active Surveillance and Infection-Control Assessments in Chicago
To identify asymptomatically colonized individuals, CDPH conducted a total of 47 PPSs at 18 unique facilities, including all Chicago LTACHs and vSNFs. The highest prevalence of C. auris colonization was identified in vSNFs (median prevalence, 66%) and LTACHs (median prevalence, 31%); C. auris colonization was nearly absent in sampled STACHs and SNFs (Table 1).
Table 1.
Median Candida auris Prevalence by Chicago Facility Type, May 2016 to December 2018
| Facility Type | Number of Facilities | Number of Surveys | C. auris Median Prevalencea (Range), % |
|---|---|---|---|
| Short-term acute-care hospitalb | 8 | 8 | 0 (0–14) |
| Long-term acute-care hospital | 4 | 18 | 31 (23–36) |
| Ventilator-capable skilled nursing facilityc | 3 | 18 | 66 (23–71) |
| Skilled nursing facilityb | 3 | 3 | 2 (0–2) |
Abbreviations: CPO, carbapenemase-producing organism; PPS, point prevalence survey.
Median prevalence and range were calculated using most recent PPS data from each facility. Candida auris and CPO prevalence was calculated as the number of patients screening positive plus the number of individuals previously known to be positive, divided by the total number of individuals listed on the ward or facility census on the day of the survey.
Excludes contact screening with <5 individuals screened on any unit or facility.
Data represent PPS conducted on ventilator-capable unit.
Infection-control assessments of LTACHs and vSNFs identified common challenges, including lack of staff member time dedicated to infection-control activities, lack of infection-surveillance systems, lack of available ABHR dispensers and PPE at point of use, and lack of adequate monitoring for compliance with HH and environmental cleaning practices. We provided mentoring and ongoing support for gap mitigation.
vSNF-A Chicago Facility Case Study
During a PPS conducted in March 2017, we screened 69 residents on the ventilator-capable unit of vSNF-A in response to the presence of a single known individual with C. auris, who had transferred to vSNF-A from an acute-care hospital; we did not identify any colonized residents other than one previously known case-patient. We conducted 3 on-site infection-control assessments and on-site reviews during 2017. Between January and October 2018, we conducted 7 C. auris PPSs at vSNF-A at a median interval of 35 days (range, 28–84 days). Candida auris prevalence increased from 43% to 71% (Figure 1). In total, 114 unique residents were surveyed for C. auris colonization during the 7 PPSs. Overall, 66 (58%) were identified to be colonized, of whom 21 (32%) had a previous negative screening result. Among 51 colonized residents who were re-screened after their initial positive C. auris result, 28 (55%) had 1 or more negative screening results following a positive result; among these, 15 (54%) had a positive screening result again 1 or more times following the negative result (Figure 2). Four PPSs included CPO screening at a median interval of 60 days (range, 28–98 days). Fifty-four (46%) of 117 residents surveyed for CPO colonization were identified to be colonized. Among 114 residents screened for C. auris and CPO, 54 (47%) were identified as co-colonized (Figure 3). Among CPO-colonized residents, 46 (85%) had the KPC gene detected, 5 (9%) had KPC and VIM genes detected, 2 (4%) had KPC and NDM genes detected, and 1 resident (2%) had an NDM gene detected.
Figure 1.
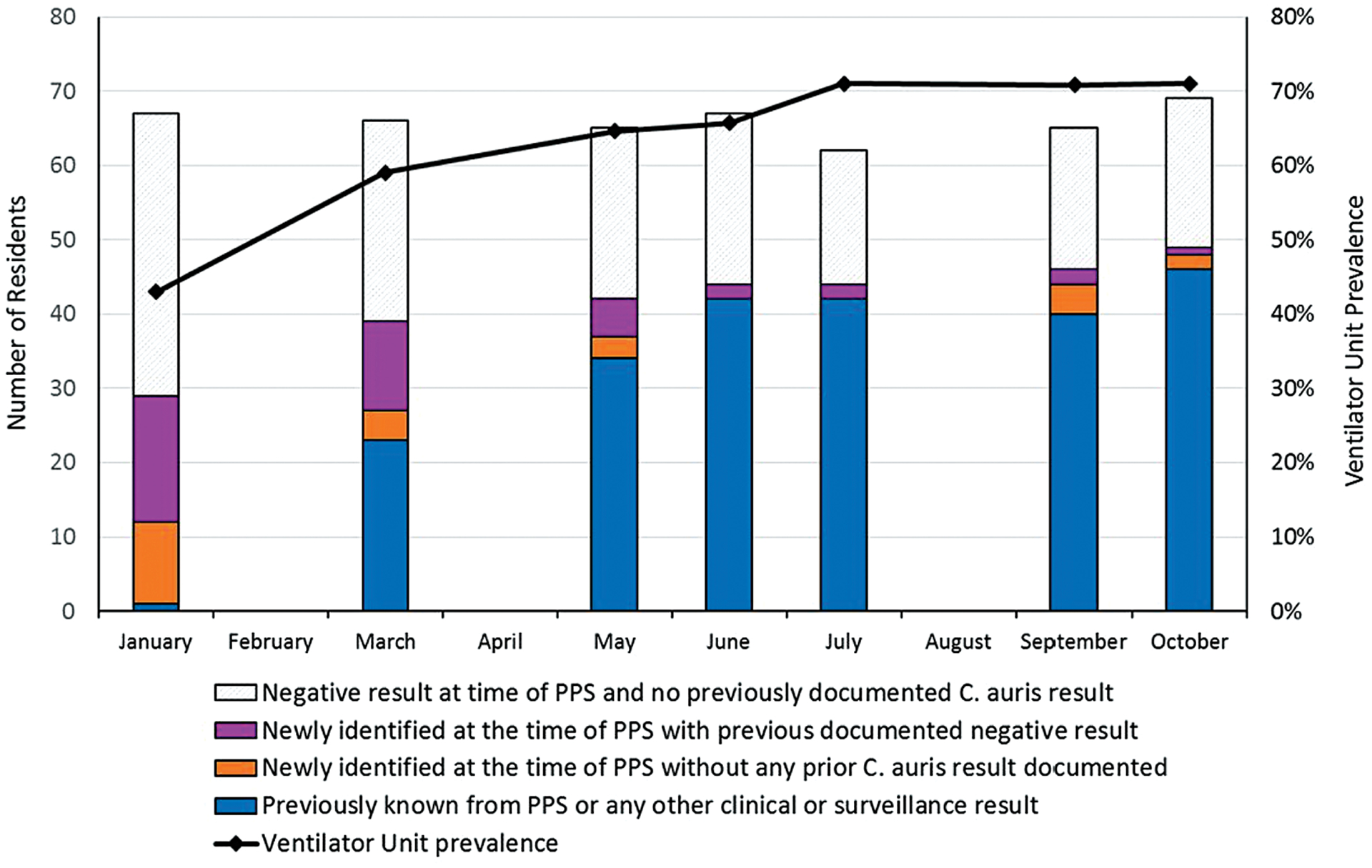
vSNF-A ventilator-capable unit Candida auris colonization status, January 2018 to October 2018. Abbreviations: PPS, point prevalence survey; vSNF, ventilator-capable skilled nursing facility.
Figure 2.
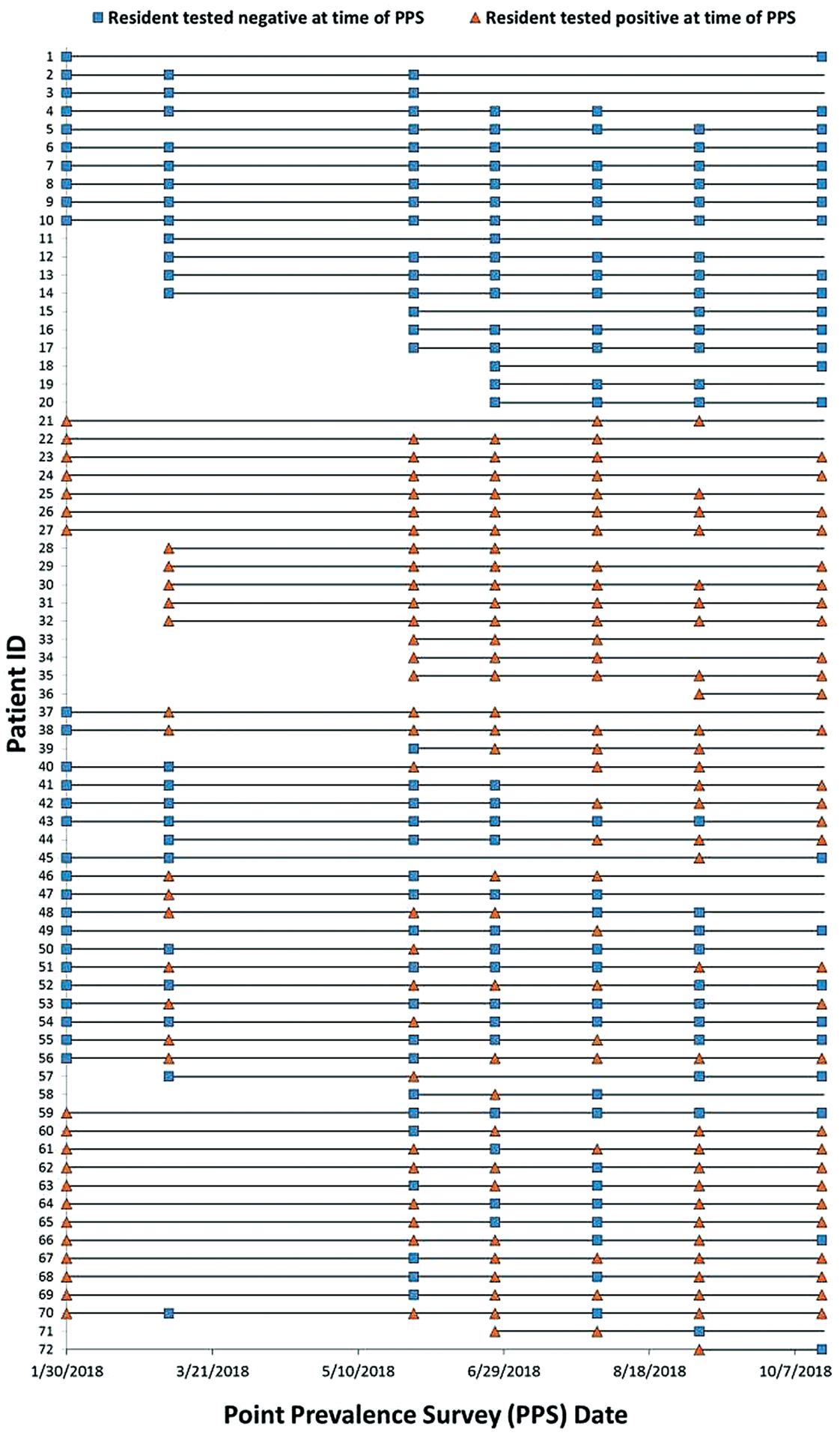
Resident with ≥2 PPS Candida auris testing results: vSNF-A, January to October 2018. Abbreviations: PPS, point prevalence survey; vSNF, ventilator-capable skilled nursing facility.
Figure 3.
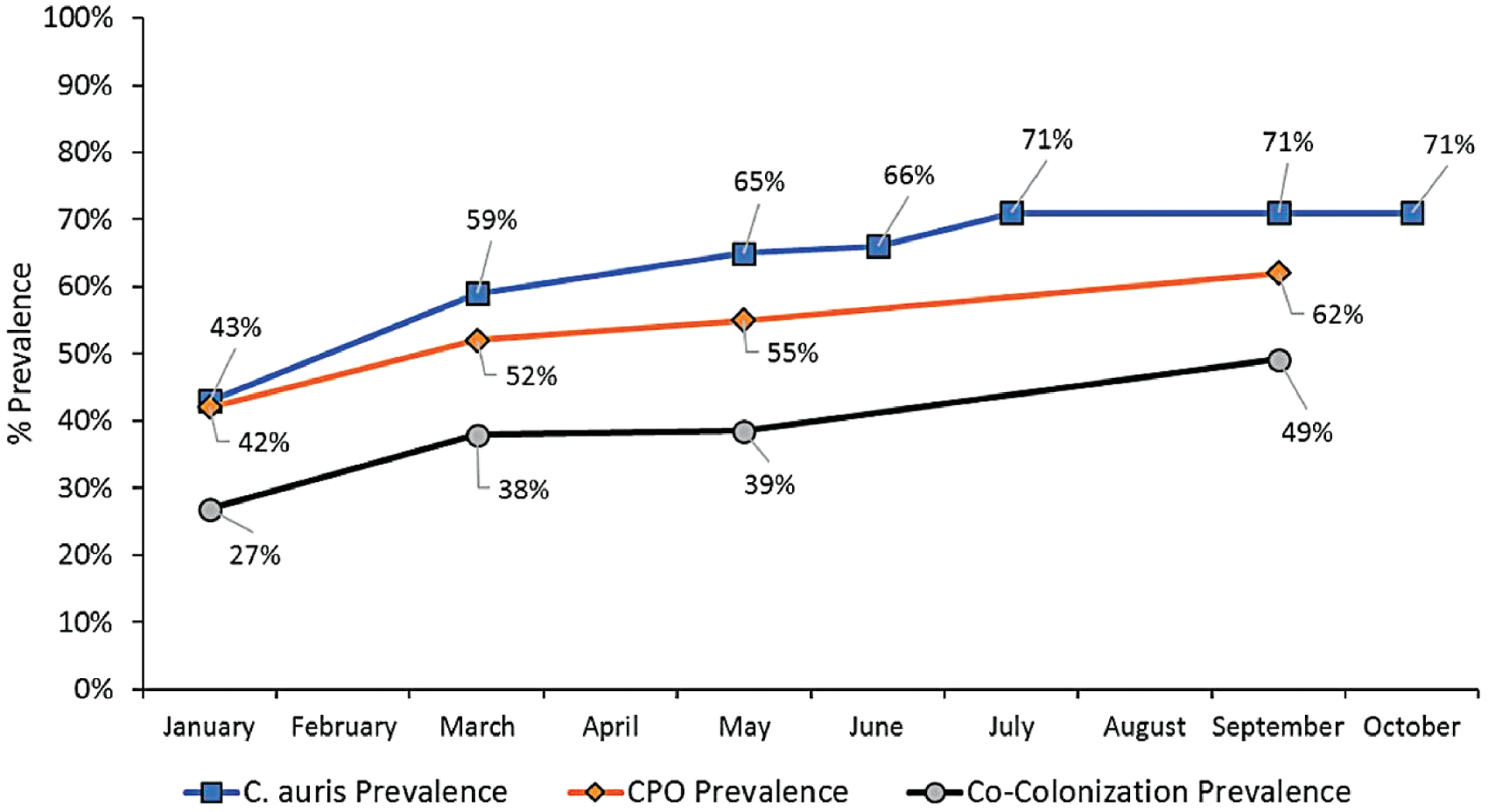
vSNF-A ventilator-capable unit Candida auris and CPO prevalence, January to October 2018. Abbreviations: CPO, carbapenemase-producing organism; vSNF, ventilator-capable skilled nursing facility.
Among 191 environmental samples collected after routine cleaning and disinfection during 4 surveys, 73 (38%) yielded C. auris. Patient-care items contaminated with C. auris included glucometers, temperature probes, mobile ultrasounds, pulse-oximeters, blood-pressure cuffs, and stethoscopes. Candida auris was also detected in samples collected from over-bed tables, bedside chairs, nursing carts, doorknobs, bedrails, and windowsills.
In February 2018, facility staff members enhanced their querying of the XDRO registry to identify residents’ C. auris and CPO history prior to their admission for Transmission-based Precaution and cohorting implementation. Between February and October 2018, vSNF-A queried a median of 38 residents (range, 9–72 residents) against the XDRO registry each month.
There were 30 multi-occupancy rooms on the ventilator-capable unit (17 double, 7 triple, 6 quadruple). Changes in C. auris and CPO carriage over time among residents who occupied these rooms required moving residents in order to maintain cohorts of the same colonization status. We assisted the facility with defining priorities to cohort residents with active infections (eg, Clostridioides difficile), those with C. auris colonization, followed by those colonized with other CPOs with emerging mechanisms of resistance (eg, NDM, VIM) (Figure 4).
Figure 4.
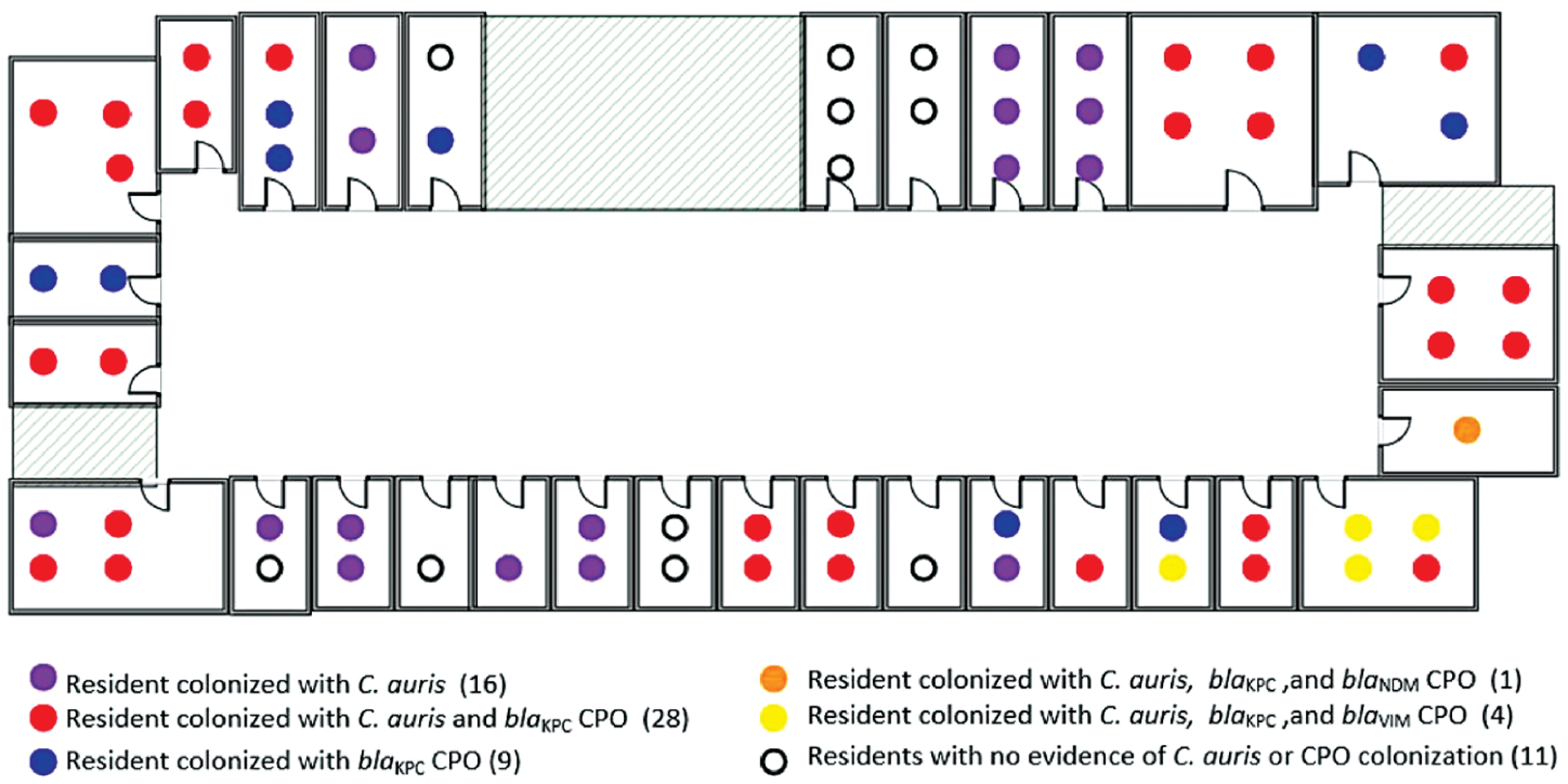
Resident Candida auris (C. auris) and CPO colonization status and room assignment: vSNF-A ventilator-capable unit, October 2018. C. auris prevalence, 71% (49 out of 69); CPO prevalence, 61% (42 out of 69). Abbreviations: CPO, carbapenemase-producing organism; vSNF, ventilator-capable skilled nursing facility.
Environmental services interventions included an increase of 1 additional full-time employee dedicated to performing environmental cleaning with sporicidal agents for all rooms on the ventilator-capable unit. Nursing and environmental services staff reviewed roles and responsibilities for cleaning and disinfection of the resident care environment and shared care items. Fluorescence residuals monitoring conducted June–August 2018 in a sample of 7 rooms indicated that 61% (199/325) of markings were appropriately removed.
Between January and March 2018, in response to CDPH recommendations, the facility increased ABHR hallway dispensers from 12 to 25 on each floor, and 1 dispenser was installed inside each of the 30 rooms on the ventilator-capable unit. Over 50 staff members were trained in appropriate HH practices during interactive educational sessions. Following on-site trainings of frontline staff, anonymous auditing provided by observers external to the facility identified higher HH compliance on room exit (75%; 219/292), than room entrance (48%; 139/292); glove and gown use compliance for residents in Contact Precautions was 73% (203/280). Of 40 residents randomly sampled, 31 (78%) had detectable CHG on the skin of their chest or axilla.
DISCUSSION
Since its emergence in May 2016, C. auris has evolved into a multi-institution outbreak in the Chicago region. Candida auris has been identified primarily among individuals with serious underlying medical conditions who have had prolonged stays in high-acuity long-term facilities. The highest C. auris prevalence has been documented on ventilator-capable units of vSNFs and in LTACHs. Candida auris prevalence in STACHs, SNFs, and non–ventilator-capable units of vSNFs remains low. Candida auris and MDRO containment is particularly challenging in vSNFs given that they are residential settings caring for individuals with complex medical needs who are highly dependent on healthcare workers for their activities of daily living. Poor adherence to core infection-control practices contributes to challenges in controlling MDRO spread in these settings.
Repeated PPSs conducted at vSNF-A demonstrated rapidly increasing C. auris prevalence over 10 months, likely due to unrecognized colonization on admission and ongoing transmission at the facility. Prevalence plateaued around 70%, perhaps reflecting achievement of a steady state of admission and discharge of residents colonized with C. auris to the facility at that time.
Collecting screening samples from all residents on the ventilator-capable unit, including those previously known to be colonized, revealed persistent C. auris colonization over long periods. Intermittent C. auris–positive and –negative screening results for the same individual demonstrate that sustained negative results in individuals previously known to have C. auris colonization or infection are rare in vSNF residents. Rescreening these individuals, absent a major improvement in clinical status that could promote decolonization, is of limited utility for infection control. Additionally, these findings continue to show that a single negative screening test for MDROs such as C. auris should not be used as a criterion for discontinuation of Transmission-based Precautions due to the high potential for false-negative results, resulting from imperfect test sensitivity, or from re-colonization due to long-term residence in the exposure setting.
Although cohorting is a recommended practice for control of emerging MDROs, we identified multiple challenges when implementing this strategy in vSNF-A. Residents colonized with C. auris or CPOs were moved to share rooms with other residents with the same MDROs. However, room movement in the absence of appropriate cleaning and disinfection may introduce opportunities for transmission of C. auris and other MDROs. Additionally, long lag times for C. auris screening test results (up to 2 weeks of laboratory turnaround time by culture-based methods) and ongoing transmission at vSNF-A introduced uncertainty as to whether residents who tested negative for MDROs at the time of the last PPS remained negative at the time of cohort re-assignment. Implementation of molecular testing in late 2018 greatly reduced turnaround time from weeks to a few days, providing a closer to real-time assessment of a resident’s C. auris status. Implementation of cohorting was additionally complicated by the presence of multiple emerging MDROs and co-colonization of individuals with different combinations of MDROs in multi-occupancy vSNF rooms where ventilator capability, residents’ gender, and care needs require consideration along with colonization status. Although cohorting can be useful in an acute outbreak with a single MDRO, in endemic settings with multiple MDROs health departments and healthcare facilities should carefully weigh benefits and drawbacks of cohorting strategies, considering MDRO prevalence, testing turnaround times, and facility infection-control and logistics capacity to safely move residents and maximize infection-prevention impact.
Multiple-occupancy rooms housing 2 or more residents are often crowded with furnishings, care equipment, and personal items, which impede changing of PPE, appropriately performing HH, and daily room cleaning. Additionally, multi-occupancy rooms seldom provide opportunities for terminal cleaning, since they are rarely unoccupied. The high rate of C. auris–positive environmental samples highlights the importance of sustained support for appropriate cleaning and disinfection practices by using the right agent, for the right contact time, applied to both inanimate surfaces in the resident care environment and care equipment. Recent work at this same vSNF found a positive correlation between the concentration of C. auris in residents’ skin swabs and the handrails of their beds. This relationship between skin colonization and environmental contamination suggests that finding an approach for source control by reducing skin burden of C. auris may be a useful strategy for controlling C. auris spread [26]. It is noteworthy that C. auris spread in vSNF-A despite unit-wide implementation of CHG bathing to control CPO and C. auris burden on the skin; the relationship between CHG bathing and C. auris skin colonization requires further evaluation.
Persistent colonization of individuals and the environment presents ongoing challenges in high-acuity long-term care settings. Improved surveillance, appropriate implementation of Transmission-based Precautions, HH, and scrupulous environmental cleaning and disinfection are likely needed to control spread. Additionally, patient movement among healthcare facilities can result in regional dissemination of MDROs and highlights the importance of appropriate infection-control practices rigorously applied across the whole network of healthcare settings [27]. Limitations of the vSNF-A investigation include the inability to determine where C. auris was acquired because of the absence of screening on admission and lack of complete resident transfer/discharge data. Future studies are needed to assess clinical characteristics that contribute to C. auris acquisition and infection and to evaluate recommendations that inform practical guidance for cohorting individuals colonized with multiple organisms in long-term care settings.
High-acuity long-term healthcare facilities represent high-priority settings for public health interventions to contain the spread of emerging healthcare-associated MDROs. Strategies are needed to improve adherence to infection-control practices, including the use of Transmission-based Precautions in nursing homes, increasing access to ABHR and PPE, improving HH compliance, and adherence to cleaning and disinfection of patient environment and shared equipment in these settings.
Acknowledgments.
The authors thank Mary Alice Lavin and Deborah Burdsall for their expertise in infection-control assessment and gap mitigation; Ellen Benson, Jinal Makhija, Mitali Shah, and Thelma Dangana for their contributions to adherence monitoring and data analysis; and Chicago healthcare facility staff members participating in C. auris surveillance and response.
Financial support.
This work was supported in part by Epidemiology and Laboratory Capacity for Infectious Diseases Cooperative Agreement numbers NU50CK000367-04-02 and NU50CK000367-05-00, funded by the Centers for Disease Control and Prevention, and the Centers for Disease Control and Prevention (SHEPheRD contract number 200-2011-42037 and Epicenter Grant Cooperative Agreement number U54CK000481)
Potential conflicts of interest.
M. Y. L. has received research support in the form of contributed product from OpGen and Sage Products (now part of Stryker Corporation) and has received an investigator-initiated grant from CareFusion Foundation (now part of Becton Dickinson). M. K. H. has been a coinvestigator on research studies that received support in the form of contributed product from Clorox, Medline, Mölnlycke, OpGen, Stryker, and Sage Products, and has received an investigator-initiated grant from Clorox. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
Publisher's Disclaimer: Disclaimer. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Vallabhaneni S, Kallen A, Tsay S, Chow N, et al. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1234–7. [DOI] [PubMed] [Google Scholar]
- 2.Tsay S, Welsh RM, Adams EH, et al. Notes from the field: ongoing transmission of Candida auris in health care facilities—United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep 2017; 66:514–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams E, Quinn M, Tsay S, et al. Candida auris in healthcare facilities, New York, USA, 2013–2017. Emerg Infect Dis 2018; 24:1816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisch J, Lansing B, Wang L, et al. New acquisition of antibiotic-resistant organisms in skilled nursing facilities. J Clin Microbiol 2012; 50:1698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pop-Vicas A, Mitchell SL, Kandel R, Schreiber R, D’Agata EM. Multidrug-resistant gram-negative bacteria in a long-term care facility: prevalence and risk factors. J Am Geriatr Soc 2008; 56:1276–80. [DOI] [PubMed] [Google Scholar]
- 6.van Buul LW, van der Steen JT, Veenhuizen RB, et al. Antibiotic use and resistance in long term care facilities. J Am Med Dir Assoc 2012; 13:568, e1–13. [DOI] [PubMed] [Google Scholar]
- 7.Schelenz S, Hagen F, Rhodes JL, et al. First hospital outbreak of the globally emerging Candida auris in a European hospital. Antimicrob Resist Infect Control 2016; 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SS, Avery TR, Song Y, et al. Quantifying interhospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol 2010; 31:1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piedrahita CT, Cadnum JL, Jencson AL, et al. Environmental surfaces in healthcare facilities are a potential source for transmission of Candida auris and other Candida species. Infect Control Hosp Epidemiol 2017; 11:1–3. [DOI] [PubMed] [Google Scholar]
- 10.Environmental Protection Agency. List K: EPA’s registered antimicrobial products effective against Clostridium difficile Spores. Available at: https://www.epa.gov/pesticide-registration/list-k-epas-registered-antimicrobial-products-effective-against-clostridium.Accessed12 May 2017.
- 11.Cadnum JL, Shaikh AA, Piedrahita CT, et al. Effectiveness of disinfectants against Candida auris and other Candida species. Infect Control Hosp Epidemiol 2017; 38:1240–3. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Interim guidance for a public health response to contain novel or targeted multidrug-resistant organisms (MDROs). Available at: https://www.cdc.gov/hai/pdfs/containment/Health-Response-Contain-MDRO-H.pdf.Accessed24 August 2019.
- 13.Tsay S, Kallen A, Jackson BR, Chiller TM, Vallabhaneni S. Approach to the investigation and management of patients with Candida auris, an emerging multidrug-resistant yeast. Clin Infect Dis 2018; 66:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Council of State and Territorial Epidemiologists. Standardized case definition for Candida auris causing clinical infection and colonization in people. Atlanta, GA: Council of State and Territorial Epidemiologists, 2017. Available at: https://wwwn.cdc.gov/nndss/conditions/candida-auris/case-definition/2018/.Accessed24 August 2019. [Google Scholar]
- 15.Trick WE, Lin MY, Cheng-Leidig R, et al. Electronic public health registry of extensively drug-resistant organisms, Illinois, USA. Emerg Infect Dis 2015; 21:1725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerins JL, Tang A, Black S, et al. Use of the Extensively Drug-resistant Organism (XDRO) registry for carbapenem-resistant Enterobacteriaceae (CRE) reporting and initiation of transmission precautions—Chicago, Illinois, 2016. Open Forum Infect Dis 2017; 4(Suppl 1):S179. [Google Scholar]
- 17.Bitterman Y, Laor A, Itzhaki S, Weber G. Characterization of the best anatomical sites in screening for methicillin-resistant Staphylococcus aureus colonization. Eur J Clin Microbiol Infect Dis 2010; 29:391–7. [DOI] [PubMed] [Google Scholar]
- 18.Hayden MK, Lin MY, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenters Program. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2015; 60:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan J, Lavin MA. The infection control assessment and response tool: is it useful during an outbreak? Am J Infect Control 2016; 44:6, S125. [Google Scholar]
- 20.Centers for Disease Control and Prevention. Hand hygiene in healthcare settings. Available at: https://www.cdc.gov/handhygiene/.Accessed24 August 2019.
- 21.Centers for Disease Control and Prevention. Tracking infections in long-term care facilities. Available at: https://www.cdc.gov/nhsn/ltc/index.html.Accessed24 August 2019.
- 22.Edmiston CE Jr, Krepel CJ, Seabrook GR, Lewis BD, Brown KR, Towne JB. Preoperative shower revisited: can high topical antiseptic levels be achieved on the skin surface before surgical admission? J Am Coll Surg 2008; 207:233–9. [DOI] [PubMed] [Google Scholar]
- 23.Welsh RM, Bentz ML, Shams A, et al. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol 2017; 55:2996–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leach L, Zhu Y, Chaturvedi S. Development and validation of a real-time PCR assay for rapid detection of Candida auris from surveillance samples. J Clin Microbiol 2018; 56:e01223–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahmad A, Spencer JE, Lockhart SR, et al. A high-throughput and rapid method for accurate identification of emerging multidrug-resistant Candida auris. Mycoses 2019; 62:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sexton J, Benz M, McPherson T, et al. Mechanisms of Candida auris transmission within the healthcare environment. ASM-Microbe 2019. [Google Scholar]
- 27.Won SY, Munoz-Price LS, Lolans K, Hota B, Weinstein RA, Hayden MK; Centers for Disease Control and Prevention Epicenter Program. Emergence and rapid regional spread of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2011; 53:532–40. [DOI] [PubMed] [Google Scholar]


