Abstract
Cones are the color-detecting photoreceptors of the vertebrate eye. Cones are specialized into subtypes whose functions are determined by the expression of color-sensitive opsin proteins. Organisms differ greatly in the number and patterning of cone subtypes. Despite these differences, thyroid hormone is an important regulator of opsin expression in most vertebrates. In this chapter, we outline how the timing of thyroid hormone signaling controls cone subtype fates during retinal development. We first examine our current understanding of cone subtype specification in model organisms and then describe advances in human stem cell-derived organoid technology that identified mechanisms controlling development of the human retina.
1. Introduction: The thyroid hormone signaling pathway
Thyroid hormones are a class of nuclear hormones that are iodinated tyrosine derivatives that have diverse functions in metabolism, growth, and development. Thyroxine (T4) is the main thyroid hormone circulating throughout an organism, carried by carrier proteins such as thyroxine binding globulin (TBG). T4 is transported throughout the body to target tissues. T4 is modified via inner or outer ring deiodination, or removal of an iodine moiety, to produce other thyroid hormone derivatives. Inner ring deiodination produces rT3, which is degraded (Gereben, Zavacki, et al., 2008). Outer ring deiodination of T4 produces triiodothyronine (T3). Inner ring deiodination of T3 produces T2, which is degraded along with rT3. T3 and T4 are both capable of binding thyroid hormone receptors, although T3 has greater affinity and is referred to as the “active form” of thyroid hormone despite the greater abundance of T4 in circulation (Samuels, Tsai, Casanova, & Stanley, 1974). This balance of abundance and affinity allows for local regulation of thyroid hormone signaling as well as global regulation through the hypothalamus/anterior pituitary gland/thyroid gland/target axis.
The generation and secretion of thyroid hormone is largely controlled by a cascade of signals through the hypothalamus/anterior pituitary gland/thyroid gland/target axis (Fig. 1). Tight regulation of the release of thyroid hormones is important for maintenance of global metabolic processes (Gelfand, Hutchinson-Williams, Bonde, Castellino, & Sherwin, 1987). The hypothalamus secretes thyrotropin-releasing hormone (TRH), which promotes thyroid stimulating hormone (TSH) release by the anterior pituitary gland (Fig. 1A). TSH acts on the thyroid to stimulate the uptake of iodide (Fig. 1B). T4 is generated when tyrosine residues in the protein thyroglobulin are iodinated and subsequently cleaved to release T4. The thyroid secretes T4, and to a lesser extent T3, which circulates throughout the organism (Fig. 1B and C). Local deiodination occurs in target tissues to either produce active T3 from T4 or degrade thyroid hormones (Chiamolera & Wondisford, 2009) (Fig. 1D–G).
Fig. 1.
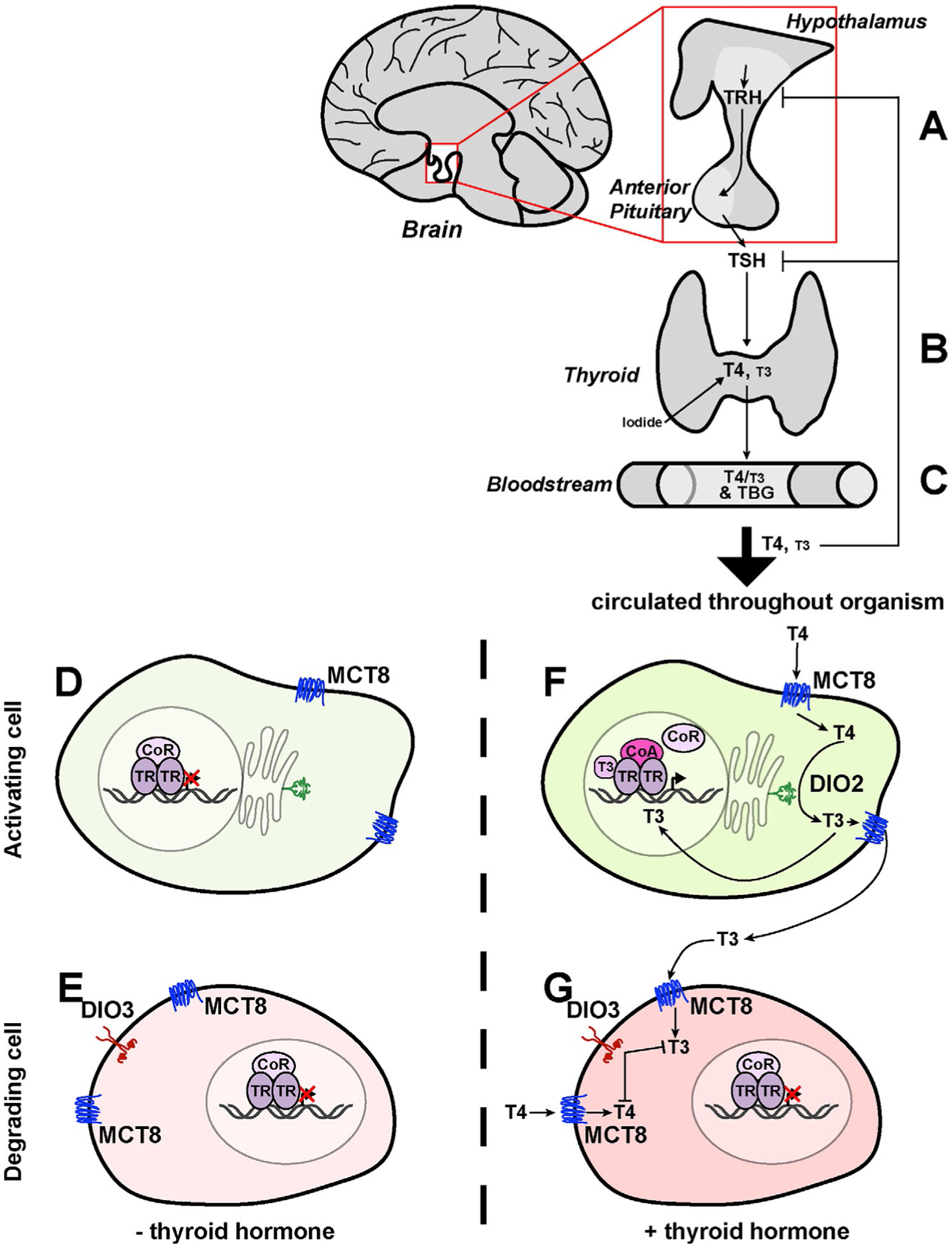
Thyroid hormone synthesis, release, circulation, and local regulation/action. (A) Activation of the hypothalamus releases TRH, which promotes release of TSH from the anterior pituitary. (B) TSH stimulates the thyroid to produce thyroid hormone (T4 and T3), which is stored or released. (C) Thyroid hormones are carried through the bloodstream by TBG. (D and E) In the absence of thyroid hormone, thyroid hormone receptors (TR) bind to TREs as dimers (here, represented as a homodimer), which can also bind to a corepressor (CoR). (F and G) In the presence of thyroid hormone, T4 and T3 enter cells through membrane transporters (here, represented by MCT8). Internally, thyroid hormone can either be activated (F) or degraded (G). (F) DIO2 can convert T4 into T3, which can be released from the cell or brought to the nucleus. T3 binding TR in the nucleus promotes CoR release and coactivator (CoA) binding to promote transcription of response genes. (G) If DIO3 is present, it degrades both T4 and T3. CoR remains bound to TR, inhibiting transcription of response genes.
Thyroid hormone is transported from the thyroid and distributed to peripheral tissues. While circulating in plasma, thyroid hormone is carried by thyroxine binding globulin (TBG) (Fig. 1C) or transthyretin (TTR). Though thyroid hormones are nuclear hormones, they cannot freely diffuse into cells. Membrane transport proteins including monocarboxylate transporters 8 and 10 (MCT8 and MCT10, respectively) bind thyroid hormones and transport them into the cytoplasm (Fig. 1D–G). MCT8 and MCT10 transport both T4 and T3, but MCT8 has a greater affinity for T4 whereas MCT10 has a greater affinity for T3. In addition, the large neutral amino acid transporters (LAT) have weak affinities for T3 and T4 but do transport thyroid hormones across cell membranes. The organic anion transporter polypeptides (OATPs) bind and transport T4 in the brain. Different tissues express different transporters for regulatory purposes (Benvenga & Robbins, 1996; Bernal, Guadano-Ferraz, & Morte, 2015; Friesema et al., 2003; Friesema, Jansen, & Visser, 2005; Korcek & Tabachnick, 1976; Palha, 2002).
Within a cell, thyroid hormones are activated or deactivated through deiodination. Deiodinases (DIOs) are integral membrane proteins characterized by cytosolic selenocysteine-containing active sites that deiodinate thyroid hormones. Deiodinase 1 (DIO1) and Deiodinase 2 (DIO2) are embedded in the ER membrane and convert T4 into active T3 (Fig. 1D and F). DIO3 is found in the plasma membrane and performs inner ring deiodination to convert T3 into T2 or convert T4 into rT3, thereby enabling their degradation (Fig. 1E and G). Deiodination regulates local levels of thyroid hormone in a careful balance between active T3 and inactive T4 (Bianco, Salvatore, Gereben, Berry, & Larsen, 2002; Galton, 2017; Gereben, Zavacki, et al., 2008; Muller et al., 2014). Feedback on the organismal scale also regulates circulating levels of thyroid hormone, as high serum thyroid hormone negatively feeds back on TRH and TSH expression to maintain global homeostatic levels (Fig. 1A–C) (Alkemade et al., 2005; Fliers, Unmehopa, & Alkemade, 2006; Larsen, 1982).
Once modified within the cell, thyroid hormone travels to the nucleus to interact with its receptors, generally referred to as thyroid hormone receptors (TRs) (Fig. 1D–G). Many vertebrates have two TR genes: THRα and THRβ, and these often have splice isoforms that are differentially expressed across tissues (Brent, 2012). Nomenclature varies between species: in model organisms, the genes are named TRα and TRβ, while in humans, these same genes are called THRα and THRβ. TRs bind to DNA at sites called thyroid hormone response elements (TREs). In the absence of thyroid hormone, TRs are often bound by corepressors (Fig. 1D). Upon binding thyroid hormone (of which T3 has a much higher affinity than T4), corepressor binding partners are exchanged for coactivators and transcription is activated (Fig. 1F).
In the absence of T3, corepressors, including nuclear receptor corepressors 1 and 2 (NCOR1, NCOR2/SMRT, respectively), repress expression at target genes (Fig. 1D and E). These proteins repress transcription by recruiting histone deacetylases to render the transcription start site inaccessible to transcriptional machinery (Sun et al., 2013; You et al., 2013). T3 binding to TRs disrupts this repressive complex and recruits local coactivators which in turn recruit histone acetylases. Histone acetylases function to increase accessibility of the start site and promote transcription (Astapova, 2016). Many of these coactivators have overlapping response elements with TREs (Oppenheimer & Schwartz, 1997; Williams, 2008), and local concentrations of the cofactors and their ligands affect how they interact with TRs (Brent, 2012). While TRs can homodimerize, most examples of transcriptional activation involve a heterodimer binding partner such as COUP TF-II or a member of the RXR class of transcriptional regulators (Butler & Parker, 1995; Koenig, 1998).
Summary: Thyroid hormones are produced by the thyroid via a signaling cascade and exist mainly as circulating T4 (inactive) and T3 (active). Thyroid hormones enter the cell via transport proteins and are modified by deiodinases to regulate local levels of thyroid hormone by either activating (DIO1, DIO2) or degrading (DIO1, DIO3) thyroid hormone. Upon entering the nucleus, T3 binds to DNA-bound TRs causing the TR to exchange its binding partner from a corepressor to a coactivator, leading to transcription initiation.
2. Thyroid hormone regulates cell fates during development
Thyroid hormone has important roles in the development of several cell types and tissues. Roles for thyroid hormone signaling have been found in oligodendrocyte differentiation, cochlear development, brain development, muscle stem cell development, and retinal development. In both cochlear and brain development, thyroid hormone signaling is suppressed early and is promoted late to temporally modulate differentiation (Billon, Tokumoto, Forrest, & Raff, 2001; Campos-Barros et al., 1999; Crantz, Silva, & Larsen, 1982; Guadano-Ferraz, Obregon, St. Germain, & Bernal, 1997; Ng et al., 2004, 2009; Oppenheimer & Schwartz, 1997; Tu et al., 1997, 1999). Dynamic thyroid hormone signaling also plays a critical role in cone subtype specification during the development of the retina, the light-detecting tissue in the eye.
3. Cones are the color-detecting photoreceptors of the retina
Vision begins in the retina, the inner layer of tissue that lines the back of the eye (Fig. 2A). The development of retinal cells occurs in a temporal progression. All seven broad cell types (six neurons and one glia; Fig. 2B) arise from a pool of retinal progenitor cells (RPCs) and are born in a series of overlapping temporal birth windows (Fig. 2C). How these fates are driven through signaling, lineage, and stochastic mechanisms remains unclear. Though RPCs are capable of differentiating into any cell type, cell fates are restricted based on developmental timing (Cepko, 2014).
Fig. 2.
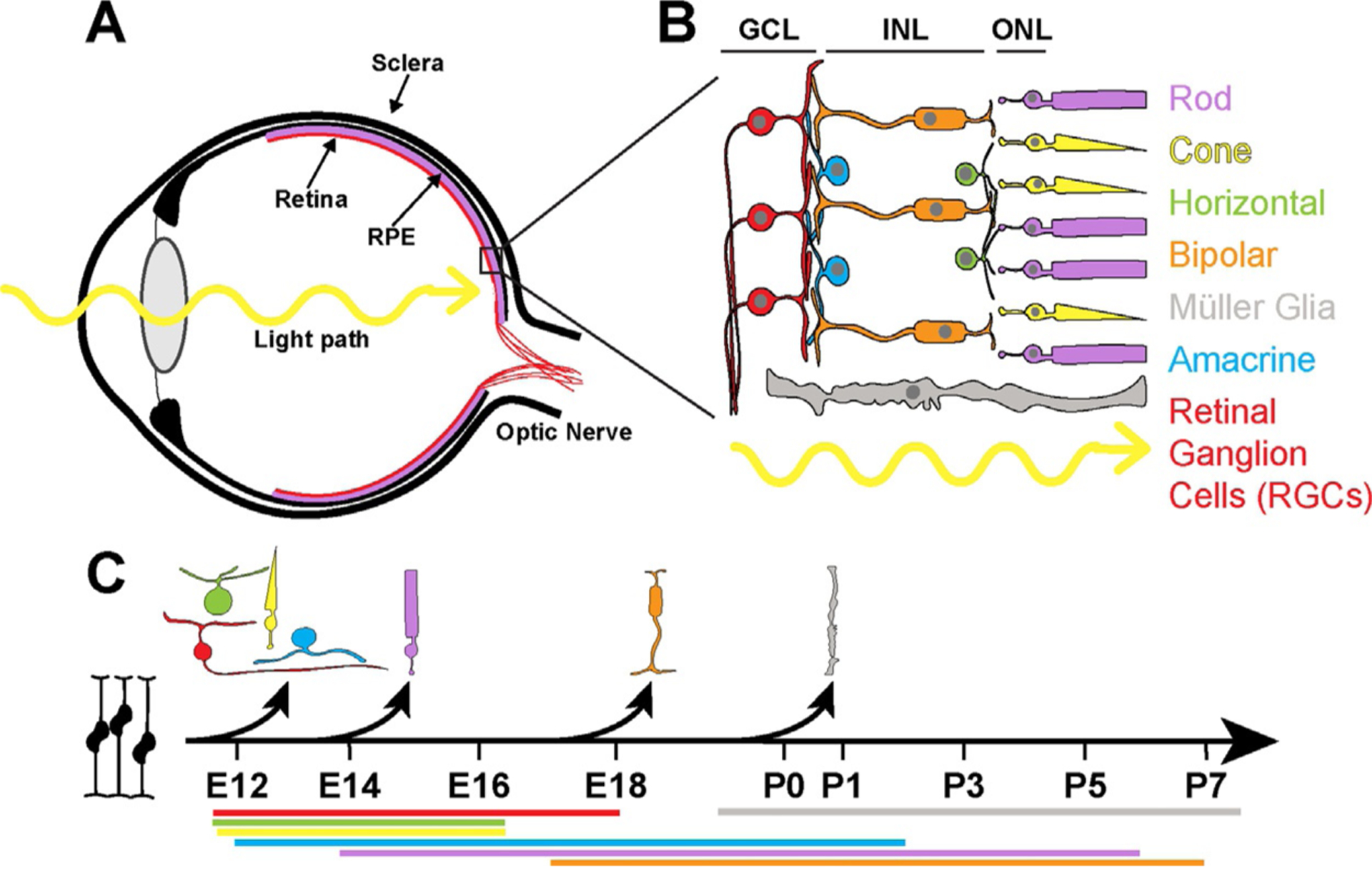
The anatomy and development of the retina. (A) The eye is comprised of a series of layers. (B) Retinal layers include: the ganglion cell layer (GCL), the inner nuclear layer (INL), and the outer nuclear layer (ONL). Müller glia span the length of the retina. Light passes through the GCL and INL before hitting the ONL and the photoreceptors. (C) The cell types of the retina differentiate from retinal precursor cells (RPCs) in a specific temporal order. The black arrows represent the onset of differentiation for each cell type. The colored bars beneath the developmental timeline depict the full birth window during which these cell types are born, color-coded to the cell type. The days listed beneath are for mouse. While the precise days and rates of development differ among organisms, the birth order is conserved. Adapted from Cepko, C. (2014). Intrinsically different retinal progenitor cells produce specific types of progeny. Nature Reviews. Neuroscience, 15(9), 615–627. https://doi.org/10.1038/nrn3767.
The immature retina has two early layers: the outer neuroblastic layer (ONBL) and inner neuroblastic layer (INBL) which contain RPCs. As RPCs become fate-restricted and start to differentiate, the immature layers further stratify into the three layers of the fully developed retina: the outer nuclear layer (ONL), the inner nuclear layer (INL), and the ganglion cell layer (GCL) (Fig. 2B). The retinal ganglion cells inhabit the GCL, the nuclei of the interneurons (amacrine cells, horizontal cells, and bipolar cells) lie in the INL, and the photoreceptors (rods and cones) are found in the ONL. The Müller glia span the retina with nuclei in the inner nuclear layer (Fig. 2B) (Masland, 2012). Additionally, astrocytes migrate from the brain to the retina through the optic nerve, a structure comprised of the axons of retinal ganglion cells. Microglia enter the retina through the CNS and blood vessels (Langmann, 2007; Ramírez, Triviño, Ramírez, & Salazar, 1998; Rashid, Akhtar-Schaefer, & Langmann, 2019; Vecino, Rodriguez, Ruzafa, Pereiro, & Sharma, 2016).
There are two main types of light-sensitive photoreceptor cells: rods and cones. Rods are responsible for low-light vision whereas cones detect color and are our primary daytime light sensors (Kawamura & Tachibanaki, 2008; Nathans & Hogness, 1984; Nathans, Thomas, & Hogness, 1986; Yokoyama, 2000). Opsin proteins in the photoreceptors are sensitive to specific wavelengths of light. Opsin expression defines three subtypes of cones in the human retina. Blue/S cones express short wavelength-sensitive S-opsin, green/M cones express medium wavelength-sensitive M-opsin, and red/L cones express long wavelength-sensitive L-opsin (Deeb, 2005; Hunt, 2001; Kainz, Neitz, & Neitz, 1998; Nathans et al., 1989, 1986). Upon sensation of light by the opsin proteins, the signal is relayed through the interneurons to the ganglion cells, which connect to and transmit visual information to the brain via the optic nerve.
Progress has been made in elucidating mechanisms of retinal development, especially for the specification of photoreceptors. Over the past three decades, work in model organisms and human retinal organoids has uncovered a role for thyroid hormone receptor-driven signaling in regulating cell fate choices during cone subtype specification. As cone subtypes are different between species, the choices are species-dependent, but all show a common theme: a tightly regulated, temporally specific change in cone subtype specification occurs upon the initiation of thyroid hormone signaling. Here, we outline our understanding of how thyroid hormone signaling specifies cone subtypes across model organisms and how human retinal organoids have been used to study this choice in cone subtype fate during human development.
Summary: The retina is the light-detecting tissue of the eye. Cone and rod photoreceptors receive this visual information through their opsins. Different opsin wavelength sensitivity defines cone subtypes.
4. Thyroid hormone specifies cone subtypes in mouse, chicken, and fish
4.1. Mouse
4.1.1. Mouse opsins are expressed in opposing dorsal/ventral gradients
In mice, expression of two cone opsins (S/blue and M/green) defines three cone subtypes: S, M, and S/M co-expressing cones. The subtypes are arranged in a dorsal-ventral gradient with S cones being concentrated ventrally and M cones being found dorsally, and a gradient of co-expression throughout the central retina (Fig. 3A) (Applebury et al., 2000; Baden et al., 2013; Calderone & Jacobs, 1995; Eldred, Avelis, Johnston, & Roberts, 2020; Haverkamp et al., 2005; Rohlich, van Veen, & Szel, 1994; Szel et al., 1994). Cone precursors become postmitotic around embryonic day (E) 11.5. S-opsin mRNA expression is first detected 4 days later at E15.5 and S-opsin protein is detectable at birth (P0), ~5.5 days later. M-opsin expression occurs later, with mRNA first detected at P7 and protein detectable approximately at P14 (Applebury et al., 2007; Fei, 2003; Fujieda, Bremner, Mears, & Sasaki, 2009; Glaschke, Glosmann, & Peichl, 2010; Katoh et al., 2010; Szel, Rohlich, Mieziewska, Aguirre, & van Veen, 1993; Wikler, Szel, & Jacobsen, 1996). Thus, the gradient of cone subtypes begins to form around P0 and is fully established by P14 (Fig. 3I and K) (Roberts, Srinivas, Forrest, Morreale de Escobar, & Reh, 2006).
Fig. 3.
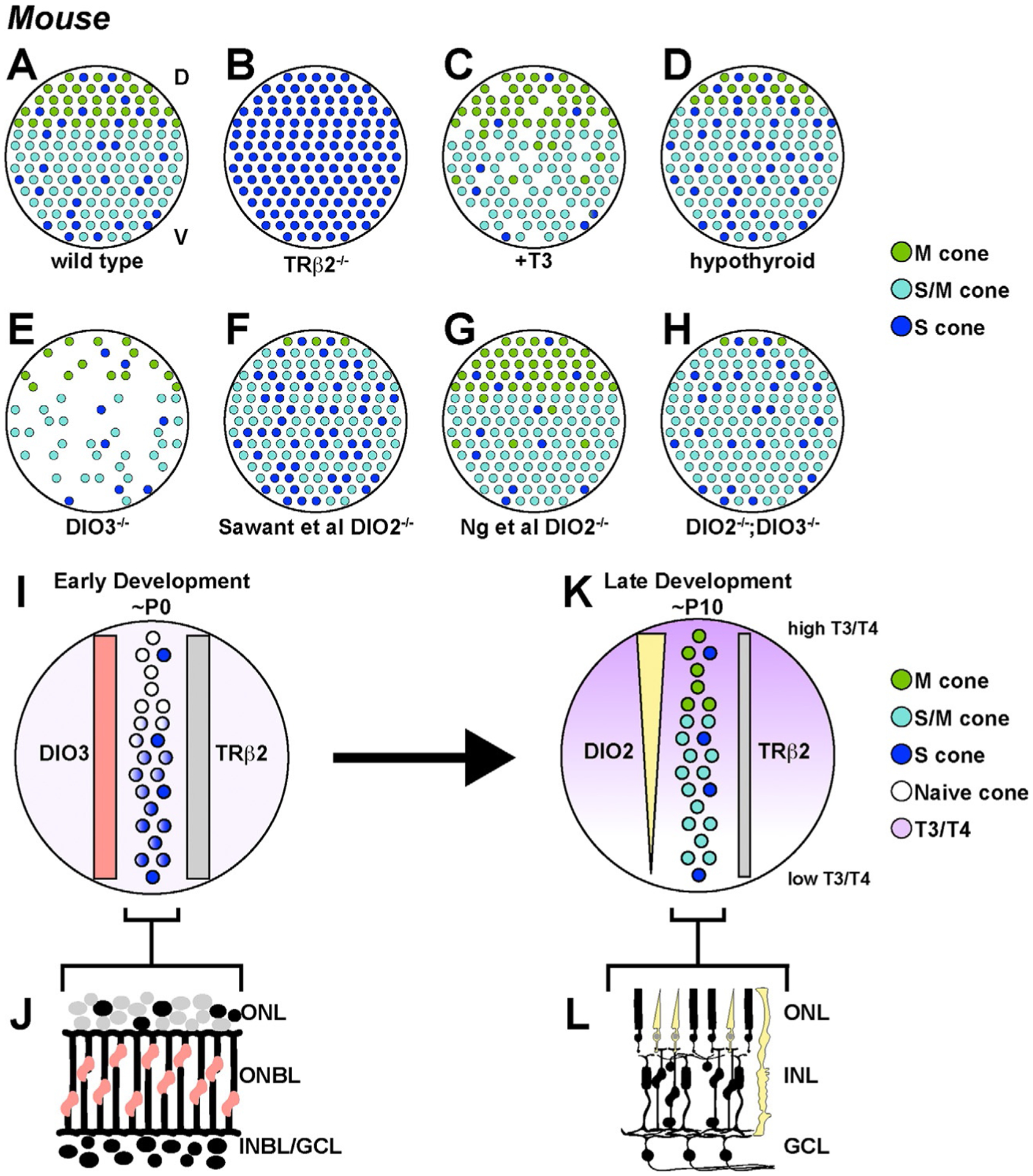
Thyroid hormone signaling regulates M and S opsin expression in mouse cones. (A–H) Phenotypes of cone subtypes in the mouse retina upon changes in thyroid hormone signaling. (A) The wild type mouse retina has a dorsal/ventral gradient of cones. The dorsal region is primarily M cones, the ventral region is primarily S cones, and the transition region contains cones that co-express M and S. (B) Trβ2 mutants display a loss of M cones and an expansion of S cones throughout the retina. (C) T3 treated retinas display a loss of cones and an expansion of M-opsin expression in the ventral region. (D) Hypothyroid retinas display an increase in S-opsin and a decrease in M-opsin expression. (E) Dio3 mutant retinas display a cone death phenotype similar to retinas treated with T3. (F and G) Dio2 mutant retinas from two studies display: (F) an increase in S-opsin and a decrease in M-opsin (Sawant et al., 2017) and (G) an increase in M-opsin and a decrease in S-opsin (Ng, Liu, St Germain, Hernandez, & Forrest, 2017). (H) Double knockout of DIO2 and DIO3 restores the cone gradient, with M only and S only cones restricted to narrower regions of the dorsal and ventral retina, respectively. (I–L) Expression patterns of thyroid hormone regulators through development inform the formation of the thyroid hormone gradient. (I and J) In early development, DIO3 and TRβ2 are expressed uniformly across the retina, with DIO3 in RPCs and TRβ2 in cones. Thyroid hormone levels are uniformly low. Here, it is assumed that the cone distribution does not change over time and that first, S-opsin is activated in S cones and future co-expressing cones in a gradient pattern, and later, M-opsin is activated in a reciprocal gradient pattern. (K and L) In late development, DIO2 is expressed in cones and Müller glia in a gradient pattern with high expression in the dorsal retina and low expression in the ventral retina. This results in a gradient of T3/T4 levels, with high T3/T4 in the dorsal region and low T3/T4 in the ventral region, promoting M-opsin expression in M cones and co-expressing cones. TRβ2 is expressed in cones but at lower levels to mediate thyroid hormone signaling.
4.1.2. TRβ2—A key regulator of cone opsin expression
How are cone subtypes in the developing retina patterned? Pioneering work in mouse identified TRβ2 as an important regulator of S- and M-opsin expression. Trβ2 mutant mice show a complete loss of M-opsin and an increase in S-opsin expression. Additionally, the gradient of S-opsin expression is lost (Fig. 3B) (Applebury et al., 2007; Eldred et al., 2020; Ng et al., 2001, 2011). These phenotypes show that TRβ2 is necessary for M-opsin expression and S-opsin repression.
While several thyroid hormone receptors are present in the retina, expression of Trβ2 is spatiotemporally dynamic during development. Trβ2 expression is detectable starting at embryonic day 13 (E13) and Trβ2-expressing cells are uniformly distributed throughout the retina in the ONBL, where developing photoreceptors are found (Applebury et al., 2007). Expression peaks around E17 and then decreases sharply at birth (P0). After birth, expression persists at a low level until P10, and then decreases further in adulthood (Fig. 3I and J) (Applebury et al., 2007; Ng et al., 2009, 2001). Together, there are two temporal phases of Trβ2 expression. The first phase includes the peak, which coincides with the end of cone precursor generation and suggests a role in the cone vs rod decision. The second phase of expression occurs during the shift from S- to M-opsin activation (Ng et al., 2009). In summary, Trβ2 is expressed in developing cone precursors and terminal cones (Fig. 3K and L) (Roberts et al., 2006).
TRβ2 requires binding to DNA, thyroid hormone, and cofactors to regulate gene expression (Brent, 2012). TRβ2 mutations that block coactivator binding have no effect on S-opsin expression but decrease M-opsin levels across the retina. This result suggests an M-opsin-specific response to T3 when corepressors cannot be exchanged with coactivators. TRβ2 mutations causing either constitutive binding of a corepressor or disruption of T3 binding, resulted in an increase in S-opsin expression in the dorsal retina and a loss of the dorsal-ventral gradient through loss of M-opsin expression in the dorsal retina (Pessoa et al., 2008). Other TRβ2 mutations that prevented ligand binding but were otherwise functional, lost all M cones and induced S cones throughout the retina (Roberts et al., 2006). These results suggest that the S- vs M-opsin decision is controlled primarily by the corepressor’s binding activity: first, when corepressor binds TRβ2 in the absence of T3, and later, upon T3 binding to TRβ2 and release of the corepressor suppression (Pessoa et al., 2008; Roberts et al., 2006).
4.1.3. Regulation of S- and M-opsin expression by thyroid hormone signaling
Expression and function of TRβ2 in cones suggest that endogenous thyroid hormone signaling regulates S- and M-opsin expression. The distribution of endogenous thyroid hormone in the mouse retina is dynamic throughout development. At the onset of S cone specification around P0, thyroid hormone is evenly distributed throughout the retina (Fig. 3I) (Roberts et al., 2006). This suggests that the gradient of S cones is generated through another mechanism that inhibits S-opsin dorsally and/or activates S-opsin ventrally. After birth, differences in thyroid hormone levels are observed between the dorsal and ventral regions of the retina. In the dorsal retina, thyroid hormone levels increase. In the ventral retina, thyroid hormone levels increase slightly until P3 and then sharply decline. By P10, a gradient of thyroid hormone is observed with high levels in the dorsal retina and low levels in the ventral retina (Fig. 3K). At this time, M cones are specified in the dorsal retina, likely due to the local increase in thyroid hormone signaling (Fig. 3A and K) (Roberts et al., 2006). This dorsal-ventral gradient of thyroid hormone levels is consistent with a role of thyroid hormone signaling in promoting M-opsin expression in the dorsal retina and restricting S-opsin expression to the ventral retina.
Mouse retinas that were treated with exogenous T3 (active thyroid hormone) to increase thyroid hormone signaling displayed fewer S-opsin-positive cones and more M-opsin-positive cones (Fig. 3C) (Roberts et al., 2006). In either genetically or pharmacologically induced hypothyroid mice (i.e., with low levels of thyroid hormone in their serum), the opposite phenotype was observed: mice showed an increase in S-opsin and a decrease in M-opsin with no change in the expression of Trβ2 (Fig. 3D) (Glaschke et al., 2010, 2011; Lu et al., 2009). M cone specification experienced a delay of approximately 10 days, but was eventually able to form the gradient (Lu et al., 2009). Treatment with T3 in hypothyroid mice rescued M-opsin distribution and slightly decreased S-opsin in the ventral region (Lu et al., 2009). Thus, thyroid hormone signaling through TRβ2 induces M-opsin and represses S-opsin, and these processes are separable.
Summary: Mouse cone opsins are expressed in opposing gradients, with S-opsin high in the ventral retina and low in the dorsal retina, and M-opsin expressed in the opposite pattern. S-opsin expressed precedes M-opsin expression during development. Thyroid hormone signaling through TRβ2 in cones and cone precursors is necessary and sufficient for M-opsin expression and S-opsin repression.
4.1.4. Thyroid hormone signaling is regulated to ensure cone survival
In addition to its role in cone opsin specification, thyroid hormone also regulates cone survival. Very high levels of T3 signaling through TRβ2 cause a loss of cones through apoptosis, with S cones being particularly susceptible (Fig. 3C) (Lu et al., 2009; Ng et al., 2010). This role of thyroid hormone signaling in cone death is independent of its role in cone subtype specification (Ma et al., 2014). Dio3−/− mutant mice, which have increased thyroid hormone levels in their serum, display a drastic loss of all cone subtypes (Fig. 3E) (Ng et al., 2017). Increasing T3 levels in mice with retinal degeneration exacerbated cone degeneration. Opposite phenotypes were observed in mice with decreased T3. When mice with retinal degeneration were exposed to thyroid hormone antagonists, cone survival improved, with higher S-opsin and lower M-opsin expression (Ma et al., 2014). Taken together, very high T3 signaling results in cone death, predominantly in S cones, whereas low T3 signaling results in a decrease in M-opsin expression.
Summary: Excess thyroid hormone induces cone apoptosis. Early DIO3 activity likely protects against cell death.
4.1.5. Deiodinases pattern thyroid hormone signaling in the mouse retina
Because TRβ2 levels are relatively uniform throughout the retina (Ng et al., 2001; Roberts, Hendrickson, McGuire, & Reh, 2005; Roberts et al., 2006), a gradient of thyroid hormone signaling appears to be generated to regulate the dorsal-ventral patterning of cone subtypes. Local thyroid hormone levels in other settings are often regulated by deiodinases (Bianco et al., 2002; Galton, 2017; Gereben, Zeold, Dentice, Salvatore, & Bianco, 2008; Muller et al., 2014), suggesting that the spatiotemporal dynamics of DIO3 and DIO2 expression may also play a role in retinal cone patterning. DIO3, which degrades T3 and T4, is highly expressed from E13 through birth, and then maintains low levels of expression (Ng et al., 2017, 2010). DIO3 is expressed in a uniform pattern across the retina and throughout all retinal layers, likely in undifferentiated RPCs and immature differentiating cells (Fig. 3I and J) (Ng et al., 2010).
DIO2, which converts T4 to active T3, displays different expression dynamics. Dio2 is very lowly expressed until about P7, at which time its expression increases rapidly (Ng et al., 2017; Sawant et al., 2017). Dio2 expression is reciprocal to Dio3: as Dio3 decreases, Dio2 increases (Ng et al., 2017, 2010). DIO2 is expressed in cones (Sawant et al., 2017) and Müller glia (Ng et al., 2017) (Fig. 3L), which contact and exchange materials with photoreceptors (Reichenbach & Bringmann, 2013). Importantly, this transition from Dio3 expression to Dio2 expression is coincident with the expression of M-opsin in the retina (Fig. 3K).
DIO2 and DIO3 are functionally important for cone subtype specification and survival. Loss of DIO3 causes an increase in thyroid hormone levels early in development, resulting in a dramatic loss of both S and M cones (Ng et al., 2017, 2010), consistent with excess T3 causing cell death (Fig. 3E). In the remaining cones, the opsin proteins are mislocalized to the cell body, pedicle, and axons, instead of the outer segments (Ng et al., 2010).
The role of DIO2 in cone subtype specification and survival remains unclear. Two groups observed different phenotypes in Dio2 knockout mice. Dio2 mutants have low levels of T3, and therefore would be expected to show a phenotype similar to hypothyroid mice, with increased S-opsin and decreased M-opsin expression. Sawant et al. reported an increase in S-opsin expression, an expanded region of co-expressing cones, and a decrease in cones expressing only M-opsin in Dio2 knockout mice (Fig. 3F) (Sawant et al., 2017). In contrast, Ng et al. observed the opposite phenotype in Dio2 knockout mice, reporting a small reduction in S-opsin expression and an increase in M-opsin expression (Fig. 3G), similar to the effect on cone opsin expression observed upon increasing T3 levels (Ng et al., 2017; Roberts et al., 2006). When double knockout Dio2; Dio3 mice were generated, the opsin expression gradient was restored (Fig. 3H) (Ng et al., 2017). The conflicting and weak phenotypes observed in Dio2 mutant mice suggest that other factors likely act with the deiodinases to determine cone opsin expression in the mouse retina. Moreover, the expression gradient observed in Dio2, Dio3 double mutant mice suggests an additional, parallel mechanism acts redundantly with thyroid hormone signaling to regulate opsin expression.
Summary: Dio3 is expressed in RPCs early in development. Dio2 is expressed later in cones and Müller glia. The switch from Dio3 to Dio2 expression correlates with the switch from the initiation of S- to M-opsin expression. Dio3 mutants display apoptosis of cones, whereas Dio2 knockouts exhibit weak, conflicting phenotypes. Early DIO3 expression may protect against cone death from excess T3 and allow for S-opsin expression, whereas late expression of DIO2 may increase local thyroid hormone signaling to repress S-opsin and promote M-opsin expression.
4.2. Chicken
4.2.1. Thyroid hormone regulators are expressed in spatiotemporal waves in chicken
Chickens have four subtypes of single cones, which are sensitive to red, green, blue, or UV light, as well as two sets of double cones, which are sensitive to long wavelengths of light and likely have a role in motion detection (Bruhn & Cepko, 1996; Govardovskii & Zueva, 1977). Each cone subtype is regularly distributed but the relative distance is different for each cone, generating a semi-random pattern (Kram, Mantey, & Corbo, 2010). The chicken also has a high acuity region at the center of the retina with dense cone photoreceptor packing, analogous to the human fovea (Bruhn & Cepko, 1996; Morris, 1982; Viets, Eldred, & Johnston, 2016).
Studies in chickens revealed temporal patterns of thyroid hormone regulation and signaling. Several thyroid hormone receptors are expressed in the chicken retina. TRα is expressed in the progenitor layer. The two isoforms of TRβ, TRβ0 and TRβ2, have different expression patterns. TRβ0 is weakly expressed later in development in the INL, while TRβ2 is expressed early in the ONBL, likely in developing photoreceptors (Fig. 4B) (Sjoberg, Vennström, & Forrest, 1992; Trimarchi, Harpavat, Billings, & Cepko, 2008). Although TRα expression likely has a role in retinal development, expression of TRβ2 in the photoreceptor layer suggests that it is the primary thyroid hormone receptor responsible for cone subtype specification in chickens (Forrest, Sjoberg, & Vennstrom, 1990; Sjoberg et al., 1992).
Fig. 4.
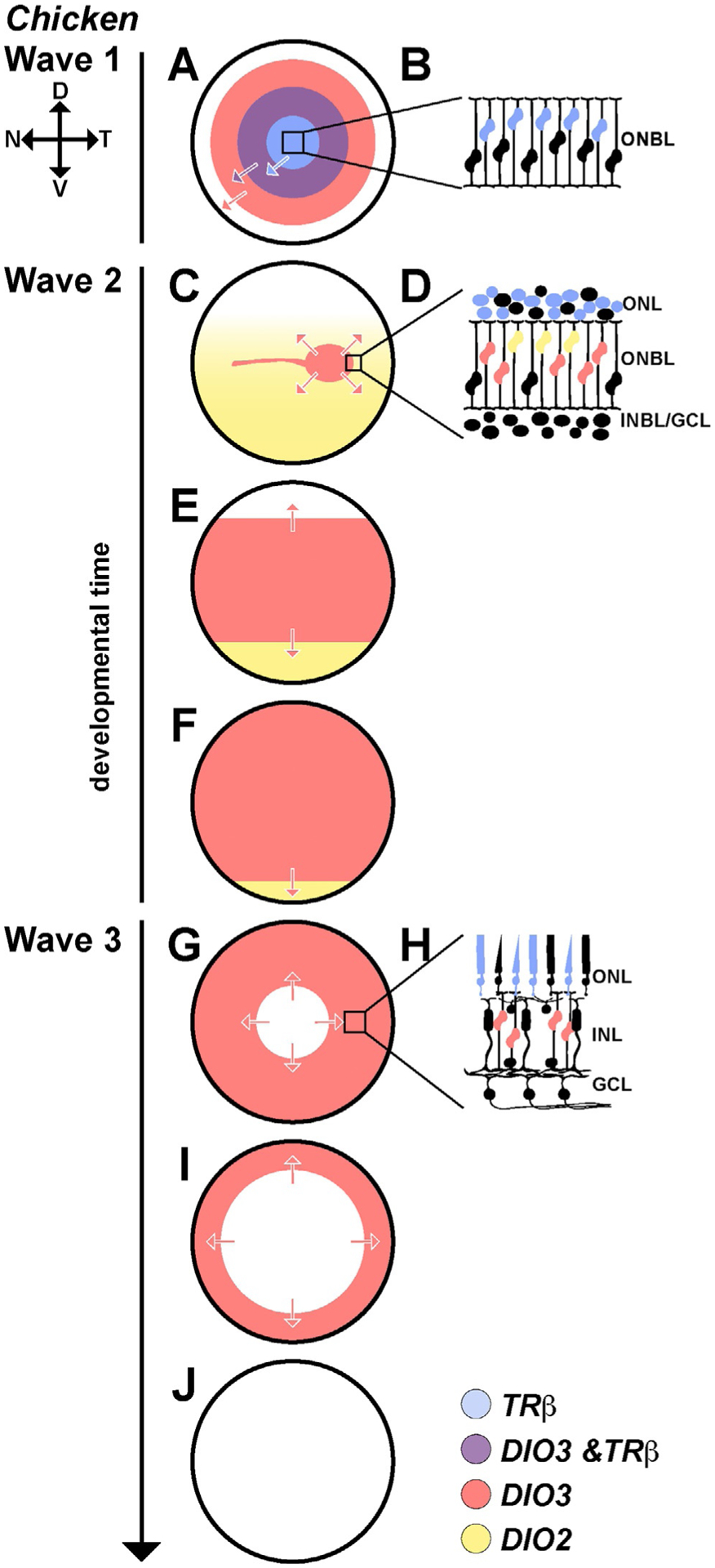
Thyroid hormone regulators are expressed in waves in the developing chicken retina. (A–J) All three waves move from center to periphery during development. (A) In wave 1, the front of the wave is led by DIO3 (red). Slightly delayed/behind DIO3 expression is TRβ (blue). At the transition, there is a region of overlap (purple) between the DIO3 and TRβ waves. (B) TRβ is expressed in a subset of progenitors, mostly in those closest to the developing photoreceptor layer. DIO3 is expressed in all cells in the wave. (C–F) In wave 2, DIO3 expression is limited to a strip running across the central retina (red), and DIO2 is expressed in the ventral retina and lowly expressed in the dorsal retina (yellow). During wave 2, this strip of DIO3 expands out toward the periphery as DIO2 expression becomes limited to the ventral periphery. (D) TRβ is expressed in developing photoreceptors (blue). DIO2 is expressed in a subset of cells in the upper ONBL (yellow). DIO3 is expressed in RPCs (red). (G, I and J) In wave 3, DIO3 expression (red) is lost from the center to the periphery, corresponding with the birth of Müller glia. (H) TRβ (blue) remains active in a subset of photoreceptors. DIO3 expression (red) is limited to the remaining RPC population. Adapted from Trimarchi, J. M., Harpavat, S., Billings, N. A., & Cepko, C. L. (2008). Thyroid hormone components are expressed in three sequential waves during development of the chick retina. BMC Developmental Biology, 8, 101. https://doi.org/10.1186/1471-213X-8-101.
In addition to the dynamic expression of the thyroid hormone receptors, the deiodinases are expressed in three distinct spatiotemporal waves corresponding to landmark events in retinal development. The first wave, during early development in Hamburger-Hamilton (HH) stages 20–26, corresponds with early retina neurogenesis. The wave moves from the central retina to the peripheral retina and involves TRα, DIO3 and TRβ2 (Fig. 4A and B). Expression of DIO3 is initiated in the center and expands to the peripheral retina in precursor cells, slightly preceding the start of neurogenesis (Fig. 4A, red). TRβ expression coincides with the wave of neurogenesis, beginning after DIO3 expression, and is limited to a subset of postmitotic cells. After a period of time where TRβ and DIO3 are both expressed (Fig. 4A, purple), DIO3 expression ceases, leaving TRβ expression in a subset of progenitor cells (Fig. 4A, blue) (Trimarchi et al., 2008).
The second wave at mid development in HH stages 29–33, includes photoreceptor differentiation and migration (Fig. 4C and D). This phase begins with high DIO2 expression in a subset of developing photoreceptors in the ventral region and low expression in the dorsal region (Fig. 4C and D, yellow). A stripe expressing DIO3 and lacking DIO2 expression runs nasal-temporal (Fig. 4C). During the second wave, DIO3 expression expands out from this central stripe to the periphery as DIO2 expression is lost (Fig. 4E and F). TRβ is expressed in developing photoreceptors during this stage (Fig. 4D, blue). DIO2 is expressed in a subset of photoreceptors, likely increasing local thyroid hormone levels (Trimarchi et al., 2008).
The final phase of deiodinase expression occurs as Müller glia are generated during late development in HH stages 35–39 (Fig. 4G–I). DIO3 expression is lost in a wave from the center to the periphery, corresponding with the differentiation of the last RPCs into Müller glia (Fig. 4G–J, red) (Trimarchi et al., 2008). As Müller glia are the final retinal cell type to differentiate, it is likely that the last wave of DIO3 expression maintains the RPC population and that loss of DIO3 expression allows for Müller glia specification. These findings in chicken were fundamental in establishing the wave-based differentiation dynamics of cone subtype specification during retinal development.
In addition to these spatiotemporal waves, roles for thyroid hormone signaling regulators have been characterized in chickens. Knockdown of MCT8, a thyroid hormone transporter, in the early chicken retina results in a decrease in L/M cones and an increase in UV/S cones, suggesting that thyroid hormone availability influences cone subtype specification (Vancamp, Bourgeois, Houbrechts, & Darras, 2019).
Summary: Expression of thyroid hormone regulators in the chicken retina follows a series of spatiotemporal waves, moving from the central retina to the periphery. Early in development, DIO3 in RPCs maintains a low thyroid hormone environment. RPCs differentiate into photoreceptor precursors, which express TRβ. A subset of TRβ positive photoreceptors also express DIO2. DIO2 expression likely generates a locally high thyroid hormone signaling environment, which may have a role in photoreceptor patterning. As DIO2 expression decreases, it is replaced by DIO3 expression, which creates a low thyroid hormone environment to maintain the remaining RPCs. In the final wave, DIO3 expression is lost, allowing Müller glia to differentiate.
4.3. Zebrafish
4.3.1. Thyroid hormone signaling controls cone fates in zebrafish
Zebrafish have four classes of cones: LWS, RH2, SWS2, and SWS1, which are homologous to red-, green-, blue-, and UV-sensitive cones of other species. There are four subtypes of RH2/green cones and two subtypes of SWS2/blue cones. LWS/red cones and RH2/green cones fuse into a cone pair (LWS/RH2 pair) (Chinen, Hamaoka, Yamada, & Kawamura, 2003; Ng et al., 2009; Raymond, Barthel, Rounsifer, Sullivan, & Knight, 1993; Vihtelic, Doro, & Hyde, 1999). The cones are arranged in a regular pattern, with two types of rows: the first is a row of the LWS/RH2 cone pair, and the second is an alternating pattern of blue/SWS2 and UV/SWS1 cones. These two rows run in parallel and alternate across the retina (Branchek & Bremiller, 1984; Raymond, Barthel, & Curran, 1995).
The differentiation of cones occurs in a “fan gradient” in which cells exit mitosis radially, from the ventral to the nasal to the dorsal and finally, to the temporal region of the retina (Easter & Malicki, 2002). Differentiation occurs asynchronously among cell types, with photoreceptors differentiating last (Easter & Malicki, 2002; Hu & Easter, 1999; Schmitt & Dowling, 1996). Cones differentiate into their subtypes in a temporal order: LWS/red cones first, RH2/green cones, SWS1/UV cones, and finally SWS2/S cones (Stenkamp, Barthel, & Raymond, 1997).
In zebrafish, trβ2 is expressed in LWS/red cones and LWS/red cone precursors. trβ2 expression is necessary and sufficient for LWS/red cone specification and repression of the SWS1/UV cone fate (Mackin et al., 2019; Suzuki et al., 2013). LWS/red and SWS1/UV cones are similar to human L/M and S cones, respectively (Ebrey & Koutalos, 2001; Vihtelic et al., 1999). Thyroid hormone signaling through trβ2 also plays a role in promoting the developmental switch in opsin expression from lws2 to lws1, two tandemly arranged long wavelength sensitive genes (Mackin et al., 2019).
Summary: In zebrafish, thyroid hormone signaling through Trβ2 regulates two choices in cone subtype specification. Thyroid hormone signaling specifies LWS/red cone subtypes while suppressing SWS1/UV cone fate, and promotes the developmental switch from lws2 to lws1 expression.
4.4. Salmonid
4.4.1. Dramatic remodeling of the cone mosaic occurs during salmonid development
Cone subtype fates in salmonids, including salmon and trout, are regulated by changes in thyroid hormone signaling that accompany several major life stage transitions. As salmonids progress through development, the retina is significantly modified during the alevin, freshwater-dwelling parr, saltwater-dwelling smolt, and adult stages (Fig. 5G). In particular, changes occur during smoltification, the transition from parr to smolt (Barron, 1986), and during the final maturation to adulthood (Beaudet, Novales Flamarique, & Hawryshyn, 1997; Novales Flamarique, 2000). Both of these events coincide with marked changes in the thyroid gland and thyroid hormone levels (Hoar, 1988; Specker, Eales, Tagawa, & Tyler, 2000). These retinal alterations involve rearrangements of cone positions and changes in opsin expression. Depending on retinal region and stage of development, salmonid retinas exhibit different permutations of cone mosaics that can be classified into three major patterns including: (1) a square mosaic, in which each unit contains an L/M double cone per side with a single cone in the center (Fig. 5A and B); (2) a row mosaic, in which the L/M double cones are turned 90 degrees relative to the square mosaic (Fig. 5C and D); and (3) a mix of square and row mosaics in a transition zone (Fig. 5E and F) (Beaudet et al., 1997; Novales Flamarique, 2000). Classification of these patterns is further subdivided based on the presence or absence of UV cones at the four corners of each unit, giving rise to six total cone mosaic patterns (Fig. 5A–F).
Fig. 5.
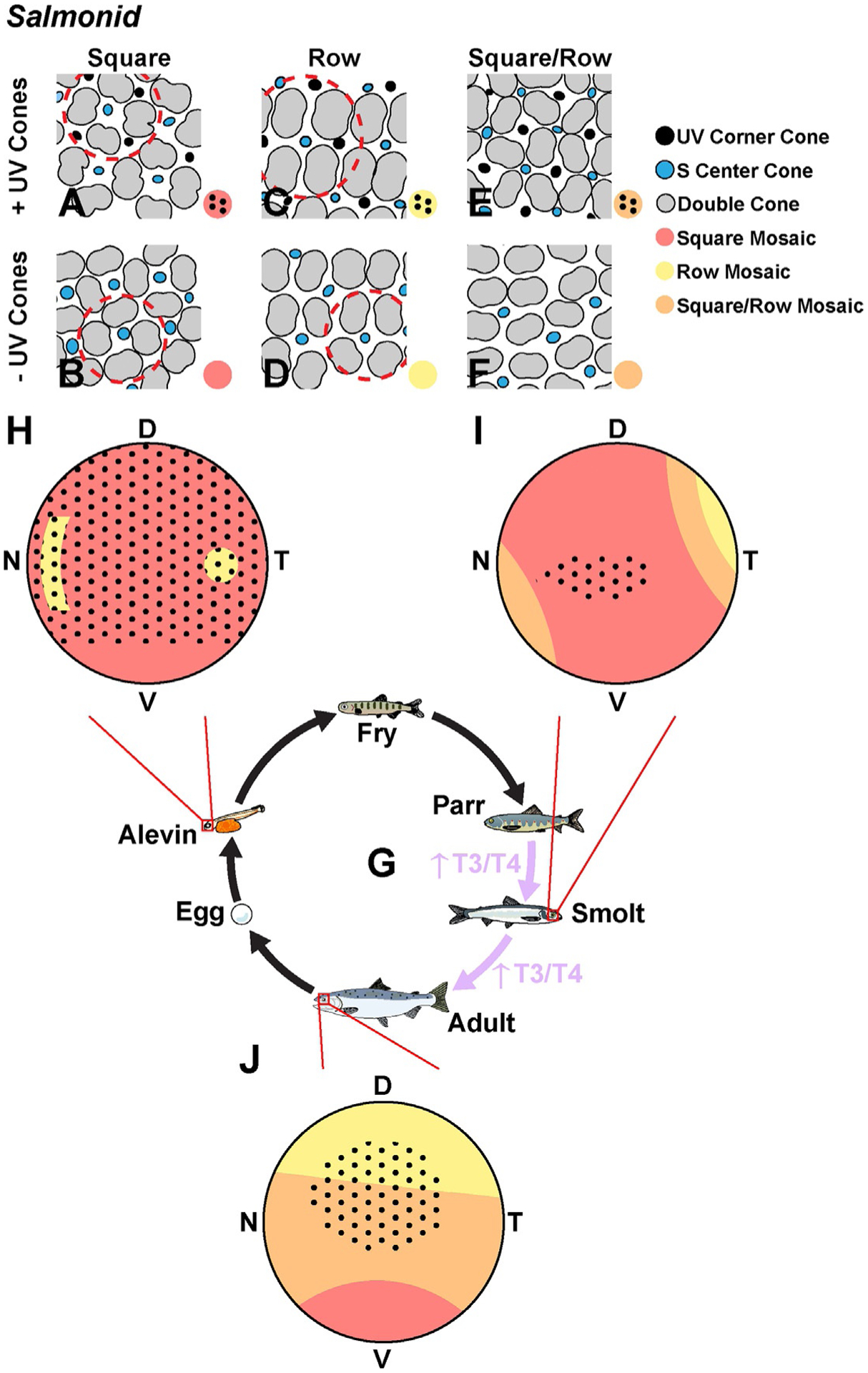
The retina of the salmonid undergoes dramatic changes during development. (A–F) Six types of retinal mosaics in the salmonid. Red dashed circles outline the unit of the mosaic pattern. (G) The lifecycle of the salmonid. (H) The alevin retina is almost entirely square mosaic with UV corner cones except for the ventral region. (I) The smolt retina displays an expansion of the row mosaic in the dorso-temporal region and only has UV corner cones in the central retina. (J) At the adult stage, there is a major expansion of the row mosaic from the dorsal into the ventral region, and the UV corner cones are limited to the central retina. Adapted from Beaudet, L., Novales Flamarique, I., & Hawryshyn, C. W. (1997). Cone photoreceptor topography in the retina of sexually mature Pacific salmonid fishes. The Journal of Comparative Neurology, 383(1), 49–59; Hawryshyn, C. W., Martens, G., Allison, W. T., & Anholt, B. R. (2003). Regeneration of ultraviolet-sensitive cones in the retinal cone mosaic of thyroxin-challenged post-juvenile rainbow trout (Oncorhynchus mykiss). The Journal of Experimental Biology, 206(Pt. 15), 2665–2673. https://doi.org/10.1242/jeb.00470; Novales Flamarique, I. (2000). The ontogeny of ultraviolet sensitivity, cone disappearance and regeneration in the sockeye salmon Oncorhynchus nerka. The Journal of Experimental Biology, 203, 1161–1172.
The cone mosaics of salmonids are highly dynamic during development. Alevin retinas have square mosaics with UV corner cones throughout much of the retina (Fig. 5A and H) (Novales Flamarique, 2000; Novales Flamarique, Sayed Ahmed, Cheng, Molday, & Devlin, 2019). This pattern deviates in the ventral-peripheral regions, which contain square mosaics without UV corner cones (Fig. 5B and H), and in the centrotemporal and nasal peripheral regions, which contain row mosaics (Fig. 5C and H). Upon smoltification, the UV-positive mosaics become restricted to the central retina (Fig. 5A and I), and row mosaics become more regularly distributed in the temporal region (Fig. 5D and I). At adulthood, retinas have row mosaics in the dorsal region, a mix of row and square mosaics in the transition zone between regions, and square mosaics in the ventral region (Fig. 5B, D, F and J). Additionally, corner UV cones reappear in the central region (Fig. 5C, E and J) (Novales Flamarique, 2000).
While several remodeling events occur in the salmonid retina, the patterning of UV corner cones has been related to changes in thyroid hormone levels during development. Consistent with the changes in UV cone constituency during development, UV sensitivity decreases during smoltification and recovers during adulthood. The loss of UV cones during smoltification was originally thought to be due to thyroid hormone-dependent apoptosis (Allison et al., 2003; Beaudet, Browman, & Hawryshyn, 1993; Bowmaker & Kunz, 1987; Flamarique & Hawryshyn, 1996; Hawryshyn, Arnold, Chaisson, & Martin, 1989; Hawryshyn, Martens, Allison, & Anholt, 2003; Kunz, 1987; Kunz, Wildenburg, Goodrich, & Callaghan, 1994; Loew & Wahl, 1991; Lyall, 1957). More recent studies identified two processes that control this change in patterning: thyroid hormone-independent apoptosis of corner cones and thyroid hormone-dependent switching of opsin expression (Cheng, Gan, & Flamarique, 2009).
4.4.2. Thyroid hormone signaling controls opsin switching in single cones
During development, thyroid hormone levels and thyroid hormone receptor expression increase at the alevin stage through smoltification, coinciding with the conversion of single cones from UV to blue fate (Cheng et al., 2009; Greenblatt, Brown, Lee, Dauder, & Bern, 1989; Jones, Rogers, Kille, & Sweeney, 2002). In the single cones, thyroid hormone signaling represses UV opsin and induces blue opsin (Cheng et al., 2009; Gan & Novales Flamarique, 2010).
Thyroid hormone signaling in the retina likely occurs through activation of trα, which is expressed in a dynamic pattern. Prior to opsin switching and smoltification, trα is expressed in all single cones across the retina. As thyroid hormone levels increase and opsin switching begins in the ventral region, a gradient of trα is established, with high expression in the ventral region and low expression in the dorsal region. As the wave of opsin switching progresses into the dorsal region, the gradient reverses, with high trα in the dorsal region and low trα in the ventral region. Once opsin switching is completed after smoltification, trα expression is turned off in all single cones (Gan & Novales Flamarique, 2010; Raine & Hawryshyn, 2009). These observations suggest that Trα mediates the switch from UV to blue opsin.
Exogenous thyroid hormone accelerates the onset of opsin expression and the expansion of cone specification in development. Additionally, after differentiation, thyroid hormone promotes switching of UV cones to blue single cones and induces trα and trβ expression. These are likely two separate processes, with the initial opsin expression occurring through an unknown thyroid hormone receptor (possibly Trβ) or another factor, and the opsin switching being mediated by Trα signaling (Gan & Novales Flamarique, 2010; Raine & Hawryshyn, 2009).
Summary: Thyroid hormone signaling drives opsin expression switching during salmonid smoltification. Thyroid hormone signaling, likely mediated by Trα, induces blue opsin expression and represses UV opsin expression in single cones.
5. Human retinal organoids are a model to study how thyroid hormone signaling specifies human cone subtypes
Extensive work in model systems laid the groundwork for understanding how thyroid hormone signaling specifies cone subtypes during retinal development. Studies in mouse identified how differential, local regulation of thyroid hormone signaling through TRβ2 controls cone opsin expression. Work in chicken revealed how regulators of thyroid hormone signaling are expressed in waves during retinal development. Experiments in fish revealed new roles for thyroid hormone signaling in cone development, including switching of opsin expression.
Thyroid hormone signaling plays a conserved role in determining opsin expression in cones despite dramatic differences in cone subtype patterning, roles of thyroid hormone receptor isoforms, developmental timing, and cell type-specific expression of regulatory proteins. Considering these differences, it is important to understand how thyroid hormone signaling specifies cone subtypes in the human retina. Our understanding of human cone subtype patterning and development largely comes from studies of human retinal tissue and clinical connections between color vision and changes in thyroid hormone level status. Recent advances in retinal organoid technology have enabled the visualization and manipulation of cone subtype specification to gain mechanistic insights. Here, we discuss our current understanding of how thyroid hormone signaling specifies cone subtypes in human retinas and retinal organoids and explore the challenges that need to be addressed in future studies.
5.1. Human cone subtypes are patterned in a series of waves
Humans have three subtypes of cones, each expressing either L-opsin (red-sensitive), M-opsin (green-sensitive), or S-opsin (blue-sensitive) (Nathans et al., 1986). L- and M-opsin proteins are extremely similar and are currently indistinguishable by immunohistochemistry, making them difficult to study independently. As a result, these subtypes are often grouped together during analysis as “L/M” cones (Gowdy & Cicerone, 1998; Hofer, Carroll, Neitz, Neitz, & Williams, 2005; Li & Roorda, 2007; Otake, Gowdy, & Cicerone, 2000; Roorda & David, 1999; Roorda, Metha, Lennie, & Williams, 2001; Rossi et al., 2011).
In the adult human retina, cone density is generally higher in the central retina and decreases toward the periphery, with a slight increase again at the outer rim of the retina. The central retina contains a structure called the macula, which is responsible for high acuity vision. The macula can be broken down into concentric sub-regions that are defined in part by photoreceptor constituency, photoreceptor density, and a physical pit structure. Moving from the center to the periphery, the central foveola contains L/M cones exclusively. Immediately outside of the foveola, the fovea contains S cones along with L/M cones. The outer edge of the macula is denoted by high cone density with sparse rods. In the peripheral retina, rods are densely packed, whereas S cones and L/M cones are distributed at lower density, with L/M cones in a greater proportion than S cones. The far peripheral outer rim of the retina is again cone-rich (Cornish, Hendrickson, & Provis, 2004; Curcio et al., 1991; Curcio, Sloan, Kalina, & Hendrickson, 1990; Provis, Dubis, Maddess, & Carroll, 2013; Xiao & Hendrickson, 2000).
In the developing human retina, cones are generated and specified in a series of waves starting from the foveola and moving out to the periphery (Fig. 6). Cones are first specified during week 11 of fetal development as S cones are generated in the central retina. S cones are then specified in a wave that spreads out to the periphery. Starting around week 14, L/M cones begin to develop at the foveola and continue out to the periphery, following the initial wave of S cone specification. At the front between developing S and L/M cones, there are cones that co-express S and L/M-opsin, and a small percentage of these co-expressing cones persist through adulthood (Cornish et al., 2004; Xiao & Hendrickson, 2000).
Fig. 6.
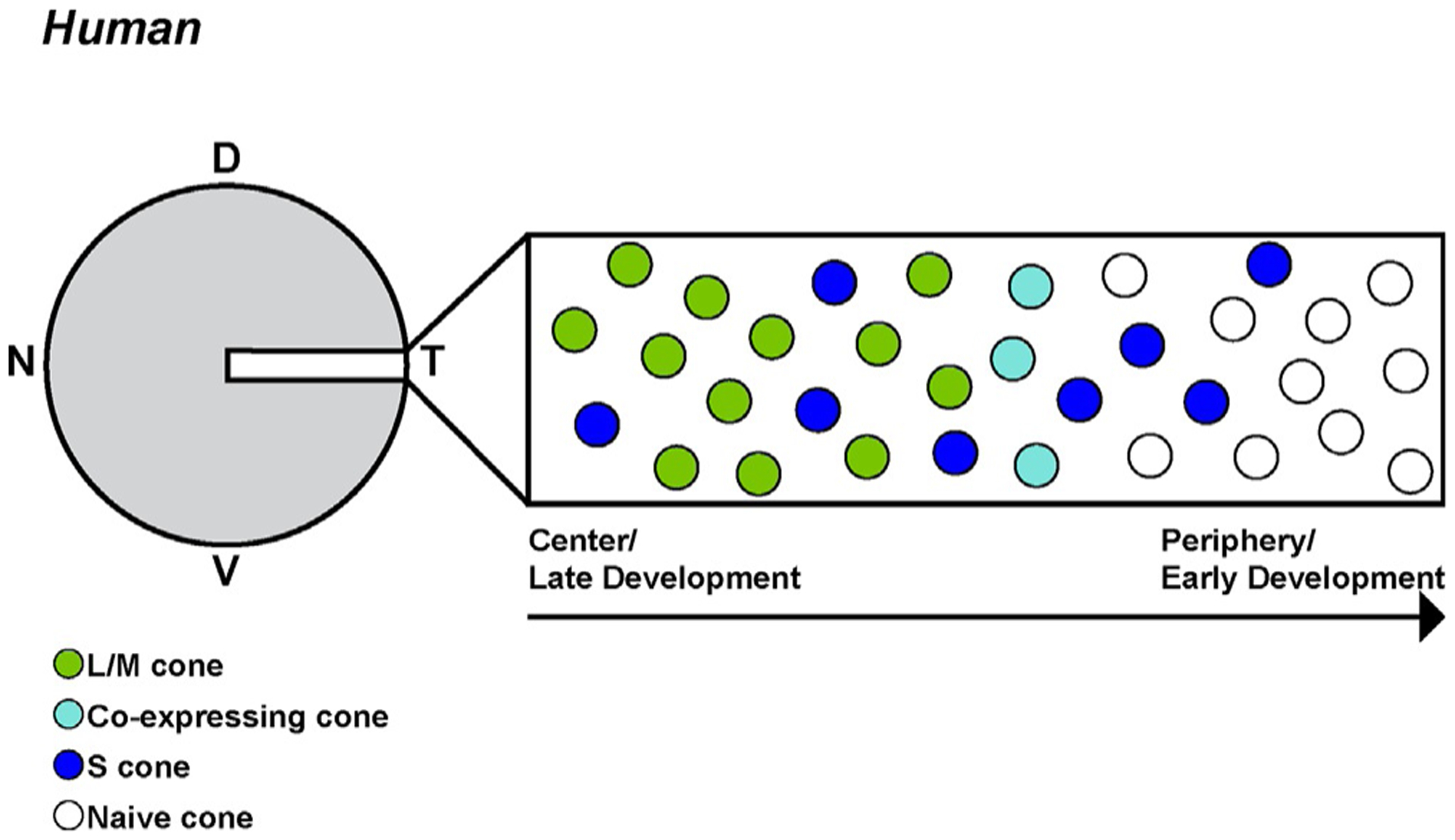
Human cones are specified in waves. A section of the developing fetal retina representing both the progression of developmental time and distance from central to peripheral retina. Note that there is significant retinal growth over time which is not described in this depiction (Xiao & Hendrickson, 2000).
Summary: Humans have three types of cones that are patterned in two waves: first, a wave of S cone specification, followed by a wave of L/M cone specification.
5.2. Clinical thyroid dysfunction correlates with color vision defects
Thyroid hormone dysfunction has been linked to changes in the proportions of cone subtypes and color perception. In one case, an infant with severe thyroid hormone resistance was found to have increased S cone function and decreased L/M cone function. Sequencing of the THRβ locus revealed compound heterozygous mutations in the ligand binding domain, suggesting a disruption of T3 binding affinity (Weiss, Kelly, Bisset, & Deeb, 2012). This was originally thought to be specific to THRβ2, but re-evaluation of the mutations revealed that the mutations affected both THRβ1 and THRβ2 isoforms (Eldred et al., 2018). These findings suggested that thyroid hormone signaling induces L and M cones and suppresses S cone specification, providing a link between thyroid hormone signaling and cone subtype fate in humans.
The first step of local thyroid hormone signaling is entry into the cell through transporters (Fig. 1F and G). The thyroid hormone membrane transporter MCT8 plays a primary role in the retina. A mutation in the SLC16A2 gene, which encodes MCT8, causes Allan-Herndon-Dudley syndrome. This syndrome is characterized by high serum T3, low serum T4, and slightly upregulated TSH. Individuals with Allan-Herndon-Dudley syndrome have several visual impairments, although it is currently unclear if these are directly due to retinal dysfunction or another non-retinal neurological defect (Schwartz & Stevenson, 2007).
While connections between disruption of thyroid hormone signaling during development and defects in color vision are just beginning to be identified, many adult humans, from 1% to 10% depending on the population, have some degree of thyroid disorder (Canaris, Manowitz, Mayor, & Ridgway, 2000; Sawin, Castelli, Hershman, McNamara, & Bacharach, 1985; Tunbridge et al., 1977). Several studies have been conducted to identify color vision defects related to these disorders. Adults with hypothyroidism (i.e., low thyroid hormone levels) have impaired color contrast sensitivity compared to euthyroid (i.e., normal thyroid hormone levels) adults. After l-thyroxine/T4 treatment returned some hypothyroid patients to euthyroid status, the color sensitivities of these patients were significantly improved. However, comparison to the euthyroid control group revealed that, while the red/green sensitivity was restored, the blue/yellow sensitivity had improved but had not been completely restored (Cakir et al., 2015; Racheva et al., 2020). These findings hint at a plasticity in opsin expression and cone function upon changes in thyroid hormone levels even after specification.
Summary: Dysfunctions in the thyroid gland and alterations in serum thyroid hormone levels have been linked to color vision defects in humans.
5.3. Human retinal organoids are a powerful model system to study human retinal development
Studies of humans and model organisms connected thyroid hormone signaling to cone subtype specification during retinal development. Yet, testing human retinal development had been hindered by the lack of an experimentally tractable human model system. Recently, human retinal organoids have emerged as a system to observe development with high temporal resolution and test mechanisms through genetic and pharmacological approaches (Nakano et al., 2012).
Human organoids are three-dimensional tissues, derived from embryonic or induced pluripotent stem cells, that can model developmental events in vitro (Fig. 7). They are small and free-floating in media which enables experimental study and manipulation. A variety of protocols have been developed for generating human retinal organoids. All approaches involve the aggregation of human stem cells (Fig. 7A) to generate a 3D structure. Aggregation is facilitated by the addition of Matrigel, which is composed of extracellular matrix components, during the first few days of differentiation (Fig. 7B). These aggregates are subjected to a series of small molecules that replicate signals that promote retinal development, by suppressing endodermal fate and promoting neural development. In the initial days of differentiation, neuronal vesicles form on the organoid (Fig. 7C), marking the start of eye field specification. Next, the neural tissue is pushed to neural retina fate, and the organoids develop optic vesicles (i.e., early retinal tissue). Successful optic vesicles are excised and cultured individually. Finally, differentiation of retinal cells is promoted, and the organoids are cultured long-term (Fig. 7D) (Capowski et al., 2016; Chichagova et al., 2019; Eiraku et al., 2011; Eldred et al., 2018; Nakano et al., 2012; Wahlin et al., 2017; Zhong et al., 2014).
Fig. 7.
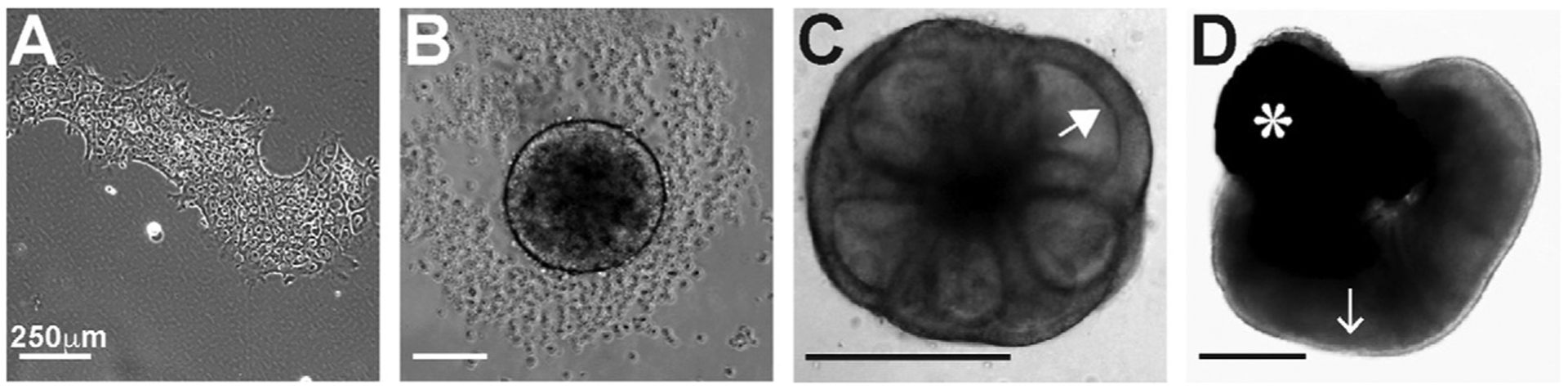
Differentiation of stem cells to human retinal organoids. (A) Stem cells colonies are cultured (iPSCs or ESCs). (B) Day 3 of differentiation. Aggregation of stem cells is almost complete. (C) Day 7 of differentiation. Neuronal vesicles, indicated with a white arrowhead, begin to differentiate. (D) Day 200 of differentiation. The lighter outer edge of the organoid, indicated by the white arrow, is retinal tissue. Photoreceptors are present on the outer edge of the organoid. RPE is also present, marked by a white asterisk.
Organoids can be cultured for several hundred days. They appear to develop on approximately the same time scale as human retinas. Importantly, they faithfully recapitulate the generation of the neuronal types of the retina, including photoreceptors with proper gene expression, morphologies, and functions (Chichagova et al., 2019; Eldred et al., 2018; Hoshino et al., 2017; Kaewkhaw et al., 2015; Nakano et al., 2012; Phillips et al., 2018; Sridhar et al., 2020; Wahlin et al., 2017; Zhong et al., 2014). Some protocols promote certain cell fates, yielding photoreceptor-rich organoids (Eldred et al., 2018; Wahlin et al., 2017). Molecular mechanisms have been interrogated through CRISPR-Cas mutagenesis of stem cells and differentiation of mutant organoids (Eldred et al., 2018; Wahlin et al., 2017), addition of pharmacological agonists and antagonists (Eldred et al., 2018), and viral infection for sparse labeling and lineage tracing (Garita-Hernandez et al., 2018, 2020; Quinn et al., 2019). Additionally, patient-derived stem cells can be differentiated into organoids to model diseases (Deng et al., 2018; Foltz & Clegg, 2019; Gao et al., 2020; Guo et al., 2019; Li et al., 2019).
Human retinal organoids have great potential to address questions about human biology. Our work identifying the role of thyroid hormone signaling in human cone subtype specification demonstrated how organoids could be used as a tractable model for studying mechanisms of human development.
Summary: Human retinal organoids are a powerful system to study the molecular mechanisms controlling the development of the human retina.
5.4. Thyroid hormone signaling regulates the choice between S and L/M cone fates in humans
The Johnston lab utilized human retinal organoid technology to investigate the mechanisms controlling S vs L/M cone subtype specification (Eldred et al., 2018). Our studies started by observing the timing of S and L/M cone development in organoids. Tracking the generation of cone subtypes in organoids over time showed that S cones are generated between days 150 and 170 of differentiation. L/M cones are specified later, between days 170 and 300. The temporality of cone specification in organoids mirrors that in the developing human retina, with S cones generated before L/M cones. The approximately 20-day delay between the onset of S and L/M cone specification is similar to the timing observed in fetal development (Hoshino et al., 2017).
The similarities between cones in human retinas and organoids extend beyond timing and opsin expression. Cones, like other neurons, have axons and cell bodies. Additionally, they have functional structures extending opposite the axons. The outer segments are farthest from the cell body and are rich in the opsin proteins that detect light. Between the outer segments and the cell body are inner segments, which are responsible for carrying out the downstream phototransduction cascade to transmit the signal to the interneurons (Richardson, 1969; Wassle, 2004). S and L/M cones are morphologically distinct and can be identified by their unique shapes and sizes of their outer and inner segments. S cones have short outer segments and thin inner segments, while L/M cones have long outer segments and wide inner segments (Curcio et al., 1991). These morphological features are recapitulated in organoids at day 200 of differentiation, showing that organoids grow cones that resemble those in humans (Eldred et al., 2018).
Across the human retina, cone subtypes are found in a ratio of approximately 13% S cones to 87% L/M cones. Human retinal organoids had a similar ratio, with 29% S cones and 71% L/M cones. Therefore, the proportion of cone subtypes is similar in human retinas and organoids, with more L/M cones than S cones (Eldred et al., 2018).
Using human retinal organoids as a model for human retinal development, we next evaluated the role of thyroid hormone signaling in human cone subtype specification. Organoids treated with T3 were comprised of almost exclusively L/M cones and few S cones, suggesting that thyroid hormone is sufficient to promote L/M cone fate and suppress S cone fate. THRβ mutant retinal organoids displayed a complete loss of L/M cones, generating an S cone-only organoid, showing that THRβ is necessary for the L/M cone fate. Interestingly, knocking out THRβ2 alone showed no changes in cone ratios, suggesting functional differences between human and mouse isoforms of the receptor. Importantly, THRβ knockouts treated with T3 still only generated S cones, showing that T3 signals through THRβ to promote L/M fate and suppress S cone fate in human cone subtype specification (Eldred et al., 2018).
As retinal organoids grow in the absence of other extrinsic signals, regulation of thyroid hormone signaling must be intrinsic to the retina. DIO3 expression peaks early in development, and then decreases. Inversely, DIO2 expression is low early in development and peaks later, coinciding with the switch from S to L/M cone subtype specification. This developmental progression resembles that observed in chick and mouse. Other thyroid hormone signaling regulators show varying expression patterns. SLC16A2 (MCT8), the thyroid hormone membrane transporter, as well as the thyroid hormone receptor cofactors NCOR2 and MED1, are expressed at constant levels throughout development. THRβ and some thyroid hormone transport genes are lowly expressed early in development but increase over time (Eldred et al., 2018). While transporters and coregulators may still play an important role in thyroid hormone signaling regulation, the temporality of deiodinase expression with DIO3 expression peaking early and DIO2 expression peaking late, correlating with the time that L/M cones begin to be specified, suggests that the deiodinases are the primary regulators of thyroid hormone levels in the developing human retina.
Taken together, these data support a model in which DIO3 degrades T4 and T3 to keep thyroid hormone levels low early in development, creating an environment permissive for specification of S cones (Fig. 8A and B). Later in development, DIO3 expression decreases and DIO2 expression increases, promoting the generation of T3 that signals through THRβ to specify L/M cones (Fig. 8C and D) (Eldred et al., 2018).
Fig. 8.
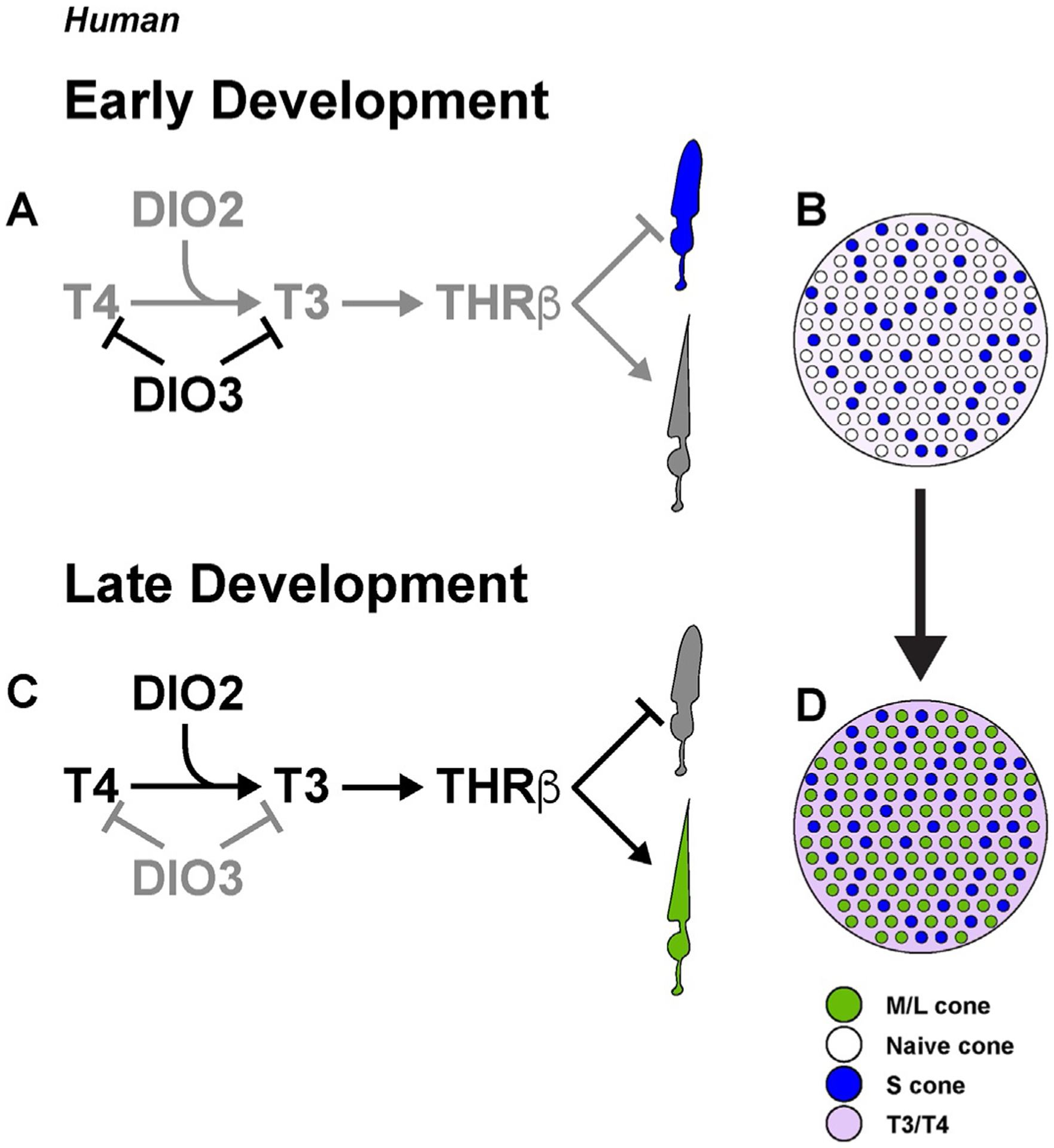
Human cone subtype specification is controlled by thyroid hormone signaling. (A) Early in retinal development, DIO3 is expressed and thyroid hormones are degraded, allowing for S cone specification. (B) The early retina is populated by S cones and unspecified naïve cones in a low-thyroid hormone environment. (C) Late in retinal development, DIO2 is expressed to promote active thyroid hormone signaling. T3 signals through THRβ to inhibit S cone fate and promote L/M cone specification. (D) The late retina is populated by both S cones and L/M cones in a high-thyroid hormone environment. Adapted from Eldred, K. C., Hadyniak, S. E., Hussey, K. A., Brenerman, B., Zhang, P. W., Chamling, X., et al. (2018). Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science, 362(6411), eaau6348. https://doi.org/10.1126/science.aau6348.
Summary: Human retinal organoids recapitulate human cone development temporally and morphologically. In humans, S cones are specified prior to M cones. T3-driven THRβ signaling promotes L/M cone specification and suppression of S cone fate. DIO3 is highly expressed early to restrict thyroid hormone signaling and allow for specification of S cones, while DIO2 is highly expressed later in development to promote thyroid hormone signaling and L/M cone specification.
5.5. Challenges in understanding human cone subtype specification
Human retinal organoids provide a powerful system to study human retinal development. As a genetically and pharmacologically tractable system, human retinal organoids are a model to address new challenges and open questions regarding cone subtype specification. How is the temporal switch between generating S and L/M cone fates regulated? Thyroid hormone signaling through THRβ is required for this process, but how does the retina temporally control thyroid hormone signaling? If the deiodinases are the primary factors controlling the timing, which cell types express the deiodinases and other important regulators? Additionally, is the dynamic expression of the deiodinases during development sufficient to drive this cone fate change? Expression and functional studies in human retinal organoids have the potential to answer these questions.
Experiments on retinal organoids in conjunction with clinical studies would impact our understanding of how differences in thyroid hormone levels affect human vision. If human cones are plastic during development as suggested by observations from fetal retinas (Cornish et al., 2004; Xiao & Hendrickson, 2000), how are S cones maintained after DIO2 begins to be expressed? There may be a distinct mechanism that prevents opsin switching in S cones late in development. Alternatively, plasticity may persist into adulthood, and hyper- or hypothyroidism may cause changes in cone subtype fates, with implications for color vision therapeutics.
Human retinal organoids provide a potential platform to not only study, but also treat, retinal diseases. Current methods involve differentiating photoreceptors from hiPSC or hESCs and transplanting them into the retinas of mouse models of retinal degeneration, which are able to restore some function (Assawachananont et al., 2014; Lamba, Gust, & Reh, 2009; Lamba, Karl, & Reh, 2009; Lamba, Karl, Ware, & Reh, 2006; Lamba et al., 2010; Reh, 2016; Singh et al., 2013); however, this technique requires proper migration and connectivity of the transplanted photoreceptors. Organoids may provide large numbers of photoreceptors and interneurons to allow for higher efficiency of transplantation (Llonch, Carido, & Ader, 2018). Organoid technology provides a tool to address questions in the context of human retinal development, and the possibility of subretinal transplantation of organoids bridges the gap between developmental studies and therapeutic use.
Summary: Human retinal organoids provide a system to address the many remaining questions about the mechanisms of cone subtype specification and the regulation of thyroid hormone signaling during human retinal development.
Acknowledgment
This work was funded by R01EY030872.
References
- Alkemade A, Vuijst CL, Unmehopa UA, Bakker O, Vennstrom B, Wiersinga WM, et al. (2005). Thyroid hormone receptor expression in the human hypothalamus and anterior pituitary. The Journal of Clinical Endocrinology and Metabolism, 90(2), 904–912. 10.1210/jc.2004-0474. [DOI] [PubMed] [Google Scholar]
- Allison WT, Dann SG, Helvik JV, Bradley C, Moyer HD, & Hawryshyn CW (2003). Ontogeny of ultraviolet-sensitive cones in the retina of rainbow trout (Oncorhynchus mykiss). The Journal of Comparative Neurology, 461(3), 294–306. 10.1002/cne.10682. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, et al. (2000). The murine cone photoreceptor: A single cone type expresses both S and M opsins with retinal spatial patterning. Neuron, 27(3), 513–523. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Farhangfar F, Glosmann M, Hashimoto K, Kage K, Robbins JT, et al. (2007). Transient expression of thyroid hormone nuclear receptor TRbeta2 sets S opsin patterning during cone photoreceptor genesis. Developmental Dynamics, 236(5), 1203–1212. 10.1002/dvdy.21155. [DOI] [PubMed] [Google Scholar]
- Assawachananont J, Mandai M, Okamoto S, Yamada C, Eiraku M, Yonemura S, et al. (2014). Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Reports, 2(5), 662–674. 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astapova I (2016). Role of co-regulators in metabolic and transcriptional actions of thyroid hormone. Journal of Molecular Endocrinology, 56(3), 73–97. 10.1530/JME-15-0246. [DOI] [PubMed] [Google Scholar]
- Baden T, Schubert T, Chang L, Wei T, Zaichuk M, Wissinger B, et al. (2013). A tale of two retinal domains: Near-optimal sampling of achromatic contrasts in natural scenes through asymmetric photoreceptor distribution. Neuron, 80(5), 1206–1217. 10.1016/j.neuron.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Barron MG (1986). Endocrine control of smoltification in anadromous salmonids. The Journal of Endocrinology, 108(2), 313–319. 10.1677/joe.0.1080313. [DOI] [PubMed] [Google Scholar]
- Beaudet L, Browman HI, & Hawryshyn CW (1993). Optic nerve response and retinal structure in rainbow trout of different sizes. Vision Research, 33(13), 1739–1746. 10.1016/0042-6989(93)90164-r. [DOI] [PubMed] [Google Scholar]
- Beaudet L, Novales Flamarique I, & Hawryshyn CW (1997). Cone photoreceptor topography in the retina of sexually mature Pacific salmonid fishes. The Journal of Comparative Neurology, 383(1), 49–59. [PubMed] [Google Scholar]
- Benvenga S, & Robbins J (1996). Altered thyroid hormone binding to plasma lipoproteins in hypothyroidism. Thyroid, 6(6), 595–600. 10.1089/thy.1996.6.595. [DOI] [PubMed] [Google Scholar]
- Bernal J, Guadano-Ferraz A, & Morte B (2015). Thyroid hormone transporters-functions and clinical implications. Nature Reviews. Endocrinology, 11(12), 690. 10.1038/nrendo.2015.186. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, & Larsen PR (2002). Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocrine Reviews, 23(1), 38–89. 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Billon N, Tokumoto Y, Forrest D, & Raff M (2001). Role of thyroid hormone receptors in timing oligodendrocyte differentiation. Developmental Biology, 235(1), 110–120. 10.1006/dbio.2001.0293. [DOI] [PubMed] [Google Scholar]
- Bowmaker JK, & Kunz YW (1987). Ultraviolet receptors, tetrachromatic colour vision and retinal mosaics in the brown trout (Salmo trutta): Age-dependent changes. Vision Research, 27(12), 2101–2108. 10.1016/0042-6989(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Branchek T, & Bremiller R (1984). The development of photoreceptors in the zebrafish, Brachydanio rerio. I. Structure. The Journal of Comparative Neurology, 224(1), 107–115. 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Brent GA (2012). Mechanisms of thyroid hormone action. The Journal of Clinical Investigation, 122(9), 3035–3043. 10.1172/JCI60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn SL, & Cepko CL (1996). Development of the pattern of photoreceptors in the chick retina. The Journal of Neuroscience, 16(4), 1430–1439. http://www.jneurosci.org/content/16/4/1430.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AJ, & Parker MG (1995). COUP-TF II homodimers are formed in preference to heterodimers with RXR alpha or TR beta in intact cells. Nucleic Acids Research, 23(20), 4143–4150. 10.1093/nar/23.20.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir M, Turgut Ozturk B, Turan E, Gonulalan G, Polat I, & Gunduz K (2015). The effect of hypothyroidism on color contrast sensitivity: A prospective study. European Thyroid Journal, 4(1), 43–47. 10.1159/000371549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone JB, & Jacobs GH (1995). Regional variations in the relative sensitivity to UV light in the mouse retina. Visual Neuroscience, 12(3), 463–468. [DOI] [PubMed] [Google Scholar]
- Campos-Barros A, Amma LL, Faris JS, Shallam R, Kelley MW, & Forrest D (1999). Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proceedings of the National Academy of Sciences of the United States of America, 97, 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, & Ridgway EC (2000). The Colorado thyroid disease prevalence study. Archives of Internal Medicine, 160(4), 526–534. 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- Capowski EE, Wright LS, Liang K, Phillips MJ, Wallace K, Petelinsek A, et al. (2016). Regulation of WNT signaling by VSX2 during optic vesicle patterning in human induced pluripotent stem cells. Stem Cells, 34(11), 2625–2634. 10.1002/stem.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C (2014). Intrinsically different retinal progenitor cells produce specific types of progeny. Nature Reviews. Neuroscience, 15(9), 615–627. 10.1038/nrn3767. [DOI] [PubMed] [Google Scholar]
- Cheng CL, Gan KJ, & Flamarique IN (2009). Thyroid hormone induces a time-dependent opsin switch in the retina of salmonid fishes. Investigative Ophthalmology & Visual Science, 50(6), 3024–3032. 10.1167/iovs.08-2713. [DOI] [PubMed] [Google Scholar]
- Chiamolera MI, & Wondisford FE (2009). Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology, 150(3), 1091–1096. 10.1210/en.2008-1795. [DOI] [PubMed] [Google Scholar]
- Chichagova V, Dorgau B, Felemban M, Georgiou M, Armstrong L, & Lako M (2019). Differentiation of retinal organoids from human pluripotent stem cells. Current Protocols in Stem Cell Biology, 50(1), e95. 10.1002/cpsc.95. [DOI] [PubMed] [Google Scholar]
- Chinen A, Hamaoka T, Yamada Y, & Kawamura S (2003). Gene duplication and spectral diversification of cone visual pigments of zebrafish. Genetics, 163(2), 663–675. https://www.ncbi.nlm.nih.gov/pubmed/12618404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish EE, Hendrickson AE, & Provis JM (2004). Distribution of short-wavelength-sensitive cones in human fetal and postnatal retina: Early development of spatial order and density profiles. Vision Research, 44(17), 2019–2026. 10.1016/j.visres.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Crantz FR, Silva JE, & Larsen PR (1982). An analysis of the sources and quantity of 3,5,3′-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology, 110(2), 367–375. 10.1210/endo-110-2-367. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB, et al. (1991). Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. The Journal of Comparative Neurology, 312(4), 610–624. 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]
- Curcio CA, Sloan KR, Kalina RE, & Hendrickson AE (1990). Human photoreceptor topography. The Journal of Comparative Neurology, 292(4), 497–523. 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- Deeb SS (2005). The molecular basis of variation in human color vision. Clinical Genetics, 67(5), 369–377. 10.1111/j.1399-0004.2004.00343.x. [DOI] [PubMed] [Google Scholar]
- Deng WL, Gao ML, Lei XL, Lv JN, Zhao H, He KW, et al. (2018). Gene correction reverses ciliopathy and photoreceptor loss in iPSC-derived retinal organoids from retinitis pigmentosa patients. Stem Cell Reports, 10(6), 2005. 10.1016/j.stemcr.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter SS Jr., & Malicki JJ (2002). The zebrafish eye: Developmental and genetic analysis. Results and Problems in Cell Differentiation, 40, 346–370. 10.1007/978-3-540-46041-1_17. [DOI] [PubMed] [Google Scholar]
- Ebrey T, & Koutalos Y (2001). Vertebrate photoreceptors. Progress in Retinal and Eye Research, 20(1), 49–94. 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature, 472(7341), 51–56. 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Eldred KC, Avelis C, Johnston RJ Jr., & Roberts E (2020). Modeling binary and graded cone cell fate patterning in the mouse retina. PLoS Computational Biology, 16(3), e1007691. 10.1371/journal.pcbi.1007691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldred KC, Hadyniak SE, Hussey KA, Brenerman B, Zhang PW, Chamling X, et al. (2018). Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science, 362(6411), eaau6348. 10.1126/science.aau6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y (2003). Development of the cone photoreceptor mosaic in the mouse retina revealed by fluorescent cones in transgenic mice. Molecular Vision, 9, 31–42. https://www.ncbi.nlm.nih.gov/pubmed/12592228. [PubMed] [Google Scholar]
- Flamarique II, & Hawryshyn C (1996). Retinal development and visual sensitivity of young Pacific sockeye salmon (Oncorhynchus nerka). The Journal of Experimental Biology, 199(Pt. 4), 869–882. [DOI] [PubMed] [Google Scholar]
- Fliers E, Unmehopa UA, & Alkemade A (2006). Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Molecular and Cellular Endocrinology, 251(1–2), 1–8. 10.1016/j.mce.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Foltz LP, & Clegg DO (2019). Patient-derived induced pluripotent stem cells for modelling genetic retinal dystrophies. Progress in Retinal and Eye Research, 68, 54–66. 10.1016/j.preteyeres.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Forrest D, Sjoberg M, & Vennstrom B (1990). Contrasting developmental and tissue-specific expression of alpha and beta thyroid hormone receptor genes. The EMBO Journal, 9(5), 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, & Visser TJ (2003). Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. The Journal of Biological Chemistry, 278(41), 40128–40135. 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- Friesema EC, Jansen J, & Visser TJ (2005). Thyroid hormone transporters. Biochemical Society Transactions, 33(Pt. 1), 228–232. 10.1042/BST0330228. [DOI] [PubMed] [Google Scholar]
- Fujieda H, Bremner R, Mears AJ, & Sasaki H (2009). Retinoic acid receptor-related orphan receptor alpha regulates a subset of cone genes during mouse retinal development. Journal of Neurochemistry, 108(1), 91–101. 10.1111/j.1471-4159.2008.05739.x. [DOI] [PubMed] [Google Scholar]
- Galton VA (2017). The ups and downs of the thyroxine pro-hormone hypothesis. Molecular and Cellular Endocrinology, 458, 105–111. 10.1016/j.mce.2017.01.029. [DOI] [PubMed] [Google Scholar]
- Gan KJ, & Novales Flamarique I (2010). Thyroid hormone accelerates opsin expression during early photoreceptor differentiation and induces opsin switching in differentiated TRalpha-expressing cones of the salmonid retina. Developmental Dynamics, 239(10), 2700–2713. 10.1002/dvdy.22392. [DOI] [PubMed] [Google Scholar]
- Gao ML, Lei XL, Han F, He KW, Jin SQ, Zhang YY, et al. (2020). Patient-specific retinal organoids recapitulate disease features of late-onset retinitis pigmentosa. Frontiers in Cell and Development Biology, 8, 128. 10.3389/fcell.2020.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garita-Hernandez M, Guibbal L, Toualbi L, Routet F, Chaffiol A, Winckler C, et al. (2018). Optogenetic light sensors in human retinal organoids. Frontiers in Neuroscience, 12, 789. 10.3389/fnins.2018.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garita-Hernandez M, Routet F, Guibbal L, Khabou H, Toualbi L, Riancho L, et al. (2020). AAV-mediated gene delivery to 3D retinal organoids derived from human induced pluripotent stem cells. International Journal of Molecular Sciences, 21(3), 994. 10.3390/ijms21030994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand RA, Hutchinson-Williams KA, Bonde AA, Castellino P, & Sherwin RS (1987). Catabolic effects of thyroid hormone excess: The contribution of adrenergic activity to hypermetabolism and protein breakdown. Metabolism, 36(6), 562–569. 10.1016/0026-0495(87)90168-5. [DOI] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, et al. (2008). Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocrine Reviews, 29(7), 898–938. 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereben B, Zeold A, Dentice M, Salvatore D, & Bianco AC (2008). Activation and inactivation of thyroid hormone by deiodinases: Local action with general consequences. Cellular and Molecular Life Sciences, 65(4), 570–590. 10.1007/s00018-007-7396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaschke A, Glosmann M, & Peichl L (2010). Developmental changes of cone opsin expression but not retinal morphology in the hypothyroid Pax8 knockout mouse. Investigative Ophthalmology & Visual Science, 51(3), 1719–1727. 10.1167/iovs.09-3592. [DOI] [PubMed] [Google Scholar]
- Glaschke A, Weiland J, Del Turco D, Steiner M, Peichl L, & Glosmann M (2011). Thyroid hormone controls cone opsin expression in the retina of adult rodents. The Journal of Neuroscience, 31(13), 4844–4851. 10.1523/JNEUROSCI.6181-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii VI, & Zueva LV (1977). Visual pigments of chicken and pigeon. Vision Research, 17(4), 537–543. [DOI] [PubMed] [Google Scholar]
- Gowdy PD, & Cicerone CM (1998). The spatial arrangement of the L and M cones in the central fovea of the living human eye. Vision Research, 38(17), 2575–2589. 10.1016/s0042-6989(97)00416-1. [DOI] [PubMed] [Google Scholar]
- Greenblatt M, Brown CL, Lee M, Dauder S, & Bern HA (1989). Changes in thyroid hormone levels in eggs and larvae and in iodide uptake by eggs of coho and chinook salmon, Oncorhynchus kisutsch and O. tschawytscha. Fish Physiology and Biochemistry, 6(5), 261–278. 10.1007/BF01881680. [DOI] [PubMed] [Google Scholar]
- Guadano-Ferraz A, Obregon MJ, St. Germain DL, & Bernal J (1997). The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proceedings of the National Academy of Sciences of the United States of America, 94, 10391–10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang P, Ma JH, Cui Z, Yu Q, Liu S, et al. (2019). Modeling retinitis pigmentosa: Retinal organoids generated from the iPSCs of a patient with the USH2A mutation show early developmental abnormalities. Frontiers in Cellular Neuroscience, 13, 361. 10.3389/fncel.2019.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G, et al. (2005). The primordial, blue-cone color system of the mouse retina. The Journal of Neuroscience, 25(22), 5438–5445. 10.1523/jneurosci.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryshyn CW, Arnold MG, Chaisson DJ, & Martin PC (1989). The ontogeny of ultraviolet photosensitivity in rainbow trout (Salmo gairdneri). Visual Neuroscience, 2(3), 247–254. 10.1017/s0952523800001164. [DOI] [PubMed] [Google Scholar]
- Hawryshyn CW, Martens G, Allison WT, & Anholt BR (2003). Regeneration of ultraviolet-sensitive cones in the retinal cone mosaic of thyroxin-challenged post-juvenile rainbow trout (Oncorhynchus mykiss). The Journal of Experimental Biology, 206(Pt. 15), 2665–2673. 10.1242/jeb.00470. [DOI] [PubMed] [Google Scholar]
- Hoar WS (1988). The physiology of smolting salmonids. Fish Physiology, 11, 275–343. [Google Scholar]
- Hofer H, Carroll J, Neitz J, Neitz M, & Williams DR (2005). Organization of the human trichromatic cone mosaic. The Journal of Neuroscience, 25(42), 9669–9679. 10.1523/JNEUROSCI.2414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Ratnapriya R, Brooks MJ, Chaitankar V, Wilken MS, Zhang C, et al. (2017). Molecular anatomy of the developing human retina. Developmental Cell, 43(6), 763–779.e764. 10.1016/j.devcel.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, & Easter SS (1999). Retinal neurogenesis: The formation of the initial central patch of postmitotic cells. Developmental Biology, 207(2), 309–321. 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Hunt DM (2001). Seeing rainbows. Biologist (London, England), 48(2), 67–71. [PubMed] [Google Scholar]
- Jones I, Rogers SA, Kille P, & Sweeney GE (2002). Molecular cloning and expression of thyroid hormone receptor alpha during salmonid development. General and Comparative Endocrinology, 125(2), 226–235. 10.1006/gcen.2001.7745. [DOI] [PubMed] [Google Scholar]
- Kaewkhaw R, Kaya KD, Brooks M, Homma K, Zou J, Chaitankar V, et al. (2015). Transcriptome dynamics of developing photoreceptors in three-dimensional retina cultures recapitulates temporal sequence of human cone and rod differentiation revealing cell surface markers and gene networks. Stem Cells, 33(12), 3504–3518. 10.1002/stem.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kainz PM, Neitz J, & Neitz M (1998). Recent evolution of uniform trichromacy in a New World monkey. Vision Research, 38(21), 3315–3320. 10.1016/s0042-6989(98)00078-9. [DOI] [PubMed] [Google Scholar]
- Katoh K, Omori Y, Onishi A, Sato S, Kondo M, & Furukawa T (2010). Blimp1 suppresses Chx10 expression in differentiating retinal photoreceptor precursors to ensure proper photoreceptor development. The Journal of Neuroscience, 30(19), 6515–6526. 10.1523/JNEUROSCI.0771-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, & Tachibanaki S (2008). Rod and cone photoreceptors: Molecular basis of the difference in their physiology. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 150(4), 369–377. 10.1016/j.cbpa.2008.04.600. [DOI] [PubMed] [Google Scholar]
- Koenig RJ (1998). Thyroid hormone receptor coactivators and corepressors. Thyroid, 8(8), 703–713. 10.1089/thy.1998.8.703. [DOI] [PubMed] [Google Scholar]
- Korcek L, & Tabachnick M (1976). Thyroxine-protein interactions. Interaction of thyroxine and triiodothyronine with human thyroxine-binding globulin. The Journal of Biological Chemistry, 251(12), 3558–3562. [PubMed] [Google Scholar]
- Kram YA, Mantey S, & Corbo JC (2010). Avian cone photoreceptors tile the retina as five independent, self-organizing mosaics. PLoS One, 5(2), e8992. 10.1371/journal.pone.0008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz YW (1987). Tracts of putative ultraviolet receptors in the retina of the two-year-old brown trout (Salmo trutta) and the Atlantic salmon (Salmo salar). Experientia, 43(11–12), 1202–1204. 10.1007/BF01945524. [DOI] [PubMed] [Google Scholar]
- Kunz YW, Wildenburg G, Goodrich L, & Callaghan E (1994). The fate of ultraviolet receptors in the retina of the Atlantic salmon (Salmo salar). Vision Research, 34(11), 1375–1383. 10.1016/0042-6989(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Gust J, & Reh TA (2009). Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell, 4(1), 73–79. 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, & Reh TA (2009). Strategies for retinal repair: Cell replacement and regeneration. Progress in Brain Research, 175, 23–31. 10.1016/S0079-6123(09)17502-7. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, & Reh TA (2006). Efficient generation of retinal progenitor cells from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 103(34), 12769–12774. 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba DA, McUsic A, Hirata RK, Wang PR, Russell D, & Reh TA (2010). Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One, 5(1), e8763. 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T (2007). Microglia activation in retinal degeneration. Journal of Leukocyte Biology, 81(6), 1345–1351. 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- Larsen PR (1982). Thyroid-pituitary interaction: Feedback regulation of thyrotropin secretion by thyroid hormones. The New England Journal of Medicine, 306(1), 23–32. 10.1056/NEJM198201073060107. [DOI] [PubMed] [Google Scholar]
- Li G, Gao G, Wang P, Song X, Xu P, Xie B, et al. (2019). Generation and characterization of induced pluripotent stem cells and retinal organoids from a Leber’s congenital amaurosis patient with novel RPE65 mutations. Frontiers in Molecular Neuroscience, 12, 212. 10.3389/fnmol.2019.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KY, & Roorda A (2007). Automated identification of cone photoreceptors in adaptive optics retinal images. Journal of the Optical Society of America. A, Optics, Image Science, and Vision, 24(5), 1358–1363. 10.1364/josaa.24.001358. [DOI] [PubMed] [Google Scholar]
- Llonch S, Carido M, & Ader M (2018). Organoid technology for retinal repair. Developmental Biology, 433(2), 132–143. 10.1016/j.ydbio.2017.09.028. [DOI] [PubMed] [Google Scholar]
- Loew ER, & Wahl CM (1991). A short-wavelength sensitive cone mechanism in juvenile yellow perch, Perca flavescens. Vision Research, 31(3), 353–360. 10.1016/0042-6989(91)90088-m. [DOI] [PubMed] [Google Scholar]
- Lu A, Ng L, Ma M, Kefas B, Davies TF, Hernandez A, et al. (2009). Retarded developmental expression and patterning of retinal cone opsins in hypothyroid mice. Endocrinology, 150(3), 1536–1544. 10.1210/en.2008-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall AH (1957). The growth of the trout retina. Journal of Cell Science, 3(98), 101–110. [Google Scholar]
- Ma H, Thapa A, Morris L, Redmond TM, Baehr W, & Ding XQ (2014). Suppressing thyroid hormone signaling preserves cone photoreceptors in mouse models of retinal degeneration. Proceedings of the National Academy of Sciences of the United States of America, 111(9), 3602–3607. 10.1073/pnas.1317041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackin RD, Frey RA, Gutierrez C, Farre AA, Kawamura S, Mitchell DM, et al. (2019). Endocrine regulation of multichromatic color vision. Proceedings of the National Academy of Sciences of the United States of America, 116(34), 16882–16891. 10.1073/pnas.1904783116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH (2012). The neuronal organization of the retina. Neuron, 76(2), 266–280. 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris VB (1982). An afoveate area centralis in the chick retina. The Journal of Comparative Neurology, 210(2), 198–203. 10.1002/cne.902100210. [DOI] [PubMed] [Google Scholar]
- Muller J, Mayerl S, Visser TJ, Darras VM, Boelen A, Frappart L, et al. (2014). Tissue-specific alterations in thyroid hormone homeostasis in combined Mct10 and Mct8 deficiency. Endocrinology, 155(1), 315–325. 10.1210/en.2013-1800. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. (2012). Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell, 10(6), 771–785. 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Nathans J, Davenport CM, Maumenee IH, Lewis RA, Hejtmancik JF, Litt M, et al. (1989). Molecular genetics of human blue cone monochromacy. Science, 245(4920), 831–838. 10.1126/science.2788922. [DOI] [PubMed] [Google Scholar]
- Nathans J, & Hogness DS (1984). Isolation and nucleotide sequence of the gene encoding human rhodopsin. Proceedings of the National Academy of Sciences of the United States of America, 81(15), 4851–4855. 10.1073/pnas.81.15.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J, Thomas D, & Hogness DS (1986). Molecular genetics of human color vision: The genes encoding blue, green, and red pigments. Science, 232(4747), 193–202. 10.1126/science.2937147. [DOI] [PubMed] [Google Scholar]
- Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, et al. (2004). Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proceedings of the National Academy of Sciences of the United States of America, 101(10), 3474–3479. 10.1073/pnas.0307402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, et al. (2009). A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology, 150(4), 1952–1960. 10.1210/en.2008-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Hurley JB, Dierks B, Srinivas M, Salto C, Vennstrom B, et al. (2001). A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nature Genetics, 27, 94–98. [DOI] [PubMed] [Google Scholar]
- Ng L, Liu H, St Germain DL, Hernandez A, & Forrest D (2017). Deletion of the thyroid hormone-activating type 2 deiodinase rescues cone photoreceptor degeneration but not deafness in mice lacking type 3 deiodinase. Endocrinology, 158(6), 1999–2010. 10.1210/en.2017-00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Lu A, Swaroop A, Sharlin DS, Swaroop A, & Forrest D (2011). Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. The Journal of Neuroscience, 31(31), 11118–11125. 10.1523/jneurosci.1709-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L, Lyubarsky A, Nikonov SS, Ma M, Srinivas M, Kefas B, et al. (2010). Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. The Journal of Neuroscience, 30(9), 3347–3357. 10.1523/jneurosci.5267-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novales Flamarique I (2000). The ontogeny of ultraviolet sensitivity, cone disappearance and regeneration in the sockeye salmon Oncorhynchus nerka. The Journal of Experimental Biology, 203, 1161–1172. [DOI] [PubMed] [Google Scholar]
- Novales Flamarique I, Sayed Ahmed A, Cheng CL, Molday RS, & Devlin RH (2019). Growth hormone regulates opsin expression in the retina of a salmonid fish. Journal of Neuroendocrinology, 31(11), e12804. 10.1111/jne.12804. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, & Schwartz HL (1997). Molecular basis of thyroid hormone-dependent brain development. Endocrine Reviews, 18(4), 462–475. 10.1210/edrv.18.4.0309. [DOI] [PubMed] [Google Scholar]
- Otake S, Gowdy PD, & Cicerone CM (2000). The spatial arrangement of L and M cones in the peripheral human retina. Vision Research, 40(6), 677–693. 10.1016/s0042-6989(99)00202-3. [DOI] [PubMed] [Google Scholar]
- Palha JA (2002). Transthyretin as a thyroid hormone carrier: Function revisited. Clinical Chemistry and Laboratory Medicine, 40(12), 1292–1300. 10.1515/CCLM.2002.223. [DOI] [PubMed] [Google Scholar]
- Pessoa CN, Santiago LA, Santiago DA, Machado DS, Rocha FA, Ventura DF, et al. (2008). Thyroid hormone action is required for normal cone opsin expression during mouse retinal development. Comparative Study Research Support, Non-U.S. Gov’t Investigative Ophthalmology & Visual Science, 49(5), 2039–2045. 10.1167/iovs.07-0908. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Jiang P, Howden S, Barney P, Min J, York NW, et al. (2018). A novel approach to single cell RNA-sequence analysis facilitates in silico gene reporting of human pluripotent stem cell-derived retinal cell types. Stem Cells, 36(3), 313–324. 10.1002/stem.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provis JM, Dubis AM, Maddess T, & Carroll J (2013). Adaptation of the central retina for high acuity vision: Cones, the fovea and the avascular zone. Progress in Retinal and Eye Research, 35, 63–81. 10.1016/j.preteyeres.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PM, Buck TM, Mulder AA, Ohonin C, Alves CH, Vos RM, et al. (2019). Human iPSC-derived retinas recapitulate the fetal CRB1 CRB2 complex formation and demonstrate that photoreceptors and Muller glia are targets of AAV5. Stem Cell Reports, 12(5), 906–919. 10.1016/j.stemcr.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racheva K, Totev T, Natchev E, Bocheva N, Beirne R, & Zlatkova M (2020). Color discrimination assessment in patients with hypothyroidism using the Farnsworth-Munsell 100 hue test. Journal of the Optical Society of America. A, Optics, Image Science, and Vision, 37(4), A18–A25. 10.1364/JOSAA.382390. [DOI] [PubMed] [Google Scholar]
- Raine JC, & Hawryshyn CW (2009). Changes in thyroid hormone reception precede SWS1 opsin downregulation in trout retina. The Journal of Experimental Biology, 212(17), 2781–2788. 10.1242/jeb.030866. [DOI] [PubMed] [Google Scholar]
- Ramírez JM, Triviño A, Ramírez AI, & Salazar JJ (1998). Organization and function of astrocytes in human retina. In Understanding glial cells (pp. 47–62). Springer. 10.1007/978-1-4615-5737-1_3. [DOI] [Google Scholar]
- Rashid K, Akhtar-Schaefer I, & Langmann T (2019). Microglia in Retinal Degeneration. Frontiers in Immunology, 10, 1975. 10.3389/fimmu.2019.01975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, & Curran GA (1995). Developmental patterning of rod and cone photoreceptors in embryonic zebrafish. The Journal of Comparative Neurology, 359(4), 537–550. 10.1002/cne.903590403. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, & Knight JK (1993). Expression of rod and cone visual pigments in goldfish and zebrafish: A rhodopsin-like gene is expressed in cones. Neuron, 10(6), 1161–1174. 10.1016/0896-6273(93)90064-x. [DOI] [PubMed] [Google Scholar]
- Reh TA (2016). Photoreceptor transplantation in late stage retinal degeneration. Investigative Ophthalmology & Visual Science, 57(5), ORSFg1–7. 10.1167/iovs.15-17659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenbach A, & Bringmann A (2013). New functions of Muller cells. Glia, 61(5), 651–678. 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- Richardson TM (1969). Cytoplasmic and ciliary connections between the inner and outer segments of mammalian visual receptors. Vision Research, 9(7), 727–731. 10.1016/0042-6989(69)90010-8. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Hendrickson A, McGuire CR, & Reh TA (2005). Retinoid X receptor (gamma) is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Investigative Ophthalmology & Visual Science, 46(8), 2897–2904. 10.1167/iovs.05-0093. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, & Reh TA (2006). Making the gradient: Thyroid hormone regulates cone opsin expression in the developing mouse retina. Proceedings of the National Academy of Sciences of the United States of America, 103(16), 6218–6223. 10.1073/pnas.0509981103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlich P, van Veen T, & Szel A (1994). Two different visual pigments in one retinal cone cell. Neuron, 13(5), 1159–1166. https://www.ncbi.nlm.nih.gov/pubmed/7946352. [DOI] [PubMed] [Google Scholar]
- Roorda AW, & David R (1999). The arrangement of the three cone classes in the living human eye. Nature, 397, 520–522. [DOI] [PubMed] [Google Scholar]
- Roorda A, Metha AB, Lennie P, & Williams DR (2001). Packing arrangement of the three cone classes in primate retina. Vision Research, 41(10–11), 1291–1306. 10.1016/s0042-6989(01)00043-8. [DOI] [PubMed] [Google Scholar]
- Rossi EA, Chung M, Dubra A, Hunter JJ, Merigan WH, & Williams DR (2011). Imaging retinal mosaics in the living eye. Eye (London, England), 25(3), 301–308. 10.1038/eye.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels HH, Tsai JS, Casanova J, & Stanley F (1974). Thyroid hormone action: In vitro characterization of solubilized nuclear receptors from rat liver and cultured GH1 cells. The Journal of Clinical Investigation, 54(4), 853–865. 10.1172/JCI107825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawant OB, Horton AM, Zucaro OF, Chan R, Bonilha VL, Samuels IS, et al. (2017). The circadian clock gene Bmal1 controls thyroid hormone-mediated spectral identity and cone photoreceptor function. Cell Reports, 21(3), 692–706. 10.1016/j.celrep.2017.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin CT, Castelli WP, Hershman JM, McNamara P, & Bacharach P (1985). The aging thyroid. Thyroid deficiency in the Framingham study. Archives of Internal Medicine, 145(8), 1386–1388. [PubMed] [Google Scholar]
- Schmitt EA, & Dowling JE (1996). Comparison of topographical patterns of ganglion and photoreceptor cell differentiation in the retina of the zebrafish, Danio rerio. The Journal of Comparative Neurology, 371(2), 222–234. . [DOI] [PubMed] [Google Scholar]
- Schwartz CE, & Stevenson RE (2007). The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Practice & Research. Clinical Endocrinology & Metabolism, 21(2), 307–321. 10.1016/j.beem.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MS, Charbel Issa P, Butler R, Martin C, Lipinski DM, Sekaran S, et al. (2013). Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proceedings of the National Academy of Sciences of the United States of America, 110(3), 1101–1106. 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoberg M, Vennström B, & Forrest D (1992). Thyroid hormone receptors in chick retinal development: Differential expression of mRNAs for a and N-terminal variant B receptors. Development, 114, 39–47. [DOI] [PubMed] [Google Scholar]
- Specker JL, Eales JG, Tagawa M, & Tyler WA (2000). Parr-smolt transformation in Atlantic salmon: Thyroid hormone deiodination in liver and brain and endocrine correlates of change in rheotactic behavior. Canadian Journal of Zoology, 78, 696–705. [Google Scholar]
- Sridhar A, Hoshino A, Finkbeiner CR, Chitsazan A, Dai L, Haugan AK, et al. (2020). Single-cell transcriptomic comparison of human fetal retina, hPSC-derived retinal organoids, and long-term retinal cultures. Cell Reports, 30(5), 1644–1659.e1644. 10.1016/j.celrep.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenkamp DL, Barthel LK, & Raymond PA (1997). Spatiotemporal coordination of rod and cone photoreceptor differentiation in goldfish retina. The Journal of Comparative Neurology, 382(2), 272–284. 10.1002/(sici)1096-9861(19970602)382:2. [DOI] [PubMed] [Google Scholar]
- Sun Z, Feng D, Fang B, Mullican SE, You SH, Lim HW, et al. (2013). Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Molecular Cell, 52(6), 769–782. 10.1016/j.molcel.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki SC, Bleckert A, Williams PR, Takechi M, Kawamura S, & Wong RO (2013). Cone photoreceptor types in zebrafish are generated by symmetric terminal divisions of dedicated precursors. Proceedings of the National Academy of Sciences of the United States of America, 110(37), 15109–15114. 10.1073/pnas.1303551110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szel A, Csorba G, Caffe AR, Szel G, Rohlich P, & van Veen T (1994). Different patterns of retinal cone topography in two genera of rodents, Mus and Apodemus. Cell and Tissue Research, 276(1), 143–150. 10.1007/BF00354793. [DOI] [PubMed] [Google Scholar]
- Szel A, Rohlich P, Mieziewska K, Aguirre G, & van Veen T (1993). Spatial and temporal differences between the expression of short- and middle-wave sensitive cone pigments in the mouse retina: A developmental study. The Journal of Comparative Neurology, 331(4), 564–577. 10.1002/cne.903310411. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Harpavat S, Billings NA, & Cepko CL (2008). Thyroid hormone components are expressed in three sequential waves during development of the chick retina. BMC Developmental Biology, 8, 101. 10.1186/1471-213X-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, et al. (1997). Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology, 138(8), 3359–3368. 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- Tu HM, Legradi G, Bartha T, Salvatore D, Lechan RM, & Larsen PR (1999). Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology, 140(2), 784–790. 10.1210/endo.140.2.6486. [DOI] [PubMed] [Google Scholar]
- Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, et al. (1977). The spectrum of thyroid disease in a community: The Whickham survey. Clinical Endocrinology, 7(6), 481–493. 10.1111/j.1365-2265.1977.tb01340.x. [DOI] [PubMed] [Google Scholar]
- Vancamp P, Bourgeois NMA, Houbrechts AM, & Darras VM (2019). Knockdown of the thyroid hormone transporter MCT8 in chicken retinal precursor cells hampers early retinal development and results in a shift towards more UV/blue cones at the expense of green/red cones. Experimental Eye Research, 178, 135–147. 10.1016/j.exer.2018.09.018. [DOI] [PubMed] [Google Scholar]
- Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, & Sharma SC (2016). Glia-neuron interactions in the mammalian retina. Progress in Retinal and Eye Research, 51, 1–40. 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Viets K, Eldred K, & Johnston RJ Jr. (2016). Mechanisms of photoreceptor patterning in vertebrates and invertebrates. Trends in Genetics, 32(10), 638–659. 10.1016/j.tig.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic TS, Doro CJ, & Hyde DR (1999). Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Visual Neuroscience, 16(3), 571–585. 10.1017/s0952523899163168. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Maruotti JA, Sripathi SR, Ball J, Angueyra JM, Kim C, et al. (2017). Photoreceptor outer segment-like structures in long-term 3D retinas from human pluripotent stem cells. Scientific Reports, 7(1), 766. 10.1038/s41598-017-00774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassle H (2004). Parallel processing in the mammalian retina. Nature Reviews. Neuroscience, 5(10), 747–757. 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- Weiss AH, Kelly JP, Bisset D, & Deeb SS (2012). Reduced L- and M- and increased S-cone functions in an infant with thyroid hormone resistance due to mutations in the THRbeta2 gene. Ophthalmic Genetics, 33(4), 187–195. 10.3109/13816810.2012.681096. [DOI] [PubMed] [Google Scholar]
- Wikler KC, Szel A, & Jacobsen AL (1996). Positional information and opsin identity in retinal cones. The Journal of Comparative Neurology, 374(1), 96–107. . [DOI] [PubMed] [Google Scholar]
- Williams GR (2008). Neurodevelopmental and neurophysiological actions of thyroid hormone. Journal of Neuroendocrinology, 20(6), 784–794. 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- Xiao M, & Hendrickson A (2000). Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. The Journal of Comparative Neurology, 425(4), 545–559. [PubMed] [Google Scholar]
- Yokoyama S (2000). Molecular evolution of vertebrate visual pigments. Progress in Retinal and Eye Research, 19(4), 385–419. 10.1016/s1350-9462(00)00002-1. [DOI] [PubMed] [Google Scholar]
- You SH, Lim HW, Sun Z, Broache M, Won KJ, & Lazar MA (2013). Nuclear receptor co-repressors are required for the histone-deacetylase activity of HDAC3 in vivo. Nature Structural & Molecular Biology, 20(2), 182–187. 10.1038/nsmb.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, et al. (2014). Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nature Communications, 5, 4047. 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]


