Abstract
Purpose:
The purpose of this study was to measure the shear bond strength (SBS) of glass ionomer cement (GIC) to artificial carious dentin with and without silver diamine fluoride (SDF) treatment.
Methods:
Permanent molars were sectioned and demineralized to create artificial carious lesions. In five groups, the demineralization of dentin, application of SDF, use of conditioner, and elapsed time between the placement of SDF and restoration were tested for differences in SBS using an UltraTester machine. Statistical analysis was done using the Kruskal-Wallis test and Tukey-Kramer multiple comparison tests.
Results:
The highest bond strength was found when GIC was placed on conditioned and demineralized dentin treated with SDF one week earlier. Treatment with SDF and use of conditioner did not statistically affect the SBS of GIC to demineralized dentin. Statistically significant increases in bond strength were found when one week elapsed between SDF application and GIC placement. The lowest bond strength was found with immediate GIC application onto SDF-treated demineralized dentin.
Conclusions:
These in vitro findings suggest that silver diamine fluoride treatment does not significantly affect the bond strength of glass ionomer cement to dentin lesions, and improved retention is obtained by allowing SDF solution to set for one week prior to GIC placement.
Keywords: SILVER DIAMINE FLUORIDE, BOND STRENGTH, GLASS IONOMER CEMENT, ATRAUMATIC RESTORATIVE TREATMENT, CARIES
Dental caries is the most common disease affecting childhood.1 Comprehensive treatment of caries in pediatric dentistry has focused on removing infected tooth structure and filling with restorative materials such as amalgam, composite, or glass ionomer cement (GIC). However, arresting caries progression with topical silver solutions also has a long history.2 As recently encouraged by the American Dental Association, arresting carious lesions is an appropriate treatment option.3 This may be a more realistic option in certain clinical scenarios, such as holding care, special needs dentistry, and the precooperative child. Better results may be achieved by combining these two treatments: applying a caries-arresting solution followed by applying a restorative material.4
Aqueous silver diamine fluoride 38 percent (SDF) has been utilized for decades to arrest caries in various countries, including China, Japan, Germany, Nepal, Brazil, Argentina, New Zealand, and Australia.5 In 2014, SDF was cleared by the FDA in the United States as a Class II medical device for the treatment of dentinal hypersensitivity.
The mechanism of action for SDF-mediated caries arrest is not fully understood and is multifactorial in nature. SDF targets both organic and inorganic components in the carious lesion. Locally, the insoluble layer formed by precipitated oxidized silver (silver phosphate, silver oxide, and silver chloride) increases remineralization, obturates dentinal tubules, and inhibits enzymes that break down the organic dentin matrix, such as matrix metalloproteinases and cathepsins.6 These phenomena increase the tooth’s resistance to acid dissolution and enzymatic digestion, while plugging of dentinal tubules decreases sensitivity.7 Concerning antibacterial effects, silver ions and possibly metallic silver inhibit bacterial enzymes such as collagenase, cell processes such as DNA replication, cell membranes, cell wall function, and biofilm formation.7–11 Furthermore, dying bacteria release silver into the environment, thus reactivating the SDF to repeatedly act on live bacteria (the so-called zombie effect).12 In vitro, SDF has an antibacterial action against Streptococcus mutans, Streptococcus sobrinus, Lactobacillus acidophilus, Lactobacillus rhamnosus, Actinomycesnaeslundii, and Enterococcus faecalis.7,13–15
Numerous studies have demonstrated the clinical effectiveness of SDF in arresting caries. In randomized controlled trials, Chu and many others have demonstrated caries arrest.16 Caries prevention has been shown by Llodra and Tan in primary and permanent teeth.17,18 Many more studies have confirmed these findings. A meta-analysis has shown that SDF was more effective than a placebo in carious lesion arrest in primary teeth.19 Additionally, research has shown a 400-fold margin of safety, normal pulpal response, and few minor adverse events such as staining of gingiva and clothes.20,21
In cavitated lesions, SDF can be used in conjunction with GIC to combine the benefits of caries arrest and a restoration.4 Modern caries management emphasizes selective caries removal. However, few studies have examined the effects of SDF on bond strength to carious lesions.
The purposes of this study were to: (1) measure the shear bond strength of a glass ionomer cement to artificial carious lesions in dentin with and without the application of silver diamine fluoride; and (2) examine the effect of conditioner use and time-lapse between SDF application and restoration placement.
Methods
Fifty-three noncarious extracted human permanent molars were used for this study. Teeth were extracted for clinical reasons only and collected without documentation or personal identifiers; thus, this study is exempt from requiring human subject board review. The teeth were randomly divided into five groups.
All extracted molars were sterilized via gamma irradiation for 24 hours.22 Then, they were sectioned along the occlusal plane to expose the dentin just gingival to the dentinoenamel junction and polished with 400-grit silicon carbide sand-paper. Samples were stored in deionized water for one week. Sectioned molars were coated with nail varnish (no. 270, Revlon, Inc., New York, N.Y., USA) to expose a three-mm by three-mm window of dentin. Specimens from groups two to five were exposed to a demineralizing solution of acetic acid to create artificial carious lesions (66 hours on a rocker in 0.05M acetate buffer containing 2.2 mM calcium and phosphate at pH 5.0), as described elsewhere.23 Previous studies have shown that this treatment creates an artificial lesion of approximately 140-μm depth and yields a reproducible flat demineralized zone consistent with standards required for measuring shear bond strength (SBS).24 All samples were then mounted in a specimen holder (2.5-cm diameter by three-cm height cylinder) using a dental microstone (Hydrock/Rapid Stone, Kerr Dental, Romulus, Mich., USA). One hour after the samples were embedded in stone, all samples were stored in 100 percent humidity.
Five groups of samples (N equals 10 to 12) were prepared with the following materials, where indicated as follows, following manufacturers’ instructions. Conditioner: GC Cavity Conditioner (GC America Inc., Alsip, Illinois, USA) (20% polyacrylic acid and 3% aluminum chloride hexahydrate) was applied with a microbrush for 10s and rinsed thoroughly. SDF: Advantage Arrest Silver Diamine Fluoride 38% (Elevate Oral Care LLC, West Palm Beach, Fla., USA) was applied with a microbrush for 10 seconds and excess removed with a cotton roll. The conditioner and SDF were applied on the flat exposed dentin surface of the sample prior to insertion into a clamp. After each sample was fixed into a clamp (Figure 1), the restorative materials were inserted in a cylindrical mold that was part of the shear bond testing clamp with dimensions 2.38 mm diameter × 3 mm height (UltraTester Bonding Clamp and Bonding Mold Inserts, Ultradent Products, Inc., South Jordan, Utah, USA). GIC: GC Fuji IX GP capsules (GC America Inc., Alsip, Ill., USA) (100 percent high viscosity glass ionomer cement) were triturated for 10 seconds and applied with a plastic instrument. The mold was secured with the provided screws with sufficient pressure to maintain a level surface and gentle and steady pressure was applied with the plastic instrument to avoid extrusion of material. Fuji IX was chosen for its indication for use in posterior restorations. In all groups, samples were incubated at 100 percent humidity at 37 degrees Celsius for 24 hours to mature the cement prior to bond strength testing. One researcher completed all bonding and SBS testing.
Figure 1.
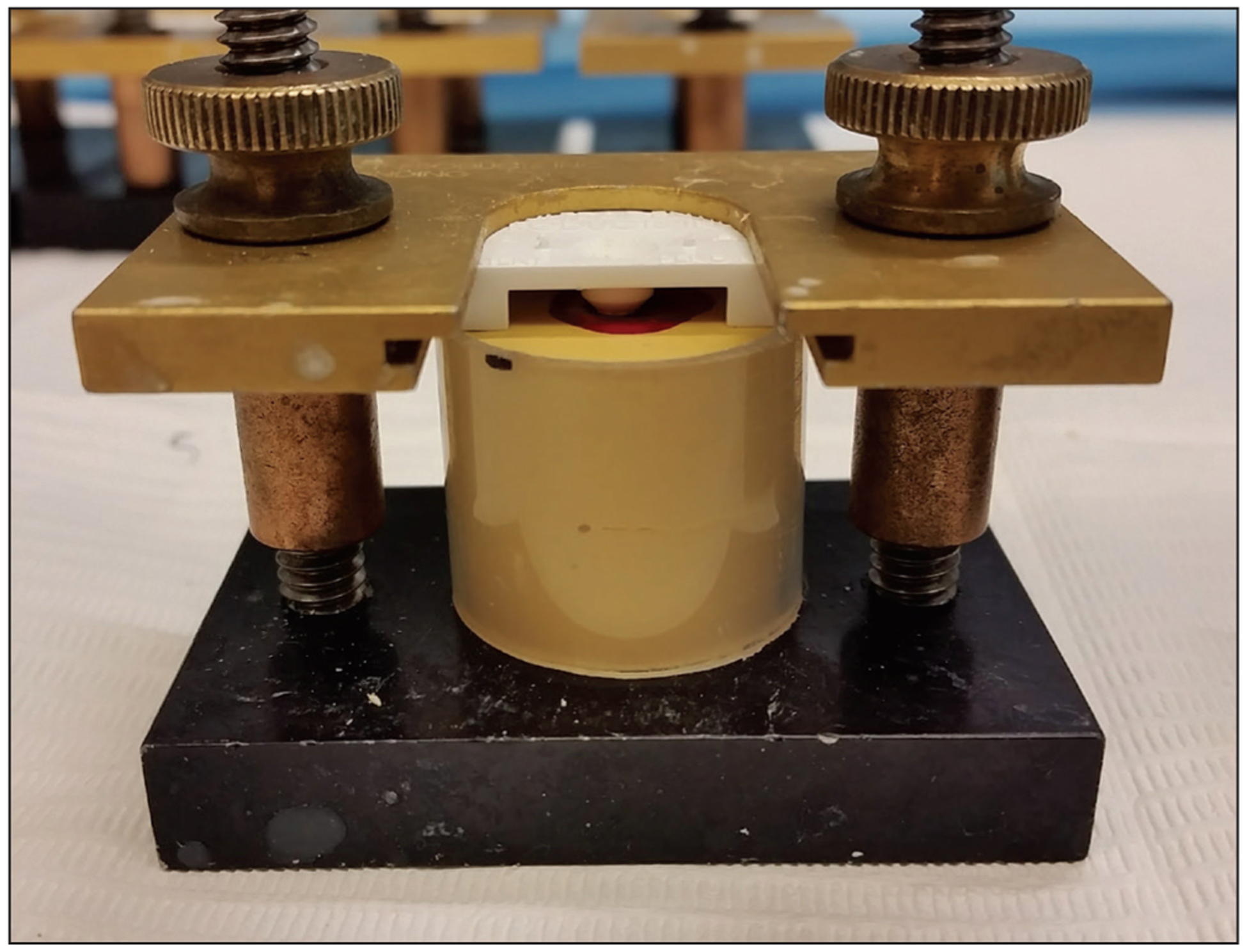
Sample mounted in UltraTester bonding clamp with mold insert.
Preparation of testing groups:
Sound dentin, conditioner, GIC.
Demineralized dentin, conditioner, GIC.
Demineralized dentin, conditioner, SDF, GIC.
Demineralized dentin, SDF, GIC.
Demineralized dentin, conditioner, SDF, GIC placed immediately after SDF.
In groups one to four, the GIC was placed one week after SDF placement. In group five, GIC was placed immediately after SDF. The SBS in MPa was measured using the UltraTester Bond Strength Testing Machine (Ultradent Products, Inc., South Jordan, Utah, USA).
A sample size of 10 was chosen per group. Since the data were not normally distributed based on a Kolmogorov-Smirnov test, the Kruskal-Wallis test was used to determine that there was a difference among groups. Post hoc Tukey-Kramer testing was used to look at pairwise comparisons at a significance level of P=0.05. Statistical analysis was performed using MATLAB (MATLAB R2017b software, Mathworks Inc., Natick, Mass., USA). The statistician was blinded to the groups.
Results
The results are summarized in Figure 2. Group one (N equals 10) had a mean SBS of 6.5 MPa (median equals 6.4, standard deviation [SD] equals 2.0). Group two (N equals 10) had a mean SBS of 10.4 (median equals 10.9±4.5 SD); there was no significant difference between sound dentin and demineralized dentin (P=0.42). Group 3 (N equals 10) had a mean SBS of 13.2 (median 13.5±3.4 SD), which is not significantly different (P=0.66) than that for group two, for which the only preparation difference was SDF treatment.
Figure 2.
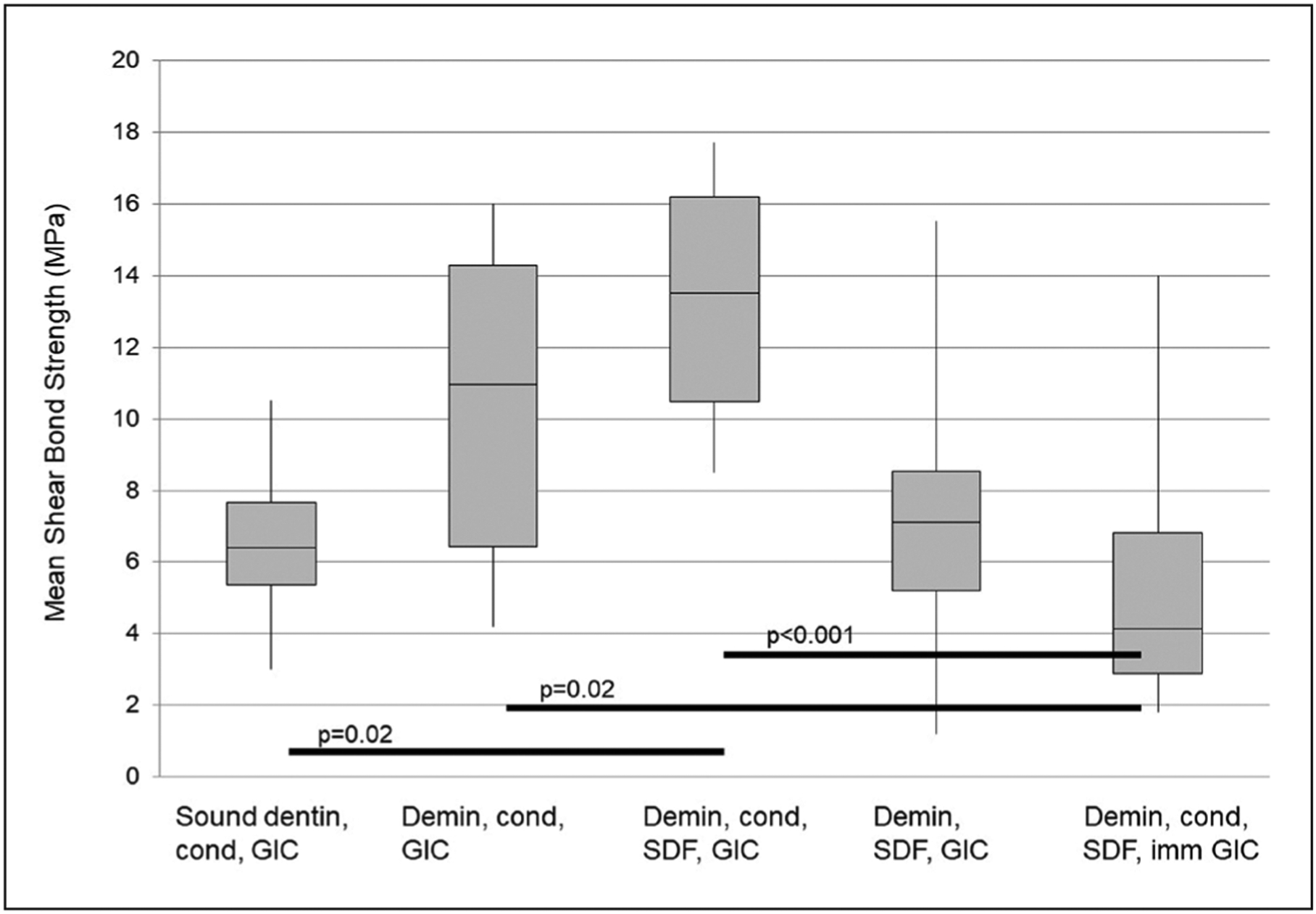
The first quartile, median, and third quartile MPa of shear bond strength. Whiskers denote minimum and maximum values. Black horizontal bars delineate all pairs of groups with statistical significance, with corresponding P-values. Pairwise comparisons were made using post hoc Tukey-Kramer testing. Group 1: sound dentin, conditioner, and glass ionomer cement. Group 2: demineralized dentin, conditioner, and GIC. Group 3: demineralized dentin, conditioner, silver diamine fluoride, and GIC. Group 4: demineralized dentin, SDF, and GIC. Group 5: demineralized dentin, conditioner, SDF, and GIC placed immediately after SDF.
Group four (N equals 11) had a mean SBS of 7.4 (median equals 7.1±3.8 SD). This was not significantly different from group three; the use of conditioner did not affect bond strength (P=0.054). Group five (N equals 12) had a mean SBS of 5.0 (median equals 4.2±3.4 SD). This was statistically significantly different from group three; the immediate placement of GIC after SDF treatment resulted in 62 percent lower bond strength than GIC placed one week after SDF (P<0.001). This group had the lowest bond strength of any GIC preparation.
Discussion
While some literature on this subject exists, there is a lack of information for researchers and clinicians.25,26 Specifically, there is insufficient evidence on the effect of SDF on bond strength in atraumatic restorative treatments (ART). The objectives of this current study were to report the findings regarding SBS with various common clinical treatments.
Sound dentin (group one; mean equals 6.5±2.0 MPa) demonstrated a mean SBS within reported literature values (4.9 to 7.2 MPa) of pure GICs applied to sound dentin.27 GIC has two-fold adhesion, utilizing both micromechanical interlocking and chemical bonding mechanisms.28 Weak polyacrylic acid in the cement and cavity conditioner initiate a demineralization process that is less potent than the etchant used for composite restorations.29 Once surface debris is removed, micro-mechanical interlocking and infiltrations by the cement occur.28 Chemically, ionic bonding occurs between the polyacrylic acid carboxylate groups and the calcium ions in both enamel and dentin.30
When comparing groups two and three, the addition of SDF in group three had no statistically significant effect on the bond strength (P=0.66). Yet the average value for SBS increased from 10.4 to 13.2 MPa when SDF was applied, thus supporting a restorative procedure of SDF-treated caries with GIC in a clinical setting. In group three, GIC was bonded one week after SDF was applied. At this time, it was assumed that SDF penetrated the dentinal tubules and formed a hardened layer on the surface comprised of silver oxide conjugates. Studies show that silver and fluoride ions penetrate 200 to 500 μm into dentin.31,32 In the oral environment, the chemical reaction products of SDF [Ag(NH3)]2F and hydroxyapatite [Ca10(PO4)6(OH)2] are hypothesized to be calcium fluoride CaF2, silver phosphate Ag3PO4, and ammonium hydroxide NH4OH.5,33 It is assumed that one week of SDF exposure forms a hardened surface layer and results in a GIC bond to this layer at a similar strength compared to non-SDF treated demineralized dentin. Although this study found no significant differences in bond strength with and without the application of SDF, a clinical difference may still exist.
There was a statistically significant difference between groups three and five (P<0.001). In group three, the GIC was bonded one week after SDF application. By contrast, GIC was bonded immediately after SDF application in group five, which had a mean of 5.0 MPa (versus 13.2 MPa for group three). When GIC was placed immediately following SDF placement, there was a statistically significant decrease in bond strength. It is possible that, when SDF is not given sufficient time to solidify fully, a layer of unreacted remnant SDF lays on the dentin surface and reduces the biomechanical adhesion to GIC. The layer of aqueous SDF, with silver, ammonia, and fluoride ions, may have affected both mechanical interlocking and chemical bonding of the GIC bond. In some clinical settings, the authors have commonly seen the practice of applying SDF in one visit and then having the patient return at a second visit to check the effectiveness of the SDF and apply a restorative material. These findings may help guide clinicians in determining the timing between SDF application and restoration.
The mean bond strength of group three (13.2 MPa) was not statistically different than that of group four (7.4 MPa), showing no difference with and without conditioner (P=0.054). The literature has shown that the addition of conditioner, which is diluted polyacrylic acid (20 percent by weight), removes the smear layer and has minimal etchant effect.34 In this study, no significant smear layer was formed, as the sample was demineralized after polishing. Therefore, the use of conditioner was not expected to affect bond strength significantly. Interestingly, a statistically nonsignificant trend toward higher bond strength was observed with the use of conditioner. Larger sample size studies are indicated to observe this phenomenon further.
Most importantly, this study’s results show that the 24-hour bond strength of GIC to demineralized dentin is significantly decreased (62 percent) when the GIC is placed immediately after SDF. When SDF is allowed to set one week before GIC placement, a statistically nonsignificant increase in bond strength is observed (27 percent). This may be due to the slow reaction and penetration kinetics of SDF, which are still ongoing when GIC is placed immediately. Clinically, these results suggest that it’s best to separate SDF treatment and GIC placement by a week or more with the optional use of conditioner.
In addition to the restoration of function and reduced food impaction in cavitated lesions, GIC placement after SDF has shown to increase resistance to marginal caries. Mei, Zhao, and colleagues have demonstrated this benefit ex vivo, which further supports SDF in atraumatic restorative treatments to prevent restorative failure.35–37
Limitations to this study include the inherent difference in shear bond strength between in vitro artificial carious lesions and clinical caries. Shear bond strength testing also has limited generalizability to ART restorations’ clinical longevity. Ideally, the longevity of SDF and ART restorations would be tested clinically. A clinical trial has recently begun comparing precisely the GIC conditions found to be most optimal in the present study in groups two and three.38
Another limitation is the use of permanent molars as opposed to primary dentition, partially due to differences in dentinal tubule structure.39 A flat surface is necessary for reproducible SBS measurements. Given the limitations and subjectivity of using clinical carious lesions that have been ground down to a flat surface into affected dentin and a small dentin surface area available on primary dentition for creating artificial carious lesions, the decision was made to use permanent molars. Lastly, the researchers did not examine the fracture surface under microscopy. Previous studies suggest that fractures in GIC may occur in the cohesive interface, resulting in a bond strength measurement that does not reflect true adhesive failure.40
Future studies could examine the time component between SDF placement and GIC bonding. In this study, the time points studied were bonding immediately and one week later. Clinically, it may be advantageous to study bonding after other times, i.e., five minutes, 10 minutes, one day. ART restorations may be used as interim care prior to definitive restoration, while the patient matures, while waiting for conscious sedation or general anesthesia, or as a definitive restoration. Therefore, another time point that is of importance for practitioners is the bond strength of GIC after several months or years. In this study, GC Fuji IX was chosen because it is indicated for posterior restorations, which is one of the more common types of ART. However, future studies should examine the effect on bond strength of composite resins, resin-modified GICs, luting cements, and zinc oxide-based materials.
Conclusions
Based on this study’s results, the following conclusions can be made:
Applying glass ionomer cement to carious lesions after treatment with 38 percent silver diamine fluoride solution is supported, as this caries-arresting agent can be used without compromising bond strength.
The use of conditioner did not significantly improve the bond strength of GIC to SDF-treated demineralized dentin.
In SDF-modified GIC restorations, stronger bond strengths may be obtained by allowing the SDF to fully dry and react for a week before placing a GIC restoration.
Acknowledgments
For their invaluable contributions to this project, the authors wish to acknowledge the California Society of Pediatric Dentistry Research Foundation; Margot Bacino, laboratory assistant, Department of Preventive and Restorative Dental Science, School of Dentistry, University of California, San Francisco, Calif., USA; Deepali Malla, international dental student, Arthur A. Dugoni School of Dentistry, University of the Pacific, San Francisco; Conrad Foo, graduate student, Department of Physics, University of California, San Diego; and NIDCR T32DE007306.
References
- 1.U.S. Public Health Service. Oral Health in America: A Report of the Surgeon General. Rockville, Md., USA: U.S. Department of Health and Human Services; 2000. [Google Scholar]
- 2.Black GV. A Work on Operative Dentistry in Two Volumes: Volume One—The Pathology of the Hard Tissues of the Teeth; Volume Two—Technical Procedures in Filling Teeth. Chicago, Ill., USA: Medico-Dental Publishing Company; 1908:248–53. [Google Scholar]
- 3.Slayton RL, Urquhart O, Araujo MWB, et al. Evidence-based clinical practice guideline on nonrestorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc 2018;149(10): 837–49. [DOI] [PubMed] [Google Scholar]
- 4.Crystal YO, Niederman R. Silver diamine fluoride treatment considerations in children’s caries management: Brief communication and commentary. Pediatr Dent 2016;38 (7):466–71. [PMC free article] [PubMed] [Google Scholar]
- 5.Fung MHT, Wong MCM, Lo ECM, Chu CH. Arresting early childhood caries with silver diamine fluoride: A literature review. J Oral Hyg Health 2013;1(3):117. [Google Scholar]
- 6.Zhao IS, Gao SS, Hiraishi N. Mechanisms of silver diamine fluoride on arresting caries: A literature review. Int Dent J 2018;68(2):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mei ML, Ito L, Cao Y, Li QL, Lo EC, Chu CH. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J Dent 2013;41(9):809–17. [DOI] [PubMed] [Google Scholar]
- 8.Youravong N, Carlen A, Teanpaisan R, Dahlén G. Metal-ion susceptibility of oral bacterial species. Lett Appl Microbiol 2011;53(3):324–8. [DOI] [PubMed] [Google Scholar]
- 9.Silver S. Bacterial interactions with mineral cations and anions: Good ions and bad. Biomineralization and Biological Metal Accumulation. St. Louis, Mo., USA: Springer, Dordrecht; 1983:439–57. [Google Scholar]
- 10.Knight GM, McIntyre JM, Craig GG, Mulyani, Zilm PS, Gully NJ. An in vitro model to measure the effect of a silver fluoride and potassium iodide treatment on the permeability of demineralized dentine to Streptococcus mutans. Aust Dent J 2005;50(4):242–5. [DOI] [PubMed] [Google Scholar]
- 11.Knight GM, McIntyre JM, Craig GG, Mulyani, Zilm PS, Gully NJ. Inability to form a biofilm of Streptococcus mutans on silver fluoride- and potassium iodide-treated demineralized dentin. Quintessence Int 2009;40(2): 155–61. [PubMed] [Google Scholar]
- 12.Wakshlak RBK, Pedahzur R, Avnir D. Antibacterial activity of silver-killed bacteria: the “zombies” effect. Sci Rep 2015;5:9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki T, Nishida M, Sobue S, Moriwaki Y. Effects of diamine silver fluoride on tooth enamel. J Osaka Univ Dent Sch 1974;14:61–72. [PubMed] [Google Scholar]
- 14.Chu CH, Mei L, Seneviratne CJ, Lo EC. Effects of silver diamine fluoride on dentine carious lesions induced by Streptococcus mutans and Actinomycesnaeslundii biofilms. Int J Paediatr Dent 2012;22(1):2–10. [DOI] [PubMed] [Google Scholar]
- 15.Hiraishi N, Yiu CK, King NM, Tagami J, Tay FR. Antimicrobial efficacy of 3.8% silver diamine fluoride and its effect on root dentin. J Endod 2010;36(6):1026–9. [DOI] [PubMed] [Google Scholar]
- 16.Chu CH, Lo EC, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese preschool children. J Dent Res 2002;81 (11):767–70. [DOI] [PubMed] [Google Scholar]
- 17.Llodra JC, Rodriguez A, Ferrer B, Menardia V, Ramos T, Morato M. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res 2005; 84(8):721–4. [DOI] [PubMed] [Google Scholar]
- 18.Tan HP, Lo EC, Dyson JE, Luo Y, Corbet EF. A randomized trial on root caries prevention in elders. J Dent Res 2010; 89(10):1086–90. [DOI] [PubMed] [Google Scholar]
- 19.Gao SS, Zhao IS, Hiraishi N, et al. Clinical trials of silver diamine fluoride in arresting caries among children: A systematic review. JDR Clin Trans Res 2016;1(3):201–10. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez E, Zegarra G, Chirinos E, et al. Short term serum pharmacokinetics of diamine silver fluoride after oral application. BMC Oral Health 2012;12(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horst JA, Ellenikiotis H, Milgrom PM. UCSF protocol for caries arrest using silver diamine fluoride: Rationale, indications, and consent. Pa Dent J (Harrisb) 2017;84(1): 16–26. [PubMed] [Google Scholar]
- 22.White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of teeth by gamma radiation. J Dent Res 1994;73(9):1560–7. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre JM, Featherstone JDB, Fu J. Studies of dental root surface caries. 1: Comparison of natural and artificial root caries lesions. Aust Dent J 2000;45(1)24–30. [DOI] [PubMed] [Google Scholar]
- 24.Chien YC, Burwell AK, Saeki K, et al. Distinct decalcification process of dentin by different cariogenic organic acids: Kinetics, ultrastructurem and mechanical properties. Arch Oral Biol 2016;63:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puwanawiroj A, Trairatvorakul C, Dasanayake AP, Auychai P. Microtensile bond strength between glass ionomer ce0 ment and silver diamine fluoride-treated carious primary dentin. Pediatr Dent 2018;40(4):291–5. [PubMed] [Google Scholar]
- 26.Kucukyilmaz E, Savas S, Akcay M, Bolukbasi B. Effect of silver diamine fluoride and ammonium hexafluorosilicate applications with and without Er:YAG laser irradiation on the microtensile bond strength in sound and caries-affected dentin. Lasers Surg Med 2016;48(1):62–9. [DOI] [PubMed] [Google Scholar]
- 27.Somani R, Jaidka S, Singh DJ, Sibal GK. Comparative evaluation of shear bond strength of various glass ionomer cements to dentin of primary teeth: An in vitro study. Int J ClinPediatr Dent 2016;9(3):192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sidhu SK, Nicolson JW. A review of glass-ionomer cements for clinical dentistry. J Funct Biomater 2016;7(3):pii:E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powis DR, Folleras T, Merson SA, Wilson AD. Improved adhesion of a glass ionomer cement to dentin and enamel. J Dent Res 1982;61:1416–22. [DOI] [PubMed] [Google Scholar]
- 30.Van Meerbeek B, Yoshida Y, Inoue S, De Munck J, van Landuyt K, Lambrechts P. Glass-ionomer adhesion: The mechanisms at the interface. J Dent 2006;34:615–7. [Google Scholar]
- 31.Chu CH, Lo EC. Microhardness of dentine in primary teeth after topical fluoride applications. J Dent 2008;36 (6):387–91. [DOI] [PubMed] [Google Scholar]
- 32.Seto J, Horst JA, Parkinson DY, Frachella JC, DeRisi JL. Silver microwires from treating tooth decay with silver diamine fluoride. bioRxiv 2017: 10.1101/152199.AccessedApril 23, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Everett FB, Hall WB, Phatak NM. Treatment of hypersensitive dentin. J Oral Ther Pharmacol 1966;2(4):300–10. [PubMed] [Google Scholar]
- 34.Van Landuyt K, De Munck J, Coutinho E, Peumans M, Lambrechts P, Van Meerbeek B. Bonding to dentin: Smear layer and the process of hybridization. In: Eliades G, Watts D, Eliades T, eds. Dental Hard Tissues and Bonding. Berlin, Heidelberg, Germany: Springer; 2005:89–122. [Google Scholar]
- 35.Mei ML, Zhao IS, Ito L, Lo EC, Chu CH. Prevention of secondary caries by silver diamine fluoride. Int Dent J 2016;66(2):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao IS, Mei ML, Burrow MF, Lo EC, Chu CH. Prevention of secondary caries using silver diamine fluoride treatment and casein phosphopeptide-amorphous calcium phosphate modified glass-ionomer cement. J Dent 2017;57:28–44. [DOI] [PubMed] [Google Scholar]
- 37.Zhao IS, Mei ML, Burrow MF, Lo EC, Chu CH. Effect of silver diamine fluoride and potassium iodide treatment on secondary caries prevention and tooth discoloration in cervical glass ionomer cement restoration. Int J Mol Sci 2007;18(2):E340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. National Library of Medicine. Outcomes of Restoring Untreated and SDF-treated Dentine Caries Lesions in Primary Teeth of Preschool Children. University of Hong Kong. U.S. National Library of Medicine, National Institutes of Health; 2018. ClinicalTrials.gov identifier: NCT03657862. [Google Scholar]
- 39.Lenzi TL, Guglielmi CA, Arana-Chavez VE, Raggio DP. Tubule density and diameter in coronal dentin from primary and permanent human teeth. Microsc Microanal 2013;19(6):1445–9. [DOI] [PubMed] [Google Scholar]
- 40.Awliya WY, Akpata ES. Effect of fluorosis on shear bond strength of glass ionomer-based restorative materials to dentin. J Prosthet Dent 1999;81(3):290–4. [DOI] [PubMed] [Google Scholar]


