Abstract
Background
The impact of bilirubin in preterm infants is poorly understood. An animal model would assist in improving understanding. The Gunn rat lacks uridine diphosphate-glucuronylsyl transferase 1 and can be made acutely hyperbilirubinemic by injection of sulfodimethoxine (sulfa), a drug that displaces bilirubin from albumin and thus increases free bilirubin.
Methods
On postnatal day (P) 5, Gunn rats either heterozygous (Nj) or homozygous (jj) for glucuronosyltransferase activity were injected with either saline or sulfa. Behavior and cerebellar weight were measured.
Results
Pups did not show any signs of acute bilirubin encephalopathy. Pup weight dropped significantly on P8 only in the jj-sulfa group. Behavior was affected only in the jj-sulfa group. Cerebellar weight was significantly less in the jj-sulfa group.
Discussion
The Gunn rat pup model may be a good model to study hyperbilirubinemia in preterm infants.
Introduction
Bilirubin is a compound produced by the catabolism of heme. It is a normal product of the breakdown of hemoglobin from red blood cells that is further catabolized and excreted. Accumulation of bilirubin can become neurotoxic as is often the case in premature infants (1). In the United States, the amount of total serum bilirubin (the sum of free bilirubin, albumin-bound bilirubin and bilirubin conjugated to glucuronic acid) exceeds concentrations of concern in almost all preterm neonates <35 weeks gestation, a condition known as hyperbilirubinemia (2). Only free bilirubin can cross the blood brain barrier and accumulate in the brain, causing injury (3). The primary brain areas affected by bilirubin in the perinatal phase are the globus pallidus, hippocampus, brainstem auditory nuclei and cerebellum (1). As a result, premature infants are particularly susceptible to dystonia and deafness associated with hyperbilirubinemia (4). Neurotoxicity can occur with lower levels of total serum bilirubin in preterm compared to term infants (5). Complicating the diagnosis and implementation of therapeutic intervention is the fact that measuring levels of bilirubin in serum does not reliably predict the likelihood of bilirubin-induced neurotoxicity (3).
In premature infants, acute bilirubin neurotoxicity may occur without obvious clinical symptoms (1). Over time, the condition can progress to chronic symptoms including motor incoordination, learning disabilities and balance disorders (6). Despite the overwhelming incidence of hyperbilirubinemia in premature infants, rodent models have focused on term equivalent ages (e.g., postnatal day (P) 16)(7). The Gunn rat (GR) is homozygous (jj) for a mutation in uridine diphosphate-glucuronylsyl transferase 1 (UGT1) and has elevated concentrations of circulating bilirubin (8). Heterozygous littermates (Nj) have reduced UGT1 (~50% of wild type), do not develop hyperbilirubinemia and serve as controls (9). The majority of studies have induced acute bilirubin encephalopathy by administration of sulfadimethoxine (sulfa) on P16 (7,9,10), a model of term newborn hyperbilirubinemia. To model preterm (24–29 weeks of gestation) human infants, we administered sulfa to P5 Gunn rat pups (11). We hypothesize that the Nj GR pups injected with sulfa on P5 will not experience acute bilirubin encephalopathy. However, we predict that jj GR pups injected with sulfa on P5 will experience long term cerebellar sequelae.
Methods
Gunn rats were obtained from the Rat Resource & Research Center and a breeding colony was established at the University of Maryland. All breeding and experimental procedures were approved by the University of Maryland Institutional Animal Care and Use Committee. Litters are the product of jj male mated with Nj females. Rats were pair housed, one female and one male. Females were checked daily for signs of a mucus plug. The day a plug was observed was designated as gestational day 0 (G0). The day of birth was designated P0. On P1, pups were genotyped for jj or Nj status. Each pup of a litter is identified as one of four groups: male/female and Nj/jj. Pups of the same sex and genotype were assigned to the different treatment groups using randomly generated numbers in Microsoft Excel. Pups were injected intraperitoneally with either 200 mg/kg of sulfa on P5 or an equivalent volume of saline. This categorization results in 8 groups: Male/female Nj saline, Male/female Nj sulfa, male/female jj saline, male/female jj sulfa.
Immediate effects
Pups were video-recorded immediately following injection. Videos were scored for movement and nursing.
Ultrasonic Vocalization
On P6, the latency and number of ultrasonic vocalizations was measured. An ultrasonic detector with a microphone was set 20 cm above the test cage. The output was recorded using Avisoft (Avisoft Bioacoustics, Berlin, Germany). Each pup was briefly removed from the dam and put in the right corner of the test cage for a 5 min test session. Three tests were conducted for each pup with 20 min between tests. Pups were returned to the dam between tests. Audio data were subsequently played back and the latency to the first vocalization and the number of USV at 40 kHz were recorded. The average of the three tests was calculated.
Negative geotaxis
Testing was conducted on P10. Pups were placed on a plane inclined at an angle of 45 degree. The testing surface was covered with a polypropylene mesh with 0.5 cm × 0.5 cm grids. The trials started with pups placed on the incline with their heads on the lowest part of the incline. The time taken to position themselves with their head at the highest part of the incline was measured. Each pup was tested three times with 20 min between tests. Pups were returned to the dam between tests. The average of the three tests was calculated.
Righting reflex
Testing was conducted on P10. Rat pups were gently placed on their back. Time taken to flip over onto their stomach was measured. Each pup was tested three times with 20 min between tests. Pups were returned to the dam between tests. The time for each test was averaged for each pup.
Rotarod
On P28–30, performance on an accelerating rotarod was tested. Pups were placed on a horizontally oriented, rotating cylinder (Roto-Rod series 8, IITC Life Science Inc, CA) suspended 15 cm above the bottom of the apparatus, which is low enough not to injure the animal, but high enough to induce avoidance of fall. The rotarod is mechanically driven at an accelerating speed of 5 rpm to a maximum speed of 45 rpm within100 seconds of initiation of the test. A soft padded surface was placed at the base of the apparatus to cushion falls. The length of time that each pup was able to remain on the rotating rod was measured each day for 3 days. The average time was calculated for the three days.
Cerebellar weights
Pups were euthanized on P30. The cerebellum was dissected free and weighed.
Statistical analysis
Mixed factor ANOVA with Genotype (2) and sulfa treatment (2) as between group factors was conducted for each dependent variable: 1) Pup weight; 2) Latency to first vocalization; 3) Number of USVs, 4) Negative geotaxis performance; 5) Righting reflex; 6) Rotarod performance and 7) Cerebellar weight. When the Genotype and sulfa interaction did not reach significance, Bonferroni post-hoc tests were used to determine whether rats treated with sulfa differ from rats treated with saline for each genotype. Table 1 shows the number of animals tested in each group for each significantly different test.
Table.
Number of animals for each test (M/F)
| USV | Negative Geotaxis | Rotarod | Cerebellar Weight | |
|---|---|---|---|---|
| Nj-saline | 14 (7/7) | 13 (7/6) | 16 (8/8) | 16 (8/8) |
| Nj-sulfa | 15 (7/8) | 14 (6/8) | 16 (8/8) | 18 (8/8) |
| jj-saline | 9 (4/5) | 7 (3/4) | 12 (5/7) | 11 (5/6) |
| jj-sulfa | 14 (7/7) | 13 (7/6) | 16 (8/8) | 16 (8/8) |
Results
Sex differences
No sex differences were found for any measure. Subsequent analyses were performed for combined males and females.
Immediate effects
Videotapes showed no observable effects on movement or nursing of pups (data not shown).
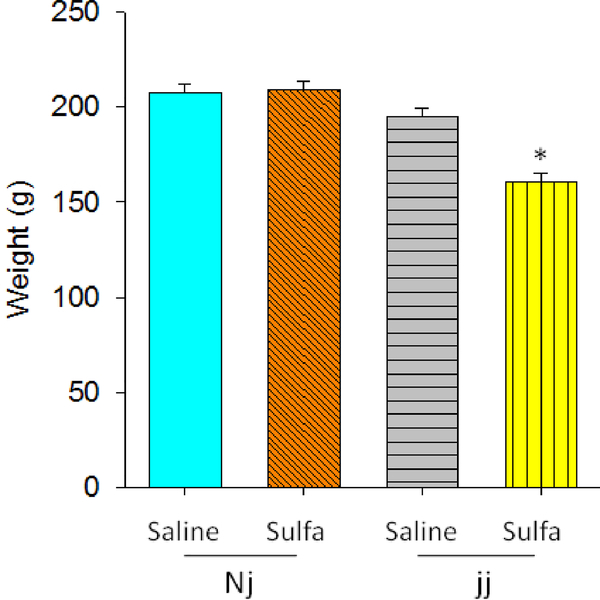
Pup Weight
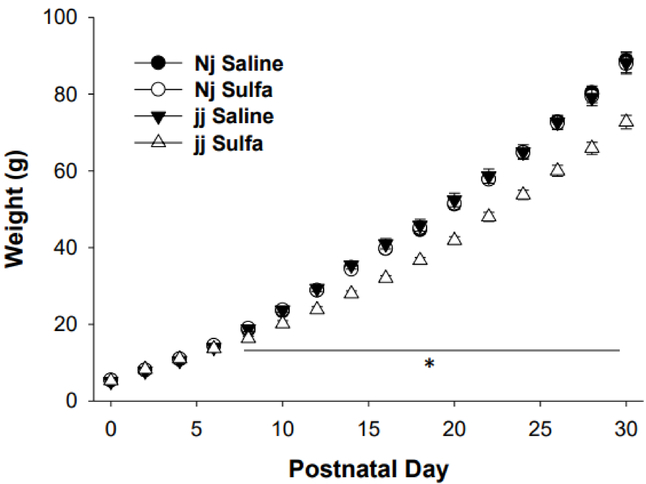
Pup weight was the same across groups until P8 when the jj sulfa pup weight fell significantly below that of the other groups. This was evident in a Genotype × sulfa treatment interaction, F(1, 60)=53.23, p<0.026. (Figure 1)
Figure 1.
Pup weight, g, mean +/− SEM, from Day of Birth (postnatal day (P) 0) until P30. Genotypes: Nj – heterozygous Gunn rat pups, jj – homozygous Gunn rat pups. Treatments: Saline – saline given on P5, sulfa – sulfodimethoxine given on P5.
USVs
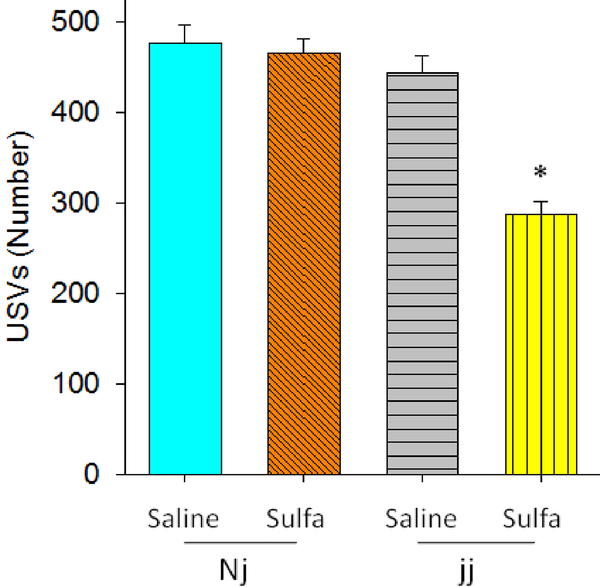
No difference in latency was found among the groups (all F values less than 1, indicating no between group differences).The frequency of the calls were also not affected. All pups expressed calls with the 40–48 kHz range. The number of USVs was affected by sulfa treatment in jj rats only. A significant effect of Genotype, F(1, 48)=36.682, p<0.0001 and sulfa treatment, F(1,484)=22.56, p<0.0001 were found. The Genotype × sulfa treatment interaction was significant, F(1,48)17.21, p<0.0001, indicating that sulfa treatment reduced the number of USVs in jj rats only, and that jj rats treated with saline did not differ from either Nj group (Figure 2).
Figure 2.
Ultrasonic vocalizations (USV) number (mean +/− SEM) between the various treatment groups measured on P6. Genotypes: Nj – heterozygous Gunn rat pups, jj – homozygous Gunn rat pups. Treatments: Saline – saline given on P5, sulfa – sulfodimethoxine given on P5.
Negative Geotaxis
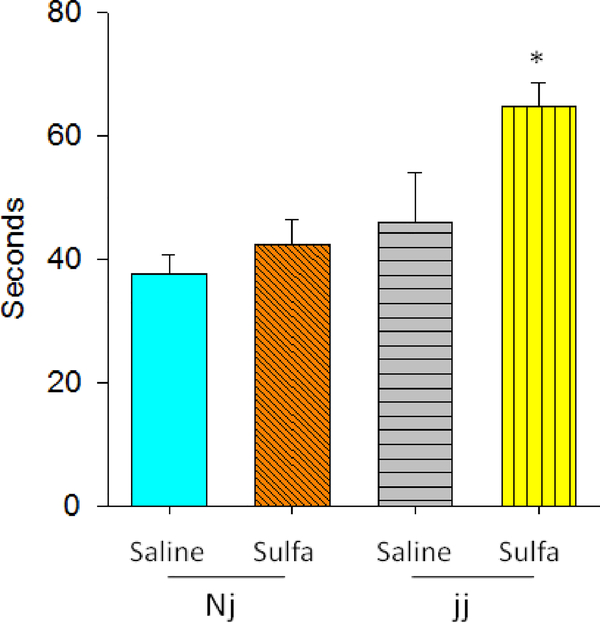
A significant effect of Genotype, F(1,43)=11.59, p<0.00 and sulfa treatment F(1,43)=6.86, p<0.01 were detected. The interaction of Genotype × sulfa was not significant, F=2.45. This is likely due to the increase in time exhibited by both Nj and jj pups treated with sulfa. Despite the lack of a significant interaction, Bonferroni post-hoc tests confirmed that jj sulfa pups were significantly slower to orient with the snout upward than jj saline pups (p<0.05). Heterozygous groups did not differ from one another (Figure 3).
Figure 3.
Negative geotaxis measured on P10. Data recorded as seconds until reached head up position, (mean +/− SEM). Genotypes: Nj – heterozygous Gunn rat pups, jj – homozygous Gunn rat pups. Treatments: Saline – saline given on P5, sulfa – sulfodimethoxine given on P5.
Righting reflex
No difference in time to right was found between the four groups (data not shown).
Rotarod
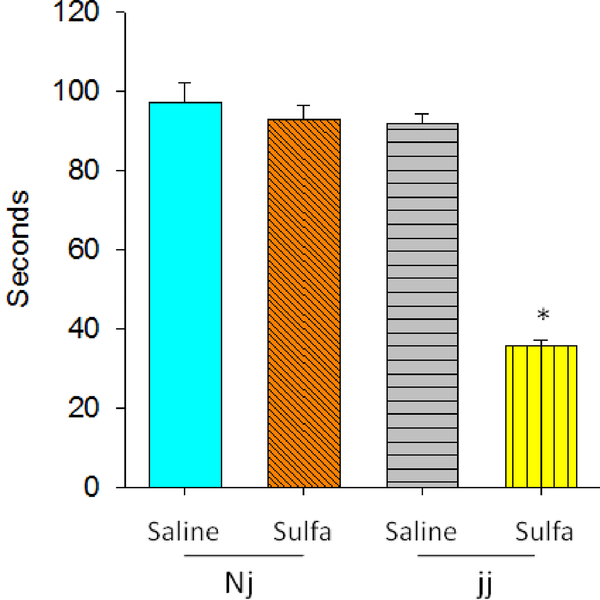
The effect of Genotype was significant, F(1,57)=69.99, p<0.0001 as was the effect of sulfa treatment, F(1,57)=68.09, p<0.0001. The Genotype × sulfa interaction was significant, F(1,57)= 49.96, p<0.0001. Like the pattern detected in emission of USVs, sulfa administration reduced the time the rat was able to stay on the accelerating rotarod only in jj pups, and not the Nj group (Figure 4).
Figure 4.
Rotarod test measured on P28, 29, 30. Data recorded as seconds from time of placement until falling, mean +/− SEM. Genotypes: Nj – heterozygous Gunn rat pups, jj – homozygous Gunn rat pups. Treatments: Saline – saline given on P5, sulfa – sulfodimethoxine given on P5. Genotypes: Nj – heterozygous Gunn rat pups, jj – homozygous Gunn rat pups. Treatments: Saline – saline given on P5, sulfa – sulfodimethoxine given on P5.
Cerebellar Weight
The effect of Genotype was significant, F(1,55)=42.7, p<0.0001 as was the effect of sulfa, F(1,55)=12.05, p<0.004. The Genotype × sulfa interaction was significant, F(1,55)=14.36, p<0.001. Administration of sulfa to jj pups significantly reduced the weight of the cerebellum but did not reduce the cerebellar weight in the Nj rats (Figure 5).
Figure 5.
Cerebellar weights measured on P30 (g, mean +/− SEM). Genotypes: Nj – heterozygous Gunn rat pups, jj – homozygous Gunn rat pups. Treatments: Saline – saline given on P5, sulfa – sulfodimethoxine given on P5.
Discussion
Our major findings from this study are that in jj rats at a stage of brain development equivalent to human preterm infants experience no immediate symptoms of acute bilirubin encephalopathy such as lethargy or poor feeding, there are no sex differences in the outcomes in the measures used here of acute hyperbilirubinemia, and behavioral deficits occur in some but not all cerebellar behaviors tested. Postnatal cerebellar development in the rat progresses rapidly in the first three weeks of life. Granule cell genesis is robust at birth and continues until at least P21 (12, 13). Between P4–8 in the rat, the granule and molecular layers increase in volume by 2–3 fold (13). Late gestation of the human is similarly characterized by rapid volume increases in the cerebellum. From gestational week 24–40, the human cerebellum volume increases 5-fold (14). This gain in volume is driven largely by both cell genesis and migration of cells from the external granule cell layer in both rats and humans, and is the primary area of growth during 30–40 weeks human gestation (13, 15, 16). Purkinje cells are just beginning to sprout dendritic trees and form synaptic contacts around P6 in the rat, an event that parallels the human at 25–30 weeks of gestation (13, 15–18). Pruning and stabilization of long-lasting contacts between granule cells and purkinje cells continues until at least P17 in the rat (19, 20). Though the developmental tempo of rats compared to humans is unlikely to progress in perfect parallel, the P4–8 day rat pup cerebellum is populating primary neural subregions and rapidly increasing in volume like the human during the second half of gestation. Based on these morphological changes, we estimate that the cerebellar development of rat pups at P5 is roughly equivalent to that of humans at 24–29 weeks of gestation. Important for the context of the data presented here is the finding that cerebellar growth during late gestation is disrupted by prematurity (21). The potential role of bilirubin in this disruption is largely overlooked.
We focused on cerebellar function because the cerebellum is particularly vulnerable to injury during this developmental stage in humans (22, 23) although these tests may involve brain regions in addition to the cerebellum. A randomized clinical trial investigated the difference between aggressive and conservative phototherapy in infants with birth weights <1000g (24). All infants in the aggressive arm were treated with phototherapy starting on the day of birth and at lower total bilirubin concentrations. A modest reduction of bilirubin was observed between the two groups (mg/dl: peak bilirubin, 7.0+1.8 vs 9.8+2.1; average daily bilirubin, 4.7+1.1 vs 6.2+1.5; aggressive vs conservative phototherapy). At 18–22 months follow up, measures relating to cerebellar function were recorded. Athetosis was significantly reduced in the aggressive phototherapy group, indicating a role for bilirubin in this movement disorder. These results imply that bilirubin, in the absence of clinical signs or symptoms, has long lasting effects on the brain.
Our findings suggest that administration of sulfa on P5 in the jj GR rat models the clinical manifestation of hyperbilirubinemia in the premature infant. We do not observe any immediate obvious symptoms of acute bilirubin encephalopathy such as decrease in movement or eating behaviors. However, the number of ultrasonic vocalizations are significantly reduced by P6 in jj GR pups treated with sulfa but not the latency to vocalize. It is unclear what behavior ultrasonic vocalization represents in preterm infants. It may be related to effects on hearing (25) or to vocal cord dystonia. Early subtle indicators of bilirubin neurotoxicity would be helpful to guide intervention, such as phototherapy. We will be measuring brainstem auditory evoked potentials in future studies to investigate a possible underlying cause of the effect of bilirubin on USV expression.
Our finding of a significant decrease in weight of the jj-sulfa group may have several explanations. First it may be a direct effect of bilirubin toxicity. Second, the jj-sulfa pups may be less able to compete with littermates for maternal milk. Third, the dam may be less attentive to this group of pups due to the drastic reduction in vocalizations. It is possible that all three reasons maybe contribute to the reduced weight.
We also do not observe any sex differences on the outcomes measured here. This finding is consistent with that in the literature for human infants. As summarized by the National Collaborative Centre for Women’s and Children’s Health, gender is not a risk factor for developing hyperbilirubinemia, nor is it a risk factor for developing sequelae following hyperbilirubinemia (26).
While we measured deficits in a number of behaviors (ultrasonic vocalization number, negative geotaxis, rotarod), there were a number of behaviors which showed no effect (ultrasonic vocalization latency, righting reflex, open field). There may be several explanations for this finding. First, the test may not be sensitive enough to detect small changes. For example, the righting reflex is very fast, usually less than 2 seconds. A delay by a fraction of a second would not be detectable in our test. Similarly, for an increase or decrease of fractions of seconds in latency to emit USV would not be detected. A second reason for the selective impact on behaviors may be the known temporal and regional vulnerability of the developing brain to hyperbilirubinemia. Developing neurons are known to be more vulnerable than mature neurons (27). It may be that the neural pathways necessary for normal execution of some behaviors are less mature at the time of sulfa injection than those involved in other behaviors. Those pathways that were still immature would be differentially affected (10). The righting reflex appears to be developed in mice by P7 whereas negative geotaxis matures at P17 (28). Hence the effect of acute hyperbilirubinemia at P5 would be expected to affect negative geotaxis more than the righting reflex as the neurons for negative geotaxis are in the process of developing (10). The underlying reason for the increased vulnerability of developing neurons remains unexplained.
There are several limitations to this study. First is the lack of measurement of total serum bilirubin and brain bilirubin in our model. While the hallmark of the hyperbilirubinemia exposure in Gunn rats is cerebellar hypoplasia, we found no significant difference between the cerebellar weights of Nj-saline and jj-saline pups. One reason is that, in this strain of Gunn rat, the bilirubin is low compared to other strains and is not high enough to reduce cerebellar weight without sulfa administration. It will be important in the future to characterize the total serum bilirubin and the brain bilirubin in this model. The second limitation is that the behaviors were limited to those involving the cerebellum. Future studies will investigate the impact on other vulnerable brain regions such as the globus pallidus, brainstem auditory nuclei and hippocampus using physiological and behavioral tests that can dissociate these neural substrates. Third, we did not measure the time course of brain bilirubin content. We suspect that it is less than that reported for Gunn rats injected on P16 as total serum bilirubin in Gunn rats peaks at approximately P16 (7), thus more bilirubin is present to displace from albumin tin enter the brain.
In summary, this model is promising as a model for preterm hyperbilirubinemia. Further characterization is necessary such as determining bilirubin concentrations in serum and brain. The mechanism of cerebellar neurotoxicity of preterm infants from bilirubin may be investigated using this animal model.
Acknowledgments
Statement of Financial Support: This study was supported by NIH/NICHD 1R21HD085061-01A1 (CFB), the Cobey Fund (CFB) and The Munro Fund (CFB).
Footnotes
Disclosure statement: The authors declare no conflicts of interest.
References
- 1.Bhutani VK, Wong RJ & Stevenson DK Hyperbilirubinemia in Preterm Neonates. Clin. Perinatol 43, 215–232 (2016). [DOI] [PubMed] [Google Scholar]
- 2.van der Schoor LW et al. Unconjugated free bilirubin in preterm infants. Early Hum. Dev 106–107, 25–32 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Hulzebos CV & Dijk PH Bilirubin-albumin binding, bilirubin/albumin ratios, and free bilirubin levels: where do we stand? Semin. Perinatol 38, 412–421 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Shapiro SM & Nakamura H Bilirubin and the auditory system. J. Perinatol 21 Suppl 1, S52–5; discussion S59–62 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Arnold C, Pedroza C & Tyson JE Phototherapy in ELBW newborns: does it work? Is it safe? The evidence from randomized clinical trials. Semin. Perinatol 38, 452–464 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Amin SB, Smith T & Timler G Developmental influence of unconjugated hyperbilirubinemia and neurobehavioral disorders. Pediatr. Res 85, 191–197 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Schutta HS & Johnson L Clinical signs and morphologic abnormalities in Gunn rats treated with sulfadimethoxine. J. Pediatr 75, 1070–1079 (1969). [DOI] [PubMed] [Google Scholar]
- 8.Takagishi Y & Yamamura H Severity of cerebellar hypoplasia is predictable from total plasma bilirubin level at 3 to 7 days of age in jaundiced Gunn rats. Lab. Anim. Sci 39, 158–160 (1989). [PubMed] [Google Scholar]
- 9.Chaniary KD et al. Quantification of gait in dystonic Gunn rats. J. Neurosci. Methods 180, 273–277 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Conlee JW & Shapiro SM Development of cerebellar hypoplasia in jaundiced Gunn rats: a quantitative light microscopic analysis. Acta Neuropathol. 93, 450–460 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Clancy B, Darlington RB & Finlay BL Translating developmental time across mammalian species. Neuroscience 105, 7–17 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Altman J & Das GD Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis. J. Comp. Neurol 126, 337–389 (1966). [DOI] [PubMed] [Google Scholar]
- 13.Altman J Morphological development of rat cerebellum and some of its mechanisms. Exp. brain Res Suppl. 6, 8–49 (1982). [Google Scholar]
- 14.Chang CH, Chang FM, Yu CH, Ko HC & Chen HY Assessment of fetal cerebellar volume using three-dimensional ultrasound. Ultrasound Med. Biol 26, 981–988 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Rakic P & Sidman RL Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J. Comp. Neurol 139, 473–500 (1970). [DOI] [PubMed] [Google Scholar]
- 16.Sidman RL & Rakic P Neuronal migration, with special reference to developing human brain: a review. Brain Res. 62, 1–35 (1973). [DOI] [PubMed] [Google Scholar]
- 17.Volpe JJ Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J. Child Neurol 24, 1085–1104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ten Donkelaar HJ, Lammens M, Wesseling P, Thijssen HOM & Renier WO Development and developmental disorders of the human cerebellum. J. Neurol 250, 1025–1036 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Watanabe M & Kano M Climbing fiber synapse elimination in cerebellar Purkinje cells. Eur. J. Neurosci 34, 1697–1710 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto K, Ichikawa R, Kitamura K, Watanabe M & Kano M Translocation of a ‘winner’ climbing fiber to the Purkinje cell dendrite and subsequent elimination of ‘losers’ from the soma in developing cerebellum. Neuron 63, 106–118 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Limperopoulos C et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics 115, 688–695 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Xoinis K, Weirather Y, Mavoori H, Shaha SH & Iwamoto LM Extremely low birth weight infants are at high risk for auditory neuropathy. J. Perinatol 27, 718–723 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Biran V, Verney C & Ferriero DM Perinatal cerebellar injury in human and animal models. Neurol. Res. Int 2012, 858929 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris BH et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N. Engl. J. Med 359, 1885–1896 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rice AC, Chiou VL, Zuckoff SB & Shapiro SM Profile of minocycline neuroprotection in bilirubin-induced auditory system dysfunction. Brain Res. 1368, 290–298 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Factors that Influence Hyperbilirubinaemia and Kernicterus. in Neonatal Jaundice NICE Guidelines No. 98 (ed. Group GD) (RCOG, 2010). [Google Scholar]
- 27.Brites D The evolving landscape of neurotoxicity by unconjugated bilirubin: role of glial cells and inflammation. Front. Pharmacol. 3, 88 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahlfors CE & Shapiro SM Auditory brainstem response and unbound bilirubin in jaundiced (jj) Gunn rat pups. Biol. Neonate 80, 158–162 (2001). [DOI] [PubMed] [Google Scholar]