Supplemental Digital Content is available in the text.
Keywords: atrial fibrillation, atrial tachycardia, coronary sinus, ethanol, iodine, vein of Marshall
Background:
Vein of Marshall (VOM) ethanol infusion is a relatively new therapeutic option for atrial tachyarrhythmias. We aimed to evaluate the feasibility, pitfalls, and complications associated with this procedure in a large cohort of patients.
Methods:
Successful ethanol infusion, VOM-related lesion extent, and serious complications were evaluated in 713 consecutive patients treated with VOM ethanol infusion.
Results:
While feasible in 88.9% of cases, VOM ethanol infusion failure mainly resulted from nonidentification (6.2%), noncannulation (1.5%), or ethanol infusion in the wrong vein (1.7%). The Vieussens valve was a helpful landmark and was visible in 63.2% of cases. Multivariable analysis identified previous coronary sinus ablation as the only predictor for nonidentification. The mean area of VOM-related endocardial scarring was 10.2±5.3 cm2. VOM dissection (10.7%), iodine leakage (3.0%), and VOM morphology without visible branches (3.0%) were associated with smaller VOM-related scarring (5.0±3.9, 6.6±3.5, and 4.7±2.3 cm2, with a P<0.0001, P<0.044, and P<0.0001, respectively). Ethanol infusion in a wrong vein was associated with less mitral line block (72.7% versus 95.8%, P=0.012). A total of 14 serious complications (2.0%) occurred: 7 tamponades, of which were 6 delayed and treated with pericardiocentesis (2 of these patients had per-procedural VOM perforation), 4 strokes, 1 anaphylactic shock, 1 atrioventricular block, and 1 left appendage isolation. Only 4 of these complications occurred during the procedure.
Conclusions:
Although limited by previous coronary sinus ablation, VOM ethanol infusion is a highly feasible treatment for atrial tachyarrhythmia, with a low rate of serious complications.
What Is Known?
The growing interest in vein of Marshall ethanol infusion calls for a better understanding of the events that can occur during this procedure.
What the Study Adds
By including the largest number of patients to date, the present work clarifies the associated feasibility, pitfalls, and complications. These data can help optimize vein of Marshall ethanol infusion.
Although highly feasible with a low complication rate, further research is required to identify patients who benefit most from vein of Marshall ethanol infusion.
Experimental and clinical studies have demonstrated that the vein of Marshall (VOM) bundle can support random reentries priming atrial fibrillation (AF), or stable reentries that are either perimitral or localized.1,2 Since it is insulated by fat, physical ablation of the VOM bundle by radiofrequency has been highly challenging since long. Chemical ablation by retrograde ethanol infusion has provided a critical step forward for its efficient elimination.3 This technique, pioneered by Valderrábano, has proved determinant for mitral isthmus block, both in terms of acute success and lesion durability.4 As a strategy beyond pulmonary vein isolation, a prospective multicenter trial has recently shown that VOM ethanol infusion decreased the likelihood of atrial tachyarrhythmias in patients with persistent AF.5 VOM ethanol infusion has thus gained growing attention as a new therapeutic option for AF. However, there are no detailed reports in a large cohort of patients on the events that occur during the procedure and their outcomes.
This study aimed to investigate the feasibility of VOM ethanol infusion, as well as its associated pitfalls and complications.
Methods
Study Population
The data that support the findings of this study are available from the corresponding author upon reasonable request. In this retrospective study, we included all cases in which VOM ethanol infusion was attempted for the treatment of AF or atrial tachycardia, from July 2017 to August 2020 in the Haut Leveque Hospital (Bordeaux, France). Informed consent was obtained from all patients. The ablation protocol was approved by our institutional ethics committee on human research.
Ablation Protocol
General Principles
Technical details of the procedure have been previously reported.6 The procedure was performed under conscious sedation, with the CARTO 3 (Biosense Webster, Diamond Bar, CA) or the Rhythmia (Boston Scientific, Cambridge, MA) mapping systems. First ablation procedures were performed in persistent AF patients and followed 3 steps as previously described: (1) VOM ethanol infusion; (2) pulmonary vein isolation; and (3) linear lesions created at the mitral, roof, and cavotricuspid isthmuses to achieve a conduction block.6 Redo ablation procedures included VOM ethanol infusion for one of these 3 reasons: (1) treating the clinical tachycardia if the VOM bundle was potentially involved (bursting focus or localized reentry at the ridge, perimitral flutter with epicardial bypass); (2) blocking the mitral line, if not yet achieved; or (3) as an adjunct therapy for AF, beyond the initial lesion set created during the first procedure. Of note, in the present work, the terms proximal or distal will not be defined by the natural blood flow but rather by the location toward the ostium or the tributaries of the vein considered, respectively.
VOM Ethanol Infusion
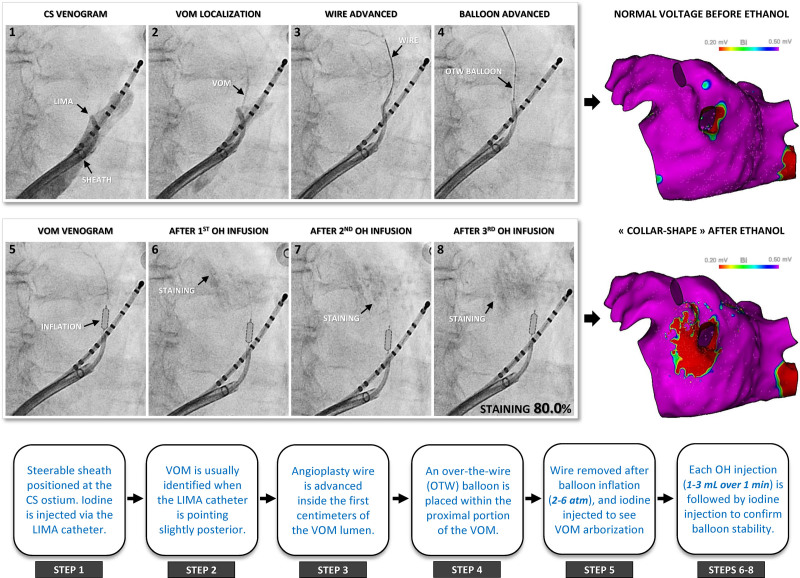
The coronary sinus (CS) was cannulated with a steerable long sheath (Agilis NxT; Abbott, St Paul, MN) inserted from the right femoral vein. An angiography catheter (5F left internal mammary artery; Medtronic, Minneapolis, MN) was proximally positioned inside the CS lumen, and contrast (iodine) was injected to localize the VOM ostium. When not identified, a CS venogram was performed by dedicated balloon occlusion. Subsequently, an angioplasty wire (Whisper 0.014, Abbott or Sion Blue 0.014, Asahi) was advanced inside the VOM lumen and used to position a preloaded over-the-wire balloon (MINI TREK, 1.5–3 mm diameter and 6–15 mm length, Abbott) within the first 15 mm of its proximal portion. After inflation at 2 to 6 atm (until some resistance was felt) and wire removal, a selective angiography was performed through the wire port to confirm balloon occlusion and visualize VOM arborization. Absolute ethanol (96%) was collected in a metal bowel. Three successive injections (1–3 mL) were slowly administered over 1 minute, with selective VOM angiogram repeated each time to confirm balloon stability (Figure 1).7
Figure 1.
Procedural steps. Venous angiographies: Successive 8 steps of vein of Marshall (VOM) ethanol infusion. Note the progressive staining that increases with each ethanol infusion, but with a preserved arborization. Electroanatomical maps: Left atrium (LA) voltage before and after ethanol infusion. A collar-shape endocardial scarring appears in the vicinity of the left inferior pulmonary vein. Stepwise diagram: key procedural details related to each step. CS indicates coronary sinus; LIMA, an angiography catheter (5F left internal mammary artery); OH, ethanol; and OTW, over the wire.
Evaluation Criteria
Feasibility
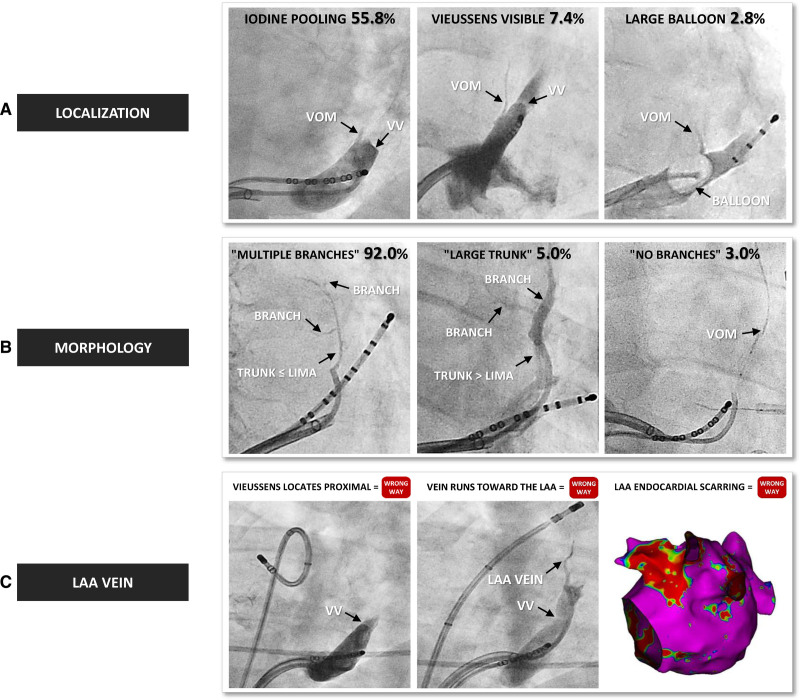
Procedural success was defined as ethanol delivered inside the VOM lumen but not in other veins. VOM identification required the 3 following features: (1) an ostium location before the valve of Vieussens (VV), as reported in anatomic series; (2) a superior course toward the left atrial (LA) ridge8; and (3) a typical lesion with a collar-shape electrical silence recorded after ethanol infusion just below, posterior to, and in front of the left inferior pulmonary vein ostium (Figure 1). VV identification was based on at least one of the 2 following features: (1) clear iodine pooling reproducibly observed at the same level of the vessel and (2) direct visualization of a translucent structure (Figure 2A). VOM arborization has been extensively described by Valderrábano et al.9 To view this varying anatomy in the perspective of the procedural impact, we distinguished 3 key morphologies: (1) multiple branches if the VOM exhibited ≥2 branches with the trunk not exceeding the diameter of the 5F left internal mammary artery; (2) large trunk if the VOM exhibited ≥2 branches but with the trunk exceeding the diameter of the 5F left internal mammary artery; and (3) no branches if the VOM was straight without visible branches (Figure 2B).6 Veins misidentified as the VOM included (1) the inferior LA vein, which originates before the VV and runs toward the posterior, or even septal wall of the LA and (2) the left atrial appendage (LAA) vein, which originates after the VV and exhibits an anterior course (Figure 2C). In case of unusual anatomy, veins that did not fit the above-mentioned definitions were labeled undefined.
Figure 2.
Feasibility.A, Tips and tricks for vein of Marshall (VOM) localization. VOM ostium always sites slightly proximal from the valve of Vieussens (VV), which is distinguished either indirectly by iodine pooling or directly as a translucent structure. A large balloon occluding the coronary sinus (CS) was sometimes helpful to visualize the VOM. B, Different types of VOM. A representative example of multiple branches, large trunk, and no branches morphologies. C, Representative example of inadvertent left atrial appendage (LAA) vein infusion. Note that none of the 3 key criteria for VOM identification were fulfilled: the VV is visible but proximal to the targeted vein, the targeted vein points toward the catheter tip placed at the LAA apex, and the endocardial scarring impacts the lateral portion of the LAA. LIMA indicates an angiography catheter (5F left internal mammary artery).
Pitfalls
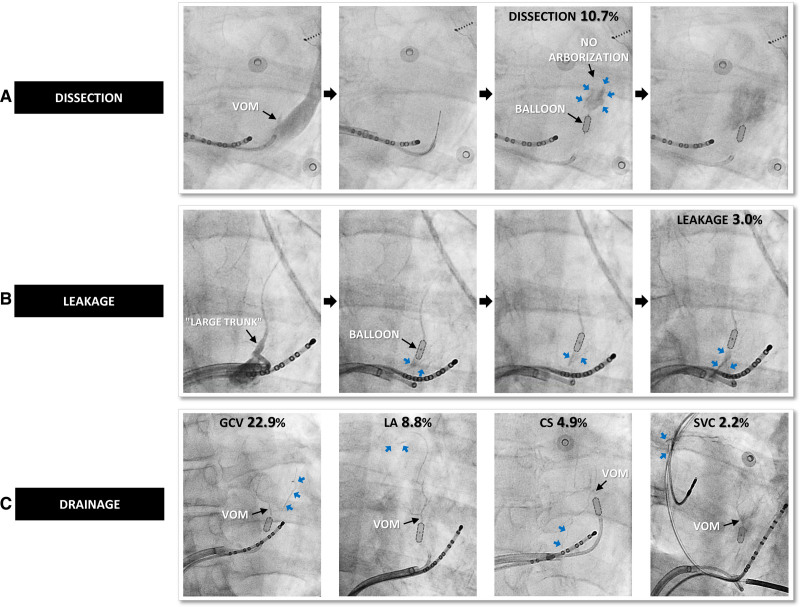
Procedural pitfall was defined as any event that is likely to mitigate the impact of VOM ethanol infusion, either in terms of tissue lesion or electrophysiological criteria. Voltage was measured immediately after VOM ethanol infusion and before any endocardial radiofrequency application at the mitral isthmus by electroanatomic mapping using peak-to-peak electrogram amplitude, with a threshold set at 0.5 mV in sinus rhythm and 0.2 mV in AF (Figure 1). Mitral line block was validated during LAA pacing by the combination of a proximal-to-distal activation of the CS catheter with a septal-to-lateral activation of the LA posterior wall and differential pacing maneuvers. Critical events were thoroughly identified, as follows: (1) VOM staining corresponded to progressive iodine diffusion due to distal extravasation inside the tissue favored by ethanol-induced fragilization of the vessel, without loss of distal vascular arborization (Figure 1); (2) VOM dissection corresponded to an abrupt iodine densification with loss of distal vascular arborization occurring before any ethanol infusion, mostly during the first selective venogram (Figure 3A); (3) mechanical leakage corresponded to a loss of iodine into the CS due to incomplete occlusion during all VOM selective angiograms (Figure 3B); and (4) anatomic drainage corresponded to a loss of iodine into the atria, the great cardiac vein, the CS, or the superior vena cava via natural vascular anastomoses with the VOM (Figure 3C).
Figure 3.
Pitfalls.A, Angiogram of a patient with vein of Marshall (VOM) dissection (blue arrows) during the first selective VOM angiogram. Note that distal VOM arborization is never visible. B, Angiogram of a patient with a large trunk morphology and a mechanical leakage (blue arrows) that is proximal from the balloon, with iodine loss into the coronary sinus (CS). C, Representative examples of the 4 types of anatomic drainage (blue arrows) via natural anastomosis between the VOM and the great cardiac vein (GCV), left atrium (LA), CS, or the superior vena cava (SVC).
Complications
All patients underwent transthoracic echocardiography within 48 hours after the procedure. A procedural complication was defined as any adverse event considered to be life-threatening or likely to impair the functional status of the patient.
Statistical Analysis
Data were analyzed using JMP12 software (SAS Institute Inc, Cary, NC). Continuous variables are presented as mean ± SD or median with interquartile range (25th–75th percentile) and analyzed using an independent Student t test or a Mann-Whitney U test, as appropriate. Categorical variables are expressed as numbers and percentages and were analyzed using the Fisher exact test or the χ2 test. Univariable and multivariable logistic regression was used to identify predictors of VOM identifiability. For the multivariable analysis, variables with a P<0.05 in the univariable analysis were selected. A P<0.05 was considered statistically significant.
Results
General Characteristics
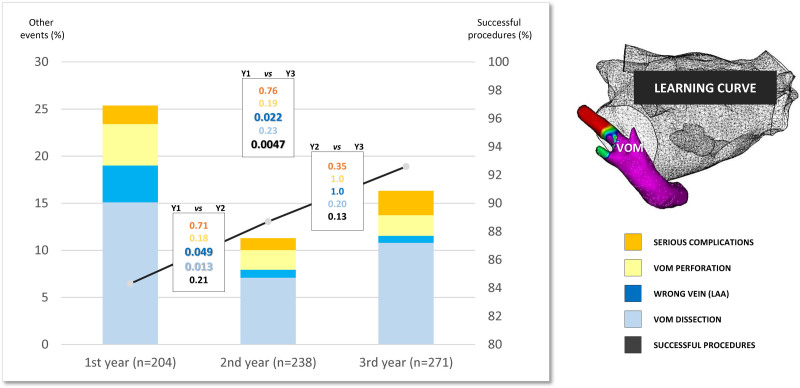
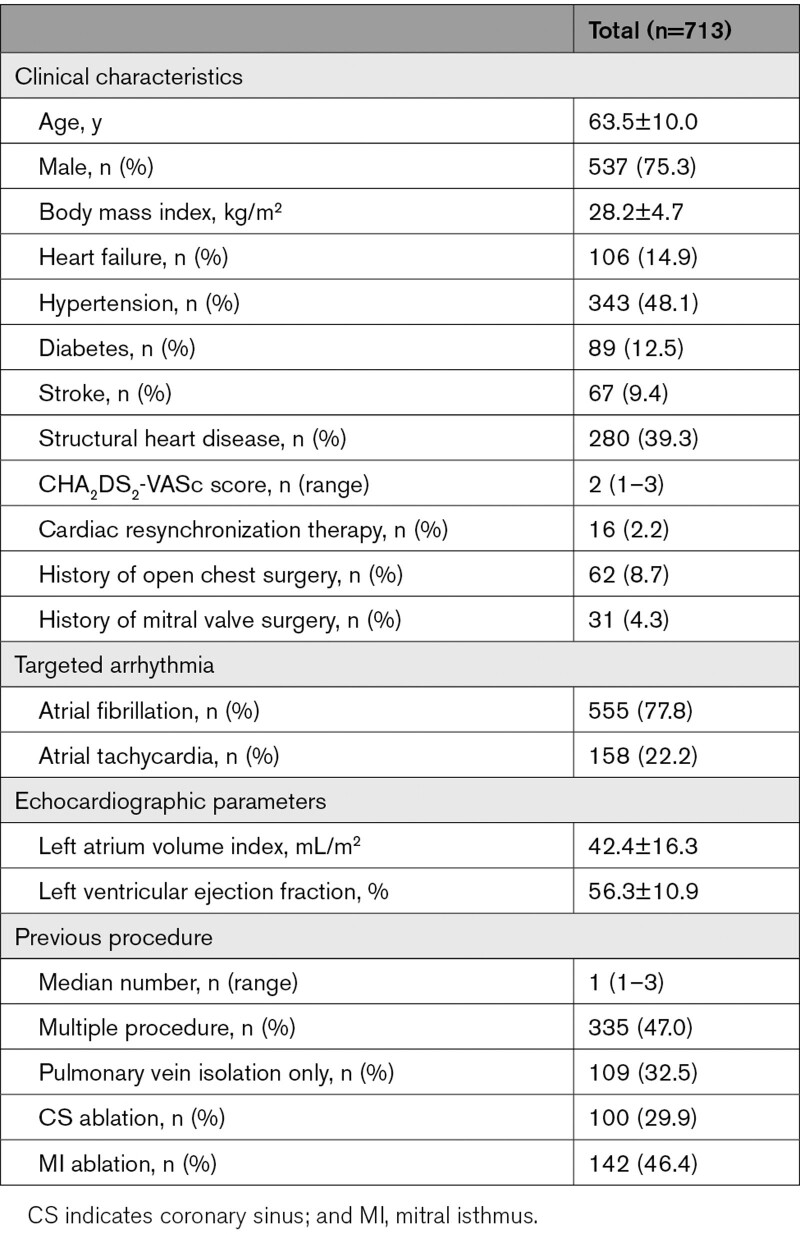
The study population comprised a total of 713 patients. Of these, VOM ethanol infusion was performed without any previous ablation procedure in 378 patients (53.0%). Among the 335 patients (47.0%) with previous AF ablation, 100 patients (14.0%) had undergone CS endovascular radiofrequency application. Ablation was indicated for AF in 555 patients (77.8%) and for atrial tachycardia in 158 patients (22.2%). Clinical characteristics are detailed in the Table. The temporal evolution of the main outcomes of this technique is summarized in Figure 4.
Figure 4.
Learning curve. Evolution of key procedural events over the years: successful vein of Marshall (VOM) ethanol infusion (black line), VOM dissection (pale blue), wrong vein ethanol infusion (dark blue), VOM perforation (yellow), and serious complications (orange). Statistical comparison with a P value is shown between each year (black-framed rectangle) and for each item (following identical color code). LAA indicates left atrial appendage.
Table.
Patient Characteristics
VOM Ethanol Infusion
Feasibility
VOM ethanol infusion was achieved in 634 patients (88.9%) after the first attempt (Figure 2). CS venogram with dedicated balloon occlusion was performed in 51 patients (7.2%) and helped identify the VOM in 20 patients (2.8%). VOM morphology was multiple branches in 583 patients (92.0%), large trunk in 32 patients (5.0%), and no branches in 19 patients (3.0%). The VV was visible in 401 patients (63.2%), either indirectly by contrast pooling in 354 patients (55.8%) or directly in 47 patients (7.4%). All the visible VV were located after the VOM ostium.
The reasons for procedure failure in the remaining 79 patients (11.1%) were (1) nonidentification of the VOM in 44 patients (6.2%); (2) noncannulation of the VOM in 11 patients (1.5%); (3) ethanol infusion inside a wrong vein (inferior LA vein: 0, LAA vein: 10, undefined vein: 2) in 12 patients (1.7%); (4) CS dissection in 6 patients (0.8%); (5) persistent left superior vena cava in 4 patients (0.6%); and (6) VOM perforation in 2 patients (0.3%).
Another procedure for atrial tachyarrhythmia recurrence was performed in 177 out of the 713 patients of the cohort: repeat VOM ethanol infusion was attempted in 59 patients. Of the 41 patients with previous VOM ethanol infusion success, the VOM could be identified in 7 patients (17.1%), and ethanol delivered in 6 patients (14.6%). Of the 18 patients with previous VOM ethanol infusion failure, ethanol could be delivered in 15 patients (83.3%). Thus, VOM could be identified in a total of 662 patients (92.8%) after multiple attempts and VOM ethanol infusion was achieved in 649 patients (91.0%). Multivariable analysis identified previous CS ablation as the only predictor for VOM nonidentification (odds ratio, 4.08 [95% CI, 1.65–10.19], P=0.0024; Table I in the Data Supplement). Neither cardiac resynchronization therapy nor mitral valve surgery was predictors.
The yearly success rate significantly increased from 84.3% in the first year to 92.6% in the third year (P=0.0047; Figure 4).
Pitfalls
Among the 634 patients where VOM ethanol infusion was successful, some procedural events were observed (Figures 1 and 3). In decreasing order of frequency, we found (1) VOM staining in 507 patients (80.0%); (2) at least one anatomic drainage in 223 patients (35.2%); (3) VOM dissection in 68 patients (10.7%); and (4) persistent mechanical leakage in 19 patients (3.0%).
Voltage in the VOM-related area could be assessed before any endocardial radiofrequency application at the mitral isthmus in 251 patients. The increase in the area of VOM-related endocardial scarring was 10.2±5.3 cm2. Patients with VOM dissection had significantly smaller endocardial scarring than those without (5.0±3.9 cm2 with versus 10.8±5.2 cm2 without, P<0.0001). Among the latter, mechanical leakage significantly decreased endocardial scarring (6.6±3.5 cm2 with versus 11.1±5.1 cm2 without, P=0.044), while anatomic drainage did not (11.2±4.8 cm2 with versus 11.1±5.3 cm2 without, P=0.84). Of note, the large trunk morphology was associated with a significantly higher incidence of mechanical leakage than other morphologies (34.4% versus 1.3%, P<0.0001) but only the no branches morphology was associated with significantly decreased endocardial scarring (4.7±2.3 cm2 with versus 11.1±5.1 cm2 without, P<0.0001).
The rate of mitral line block in the 477 patients with first mitral line attempt was 95.8% at the end of the procedure, without significant difference with regard to VOM dissection (96.1% with versus 95.8% without, P=1.0), mechanical leakage (88.9% with versus 95.9% without, P=0.32), or VOM morphology (100% for no branches VOM versus 95.7% for others VOM morphologies, P=1.0). The area of VOM-related endocardial scarring was larger in patients with mitral line block than those without, but not statistically significant (10.4±5.4 versus 7.3±3.7 cm2, P=0.065). Ethanol infusion inside a wrong vein was the only event significantly associated with a lower rate of mitral line block (72.7% versus 95.8%, P=0.012).
The yearly incidence of VOM dissection significantly decreased from 15.1% in the first year to 7.1% in the second year (P=0.013; Figure 4).
Complications
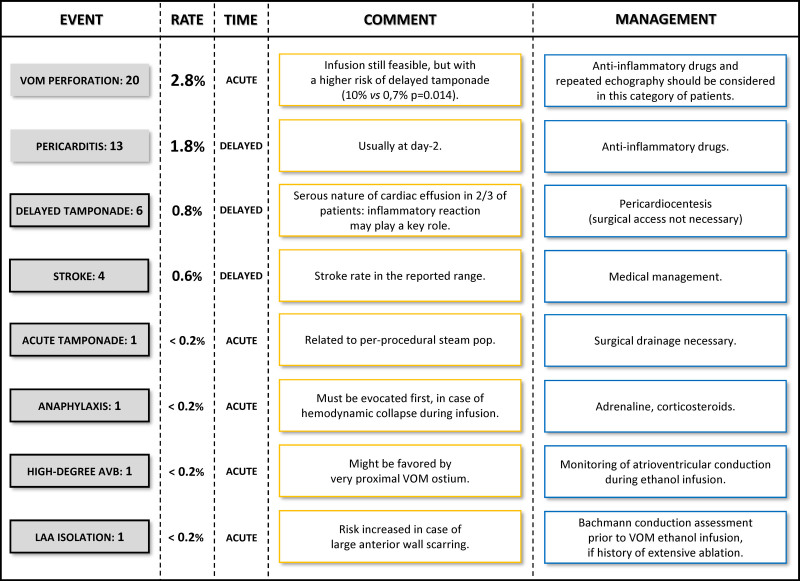
After a mean follow-up of 9.5±8.3 months, the following adverse events were reported: (1) VOM perforation in 20 patients (2.8%), defined as iodine extravasation into the pericardial space; (2) pericarditis in 13 patients (1.8%), defined as chest pain and limited pericardial effusion; (3) cardiac tamponade in 7 patients (1.0%); (4) stroke in 4 patients (0.6%); (5) anaphylactic shock in 1 patient; (6) high-degree atrioventricular block in 1 patient; and (7) LAA isolation in 1 patient (Figure 5). In total, serious complications occurred during the procedure in 4 patients (0.6%) and were delayed in 10 patients (1.4%).
Figure 5.
Complications. Column 1: type and number of minor (no frame) and serious (black framed) events. Column 2: event rate. Column 3: event timing, considered acute if it occurs during the procedure, or delayed if it occurs after the procedure. Column 4: comment related to vein of Marshall (VOM) ethanol infusion. Column 5: specific management related to VOM ethanol infusion. LAA indicates left atrial appendage.
Regarding cardiac tamponade, pericardiocentesis was performed during the procedure in 1 patient, after 1 week in 1 patient, and after at least 14 days (14–106 days) in 5 patients (0.7%). During pericardiocentesis, cardiac effusion was serous in 4 patients (0.6%). The rate of cardiac tamponade was significantly higher in patients with VOM perforation than in patients without VOM perforation (10% versus 0.7%, P=0.014). The rate of VOM perforation (28.6% versus 1.9%, P=0.0089) and the age at the time of the procedure (70.4±4.6 versus 63.3±10.2 years, P=0.0059) were significantly higher in patients with than in those without cardiac tamponade (Table II in the Data Supplement).
The anaphylactic shock started with a coughing spell during the first ethanol injection, followed by a cutaneous rash and a complete electromechanical dissociation in <1 minute. Resuscitation maneuvers, including external cardiac massage, intravenous adrenaline, systemic corticosteroid, intubation with mechanical ventilation, and nebulization with a β2-agonist, resulted in rapid and complete recovery. The high-degree atrioventricular block (2:1) occurred during the first ethanol infusion and persisted for 3 days, finally leading to pacemaker implantation. The LAA isolation also occurred during the first ethanol injection. Stroke occurred in 4 patients (0.6%), within 24 hours in 3 patients, and several weeks later in another patient. None of the ethanol deliveries in the LAA vein resulted in complete scarring of the LAA, and no thrombus was observed during follow-up.
Over the 3 years, the yearly incidence of VOM perforation and serious complications did not statistically differ (Figure 4).
Discussion
The present study is the first to extensively characterize the VOM ethanol infusion procedure in a large cohort of patients. The main findings are (1) its high feasibility with a success rate of 88.9% during the first attempt, while previous CS ablation is the only predictor identified for failure; (2) VOM dissection, mechanical leakage, no branches morphology, and ethanol infusion inside a wrong vein are the 4 pitfalls reducing its impact on tissue lesion extent or mitral line block; (3) serious acute complications during the procedure, although challenging to anticipate, are rare (0.6%); and (4) experience in performing the procedure ensures a progressive increase in success rate and decrease in VOM dissection.
Feasibility
Recent studies have shed light on the therapeutic benefits of VOM ethanol infusion during persistent AF ablation.5,6,8 However, very few have focused on the technical details that guarantee its feasibility.9,10 With a total success rate of 91% in the largest series to date, the present work points out the importance of VOM identification. Its ostium is characterized by a variable absolute distance from the CS ostium (range of 5–50 mm) but a consistent relative position to the VV (within 1 cm proximal).11,12 The VV is, therefore, a key anatomic marker. Although frequently found in anatomic series (74% to 87%), the VV exhibits different morphologies that may not all be suitable for angiographic identification.11,13 However, with proximal iodine injection, the VV was visible in almost two-thirds of patients, making it very helpful to circumscribe the area of interest. VOM identification can be further facilitated by (1) CS venogram with dedicated balloon occlusion, which ensured VOM identification in 2.8% of patients; (2) placing a catheter at the posterior LAA edge to help distinguish the VOM from the LAA vein in case of equivocal course; and (3) suspended endocardial scarring remote from the inferior ridge should caution of ethanol infusion inside a wrong vein and prompt for a reappraisal of the initial venous angiography. Together, the increasing success rate over the years along with a good success rate (83.3%) of repeat VOM ethanol infusion after a previous failure suggest that this procedure improves with experience.
Of interest, previous CS ablation was the only predictor for procedure failure, probably due to lesion-induced VOM stenosis. This advocates the need for integrating VOM ethanol infusion at an early stage of the ablation strategy, if necessary, to increase the chances of success.
Pitfalls
Regarding lesion extent, a restriction of either the spatial distribution or the distributed amount of ethanol was found to have the potential to limit its penetration into the tissue. VOM staining proved to be an innocuous event inherent to progressive tissue penetration. Anatomic drainage—due to anastomosis variants—did not hinder the large distribution of a sufficient amount of ethanol. By contrast, 3 pitfalls were significantly associated with smaller VOM-related endocardial scarring: (1) no branches morphology; (2) VOM dissection, and (3) mechanical leakage. The absence of branches was not a modifiable factor and was previously identified by Valderrábano et al. as a cause of limited scarring.9 The remaining 2 pitfalls were significantly reduced with growing experience. VOM dissection was circumvented by not overinflating the balloon or pushing the wire too far inside the lumen. Mechanical leakage—especially in the large trunk morphology—could be corrected either by the distal repositioning of the balloon where the vessel diameter seemed more suitable, or by using a larger balloon (diameter, 2.5–3 mm) maintained at the proximal portion of the VOM. Of note, the poor success rate (14.6%) of repeat VOM ethanol infusion after previous success at the first attempt proves that it must be seen as a single-shot procedure. Therefore, even when VOM dissection occurs, it is preferable to carry on with the ethanol infusion.
Regarding electrophysiological effect, none of the above pitfalls significantly altered the rate of mitral line block; on the contrary, ethanol infusion inside a wrong vein did. This emphasizes that the location—beyond the amount or the extent—of ethanol into the tissue is a crucial determinant. This may reflect the preferential location of the VOM bundle at the ridge. Its epicardial course is both protected against endocardial radiofrequency by fat and not directly accessible by conventional means. A suspended and upper endocardial scarring, as found in LAA vein ethanol infusion, will not help eliminate it and will thus make mitral line block still difficult to achieve.
Complications
In total, serious complications occurred in 14 patients (2.0%), which is the usual range previously reported (Figure 5).14 Cardiac tamponade, which had been previously described, occurred in 7 patients (1.0%).15 Since the majority of patients had a delayed onset and a serous nature, an inflammatory reaction was considered the most frequent underlying mechanism. The higher rate of cardiac tamponade observed in patients with VOM perforation evokes a causal relationship between the inflammatory reaction and the inadvertent drainage of ethanol in the pericardial space. Thus, anti-inflammatory drugs and a close follow-up with repeated echocardiography should be proposed after ethanol infusion inside a perforated VOM.
Of importance, 3 patients (0.4%) experienced a serious complication, previously undescribed in the setting of VOM ethanol infusion. An anaphylactic shock occurred and could have been lethal if it had not been diagnosed and treated immediately. Therefore, anaphylaxis should in priority be evocated in case of hemodynamic collapse. High-degree atrioventricular block occurred in a patient with a very proximal VOM. We hypothesized that a septal branch (not visible) probably damaged the conduction system. A more distal balloon position might be advised in such proximal VOM, to limit the risk of impacting the septum. Of note, the patient had previously undergone an aortic valve replacement surgery that may have fragilized nodo-Hissian conduction. However, careful monitoring of atrioventricular conduction during ethanol infusion should be the rule in all cases. LAA isolation occurred in a patient with multiple procedures based on repeated electrical substrate ablation at the anteroseptal wall. If extensive scarring is suspected, careful LA mapping should be performed before VOM ethanol infusion, to confirm that conduction to the LAA is still effective via the Bachmann bundle.
Limitations
The present work was an observational single-center study, conducted in a high-volume institution that performs over 200 VOM ethanol infusions per year. The rate of success and complication can be affected by the time invested in the procedure, the number of venograms attempted, and the operator’s experience. Although the reported results improved over time, VOM ethanol infusion rapidly proves effective.
Conclusions
VOM ethanol infusion is highly feasible, with an excellent success rate after the first attempt. VOM dissection, mechanical drainage, and the no branches morphology are the only pitfalls limiting the expected effect. Serious complications are rare, and the majority were due to delayed cardiac tamponade related to an inflammatory reaction. The rate of success and dissection progressively improves with experience.
Sources of Funding
This work was supported by the Mochida Memorial Foundation for Medical and Pharmaceutical Research, a grant from the SENSHIN Medical Research Foundation, and the Agence Nationale de la Recherche (grant no. IHU LIRYC ANR-10-IAHU-04).
Disclosures
Drs Haïssaguerre, Hocini, Jais, Derval, and Pambrun have received lecture fees from Biosense Webster. Dr Jais received consulting fees from Boston Scientific. Dr Sacher has received lecture fees from Biosense Webster, Abbott, and Medtronic. The other authors report no conflicts.
Supplemental Material
Supplemental Tables I–II
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AF
- atrial fibrillation
- CS
- coronary sinus
- LA
- left atrium
- LAA
- left atrial appendage
- VOM
- vein of Marshall
- VV
- valve of Vieussens
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCEP.121.010001.
For Sources of Funding and Disclosures, see page 727.
Contributor Information
Tsukasa Kamakura, Email: tkamakura@live.jp.
Nicolas Derval, Email: dervalnicolas@gmail.com.
Josselin Duchateau, Email: josselin.duchateau@chu-bordeaux.fr.
Arnaud Denis, Email: arnaud.denis@lilo.org.
Takashi Nakashima, Email: takashin727jw@yahoo.co.jp.
Takamitsu Takagi, Email: takamitsu.tkg@gmail.com.
F. Daniel Ramirez, Email: dramirez@ottawaheart.ca.
Clémentine André, Email: clementine2andre@gmail.com.
Philipp Krisai, Email: philipp.krisai@usb.ch.
Yosuke Nakatani, Email: yosuke3gbst@yahoo.co.jp.
Romain Tixier, Email: romain.tixier@chu-bordeaux.fr.
Rémi Chauvel, Email: remi.chauvel@chu-bordeaux.fr.
Ghassen Cheniti, Email: ghassen.chniti@gmail.com.
Kengo Kusano, Email: kusanokengo@hotmail.com.
Hubert Cochet, Email: hubert.cochet@chu-bordeaux.fr.
Frédéric Sacher, Email: frederic.sacher@chu-bordeaux.fr.
Mélèze Hocini, Email: meleze.hocini@chu-bordeaux.fr.
Pierre Jaïs, Email: pierre.jais@chu-bordeaux.fr.
Michel Haïssaguerre, Email: michel.haissaguerre@chu-bordeaux.fr.
References
- 1.Morita H, Zipes DP, Morita ST, Wu J. The role of coronary sinus musculature in the induction of atrial fibrillation. Heart Rhythm. 2012;9:581–589. doi: 10.1016/j.hrthm.2011.11.041 [DOI] [PubMed] [Google Scholar]
- 2.Vlachos K, Denis A, Takigawa M, Kitamura T, Martin CA, Frontera A, Martin R, Bazoukis G, Bourier F, Cheniti G, et al. The role of Marshall bundle epicardial connections in atrial tachycardias after atrial fibrillation ablation. Heart Rhythm. 2019;16:1341–1347. doi: 10.1016/j.hrthm.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 3.Valderrábano M, Chen HR, Sidhu J, Rao L, Ling Y, Khoury DS. Retrograde ethanol infusion in the vein of Marshall: regional left atrial ablation, vagal denervation and feasibility in humans. Circ Arrhythm Electrophysiol. 2009;2:50–56. doi: 10.1161/CIRCEP.108.818427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashima T, Pambrun T, Vlachos K, Goujeau C, André C, Krisai P, Ramirez FD, Kamakura T, Takagi T, Nakatani Y, et al. Impact of Vein of Marshall ethanol infusion on mitral isthmus block: efficacy and durability. Circ Arrhythm Electrophysiol. 2020;13:e008884. doi: 10.1161/CIRCEP.120.008884 [DOI] [PubMed] [Google Scholar]
- 5.Valderrábano M, Peterson LE, Swarup V, Schurmann PA, Makkar A, Doshi RN, DeLurgio D, Athill CA, Ellenbogen KA, Natale A, et al. Effect of catheter ablation with vein of marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the VENUS randomized clinical trial. JAMA. 2020;324:1620–1628. doi: 10.1001/jama.2020.16195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pambrun T, Denis A, Duchateau J, Sacher F, Hocini M, Jaïs P, Haïssaguerre M, Derval N. MARSHALL bundles elimination, Pulmonary veins isolation and Lines completion for anatomical ablation of persistent atrial fibrillation: MARSHALL-PLAN case series. J Cardiovasc Electrophysiol. 2019;30:7–15. doi: 10.1111/jce.13797 [DOI] [PubMed] [Google Scholar]
- 7.Kitamura T, Vlachos K, Denis A, Andre C, Martin R, Pambrun T, Duchateau J, Frontera A, Takigawa M, Thompson N, et al. Ethanol infusion for Marshall bundle epicardial connections in Marshall bundle-related atrial tachycardias following atrial fibrillation ablation: the accessibility and success rate of ethanol infusion by using a femoral approach. J Cardiovasc Electrophysiol. 2019;30:1443–1451. doi: 10.1111/jce.14019 [DOI] [PubMed] [Google Scholar]
- 8.Derval N, Duchateau J, Denis A, Ramirez FD, Mahida S, André C, Krisai P, Nakatani Y, Kitamura T, Takigawa M, et al. Marshall bundle elimination, Pulmonary vein isolation, and Line completion for anatomical ablation of persistent atrial fibrillation (Marshall-PLAN): Prospective, single-center study. Heart Rhythm. 2021;18:529–537. doi: 10.1016/j.hrthm.2020.12.023 [DOI] [PubMed] [Google Scholar]
- 9.Valderrábano M, Morales PF, Rodríguez-Mañero M, Lloves C, Schurmann PA, Dave AS. The human left atrial venous circulation as a vascular route for atrial pharmacological therapies: effects of ethanol infusion. JACC Clin Electrophysiol. 2017;3:1020–1032. doi: 10.1016/j.jacep.2017.02.022 [DOI] [PubMed] [Google Scholar]
- 10.Tuan TC, Tai CT, Lin YK, Hsieh MH, Tsai CF, Ding YA, Chen SA. Use of fluoroscopic views for detecting Marshall’s vein in patients with cardiac arrhythmias. J Interv Card Electrophysiol. 2003;9:327–331. doi: 10.1023/a:1027435208576 [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira IM, Scanavacca MI, Correia AT, Sosa EA, Aiello VD. Anatomic relations of the Marshall vein: importance for catheterization of the coronary sinus in ablation procedures. Europace. 2007;9:915–919. doi: 10.1093/europace/eum175 [DOI] [PubMed] [Google Scholar]
- 12.von Lüdinghausen M, Ohmachi N, Besch S, Mettenleiter A. Atrial veins of the human heart. Clin Anat. 1995;8:169–189. doi: 10.1002/ca.980080302 [DOI] [PubMed] [Google Scholar]
- 13.von Ludinghausen M, Ohmachi N, Boot C. Myocardial coverage of the coronary sinus and related veins. Clin Anat. 1992;5:1–15 [Google Scholar]
- 14.Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts-Thomson KC, Brooks AG, Sanders P. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol. 2013;6:1082–1088. doi: 10.1161/CIRCEP.113.000768 [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Tanaka A, Morimoto SI, Hasegawa S, Ishiguro N, Kametani R, Hattori H, Shibata N. Potential complications in patients undergoing an ethanol injection into the vein of Marshall. J Cardiovasc Electrophysiol. 2019;30:2743–2750. doi: 10.1111/jce.14221 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.