Summary
Background
Residents of long-term care facilities (LTCFs) have been prioritised for COVID-19 vaccination because of the high COVID-19 mortality in this population. Several countries have implemented an extended interval of up to 12 weeks between the first and second vaccine doses to increase population coverage of single-dose vaccination. We aimed to assess the magnitude and quality of adaptive immune responses following a single dose of COVID-19 vaccine in LTCF residents and staff.
Methods
From the LTCFs participating in the ongoing VIVALDI study (ISRCTN14447421), staff and residents who had received a first dose of COVID-19 vaccine (BNT162b2 [tozinameran] or ChAdOx1 nCoV-19), had pre-vaccination and post-vaccination blood samples (collected between Dec 11, 2020, and Feb 16, 2021), and could be linked to a pseudoidentifier in the COVID-19 Data Store were included in our cohort. Past infection with SARS-CoV-2 was defined on the basis of nucleocapsid-specific IgG antibodies being detected through a semiquantitative immunoassay, and participants who tested positive on this assay after but not before vaccination were excluded from the study. Processed blood samples were assessed for spike-specific immune responses, including spike-specific IgG antibody titres, T-cell responses to spike protein peptide mixes, and inhibition of ACE2 binding by spike protein from four variants of SARS-CoV-2 (the original strain as well as the B.1.1.7, B.1.351, and P.1 variants). Responses before and after vaccination were compared on the basis of age, previous infection status, role (staff or resident), and time since vaccination.
Findings
Our cohort comprised 124 participants from 14 LTCFs: 89 (72%) staff (median age 48 years [IQR 35·5–56]) and 35 (28%) residents (87 years [77–90]). Blood samples were collected a median 40 days (IQR 25–47; range 6–52) after vaccination. 30 (24%) participants (18 [20%] staff and 12 [34%] residents) had serological evidence of previous SARS-CoV-2 infection. All participants with previous infection had high antibody titres following vaccination that were independent of age (rs=0·076, p=0·70). In participants without evidence of previous infection, titres were negatively correlated with age (rs=–0·434, p<0·0001) and were 8·2-times lower in residents than in staff. This effect appeared to result from a kinetic delay antibody generation in older infection-naive participants, with the negative age correlation disappearing only in samples taken more than 42 days post-vaccination (rs=–0·207, p=0·20; n=40), in contrast to samples taken after 0–21 days (rs=–0·774, p=0·0043; n=12) or 22–42 days (rs=–0·437, p=0·0034; n=43). Spike-specific cellular responses were similar between older and younger participants. In infection-naive participants, antibody inhibition of ACE2 binding by spike protein from the original SARS-CoV-2 strain was negatively correlated with age (rs=–0·439, p<0·0001), and was significantly lower against spike protein from the B.1.351 variant (median inhibition 31% [14–100], p=0·010) and the P.1 variant (23% [14–97], p<0·0001) than against the original strain (58% [27–100]). By contrast, a single dose of vaccine resulted in around 100% inhibition of the spike–ACE2 interaction against all variants in people with a history of infection.
Interpretation
History of SARS-CoV-2 infection impacts the magnitude and quality of antibody response after a single dose of COVID-19 vaccine in LTCF residents. Residents who are infection-naive have delayed antibody responses to the first dose of vaccine and should be considered for an early second dose where possible.
Funding
UK Government Department of Health and Social Care.
Introduction
Long-term care facilities (LTCFs) accommodate residents with enhanced care needs and support many older people with conditions such as frailty or dementia. The COVID-19 pandemic has had a substantial impact on many LTCFs, and mortality in vulnerable older residents has been among the highest observed in any demographic group.1 As such, several approaches have been taken to reduce transmission, including regular screening for infection and minimisation of external visits.
Research in context.
Evidence before this study
Residents within long-term care facilities (LTCFs) are at high clinical risk following SARS-CoV-2 infection and have been prioritised for vaccination in the UK. Studies on the efficacy of immune responses elicited after COVID-19 vaccination within this population are now required to guide appropriate vaccine policy. We searched for the terms “COVID-19” AND “vaccine immune” OR “vaccine efficacy” AND “care homes” OR “long term care facilities”, “humoral response to vaccine”, “cellular response to vaccine” OR “older people” on Ovid MEDLINE and MedRxiv. We identified one preprint article that studied infection rates following single or dual vaccination within LTCFs in Denmark and reported that single vaccination did not provide protection for residents in the intervening 24-day period before the second dose. Three preprint reports evaluated the clinical effectiveness of vaccination in older adults in the community, but none of these studies investigated the potential immune correlates of protection.
Added value of this study
We did a detailed immunological study of 89 staff and 35 residents within LTCFs following their first dose of either the BNT162b2 or ChAdOx1 nCoV-19 COVID-19 vaccines. Antibody and cellular responses to the SARS-CoV-2 spike protein immunogen were assessed at different timepoints post-vaccination. Around a quarter of LTCF staff and residents were found to have had previous natural infection with SARS-CoV-2, and this history of infection had a profound impact on vaccine response. Individuals with previous natural infection developed rapid and high titre antibody responses that bound strongly to viral variants of concern and were independent of age. By contrast, in people without previous natural infection we found that antibody responses were detectable within 99% of staff and 79% of residents but were 8·2-times lower within residents. This apparent lower response in residents resulted from slower kinetics of antibody generation within older people such that similar antibody levels to younger staff were seen only beyond 42 days after vaccine. Antibodies from older individuals were also less effective at binding to spike protein from viral variants. Cellular responses against the spike protein were similar in all age groups and no differences were observed in relation to immune responses to the two vaccine types.
Implications of all the available evidence
The evidence indicates that SARS-CoV-2 infection status is a strong determinant of immune responses after a single dose of COVID-19 vaccine in LTCF staff and residents. People with a history of SARS-CoV-2 infection are likely to develop strong clinical protection after a single dose, whereas older LTCF residents who have remained infection-naive show delayed kinetics of antibody response within the first 42 days post-vaccination. It will be important to assess whether this delay is associated with any enhanced risk of infection during this period. The ability of post-vaccination sera to bind to viral variants of concern is also impaired in people without previous SARS-CoV-2 infection, suggesting that LTCF residents without previous infection might benefit from early delivery of a second dose. Further studies are needed to assess immune responses after the second dose of vaccine and how these results might be used to guide disease control measures.
The introduction of COVID-19 vaccines has proven highly effective in reducing infection-related mortality in many demographic subgroups.2 In the UK, staff and residents within LTCFs have been prioritised for vaccine delivery, which has been associated with reductions in the incidence and clinical severity of SARS-CoV-2 infection. Given the clinical vulnerability of LTCF residents to COVID-19, there is considerable interest in understanding the immune correlates of vaccine protection within this group. However, older and more frail populations are often under-represented in vaccine trials,3, 4, 5, 6 making the extrapolation of data from registration studies directly to resident populations in LTCFs difficult.7, 8, 9
Although most COVID-19 vaccine schedules comprise the administration of two doses, several countries such as the UK have adopted a policy of delaying the second dose to increase the proportion of the population vaccinated with at least one dose. Real-world evidence suggests that a single dose provides over 80% protection against hospitalisation in older people and LTCF residents relative to unvaccinated individuals.2, 10 However, little information is available regarding the immune response to a single dose of vaccine in staff and residents within the care home setting. In particular, there is concern that vaccine-induced immunity might be impaired in LTCF residents as a result of immune senescence, which is apparent from the general increased risk of infection and the attenuated efficacy of vaccines (such as the annual influenza vaccine) in older people.11 A further concern relates to the ability of vaccination to protect against SARS-CoV-2 variants of concern, such as lineage B.1.1.7 (also known as the alpha variant, initially reported in the UK),12 B.1.351 (the beta variant, initially reported in South Africa),13 and P.1 (the gamma variant, initially reported in Brazil),14 which contain mutations in the viral spike protein.
In this study, we aimed to ascertain the humoral and cellular immune responses following a single dose of either the BNT162b2 mRNA vaccine (tozinameran; developed by Pfizer–BioNTech) or ChAdOx1 nCoV-19 vaccine (Oxford University–AstraZeneca).
Methods
Study design and participants
The VIVALDI study is an ongoing prospective cohort study that was set up in May, 2020, to investigate SARS-CoV-2 transmission, infection outcomes, and immunity in residents and staff in LTCFs in England that provide residential or nursing care for adults aged 65 years and older.15 In this Article, we report the results of our investigation into the immune responses of staff and residents at LTCFs participating in the VIVALDI study, following a single dose of the BNT162b2 or ChAdOx1 nCoV-19 vaccines (the clinical standard of care in the UK at the time of the study).
Eligible LTCFs were identified by the senior management team of each care provider or by the National Institute for Health Research Clinical Research Network. Pseudonymised clinical and demographic data were retrieved for staff and residents from participating LTCFs through national surveillance systems. All participants provided written informed consent. If residents lacked the capacity to consent, a personal or nominated consultee was identified to act on their behalf. Demographic data comprising age, sex, address, and whether the individual was a staff member or resident was obtained for all participants.
Ethical approval for this study was obtained from the South Central—Hampshire B Research Ethics Committee (reference 20/SC/023).
Inclusion criteria and data linkage
Staff and residents were eligible for inclusion if it was possible to link them to a pseudoidentifier in the COVID-19 Data Store (established as part of the national pandemic response), which enabled linkage to vaccination records. Only participants who had undergone their first dose of vaccination and two rounds of blood sampling (before and after vaccination) could be included. Those who had had both doses of vaccine before the second round of sampling were excluded from this study. Past infection with SARS-CoV-2 was defined on the basis of results from the semiquantitative ARCHITECT immunoassay (Abbott, Maidenhead, UK) for SARS-CoV-2 nucleocapsid-specific antibodies, using thresholds and methods outlined below. Samples from individuals who were negative for anti-nucleocapsid IgG antibodies in their pre-vaccination sample but positive when tested a second time were considered likely to have had natural SARS-CoV-2 infection between testing rounds and were therefore excluded. Because of a lack of mass testing in the UK in the first wave of the pandemic, it was not possible to ascertain when individuals had been infected with SARS-CoV-2.
The results of anti-nucleocapsid IgG antibody tests were submitted to the COVID-19 Data Store and linked to routinely held data on age, sex, role (staff or resident), and LTCF (obtained through the national SARS-CoV-2 testing programme), and to vaccination status (derived from the National Immunisations Management System). These records are linked to each LTCF via their unique Care Quality Commission location identification, allocated by the Care Quality Commission, which regulates all providers of health and social care in the UK. Linkage of vaccination records to antibody test results was done by NHS England with an algorithm based on an individual's National Health Service (NHS) number to generate a common pseudoidentifier. The linked dataset was analysed in the UCL Data Safe Haven (University College London, London, UK) and vaccination status linked to the laboratory identifier was shared securely with the research team at the University of Birmingham (Birmingham, UK).
Sample collection and preparation
Blood sampling was offered to all participants at two timepoints: first, between Dec 11 and 18, 2020, for the pre-vaccine sample; and second, between Feb 1 and 16, 2021, for the post-vaccine sample. These dates were chosen to coincide with roll-out of the national vaccination programme in LTCFs in England from Dec 8, 2020, onwards. At each round, two blood samples (collected into a serum tube and a 5 mL sodium heparin tube) were obtained from residents and staff. The sodium heparin tube was sent to the Department of Immunology and Immunotherapy of the University of Birmingham to be processed, and the serum tube to The Doctors Laboratory (London, UK) for SARS-CoV-2 antibody testing with the Abbott ARCHITECT anti-nucleocapsid IgG immunoassay.
Samples were processed within 24 h of receipt. Lymphocyte viability has been shown to remain high if processing occurs within this timeframe.16 Blood samples were spun at 300 × g for 5 min. Plasma was removed and spun at 500 × g for 10 min before storage at −80°C, and the remaining blood was separated with use of a SepMate density gradient centrifugation tube (Stemcell Technologies, Cambridge, UK). The resulting layer of peripheral blood mononuclear cells (PBMCs) was washed twice with RPMI 1640 medium and rested overnight in RPMI 1640 medium containing 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin in a humidified incubator at 37°C with 5% CO2.
T-cell responses
T-cell responses of post-vaccination samples were determined using a Human IFN-γ ELISpotPRO kit (Mabtech, Stockhom, Sweden). Peptide mixes containing 15-mer peptides overlapping by ten amino acids from either the S1 or S2 domain of the SARS-CoV-2 spike protein were purchased from Alta Biosciences (Birmingham, UK). Before being assayed, isolated PBMCs were rested overnight in RPMI 1640 medium containing 10% FBS and 1% penicillin–streptomycin. 2–3 × 105 PBMCs were stimulated in duplicate with peptide mixes (2 ng per peptide), with a monoclonal anti-human CD3 antibody (catalogue number 3605-1-50; MabTech) used as a positive control and dimethyl sulfoxide (DMSO) used as a negative control. Supernatants were harvested and stored at −80°C. Following development of the plates using the kit reagents, spot counts were read using a Bioreader 5000 (BioSys, Frankfurt, Germany). Mean spot counts in DMSO-treated negative control wells were deducted from the means to generate normalised spot counts for all other treated wells. Cutoff values were determined previously by Zuo and colleagues.17
Anti-nucleocapsid protein IgG antibody assay
Blood samples were tested for anti-nucleocapsid IgG antibodies with the Abbott ARCHITECT system, a semiquantitative chemiluminescent microparticle immunoassay (performed by The Doctors Laboratory). An index value cutoff of 0·8 was used to classify samples as antibody positive (≥0·8) or antibody negative (<0·8).18, 19
Anti-spike protein IgG antibody assay
Quantitative IgG antibody titres against the trimeric SARS-CoV-2 spike protein were measured with a multiplex serology assay (V-PLEX SARS-CoV-2 Panel 2 [IgG] kit, catalogue number K15384U; Meso Scale Discovery, Rockville, MD, USA), in accordance with the manufacturer's instructions. Briefly, 96-well plates were blocked using kit reagents. After washing, samples were diluted 1:5000 in diluent and added to the wells together with the reference standard and internal controls from the assay kit. Subsequently, incubation plates were washed and anti-IgG detection antibodies added. Plates were washed and read immediately with a MESO QuickPlex SQ 120 system (Meso Scale Discovery). Data were generated by Methodological Mind software (version 1.0.36) and analysed with Discovery Workbench software (version 4.0; Meso Scale Discovery). Presented data were adjusted for any sample dilutions.
ACE2 binding assay
Quantitative inhibition of ACE2 binding by trimeric SARS-CoV-2 spike protein from variants of concern and from the original strain identified in Wuhan, China, was measured with a V-PLEX SARS-CoV-2 Panel 7 (ACE2) Kit (catalogue number K15440U; Meso Scale Discovery) in accordance with the manufacturer's instructions. Briefly, 96-well plates were blocked using reagents from the kit. After washing, samples were diluted 1:10 in diluent and were added to plates along with reference standards. After incubation, detection protein (SULFO-TAG Human ACE-2 Protein, included with the kit) was added to the plate and incubated for 1 h. Plates were washed immediately before reading with the MESO QuickPlex SQ 120 system. Data were generated by Methodological Mind software (1.0.36) and analysed with Discovery Workbench software (version 4.0). Presented data were adjusted for any sample dilutions.
Statistical analysis
All data were checked for normal and logarithmic distribution with use of the Kolmogorov–Smirnov test of distance. Comparative analyses of median values between two groups were done with the Mann-Whitney U test. Three or more groups were compared with the Kruskal-Wallis test, and multiple comparisons were done with the uncorrected Dunn's test for non-parametric data. Spearman's rank correlation coefficients were calculated to assess correlations of time or age with antibody titres or inhibition assay results. Analyses were done with GraphPad Prism software (version 9.1.0).
The VIVALDI study is registered with ISRCTN (ISRCTN14447421).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Plasma samples were collected from 124 staff or residents at 14 LTCFs across England. These participants were chosen because they had samples available before and after vaccination. Specifically, matched samples were taken before vaccination (Dec 11–18, 2020) and 6–52 days after vaccination (Feb 1–6, 2021) with either BNT162b2 or ChAdOx1 nCoV-19 vaccine. Characteristics of the cohort are provided in the table.
Table.
Cohort characteristics
| Participants (n=124) | |||
|---|---|---|---|
| History of SARS-CoV-2 infection* | |||
| Yes | 30 (24%) | ||
| No | 94 (76%) | ||
| Role | |||
| Resident | 35 (28%) | ||
| Staff | 89 (72%) | ||
| Age, years | |||
| Median (IQR) | 56·0 (42·0–66·0) | ||
| Residents | 87·0 (77·0–90·0) | ||
| Staff | 48·0 (35·5–56·0) | ||
| ≥80 | 24 (19%) | ||
| 65–79 | 11 (9%) | ||
| ≤64 | 89 (72%) | ||
| Sex | |||
| Female | 110 (89%) | ||
| Male | 14 (11%) | ||
| Vaccine received | |||
| ChAdOx1 nCoV-19 | 26 (21%) | ||
| BNT162b2 | 98 (79%) | ||
| Mean number of participants per long-term care facility† | 8·9 (8·1) | ||
| Interval between blood tests, days | 49 (49–50) | ||
| Interval between vaccine dose one and blood test two, days | 40 (25–47) | ||
Data are n (%), median (IQR), or mean (SD).
Based on presence of SARS-CoV-2 nucleocapsid-specific IgG antibody responses.
Across 14 long-term care facilities.
As high rates of SARS-CoV-2 infection have been reported in some LTCFs, we initially determined the prevalence of previous natural infection within staff and residents. Nucleocapsid-specific IgG antibody responses (indicating previous natural infection) were detected in 30 (24%) of 124 participants.
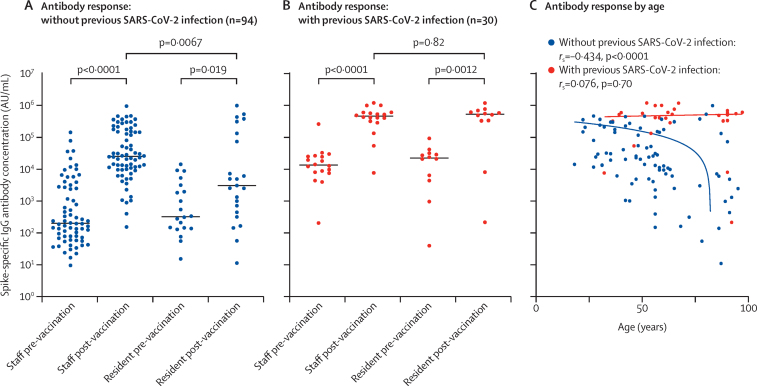
Because COVID-19 vaccines induce spike-specific antibodies, we next assessed the prevalence and magnitude of these antibodies with use of a multiplex serology assay. In staff with no history of natural infection (n=70), the median spike-specific responses increased 127-times, from 202 AU/mL (73–2809 AU/ml) before vaccination to 25 651 AU/mL (10 013–161 212) after vaccination (p<0·0001). In residents without previous infection (n=23), these values were 327 AU/mL (140–2898) before vaccination and 3102 AU/mL (449–135 455) after vaccination (p=0·019)—a 9·5-times increase. Notably, these final values in residents were 8·2-times lower than those seen in staff (p=0·0067; figure 1A).
Figure 1.
SARS-CoV-2 spike-specific IgG antibody responses after a single dose of COVID-19 vaccine
(A, B) SARS-CoV-2 spike-specific IgG antibody concentrations in blood samples from long-term care facility staff and residents without (A) and with (B) evidence of previous SARS-CoV-2 infection (based on anti-nucleocapsid IgG antibody assays). Median values are indicated by solid horizontal lines. p values were calculated by Kruskal-Wallis test. Antibody geometric means are shown in the appendix (p 1). (C) SARS-CoV-2 spike-specific IgG antibody concentrations in blood samples from staff and residents by age and previous infection status. rs and p values are from Spearman's rank correlation analysis.
Within staff with serological evidence of previous infection (n=18), the median IgG antibody titre before vaccination was 13 719 AU/mL (IQR 8077–25 869), and this value was 34-times higher (462 935 AU/mL [316 566–603 527]) after vaccination (p<0·0001). In residents with previous infection (n=12), these titres were 22 827 AU/mL (5005–31 712) before vaccination and 534 184 AU/mL (334 847–676 097) after vaccination (p=0·0012), equivalent to a 23-times increase (figure 1B). These data showed little evidence of any potential effect of immune senescence on the COVID-19 vaccine response within LTCF residents who have had previous natural infection, although this sample size is modest and the findings might represent a survivor effect within this cohort.
The magnitude of antibody response after vaccination was then assessed in relation to age in participants with and without previous infection. Within all participants not previously infected with SARS-CoV-2 (n=94), the magnitude of antibody response after vaccination was negatively correlated with increasing age (rs=–0·434, p<0·0001), and was most notably decreased in those older than 70 years, whereas no such effect was seen in previously infected participants (n=30; rs=0·076, p=0·70; figure 1C).
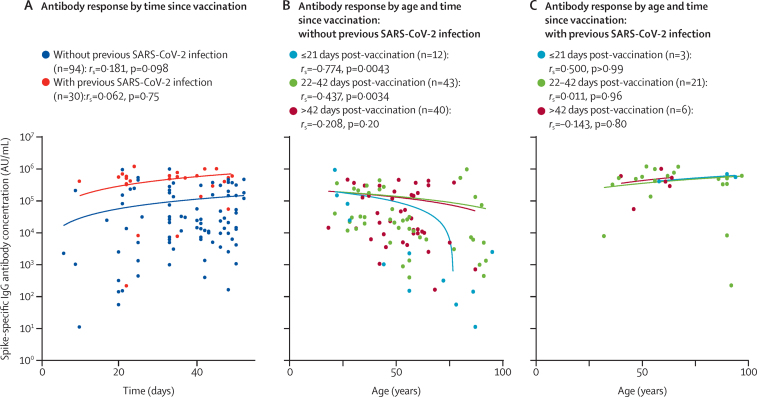
We then examined kinetics of the antibody response to the first dose of COVID-19 vaccination. Considerable differences were observed between participants who had previously been infected with SARS-CoV-2 and those who had not. In participants who had no previous infection with SARS-CoV-2, titres of IgG antibodies against spike protein increased with time since first vaccination (rs=0·181, p=0·098), whereas this correlation was not seen in participants with previous infection (rs=0·062, p=0·75), suggesting that the response peaked sooner (figure 2A).
Figure 2.
Kinetics of spike-specific antibody responses after a single dose of COVID-19 vaccine
(A) SARS-CoV-2 spike-specific IgG antibody concentrations over time after receipt of a first dose of COVID-19 vaccine. (B, C) SARS-CoV-2 spike-specific IgG concentrations by age in three subgroups according to time of analysis post-vaccination (≤21 days, 22–42 days, or >42 days), in participants without (B) or with (C) evidence of previous SARS-CoV-2 infection (based on anti-nucleocapsid IgG antibody assays). rs and p values are from Spearman's rank correlation analysis.
In participants without a history of SARS-CoV-2 infection, age had a substantial effect on the kinetics of antibody generation. In the subgroup of samples taken within the first 21 days post-vaccination (n=12), there was a strong negative correlation between older age and antibody concentration (rs=–0·774, p=0·0043), whereas this effect was weaker at 22–42 days post-vaccination (rs=–0·437, p=0·0034; n=43) and was no longer present beyond 42 days (rs=–0·207, p=0·20; n=40), with similar antibody titres reached regardless of age by this timepoint (figure 2B). This kinetic delay in antibody generation appears to develop around age 50 years in those without a history of SARS-CoV-2 infection, with marked decline in those aged 70 years and older. By contrast, in participants previously infected with SARS-CoV-2, the rate of antibody generation was rapid in all participants and had no apparent correlation with age (figure 2C), indicating that immune memory from previous infection can overcome the delay in antibody generation observed in older people without a history of infection.
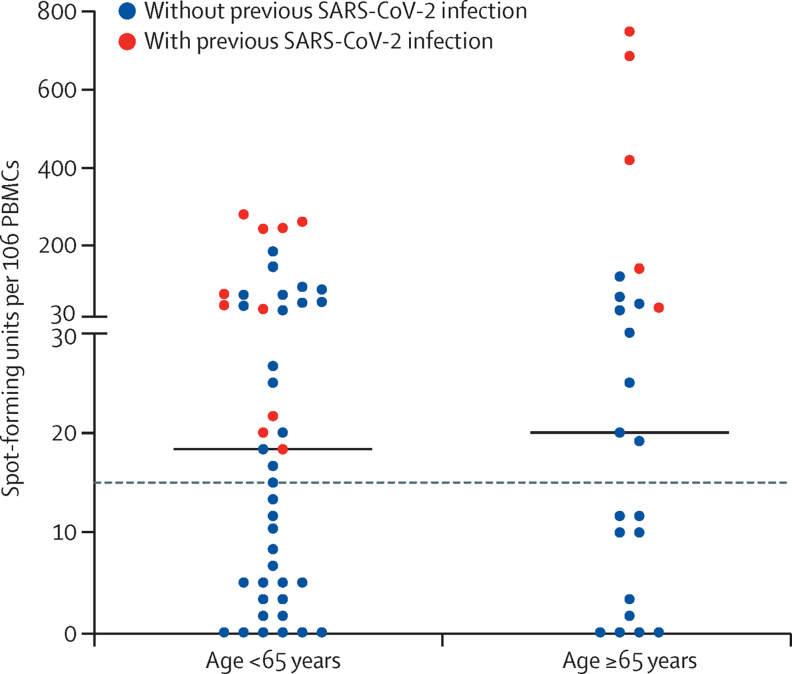
A history of SARS-CoV-2 infection was also found to markedly alter the profile of cellular immunity against SARS-CoV-2 after vaccination. Among participants without a history of infection, positive T-cell responses (defined as >15 spot-forming units [SFU] per 106 PBMCs) to spike protein peptide stimulation were detected in 15 (45%) of 35 participants younger than 65 years and eight (44%) of 18 participants aged 65 years and older. In both age categories, the magnitude of the responses was modest, at 12 SFU per 106 PBMCs (IQR 3–45; n=35) in those younger than 65 years and 12 SFU per 106 PBMCs (1–31; n=18; p=0·82) in those aged 65 years or older on the IFN-γ ELISpotPro Assay. By contrast, in previously infected participants, spike-specific responses were detectable in 100% of staff and residents, and were of larger magnitude (61 SFU per 106 PBMCs [21–247; n=10] in those aged <65 years and 418 SFU per 106 PBMCs [90–748; n=5] in those aged ≥65 years), but still showed no difference between age groups (p=0·075; figure 3).
Figure 3.
Spike-specific cellular immune responses after a single dose of COVID-19 vaccine
T-cell responses (spot-forming units per 106 PBMCs) against spike protein as assessed by the IFN-γ ELISpotPRO assay in long-term care facility staff and residents younger than 65 years (n=89) and 65 years and older (n=35) with and without evidence of previous SARS-CoV-2 infection (based on anti-nucleocapsid IgG antibody assays). Median values per age category are indicated by solid horizontal lines (p=0·79 for age <65 years vs ≥65 years, assessed by Mann-Whitney U test). The dashed horizontal line indicates the cutoff for a positive response (>15 spot-forming units per 106 PBMCs). PBMCs=peripheral blood mononuclear cells.
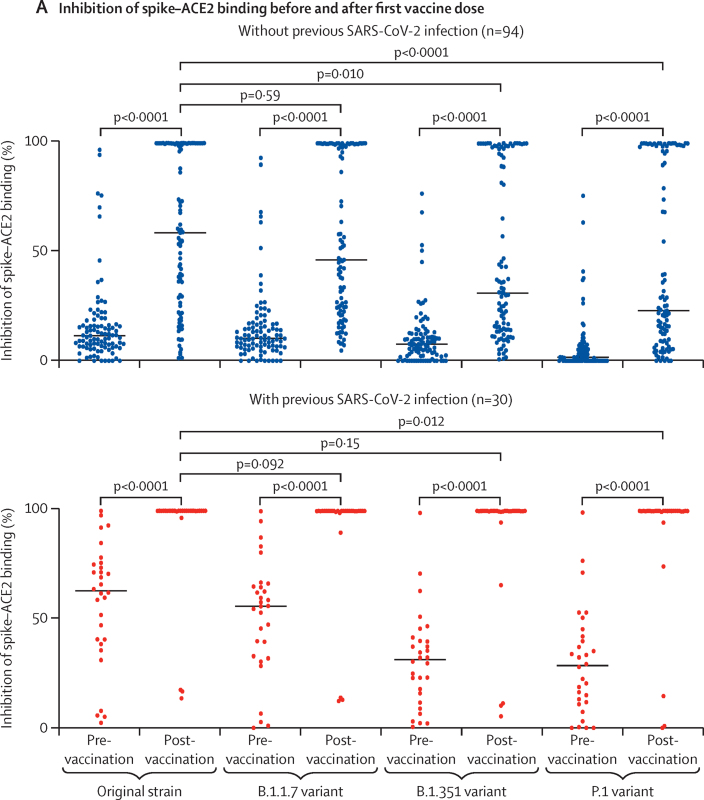
Ageing can be associated with reduced functional antibody activity even in the presence of normal antibody concentrations. In addition, the efficacy of vaccination to prevent infection with viral variants of concern is a crucial public health question. As such, we next assessed the relative avidity of post-vaccination serum in binding spike protein factor for the original strain of SARS-CoV-2 (first identified in Wuhan) and three other viral variants (B.1.1.7, B.1.351, and P.1). To do this, we used an inhibition assay in which serum was used to block the binding of spike protein to labelled ACE2. In participants without a history of infection, the median relative inhibition of binding of ACE2 by spike protein was 12% (IQR 7–17) against the original strain, 10% (6–17) against B.1.1.7, 8% (2–13) against B.1.351, and 2% (0–6) against P.1 before vaccination, and all values were significantly increased after a single dose of vaccine (all p<0·0001; figure 4A). Post-vaccination, median inhibition of ACE2 binding by spike protein from the original SARS-CoV-2 strain was 59% (27–100), and this value was similar to that of the B.1.1.7 variant spike protein (46% [23–100], p=0·59; figure 4A). However, compared with the original strain, inhibition of the spike–ACE2 interaction after vaccination was significantly lower with spike protein from the B.1.351 variant (median inhibition 31% [14–100], p=0·010) and the P.1 variant (23% [9–98], p<0·0001; figure 4A).
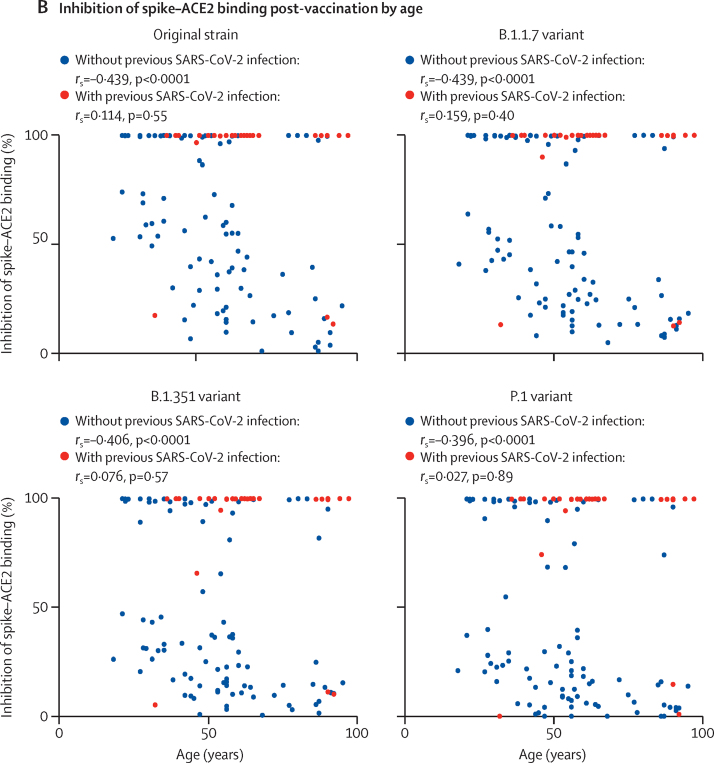
Figure 4.
Serological inhibition of ACE2 binding by spike protein from the original SARS-CoV-2 strain and the B.1.1.7, B.1.351, and P.1 variants
(A) Inhibition of spike–ACE2 binding before and after a single dose of COVID-19 vaccine in long-term care facility staff and residents without and with evidence of previous SARS-CoV-2 infection (based on anti-nucleocapsid IgG antibody assays). Median values are indicated by solid horizontal lines. Post-vaccination, for all SARS-CoV-2 lineages tested, inhibition of spike–ACE2 binding was significantly higher in individuals with previous SARS-CoV-2 infection than in those without (all p<0·0001). (B) Inhibition of spike–ACE2 binding after a single dose of COVID-19 vaccine in staff and residents by age and previous infection status. rs and p values are from Spearman's rank correlation analysis.
In participants who had been infected with SARS-CoV-2 previously, median inhibition of spike–ACE2 binding before vaccination was 63% (40–75) against the original strain, 56% (33–65) against B.1.1.7, 31% (15–40) against B.1.351, and 28% (12–43) against P.1. After a single dose of vaccine, inhibition of the spike–ACE2 interaction was substantially enhanced, reaching median and IQR values of 100% against all variants (figure 4A). The dynamic range of the assay is limited at this plateau and no further delineation was observed between variants. Notably, these observations show that, in previously SARS-CoV-2-infected individuals, a high level of inhibition of ACE2–spike interactions could be achieved for all variants after vaccination, despite the antibodies elicited through natural infection (ie, before vaccination) showing impaired inhibitory capacity against the B.1.351 and P.1 variants compared with the original strain. By comparison, in infection-naive participants, the levels of inhibition achieved after a single dose of vaccine were significantly lower for all variants (p<0·0001 vs previously infected participants); in particular, only a moderate level of inhibition against spike protein from the B.1.351 and P.1 variants was achieved in infection-naive participants after vaccination.
Serological inhibition of spike–ACE2 binding against viral variants was also assessed in relation to participant age. In participants without a history of SARS-CoV-2 infection, a marked decrease in inhibition was associated with increasing age, and this pattern was observed for the original strain (rs=–0·439, p<0·0001) and the B.1.1.7 (rs=–0·439, p<0·0001), B.1.351 (rs=–0·406, p<0·0001), and P.1 variants (rs=–0·396, p<0·0001; figure 4B). However, this negative effect of age was overcome in participants with previous SARS-CoV-2 infection (figure 4B), reinforcing the observation that natural infection can overcome the influence of ageing and immune senescence in relation to antibody avidity in spike binding.
Discussion
It is imperative that vaccine protocols are optimised to deliver strong clinical protection in staff and residents of long-term care facilities. This analysis of adaptive immunity following single-dose vaccination identified a range of novel features within this population—most notably the substantial influence of previous natural infection on the profile of the immune response to a single COVID-19 vaccine—that have implications for vaccine delivery.
24% of participants in this study showed evidence of previous SARS-CoV-2 infection. This rate is higher than the background level within the population, but is not surprising given the high rates of infection that have been reported within some facilities.20 Previous infection increased the magnitude and quality of the adaptive immune response after a single dose of vaccine. In particular, antibody titres were 34-times higher after vaccination in previously infected staff and 23-times higher in previously infected residents compared with before vaccination. These enhanced responses in those with a history of infection have been observed previously, but the magnitude of the effect in younger health-care workers is more modest than that observed in older residents.21, 22 In addition to the high antibody titres in previously infected participants, the functional activity of antibodies was also greatly enhanced, with almost complete inhibition of binding of spike protein from viral variants to the ACE2 receptor following vaccination. This finding concurs with those of previous studies in health-care workers, which showed that people with a history of SARS-CoV-2 infection have 15-times higher humoral responses after vaccination against B.1.351 compared to those with no previous infection.23 Our study also showed that previous infection abrogated any negative influence of ageing or immune senescence on the magnitude or quality of vaccine-induced immune responses. However, it is important to note that vaccination remained beneficial, even in previously naturally infected participants. These observations augur well for potential protection against viral variants of concern within previously infected and single-vaccinated people.
The reasons for the strong enhancement of humoral immunity by previous natural infection are yet to be determined. One explanation might relate to an adjuvant effect of inflammation during natural infection. This inflammation is likely to increase adaptive immune responses, which are elevated in patients with more severe clinical symptoms.24 However, many people have asymptomatic SARS-CoV-2 infection, in which this effect might be less pronounced. SARS-CoV-2-specific immune responses following natural infection are much broader than the focused spike-specific responses that are induced after vaccination. As such, enhanced cellular immunity might act to support the expansion and maturation of spike-specific B-cell responses. In addition, we found that previous natural infection acted to increase antibody binding to viral variants of concern, and it is possible that the increased duration and breadth of adaptive immunity leads to somatic hypermutation of immunoglobulin genes within spike-specific B cells, with associated increased affinity of binding. One confounding factor is that this study only included survivors of SARS-CoV-2 infection, and, because the mortality rate within LTCF residents was high, there might have been a bias towards the selection of individuals with stronger pre-existing immune capacity.
Adaptive immune responses in participants without previous SARS-CoV-2 infection were more modest than in those with previous infection. Spike-specific antibodies were detected in the majority of staff, but median responses were around 8-times lower in residents. However, one intriguing observation was that this relative suppression reflected a slower kinetic development of spike-specific antibodies within older individuals. This phenomenon appeared to develop around the age of 50 years but was strongly enhanced in individuals over 70 years of age, in whom it took up to 42 days to reach antibody levels that were similar to those of younger participants. The reasons for this observation are not clear, but might include a reduced spike-specific B-cell repertoire that requires more time to reach peak antibody development.8, 9 In addition, age-induced impairment of immune cellular proliferation might limit clonal expansion.25 To our knowledge, this phenomenon has not been reported previously after vaccine responses in older people, possibly reflecting a later assessment of vaccine response in most clinical trials. Other analyses as part of the VIVALDI study have shown that the hazard ratio for infection after the first vaccine dose within LTCF residents falls to 44% at 28–34 days, and then further to 38% at 35–48 days, providing some epidemiological support for our immunological observations.2
The emergence of SARS-CoV-2 variants with mutations in the spike protein is a potential threat to the success of vaccine programmes. Although antibody responses were detectable in most participants after a single dose of vaccine, there are some concerns that these antibodies might provide only moderate protection against viral variants of concern. Spike protein from the B.1.1.7 variant was inhibited at a similar level to the original strain of SARS-CoV-2, whereas inhibition of binding to spike protein from the B.1.351 or P.1 variants was 2–3-times lower.
Our study also allowed investigation of the cellular immune response to spike protein in LTCF residents. Notably, cellular responses became detectable in many participants and were similar between younger and older participants, which is reassuring in relation to T cells' potential ability to lyse viral infected cells and support antibody development over time. Cellular responses were substantially enhanced in donors with previous infection, in line with observations in other settings.23
Participants in this study received one dose of either the BNT162b2 or ChAdOx1 nCoV-19 vaccine, but no statistical difference in relation to immune response was observed between vaccines (data not shown).
The limitations of this study include the fact that this was an observational study and not a clinical trial, as well as the lack of detailed medical information or prescribing history for LTCF residents and staff. 110 (89%) participants were female, but this proportion is broadly representative of staff and residents in LTCFs. In addition, established immune correlates of protection against SARS-CoV-2 remain unknown. An important future area of research would be to ascertain whether any of the cells induced by vaccination are cytolytic T cells that have the potential to clear the virus.
In conclusion, our study shows that many staff and residents in LTCFs have previously had natural infection with SARS-CoV-2, and that this infection status has a major impact on the profile of the immune response to a single dose of vaccine and markedly enhances adaptive immune responses. By contrast, vaccine responses in infection-naive individuals are slower to develop in older residents, and show impaired ability to neutralise viral variants of concern when compared with the original strain of SARS-CoV-2. As such, expedited second vaccine dose administration is advisable to deliver effective immune protection to infection-naive LTCF residents
For more on the VIVALDI study see https://www.isrctn.com/ISRCTN14447421
For more on the COVID-19 Data store see https://data.england.nhs.uk/covid-19/
Data sharing
Deidentified test results and limited meta-data will be made available for use by researchers in future studies, subject to appropriate research ethical approvals, once the VIVALDI study cohort has been finalised. These datasets will be accessible via the Health Data Research UK Gateway at https://www.healthdatagateway.org/.
Declaration of interests
AI-S is an employee of the UK Department of Health and Social Care, which funded the study. AH is a member of the New and Emerging Respiratory Virus Threats Advisory Group at the Department of Health. AC reports grants from the UK Department of Health and Social Care during the conduct of the study. LS reports grants from the UK Department of Health and Social Care during the conduct of the study and is a member of the Social Care Working Group, which reports to the Scientific Advisory Group for Emergencies. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the staff and residents in the LTCFs that participated in this study, and Mark Marshall at NHS England who pseudonymised the electronic health records. This report is independent research funded by the UK Department of Health and Social Care (COVID-19 surveillance studies). AH is supported by Health Data Research UK (grant number LOND1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation, and the Wellcome Trust. LS is funded by a National Institute for Health Research Clinician Scientist Award (CS-2016-007). MK is funded by a Wellcome Trust Clinical PhD Fellowship (222907/Z/21/Z). The views expressed in this publication are those of the authors and not necessarily those of the NHS, Public Health England, or the Department of Health and Social Care.
Contributors
LS, AC, AH, MK, GT, and PM conceptualised the study. MK, BA, CF, RB, GT, CB, UA, SH, ATJ, DB, MSB, MA, and ES contributed to project administration. GT, TL, PS, NK, and MK contributed to data curation and validation. MS contributed to database matching. LS, AH, AC, and PM contributed to acquisition of funding. GT, PM, TL, and MK wrote the original draft of the manuscript. All authors contributed to the review and editing of the manuscript. All authors had full access to all the data in the study, and GT, TL, NK, PS, and PM accessed and verified the data underlying the study. PM and GT have shared responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Dutey-Magni PF, Williams H, Jhass A. COVID-19 infection and attributable mortality in UK care homes: cohort study using active surveillance and electronic records (March–June 2020) medRxiv. 2021 doi: 10.1101/2020.07.14.20152629. published online March 9. (preprint, version 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrotri M, Krutikov M, Palmer T. Vaccine effectiveness of the first dose of ChAdOx1 nCoV-19 and BNT162b2 against SARS-CoV-2 infection in residents of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00289-9. published online June 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koff WC, Schenkelberg T, Williams T. Development and deployment of COVID-19 vaccines for those most vulnerable. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abd1525. [DOI] [PubMed] [Google Scholar]
- 4.Koff WC, Williams MA. COVID-19 and immunity in aging populations—a new research agenda. N Engl J Med. 2020;383:804–805. doi: 10.1056/NEJMp2006761. [DOI] [PubMed] [Google Scholar]
- 5.Prendki V, Tau N, Avni T. A systematic review assessing the under-representation of elderly adults in COVID-19 trials. BMC Geriatr. 2020;20:538. doi: 10.1186/s12877-020-01954-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021;50:279–283. doi: 10.1093/ageing/afaa274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Fulop T, Pawelec G, Castle S, Loeb M, Loeb M. Immunosenescence and vaccination in nursing home residents. Clin Infect Dis. 2009;48:443–448. doi: 10.1086/596475. [DOI] [PubMed] [Google Scholar]
- 9.Pereira B, Xu X-N, Akbar AN. Targeting inflammation and immunosenescence to improve vaccine responses in the elderly. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.583019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collier DA, Ferreira IATM, Kotagiri P. Age-related heterogeneity in immune responses to SARS-CoV-2 vaccine BNT162b2. medRxiv. 2021 doi: 10.1101/2021.02.03.21251054. published online June 25. (preprint, version 6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry C, Zheng NY, Huang M. Influenza virus vaccination elicits poorly adapted B cell responses in elderly individuals. Cell Host Microbe. 2019;25:357. doi: 10.1016/j.chom.2019.01.002. 66.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giles B, Meredith P, Robson S, Smith G, Chauhan A. The SARS-CoV-2 B.1.1.7 variant and increased clinical severity—the jury is out. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00356-X. published online June 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tegally H, Wilkinson E, Giovanetti M. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020 doi: 10.1101/2020.12.21.20248640. published online Dec 22. (preprint). [DOI] [Google Scholar]
- 14.Imai M, Halfmann PJ, Yamayoshi S. Characterization of a new SARS-CoV-2 variant that emerged in Brazil. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2106535118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krutikov M, Palmer T, Donaldson A. Study protocol: Understanding SARS-Cov-2 infection, immunity and its duration in care home residents and staff in England (VIVALDI) [version 2; peer review: 2 approved] Wellcome Open Res. 2021;5:232. doi: 10.12688/wellcomeopenres.16193.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson WC, Smolkin ME, Farris EM. Shipping blood to a central laboratory in multicenter clinical trials: effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J Transl Med. 2011;9:26. doi: 10.1186/1479-5876-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuo J, Dowell A, Pearce H. Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection. bioRxiv. 2020 doi: 10.1101/2020.11.01.362319. published online Nov 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ainsworth M, Andersson M, Auckland K. Performance characteristics of five immunoassays for SARS-CoV-2: a head-to-head benchmark comparison. Lancet Infect Dis. 2020;20:1390–1400. doi: 10.1016/S1473-3099(20)30634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan A, Pepper G, Wener MH. Performance characteristics of the Abbott Architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58:e00941–e001020. doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krutikov M, Palmer T, Tut G. Incidence of SARS-CoV-2 infection according to baseline antibody status in staff and residents of 100 long term care facilities (VIVALDI study) medRxiv. 2021 doi: 10.1101/2021.03.08.21253110. published online March 10. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krammer F, Srivastava K, Alshammary H. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manisty C, Otter AD, Treibel TA. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angyal A, Longet S, Moore S. T-cell and antibody responses to first BNT162b2 vaccine dose in previously SARS-CoV-2-infected and infection-naive UK healthcare workers: a multicentre, prospective, observational cohort study. SSRN. 2021 doi: 10.2139/ssrn.3820576. published online April 13. (preprint, version 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]
- 25.Weng N. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified test results and limited meta-data will be made available for use by researchers in future studies, subject to appropriate research ethical approvals, once the VIVALDI study cohort has been finalised. These datasets will be accessible via the Health Data Research UK Gateway at https://www.healthdatagateway.org/.