Abstract
Throughout the COVID-19 pandemic, many have worried that the additional burden of seasonal influenza would create a devastating scenario, resulting in overwhelmed healthcare capacities and further loss of life. However, many were pleasantly surprised: the 2020 Southern Hemisphere and 2020–2021 Northern Hemisphere influenza seasons were entirely suppressed. The potential causes and impacts of this drastic public health shift are highly uncertain, but provide lessons about future control of respiratory diseases, especially for the upcoming influenza season.
Keywords: COVID-19, Influenza, Behavior, Nonpharmaceutical intervention, Vaccination, United States, Europe, Australia
The COVID-19 pandemic has altered almost every aspect of public health since its global spread in early 2020. One such consequence of COVID-19 has been altered circulation of other respiratory pathogens, like influenza. Seasonal influenza causes 3–5 million cases of severe illness and kills 260,000–650,000 people globally each year [44]. The added strain of even a mild influenza season to a healthcare system already pushed beyond its limits by the COVID-19 pandemic was a devastating prospect that worried many. Fortunately, influenza cases were unprecedentedly low in the 2020 influenza season in the Southern Hemisphere and in the 2020–2021 influenza season in the Northern Hemisphere [26], [34], [39], [40]. The lack of additional healthcare burden due to influenza left healthcare capacity to be focused towards the continued surge of COVID-19, likely preserving many lives. With some distance now from the last influenza season, we must heed the many lessons learned and relearned about influenza dynamics and control over the last year so we can be better prepared for future influenza outbreaks.
At the outset of the COVID-19 pandemic in early 2020, the Northern Hemisphere 2019–2020 influenza season was still in full force in the US, but rapidly declined during March 2020. Some have proposed that the pandemic and the ensuing nonpharmaceutical interventions led to this decrease in influenza transmission [34]. While this is a possible explanation, behavioral data now available for the first months of 2020 show little change from baseline during this period (Figure S2). Instead, unusual viral dynamics could explain the early and rapid decline of the 2019–2020 flu season, in which unusually high and early flu B circulation was followed by a wave of flu A circulation [4]. This sits in contrast to the routine pattern of initial influenza A circulation followed by low flu B circulation which brings a gradual end to most flu seasons (Figure S3). In fact, we find that if the influenza A wave is considered independently and controlled for changes in healthcare-seeking, the US 2019–2020 flu season did not deviate significantly from expectations after the declaration of the COVID-19 emergency (Figure S1).
While the Northern Hemisphere 2019–2020 influenza season may not have been affected by the COVID-19 pandemic, for the influenza season starting in 2020, countries in the Southern Hemisphere, which typically experience their influenza season in May-October, reported drastically reduced influenza circulation [34]. The Northern Hemisphere has also remarkably experienced almost no influenza during the typical timing of the 2020–2021 season, with the US and EU reporting only 0.15–0.2% of samples as positive for flu (compared to around 18–23% in 2019–2020) [8], [17]. As a comparative case study, we can consider the US, France, Australia: 3 countries that are socially similar, have high data availability and experience similar influenza seasons by hemisphere [12]. All three settings suppressed the influenza season in 2020–2021, and a number of explanations may be behind this. One potential explanation for this pattern is viral competition between SARS-CoV-2 and influenza. This competition could occur through multiple mechanisms, such as immune interactions, viral competition and a reduced susceptible pool due to isolation [32], [38].
Another potential explanation for the decrease in the 2020 influenza burden is increased influenza vaccination. In order to respond to the pandemic, many countries increased and accelerated the distribution of influenza vaccine doses: a 36% increase in Australia, a 13% increase in France, and an 11% increase in the United States [7], [23]. However, this increase in distributed doses may not have necessarily translated to an increase in vaccine coverage. In France and other European countries, exceptionally high demand in the early phase of the flu vaccination campaign reportedly led to shortages of vaccine doses [5], [37]. Social distancing measures in the US and Europe at the start of the winter wave may have disrupted access to routine vaccination settings such as primary care providers, workplaces and schools, or increased demand due to disease awareness may have limited the protection of vulnerable groups. Even if high vaccine coverage was achieved on average, stark heterogeneities exist spatially, by age group (e.g. the US state of Iowa estimates a 20 percent decline in flu vaccination in the 65 + age group [24]), and by race and ethnicity (e.g. it is estimated that flu vaccination coverage among Black, non-Hispanic children in the US decreased 8 percentage points from the previous year [10]). This heterogeneous patchwork of influenza vaccine protection makes populations vulnerable and will further exacerbate existing health disparities amplified by the COVID-19 pandemic [2], [46].
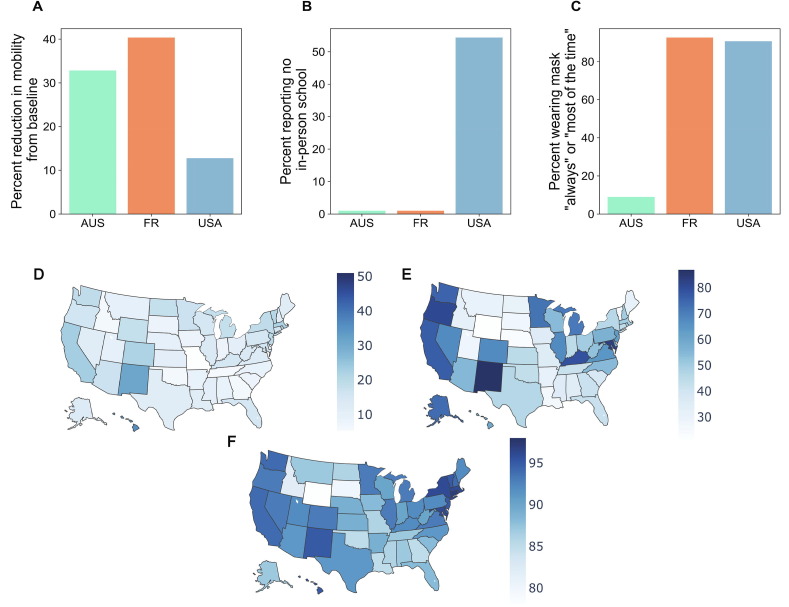
While viral competition and increased vaccination may have contributed, we propose that the most influential process in suppressing the influenza epidemic of 2020–2021 were the significant behavioral interventions in place due to the COVID-19 pandemic. Nonpharmaceutical interventions such as closures of schools and non-essential businesses, telework, restriction on gathering size, and mask-wearing have been key public health tools for limiting the impact of the COVID-19 pandemic [16], [22]. Given the shared transmission route between SARS-CoV-2 and influenza, the same protective behaviors could greatly limit influenza transmission [11], [13], [40]. In fact, far less mitigation effort is required to control a disease with a lower R0, or a less transmissible disease, like flu (R0 = 1–2), compared with a disease with a higher R0, like COVID-19 (R0 = 2–5) [3], [35]. Many non-pharmaceutical interventions, like stay-at-home orders and remote work guidelines, reduce mobility and contact, diminishing transmission potential. Both Australia and France had stringent social distancing policies in place at the typical start of their influenza seasons, resulting in drastic reductions in mobility (Fig. 1 A). The US, however, showed mobility levels that were close to a pre-pandemic baseline at the start of the influenza season. Similarly, pandemic-related school closures may have affected influenza transmission. Since school-aged children are of particular importance for influenza transmission, school closures are thought to have significant impact on influenza dynamics by reducing contacts of children and homogenizing mixing across age groups [6], [14], [18], [29]. The US had widespread school closures and distance learning throughout the 2020–2021 influenza season (Fig. 1B). Conversely, France and Australia did not: some areas of Australia implemented distance learning in April, but resumed in-person schooling prior to the typical start of the influenza season [30], and France also maintained in-person schooling during the entire influenza season. Another measure that many have taken to reduce the spread of COVID-19 that impacts all respiratory-transmitted viruses is mask-wearing. Mask-wearing data shows that both the US and France had high uptake of mask-wearing during the start of their influenza seasons, however, Australia had much lower levels (Fig. 1C).
Fig. 1.
Australia (green), France (orange), and the US (blue) implemented different behavioral interventions with different levels of adherence during the timeframe of their typical influenza seasons. A) Mobility data collected by Google, based on Google application-based location services that shows how location visits differ compared to a pre-pandemic baseline [21], demonstrates that Australia and France had a high percent reduction in mobility to retail and recreation locations, compared to the baseline, while the US showed a more modest reduction in the same mobility, during April-June 2020 for Australia and November- mid-December 2020 for France and the US, capturing the start of their respective influenza seasons. B) A social media survey of Facebook users across over 200 countries starting in 2020 demonstrated that approximately half of US respondents with school-aged children were participating in no in-person schooling from November 1-mid-December 2020 [15]. Australia and France did not have widespread school closures at the start of their influenza seasons. C) The Facebook survey also demonstrated that France and the US had high levels of mask-wearing during November 1-mid-December 2020. Australia had much lower mask-wearing uptake at the start of the influenza season in April-June 2020. Adherence to protective behaviors was spatially heterogeneous across the US as measured by (D) Google mobility data showing the percent reduction in mobility to retail and recreation locations from November-mid-December 2020; (E) the Facebook survey data showing that reports of no in-person schooling; and (F) the Facebook data showing reports of wearing a mask “always” or “most of the time”. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Each of the case study countries- the US, France, and Australia- exhibited no influenza season, but implemented different non-pharmaceutical interventions with varying levels of adherence. Reported variations partly stem from differences in strategic policies adopted to control the COVID-19 pandemic, aiming towards SARS-CoV-2 elimination (Australia) vs. mitigation (the US, France) [33]. Additionally, for all three interventions (restrictions on mobility, school closure, mask use), uptake were also spatially heterogeneous (Fig. 1D-F), driven by variation in policy strength and policy response [20], [42]. This scenario presents an opportunity to better understand the relative effects of individual interventions and the role of layering multiple partially-efficacious interventions to suppress influenza and other respiratory diseases. It will be crucial for such studies to be conducted at a fine spatial scale to capture spatial heterogeneities in infection dynamics as well as variation in human behavior [27], [31], and simultaneously to consider global dynamics to capture the shift in viral circulation and lack of viral introductions normally mediated by human mobility.
As we all breathe a collective sigh of relief for the dodged influenza season, it’s important to look ahead beyond this temporary respite. Given the lower transmission potential of influenza and significant population immunity from past infections or vaccination, we know that less collective action is necessary to stop flu transmission than COVID-19. The pandemic has demonstrated the effectiveness of several policies and social norms that should be sustained to continue to reduce influenza and other respiratory infections. Universal paid sick leave, particularly in the US, should be a focus for policy; facilitating infected individuals to remain home is shown to reduce future respiratory-disease transmission [36], [43], [46]. Additionally, during the pandemic, many workplaces have created structures that allow for remote work for non-essential jobs and flexible work schedules; maintaining this flexibility could be a means to reduce overall contact and thus reduce transmission during influenza season. The pandemic has improved acceptance of mask-wearing, increased understanding of the importance of vaccination, and increased awareness and adherence to protective behaviors which could be leveraged for disease control efforts in the future. On the other hand, the sustained behavioral changes during the pandemic or recent COVID-19 vaccination may increase apathy towards social distancing measures, fear of social stigma against mask-wearing, or hesitancy towards vaccination for influenza [41]. While some hygienic behaviors recommended during the 2009 H1N1 influenza pandemic remained as a residual effect once the pandemic was over, overall adoption of preventive measures sharply declined during the first post-pandemic seasonal influenza [19], and a similar effect was observed after the SARS outbreak in 2003 [28].
Beyond behavioral intervention, we must remain vigilant given the significant perturbation to influenza viral dynamics and host ecology. As COVID-19 cases become suppressed through vaccination and behavioral interventions are lifted, the risk of other respiratory infections will be restored. Indeed, an uptick in cases of respiratory syncytial virus has already been observed in the US [9]. The lack of natural immunity during the last influenza season also means a shift in the immune landscape and a buildup of susceptible individuals, with potential consequences for the timing and severity of future outbreaks [1]. Complicating matters further, influenza vaccine strains are typically based on viral surveillance from past influenza circulation in both hemispheres. A lack of influenza circulation last season will reduce our confidence in the strains that are likely to circulate next season, potentially resulting in strain mismatch between the vaccine and circulating strains [45]. Despite these challenges in vaccine composition which may result in a low efficacy influenza vaccine, encouraging widespread vaccine uptake in fall 2021 will be our best bet to reduce both influenza disease burden and antigenic evolution [25], [47]. In general, the influenza virus promises unpredictability over all else in the transformed post-pandemic viral landscape and we must be prepared.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Reseau Sentinelles and IQVIA France for providing us access to flu vaccine sale data. This research is based on survey results from Carnegie Mellon University’s Delphi Group. Research reported in this publication was supported by the National Institute Of General Medical Sciences of the National Institutes of Health under Award Number R01GM123007. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We also acknowledge support from the PhRMA Foundation, the Chateaubriand Fellowship Program, and the Georgetown Global Health Initiative. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.05.049.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Baker R, Park S.W., Yang W., Vecchi G.A., Metcalf C.J.E., Grenfell B. The impact of COVID-19 non-pharmaceutical interventions on the future dynamics of endemic. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(48):30547–30553. doi: 10.1101/2020.06.22.20137588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassett M.T., Chen J.T., Krieger N. Variation in racial/ethnic disparities in COVID-19 mortality by age in the United States: A cross-sectional study. PLoS Med. 2020;17(10):1–14. doi: 10.1371/journal.pmed.1003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biggerstaff M., Cauchemez S., Reed C., Gambhir M., Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: A systematic review of the literature. BMC Infect Dis. 2014;14(1):1–20. doi: 10.1186/1471-2334-14-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borchering R.K., Gunning C.E., Gokhale D.V., Weedop K.B., Saeidpour A., Brett T.S., et al. Anomalous influenza seasonality in the United States and the emergence of novel influenza B viruses. Proceedings of the National Academy of Sciences of the United States of America. 2021;118(5) doi: 10.1073/pnas.2012327118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bubola E. The New York Times; 2020. A reality check for Italy’s vaccination hopes: a flu-shot logjam. [Google Scholar]
- 6.Cauchemez S., Valleron A.J., Boëlle P.Y., Flahault A., Ferguson N.M. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008;452(7188):750–754. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. (2020). Seasonal influenza vaccine supply & distribution. Retrieved from https://www.cdc.gov/flu/prevent/vaccine-supply-distribution.htm
- 8.Centers for Disease Control and Prevention. (2021a). Weekly U.S. influenza surveillance report. Retrieved from https://www.cdc.gov/flu/weekly/index.htm
- 9.Centers for Disease Control and Prevention. (2021b). RSV national trends. Retrieved from https://www.cdc.gov/surveillance/nrevss/rsv/natl-trend.html
- 10.Centers for Disease Control and Prevention. (2021c). Weekly national flu vaccination dashboard. Retrieved from https://www.cdc.gov/flu/fluvaxview/dashboard/vaccination-dashboard.html
- 11.Chiu N.C., Chi H., Tai Y.L., Peng C.C., Tseng C.Y., Chen C.C., et al. Impact of wearing masks, hand hygiene, and social distancing on influenza, enterovirus, and all-cause pneumonia during the coronavirus pandemic: Retrospective national epidemiological surveillance study. Journal of Medical Internet Research. 2020;22(8) doi: 10.2196/21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowell G., Miller M.A., Viboud C. Seasonal influenza in the United States, France, and Australia: Transmission and prospects for control. Epidemiol Infect. 2008;136(6):852–864. doi: 10.1017/S0950268807009144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowling B.J., Ali S.T., Ng T.W.Y., Tsang T.K., Julian C.M. Impact assessment of non-pharmaceutical interventions against COVID-19 and influenza in Hong Kong: an observational study. The Lancet Public Health. 2020;5:e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Luca G., Kerckhove K. Van, Coletti P., Poletto C., Bossuyt N., Hens N., et al. The impact of regular school closure on seasonal influenza epidemics: A data-driven spatial transmission model for Belgium. BMC Infect Dis. 2018;18(1):1–16. doi: 10.1186/s12879-017-2934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delphi Group https://cmu-delphi.github.io/delphi-epidata/symptom-survey/
- 16.Di Domenico L., Pullano G., Sabbatini C.E., Boëlle P.Y., Colizza V. Impact of lockdown on COVID-19 epidemic in Île-de-France and possible exit strategies. BMC Medicine. 2020;18(1):1–13. doi: 10.1186/s12916-020-01698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Centre for Disease Prevention and Control. (2021). Influenza virus characterisation, Summary Europe, February 2021. ECDC Surveillance Report, (February 2021). Retrieved from https://www.ecdc.europa.eu/sites/default/files/documents/Influenza-characterisation-repo rt-February-2021.pdf
- 18.Ewing A., Lee E.C., Viboud C., Bansal S. Contact, travel, and transmission: The impact of winter holidays on influenza dynamics in the United States. Journal of Infectious Disease. 2017;215(5):732–739. doi: 10.1093/infdis/jiw642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Continente X., Serral G., López M.J., Pérez A., Nebot M. Long-term effect of the influenza A/H1N1 pandemic: attitudes and preventive behaviours one year after the pandemic. Eur J Pub Health. 2013;23(4):679–681. doi: 10.1093/eurpub/ckt068. [DOI] [PubMed] [Google Scholar]
- 20.Garnier R., Benetka J.R., Kraemer J., Bansal S. Socioeconomic disparities in social distancing during the COVID-19 pandemic in the United States: Observational study. Journal of Medical Internet Research. 2021;23(1) doi: 10.2196/24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Google LLC. Google COVID-19 community mobility reports. https://www.google.com/covid19/mobility/ Accessed: 2/24/2021.
- 22.Haug N., Geyrhofer L., Londei A., Dervic E., Desvars-Larrive A., Loreto V., et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nature Human. Behaviour. 2020;4(December) doi: 10.1038/s41562-020-01009-0. [DOI] [PubMed] [Google Scholar]
- 23.Hunt, G. (2020). Record flu vaccines in 2020 to protect Australians. Retrieved from https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/record-flu-vaccines-in-2020-to-protect-australians
- 24.Iowa Department of Public Health. (2020). Influenza vaccine data. Retrieved from https://tracking.idph.iowa.gov/Health/Immunization/Influenza-Vaccine/InfluenzaVaccine-Data
- 25.Kramer S., Bansal S. Assessing the use of antiviral treatment to control influenza. Epidemiol Infect. 2014;143:1–11. doi: 10.1017/S0950268814002520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagacé-Wiens P., Sevenhuysen C., Lee L., Nwosu A., Smith T. Impact of nonpharmaceutical interventions on laboratory detections of influenza A and B in Canada. CCDRCANADA. 2021;47(3):142–148. doi: 10.14745/ccdr.v47i03a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee E.C., Arab A. MedRxiv. The Preprint Server for Health Sciences; 2021. Spatial aggregation choice in the era of digital and administrative surveillance data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung G.M., Ho L.M., Chan S.K., Ho S.Y., Bacon-Shone J., Choy R.Y., et al. Longitudinal assessment of community psychobehavioral responses during and after the 2003 outbreak of severe acute respiratory syndrome in Hong Kong. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;40(12):1713–1720. doi: 10.1086/429923. [DOI] [PubMed] [Google Scholar]
- 29.Litvinova M., Liu Q.H., Kulikov E.S., Ajelli M. Reactive school closure weakens the network of social interactions and reduces the spread of influenza. PNAS. 2019;116(27):13174–13181. doi: 10.1073/pnas.1821298116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macartney K., Quinn H.E., Pillsbury A.J., Koirala A., Deng L., Winkler N., et al. NSW COVID-19 Schools Study Team. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. The Lancet Child and Adolescent Health. 2020;4(11):807–816. doi: 10.1016/S2352-4642(20)30251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masters N.B., Eisenberg M.C., Delamater P.L., Kay M., Boulton M.L., Zelner J. Fine-scale spatial clustering of measles nonvaccination that increases outbreak potential is obscured by aggregated reporting data. PNAS. 2020;117(45):28506–28514. doi: 10.1073/pnas.2011529117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nickbakhsh S., Mair C., Matthews L., Reeve R., Johnson P.C.D., Thorburn F., et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. PNAS. 2019;116(52):27142–27150. doi: 10.1073/pnas.1911083116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliu-Barton M., Pradelski B., Aghion P., Artus P., Kickbusch I., Lazarus J.V., et al. SARS-CoV-2 elimination, not mitigation, creates best outcomes for health, the economy, and civil liberties. The Lancet. 2021 doi: 10.1016/S0140-6736(21)00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen S.J., Azziz-Baumgartner E., Budd A.P., Brammer L., Sullivan S., Pineda R.F., et al. Decreased influenza activity during the COVID-19 pandemic — United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen E., Koopmans M., Go U., Hamer D.H., Petrosillo N., Castelli F., et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pichler S., Wen K., Ziebarth N.R. COVID-19 emergency sick leave has helped flatten the curve in the United States. Health Aff. 2020;39(12):2197–2204. doi: 10.1377/hlthaff.2020.00863. [DOI] [PubMed] [Google Scholar]
- 37.Roger, E. Vaccin contre la grippe : pourquoi les pharmacies sont-elles en rupture de stock? Europe1 2020. Retrieved from https://www.europe1.fr/societe/vaccin-contre-la-grippe-que-sait-on-de-la-rupture-de-stock-dans-les-pharmacies-4005002
- 38.Rohani P., Green C.J., Mantilla-Beniers N.B., Grenfell B.T. Ecological interference between fatal diseases. Nature. 2003;422(6934):885–888. doi: 10.1038/nature01542. [DOI] [PubMed] [Google Scholar]
- 39.Sohn S., Hong K., Chun C. Decreased seasonal influenza during the COVID-19 pandemic in temperate countries. Travel Med Infect Dis. 2021;May-June(41):102057. doi: 10.1016/j.tmaid.2021.102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soo R.J.J., Chiew C.J., Ma S., Pung R., Lee V., Lee V.J. Decreased influenza incidence under COVID-19 control measures. Singapore. Emerging Infectious Diseases. 2020;26(8):1933–1935. doi: 10.3201/eid2608.201229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teasdale E., Santer M., Geraghty A.W.A., Little P., Yardley L. Public perceptions of non-pharmaceutical interventions for reducing transmission of respiratory infection: Systematic review and synthesis of qualitative studies. BMC Public Health. 2014;14(1):1–17. doi: 10.1186/1471-2458-14-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valdano E., Lee J., Bansal S., Rubrichi S., Colizza V. Highlighting socio-economic constraints on mobility reductions during COVID-19 restrictions in France can inform effective and equitable pandemic response. Journal of travel medicine. 2021;045 doi: 10.1093/jtm/taab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazquez J., Islam T., Beller J., Fiori K., Correa R., Correa D.J. Expanding paid sick leave as a public health tool in the COVID-19 pandemic. J Occup Environ Med. 2020;62(10):e598–e599. doi: 10.1097/JOM.0000000000001998. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Influenza (seasonal). 2018. Retrieved from https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- 45.World Health Organization. Recommended composition of influenza virus vaccines for use in the 2021- 2022 northern hemisphere influenza season. 2021. Retrieved from https://www.who.int/influenza/vaccines/virus/recommendations/202102_recommendation.pdf?ua=1
- 46.Zipfel C.M., Colizza V., Bansal S. Health inequities in influenza transmission and surveillance. PLoS Comput Biol. 2021;17(3):1–23. doi: 10.1371/journal.pcbi.1008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wen F.T., Malani A., Cobey S. The beneficial effects of vaccination on the evolution of seasonal influenza. bioRxiv. 2020;162545 doi: 10.1101/162545. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.