Abstract
Mounting evidence has shown disrupted brain network architecture across the psychosis spectrum. However, whether these changes relate to the development of psychosis is unclear. Here, we used graph theoretical analysis to investigate longitudinal changes in resting-state brain networks in samples of 72 subjects at clinical high risk (including 8 cases who converted to full psychosis) and 48 healthy controls drawn from the North American Prodrome Longitudinal Study (NAPLS) consortium. We observed progressive reduction in global efficiency (P = 0.006) and increase in network diversity (P = 0.001) in converters compared with non-converters and controls. More refined analysis separating nodes into nine key brain networks demonstrated that these alterations were primarily driven by progressively diminished local efficiency in the default-mode network (P = 0.004) and progressively enhanced node diversity across all networks (P < 0.05). The change rates of network efficiency and network diversity were significantly correlated (P = 0.003), suggesting these changes may reflect shared neural mechanisms. In addition, change rates of global efficiency and node diversity were significantly correlated with change rate of cortical thinning in the prefrontal cortex in converters (P < 0.03) and could be predicted by visuospatial memory scores at baseline (P < 0.04). These results provide preliminary evidence for longitudinal reconfiguration of resting-state brain networks during psychosis development and suggest that decreased network efficiency, reflecting an increase in path length between nodes, and increased network diversity, reflecting a decrease in the consistency of functional network organization, may be implicated in the progression to full psychosis.
Keywords: Psychosis, Clinical high risk, Brain network, Resting state, Graph theory
1. Introduction
Substantial evidence has pointed to the disorganization of brain network architecture across psychotic disorders. The most consistent findings in patients include altered network connectivity (Baker et al., 2014; Cao et al., 2016; Lynall et al., 2010; Satterthwaite et al., 2015; Zhu et al., 2016), network efficiency (Leow et al., 2013; Liu et al., 2008; Lo et al., 2015; Lynall et al., 2010; Sheffield et al., 2017; Wang et al., 2012; Yan et al., 2015; Zhu et al., 2016) and network clustering (Leow et al., 2013; Liu et al., 2008; Lo et al., 2015; Lynall et al., 2010; Zhu et al., 2016), suggesting disrupted information integration and segregation of brain systems. Moreover, altered network properties have been found to be related to genetic risk for psychotic disorders (Lo et al., 2015; Yan et al., 2015), associated with severity of clinical symptoms (Wang et al., 2012; Zhu et al., 2016) and cognition (Lynall et al., 2010; Sheffield et al., 2017; Yan et al., 2015), and predictive of antipsychotic response (Crossley et al., 2017; Ganella et al., 2016). These lines of evidence suggest that changes in network integration and segregation may underlie the development of psychosis. However, whether these changes predict and potentially contribute to the onset of psychosis remains unclear.
Answering this question requires longitudinal observation of individuals at clinical high risk (CHR) prior to onset. Using this strategy, previous studies have identified progressive loss of gray matter in CHR subjects who converted to full psychosis compared with those who did not, involving regions that are critical to cognitive and social functioning, such as the dorsolateral and medial prefrontal cortex (Cannon et al., 2015; Pantelis et al., 2003; Sun et al., 2009), temporal cortex (Pantelis et al., 2003; Takahashi et al., 2009; Ziermans et al., 2012) and cingulate cortex (Pantelis et al., 2003; Ziermans et al., 2012). The progressive declines in gray matter volume and thickness are likely to be a result of excessive loss of neuropil (dendrites and synapses) during adolescence and early adulthood, which in turn, may lead to aberrant synaptic and neurotransmitter functioning that underlie abnormalities in brain connectivity and network configuration (Cannon, 2015). Thus far, a critical piece of information is still missing in this model that directly links longitudinal changes in functional brain connectivity to the development of psychosis.
In the present study, using the data from the second phase of the North American Prodrome Longitudinal Study (NAPLS-2) consortium (Addington et al., 2012), we report on preliminary results of longitudinal changes in resting-state brain network architecture related to the conversion to psychosis. Here, 72 subjects at CHR, including 8 individuals who converted to psychosis, and 48 demographically comparable healthy participants underwent functional magnetic resonance imaging (fMRI) scans at both baseline and follow-up. We have previously shown that brain network measures derived from fMRI data are highly reliable both across time (Cao et al., 2014) and across scanner (Cao et al., 2018), making them particularly suitable for the multisite longitudinal design as used here. We hypothesized that converters would show progressive alterations and higher change rates in functional network properties compared to non-converters and controls, in particular measures assessing network connectivity, network integration and network segregation.
2. Methods and materials
2.1. Subjects
A sample of 120 subjects (72 clinical high risk (CHR) individuals, 48 healthy controls (HC, age 19.95 ± 4.66 years, 28 males)) with available baseline and follow-up resting-state fMRI data was included in this study. During follow-up, 8 CHR subjects converted to psychosis (CHR-C, age 17.88 ± 4.39 years, 5 males), and 64 subjects did not convert (CHR-NC, age 19.55 ± 3.92 years, 39 males). The subjects were recruited as part of the NAPLS-2 consortium from eight study sites across the United States and Canada. The sample used in the present study was drawn from a larger dataset with baseline scans as previously reported (Anticevic et al., 2015) (435 subjects in total, 27 converters, 245 non-converters and 163 controls). Notably, no significant differences were found between the current sample and those with baseline data only in terms of demographic and clinical measures (Table 1, P > 0.33). The study protocol was reviewed and approved by the institutional review boards at each site. All participants provided written informed consent.
Table 1.
Characteristics of the studied sample.
| Converters (n = 8) | Non-converters (n = 64) | Controls (n = 48) | P value | |
|---|---|---|---|---|
| Demographic variables | ||||
| Age (years) | 17.88 ± 4.39 | 19.55 ± 3.92 | 19.95 ± 4.66 | 0.44 |
| Sex (M/F) | 5/3 | 39/25 | 28/20 | 0.91 |
| Education (years) | 10.88 ± 2.85 | 12.15 ± 2.55 | 13.02 ± 3.24 | 0.09 |
| IQ | 101.25 ± 15.71 | 110.27 ± 16.29 | 114.53 ± 14.20 | 0.08 |
| Clinical variables | ||||
| Baseline SOPS – positive | 12.38 ± 3.62 | 12.24 ± 4.20 | 1.51 ± 2.05 | <0.001 (overall) 0.92 (CHR-C vs CHR-NC) |
| Follow-up SOPS – positive | 18.00 ± 6.74 | 7.32 ± 4.90 | 0.81 ± 1.71 | <0.001 (overall) <0.001 (CHR-C vs CHR-NC) |
| Baseline SOPS – negative | 9.50 ± 5.12 | 12.24 ± 6.37 | 1.28 ± 1.85 | <0.001 (overall) 0.15 (CHR-C vs CHR-NC) |
| Follow-up SOPS – negative | 13.13 ± 6.38 | 8.98 ± 7.03 | 1.50 ± 2.23 | <0.001 (overall) 0.15 (CHR-C vs CHR-NC) |
| Baseline SOPS – disorganization | 8.00 ± 3.30 | 5.16 ± 3.30 | 0.68 ± 1.06 | <0.001 (overall) 0.01 (CHR-C vs CHR-NC) |
| Follow-up SOPS – disorganization | 9.75 ± 4.80 | 3.98 ± 3.37 | 0.56 ± 1.07 | <0.001 (overall) <0.001 (CHR-C vs CHR-NC) |
| Baseline SOPS – general | 7.50 ± 3.63 | 8.98 ± 4.37 | 1.53 ± 2.29 | <0.001 (overall) 0.28 (CHR-C vs CHR-NC) |
| Follow-up SOPS – general | 8.88 ± 4.79 | 6.16 ± 4.53 | 1.10 ± 1.80 | <0.001 (overall) 0.16 (CHR-C vs CHR-NC) |
| Baseline antipsychotics (% medicated) | 25 | 20 | 0 | <0.001 (overall) 0.40 (CHR-C vs CHR-NC) |
| Follow-up antipsychotics (% medicated) | 50 | 17 | 0 | <0.001 (overall) <0.001 (CHR-C vs CHR-NC) |
| Baseline antipsychotics (CPZ equivalent dosage) | 70.31 ± 131.26 | 44.89 ± 109.25 | 0 | <0.001 (overall) 0.55 (CHR-C vs CHR-NC) |
| Follow-up antipsychotics (CPZ equivalent dosage) | 50.00 ± 111.80 | 44.76 ± 120.96 | 0 | <0.001 (overall) 0.93 (CHR-C vs CHR-NC) |
| Cognitive variables | ||||
| Baseline BVMT-R | 19.00 ± 9.05 | 27.01 ± 5.80 | 27.66 ± 4.99 | 0.001 (overall) 0.001 (CHR-C vs CHR-NC) |
| Follow-up BVMT-R | 22.38 ± 6.82 | 27.11 ± 5.90 | 28.25 ± 4.13 | 0.02 (overall) 0.06 (CHR-C vs CHR-NC) |
| Baseline HVLT-R | 22.15 ± 7.08 | 27.34 ± 4.50 | 27.68 ± 4.11 | 0.006 (overall) 0.008 (CHR-C vs CHR-NC) |
| Follow-up HVLT-R | 23.63 ± 4.63 | 27.49 ± 4.73 | 28.77 ± 3.74 | 0.008 (overall) 0.06 (CHR-C vs CHR-NC) |
| Image variables | ||||
| Interscan interval (months) | 8.63 ± 4.41 | 13.19 ± 5.31 | 11.71 ± 3.31 | 0.02 (overall) 0.03 (CHR-C vs CHR-NC) |
| Interscan FD change | 0.10 ± 0.13 | 0.07 ± 0.07 | 0.05 ± 0.06 | 0.11 |
Abbreviations: SOPS = Scale of Prodromal Symptoms; CPZ = Chlorpromazine; BVMT-R = Brief Visuospatial Memory Test-revised; HVLT-R = Hopkins Verbal Learning Test-Revised; FD = frame-wise displacement.
CPZ equivalent doses were calculated according to Leucht et al. (2014).
The participants were evaluated using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID, First et al., 2002) and the Structured Interview for Prodromal Syndromes (SIPS, McGlashan et al., 2001) at each assessment point by clinicians. At each assessment point, prodromal symptom severity was quantified using the Scale of Prodromal Symptoms (SOPS, McGlashan et al., 2001). Memory and learning abilities were evaluated using the Brief Visuospatial Memory Test-Revised (BVMT-R, Benedict, 1997) and the Hopkins Verbal Learning Test-Revised (HVLT-R, Brandt and Benedict, 1998). See Table 1 and Supplementary materials for sample details.
2.2. Imaging paradigm and data acquisition
All participants underwent a 5-min eyes-open resting-state scan. See Supplementary materials for details on data acquisition.
2.3. Data processing
The entire processing pipeline followed that of previously published work (Cao et al., 2016; Cao et al., 2017; Cao et al., 2014). In brief, mean time series for each of the 90 nodes defined by the Automated Anatomical Labelling (AAL) atlas (Tzourio-Mazoyer et al., 2002) were extracted from the preprocessed data and further corrected for physiological and scanner noises. Pairwise Pearson correlation coefficients were calculated between the processed time series of each node, resulting in a 90 × 90 two-dimensional correlation matrix for each subject at each scan point (see Supplementary materials).
We quantified two connectivity metrics describing the characteristics of the derived correlation matrices: node strength and node diversity (Lynall et al., 2010). Node strength is the average connectivity between a given node and all other nodes in the network, reflecting how strongly the node is connected to others. Node diversity is the connectivity variance between a given node and all other nodes, reflecting how homogeneous the connectivity is for that node. These two metrics were then further averaged across the 90 nodes to generate a global measure.
To build weighted brain graphs, the derived correlation matrices were further thresholded into 31 densities ranging from 0.10 to 0.40 with an increment interval of 0.01. At each density, only the given proportion of edges ranked by connectivity strength were kept as true internode connections in the matrix, thereby generating a weighted adjacency matrix. This procedure ensured that the number of edges in the network was exactly the same across individuals at each threshold. Three graph metrics were subsequently computed assessing the integration and segregation of the derived weighted networks: global efficiency, transitivity and small-worldness. Global efficiency is a measure of network integration, defined as the average inverse of the shortest path length between all pairs of nodes in the network. Transitivity quantifies network segregation as the normalized global measure of network clustering. Small-worldness is an index assessing the combination of network segregation and network integration (computed from 100 randomizations). After computation, these measures were averaged across all densities to ensure that results were not biased by a single threshold.
As we have previously shown (Cao et al., 2018; Cao et al., 2014), all examined connectivity and graph metrics are highly reliable across scanners and sessions, making the investigation of their longitudinal changes feasible. Here, following our previous work (Cannon et al., 2015), we quantified the change rates for each of the examined measures for each subject. Change rate was defined as
where M(FU) and M(BL) are network measures at follow-up and at baseline, respectively, and T is the time interval between the two scans (in month). As a result, change rate reflects the percentage of change for a given measure per month. This approach was preferred to repeated-measures analysis of variance (ANOVA) because the interscan interval varied across subjects.
2.4. Statistical analysis
We employed an analysis of covariance (ANCOVA) model to test the differences in the examined metrics between the three groups at both baseline and follow-up. Here, the five baseline connectivity and graph metrics were entered as dependent variables in the baseline analysis, and their change rates were entered as dependent variables in the follow-up analysis. Group was given as independent variable, and age, sex, site, frame-wise displacement (for baseline analysis) and interscan change in frame-wise displacement (for follow-up analysis) were included as covariates. Significance was set at two-tailed P < 0.05 after false-discovery rate (FDR) correction for multiple comparisons of the five metrics.
All metrics with significant group differences were then examined for potential associations with structural, clinical and cognitive variables using Pearson correlations. Specifically, according to the psychosis onset model described previously, if progressive loss of gray matter drives changes in brain connectivity and brain networks, the change rates of cortical thickness and network measures should be highly correlated. We tested this hypothesis using data on cortical thickness for bilateral prefrontal cortex as previously described (see Cannon et al., 2015 for details), whose change rates were calculated using the same formula as above. We also probed potential associations of change rates of functional network measures with clinical symptoms and memory ability at baseline, as these baseline variables have previously been shown as predictive of conversion to psychosis (Cannon et al., 2016; Seidman et al., 2016). Clinical symptoms were quantified by the summed scores of each domain in the SOPS (positive, negative, disorganization, general), visuospatial and verbal memory abilities were assessed using the total recall score in the BVMT-R and the total recall score in the HVLT-R, respectively.
3. Results
3.1. Group differences at baseline
At baseline, there were no significant differences between converters, non-converters and controls for all examined metrics (PFDR > 0.10) in the present sample with both baseline and follow-up scans (N = 120). The results remained non-significant when including all subjects with baseline scans (N = 435, PFDR > 0.79).
3.2. Group differences in change rates
We observed significant group differences in change rates of two examined metrics: global efficiency (PFDR = 0.006) and average node diversity (PFDR = 0.001). Post-hoc analyses showed that the change rate in global efficiency was significantly different between converters and controls (P = 0.004, Hedge’s g = 1.12), and approaching significance in the contrast between converters and non-converters (P = 0.052, Hedge’s g = 1.05). Here, unlike the groups of controls and non-converters that had positive mean change rates, as a group converters had a negative change rate (Fig. 1A). Moreover, post-hoc analyses for average node diversity showed that converters had significantly larger positive change rates than both non-converters (P = 0.001, Hedge’s g = 1.27) and controls (P < 0.001, Hedge’s g = 1.28, Fig. 1B), suggesting that conversion to psychosis may be associated with a progressive decrease in global efficiency but also a progressive increase in node diversity, reflecting less integration and consistency in functional network organization.
Fig. 1.
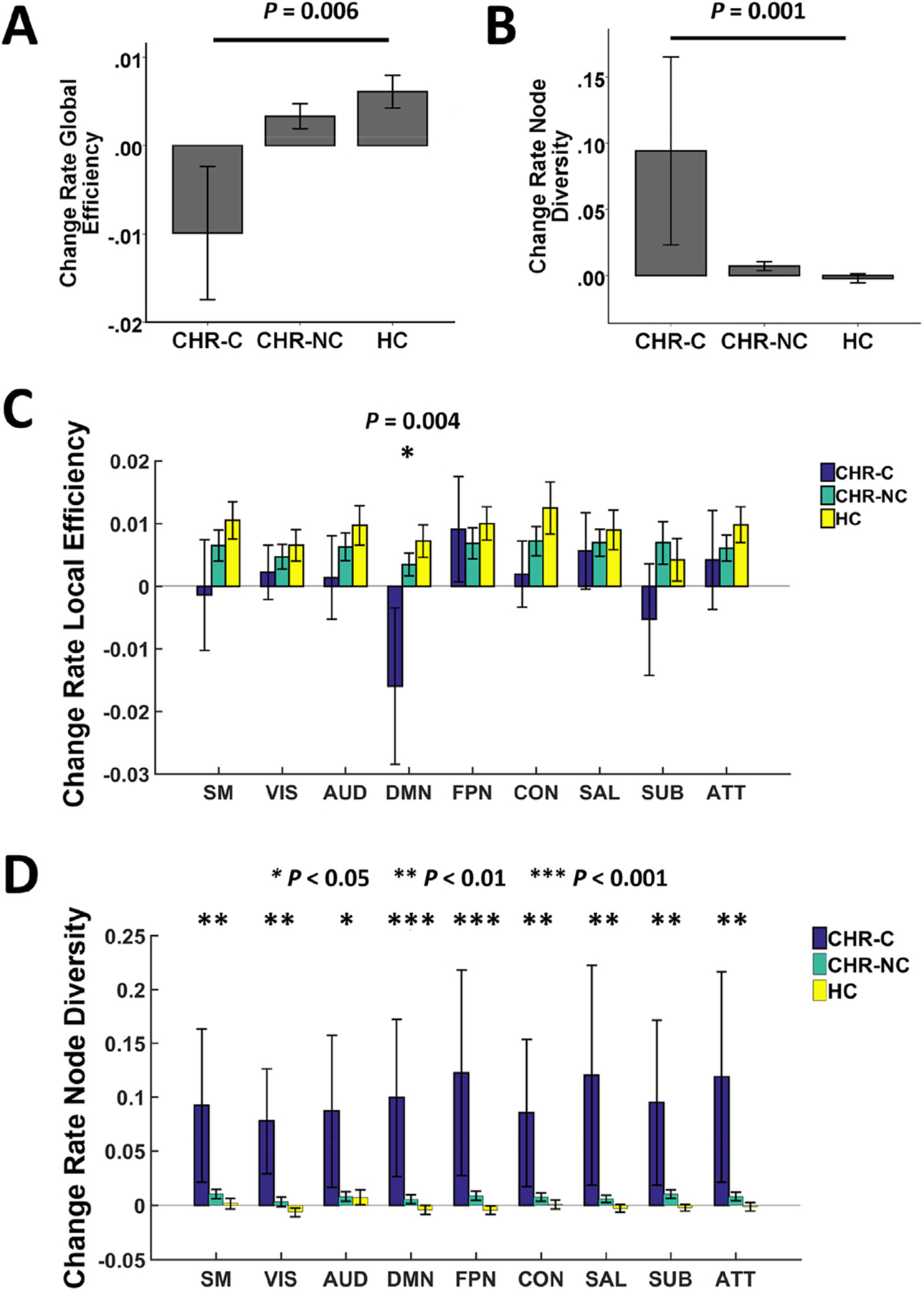
Association between change rates of network measures and conversion to psychosis. Converters showed progressively reduced global efficiency compared with non-converters and controls (Panel A), which was primarily driven by changes of local efficiency in the default-mode network (Panel C). In contrast, the progressive increase in node diversity observed in converters (Panel B) was distributed across the whole brain (Panel D). CHR-C = converters; CHR-NC = non-converters; HC = healthy controls; SM = sensorimotor network; VIS = visual network; AUD = auditory network; DMN = default-mode network; FPN = frontoparietal network; CON = cingulo-opercular network; SAL = salience network; SUB = subcortical network; ATT = attention network. The overall effects remained significant when excluding an outlier in the converter group.
To further ensure that our finding on network efficiency was not driven by a single threshold during network construction, we compared the change rates of global efficiency between groups for each of the 31 densities used in the study (from 0.10 to 0.40). The results showed significant group effects on vast majority of densities (27 out of 31 densities, P < 0.048, see Fig. S1), suggesting that the observed differences are not driven by any potential nuisance densities.
Given the results just summarized, we further investigated which brain systems were particularly involved in the differences between groups. For this, we calculated change rates of local efficiency and node diversity for each of nine well-established networks (Power et al., 2011): sensorimotor, visual, auditory, default-mode, frontoparietal, cingulo-opercular, salience, subcortical and attention. Here, each of the 90 nodes was assigned to one or more systems based on Power et al. (2011). Of note, Power’s study employed a different atlas with 264 nodes. As a result, some of the nodes in our study have been assigned to more than one network (in case different subregions of that node were allocated into different networks in Power’s study, see Table S2 for details). Change rates of local efficiency and node diversity were computed for each node and then averaged across nodes for each of the nine networks. The same ANCOVA model described above was used to test the group differences of the derived measures for each network, and significance was set at P < 0.05 after FDR correction for nine networks.
Our results revealed significant group differences in the change rate of local efficiency in the default-mode network (PFDR = 0.004, Fig. 1C). Similar to the pattern observed for global efficiency, on average converters showed a negative change rate in local efficiency in the default-mode network, compared with positive change rates among non-converters and controls. In contrast, no significant group differences were detected for other networks when controlling for multiple comparisons, suggesting that the progressive decrease of global efficiency was primarily driven by the local efficiency change in the default-mode network.
The analysis of change rate of node diversity demonstrated significant group differences for all of the nine examined networks (PFDR < 0.046, Fig. 1D). In particular, while non-converters and controls did not show significant changes in node diversity from baseline to follow-up (change rates approximately equaled to 0), converters showed significantly positive change rates for all nine networks, indicating that the progressive increase of node diversity in converters is not circumscribed but widely distributed across the whole brain.
3.3. Association between change rates
We then investigated whether the change rates of these two measures reflected independent phenomena or shared neural mechanisms. Our analysis revealed significant negative correlations between change rate of network efficiency and change rate of network diversity both in the whole sample (R = −0.57, P < 0.001) and in the converter group (R = −0.89, P = 0.003, Fig. 2), suggesting that these two functional alterations are highly dependent and possibly reflect shared underlying mechanisms.
Fig. 2.
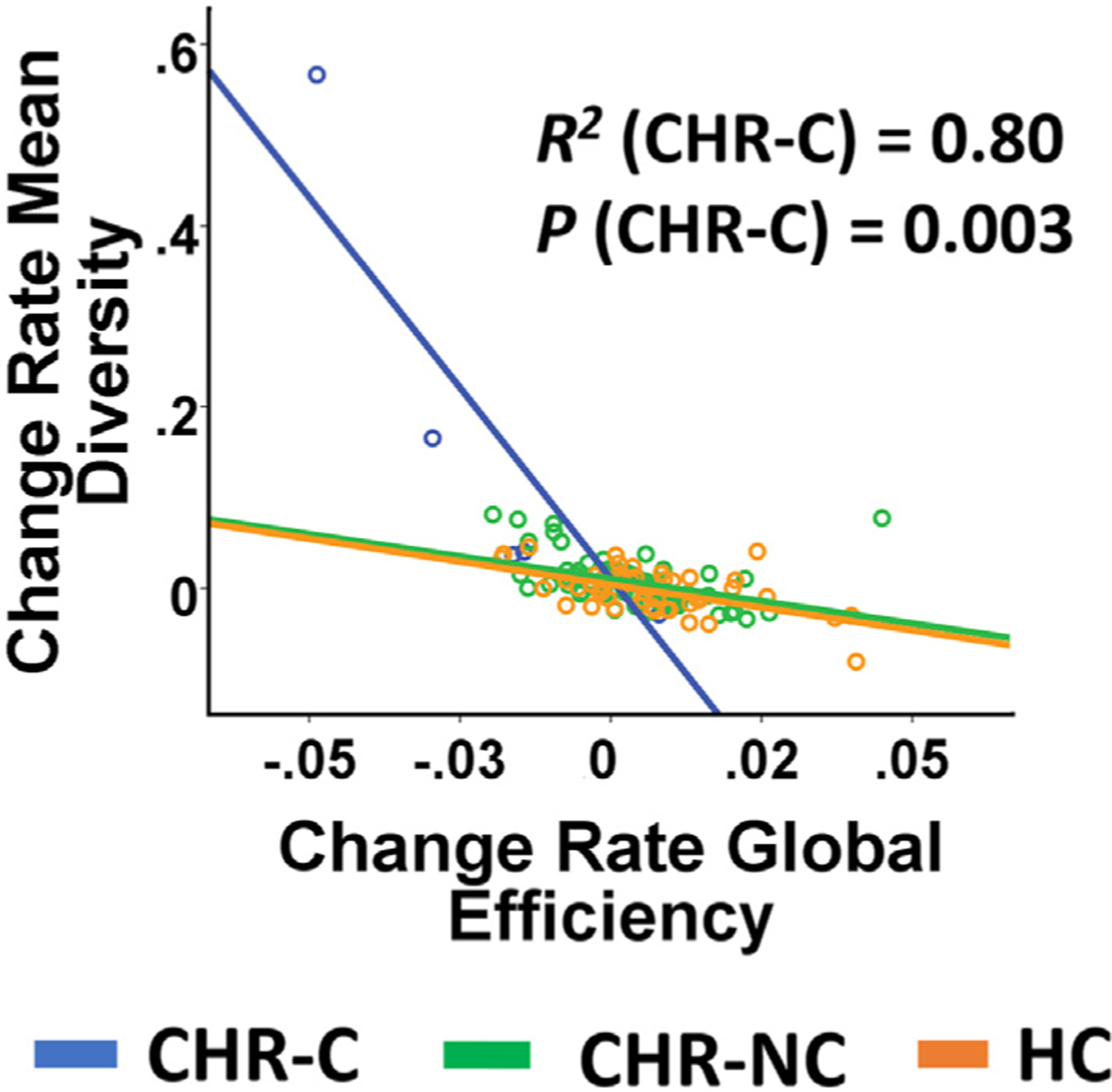
Association between change rate of network efficiency and change rate of network diversity stratified by outcome group. Two measures were significantly correlated with each other in converters and across all subjects. This association remained significant after excluding the outlier in the converter group (the subject at the top left of the graph).
3.4. Association with cortical thickness
Correlation analyses identified significant correlations between rate of cortical thinning in the prefrontal region and increasing mean node diversity over time (R = −0.28, P = 0.002 and R = −0.40, P < 0.001 for left and right hemisphere, respectively, Fig. 3A, B). Within the CHR-C group, change rates of both global efficiency and node diversity were significantly correlated with change rates of cortical thickness in both hemispheres (R2 > 0.56, P < 0.03 and R2 > 0.81, P < 0.003 for global efficiency and mean node diversity, respectively, Fig. 3A–D). In contrast, no significant correlations were found within the CHR-NC and HC groups.
Fig. 3.
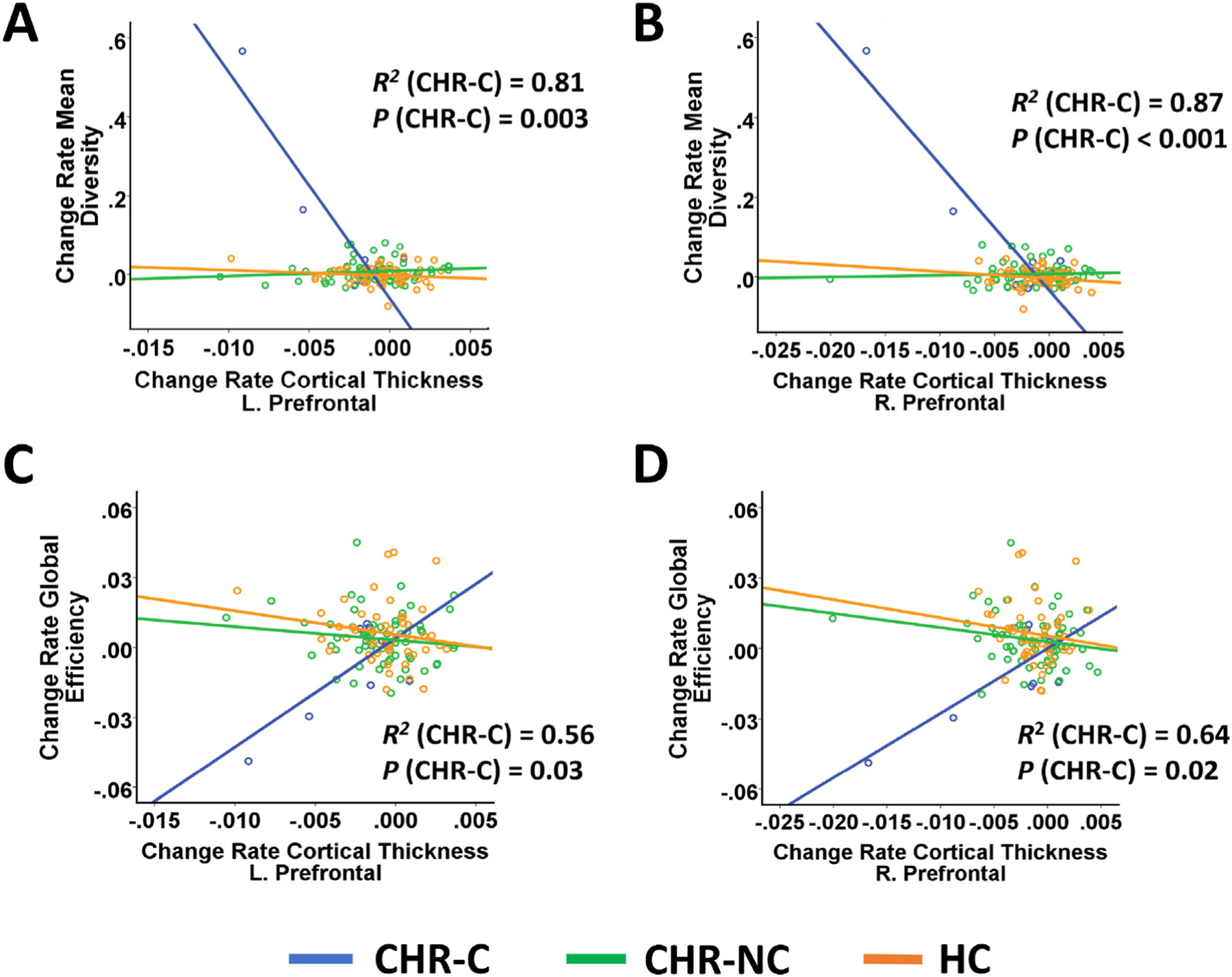
Association between change rates of network measures and change rates of cortical thickness in the prefrontal cortex stratified by outcome group. Change rates of both node diversity (Panel A & B) and global efficiency (Panel C & D) were significantly correlated with change rates of cortical thickness in bilateral prefrontal cortex in converters. However, these associations became non-significant after excluding the outlier in the converter group.
3.5. Associations with clinical symptoms and memory ability
In the whole sample, a significant positive correlation was found between change rate of mean node diversity and baseline disorganization symptoms (R = 0.21, P = 0.02). Within the group of CHR-C, trend-level negative correlations were observed between change rates of both metrics and baseline negative symptoms, with relatively large effect sizes (all R2 > 0.23, Fig. S2).
Change rates of both metrics were significantly correlated with BVMT-R total recall scores at baseline (R = 0.18, P = 0.04 for global efficiency and R = −0.37, P < 0.001 for mean node diversity, Fig. 4A, B). The correlations were also significant when analyzed within the CHR-C group (R2 = 0.52, P = 0.04 for global efficiency and R2 = 0.64, P = 0.02 for mean node diversity) but not within the CHR-NC and HC groups. These findings suggest that baseline memory ability is predictive of change rates of resting-state network measures in converters.
Fig. 4.
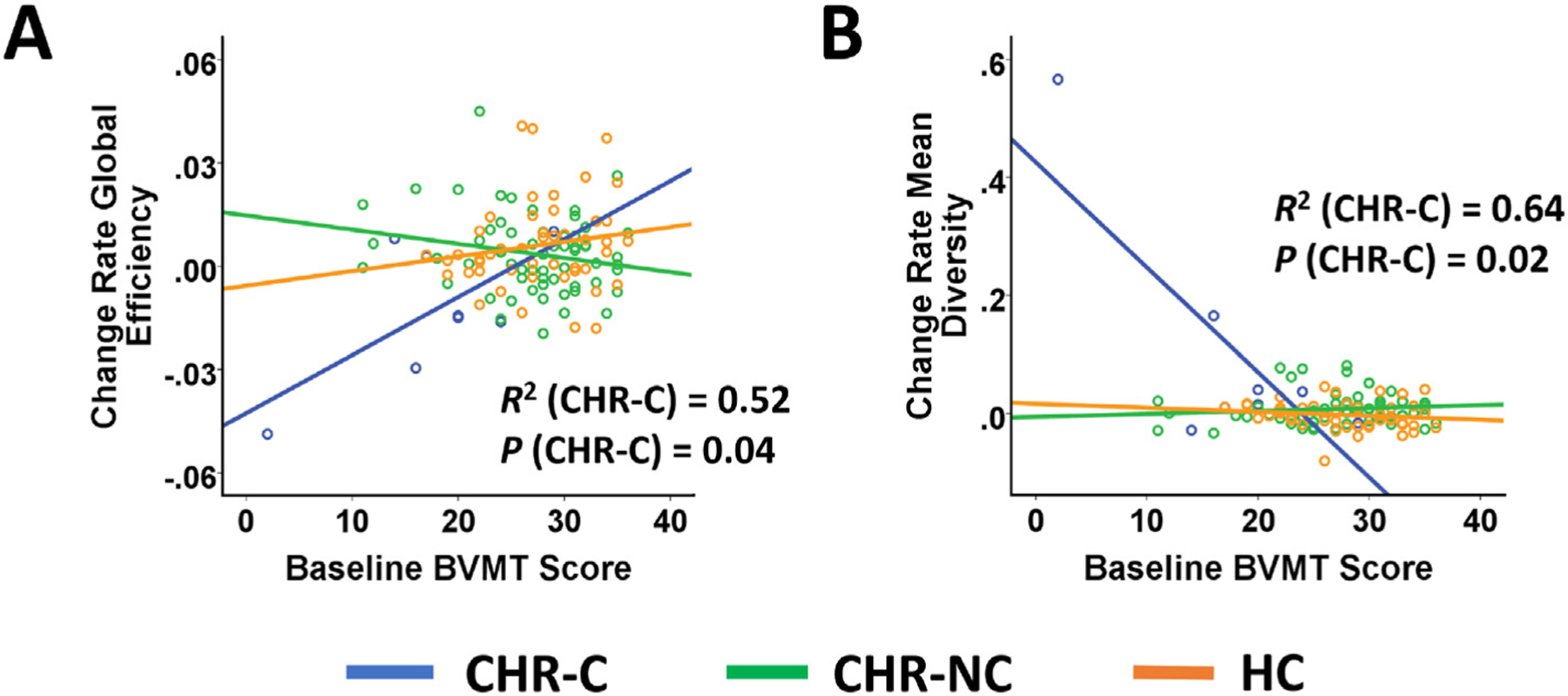
Association between change rates of network measures and baseline visuospatial memory ability as quantified by the Brief Visuospatial Memory Test (BVMT) total recall scores. Baseline BVMT scores were significantly correlated with change rates of global efficiency and node diversity in converters. However, these associations became non-significant after excluding the outlier in the converter group.
3.6. Results after excluding outlier in the converter group
Given the small sample size of this study, the reported findings may be vulnerable to outliers in our sample. To address this issue, we repeated the entire statistical process after excluding potential outliers in each group. Here, an outlier was defined as a subject whose measurements are larger than two standard deviations from the group mean. This resulted in removal of one subject from the converter group. After excluding this subject from the analyses, we still observed significant group effects of change rates of network efficiency and network diversity at P < 0.05 level (Puncorrected = 0.04 for both measures), although these findings cannot survive multiple correction for all five examined properties. Specifically, similar to results summarized above, converters showed decreased change rate of global efficiency (P = 0.03, Hedge’s g = 0.82) and increased change rate of network diversity (P = 0.01, Hedge’s g = 0.96) compared with controls. The change rates of both measures were significantly correlated in converters (R = −0.92, P = 0.003) and in the whole sample (R = −0.54, P < 0.001). However, the associations of these measures with cortical thinning rate and baseline memory scores were no longer significant with this outlier excluded. Summary of results after outlier exclusion was presented in Table S1.
4. Discussion
Using resting-state fMRI, this multisite longitudinal study found that CHR subjects who converted to psychosis showed a progressive decrease in global efficiency and increase in network diversity from baseline to the point of conversion. These effects were primarily driven by progressive changes in local efficiency in the default-mode network (DMN) and changes in node diversity across the whole brain. Moreover, the identified alterations were correlated with each other and with progressive gray matter changes in the prefrontal cortex in converters, and could be predicted by subjects’ memory scores at baseline. These results provide preliminary evidence for functional network reorganization during the progression from a prodromal to fully psychotic state.
Deficits in network efficiency are among the most consistent findings in schizophrenia and other psychotic disorders and may serve as a transdiagnostic biomarker for the psychosis spectrum (Sheffield et al., 2017). Such deficiency has been reported in functional networks during resting state (Liu et al., 2008; Sheffield et al., 2017; Zhu et al., 2016) and active tasks (Wang et al., 2010; Yu et al., 2011), as well as in structural networks constructed from diffusion tensor imaging (DTI) (Wang et al., 2012; Yan et al., 2015), and are associated with severity of psychotic symptoms (Wang et al., 2012; Zhu et al., 2016) and cognitive ability (Sheffield et al., 2017; Yan et al., 2015), suggesting a robust network-based biological trait underlying psychosis. In line with these findings, our results further showed a progressive decrease of resting-state network efficiency in converters and correlations between this change and gray matter loss in the prefrontal cortex. These results suggest that increasingly diminished network integration is implicated in the development of psychosis, which may be explained, at least in part, by changes in gray matter structure. Moreover, several lines of evidence have further suggested that declines in network efficiency may relate to aberrant synaptic and neurotransmitter functioning. This interpretation is supported by the fact that the administration of a N-methyl-D-aspartic acid (NMDA) receptor antagonist can induce chronic disruption of brain global efficiency in animals that resembles findings in patients with schizophrenia (Dawson et al., 2014), and that lower global efficiency at baseline is associated with worse response to antipsychotic medication at follow-up (Crossley et al., 2017). Since glutamate receptor-mediated neural plasticity is pivotal to synaptic pruning (Bear and Malenka, 1994), which can be further regulated by dopamine signaling (Calabresi et al., 2007), these findings echo the prevailing model interpreting the onset of psychosis and further suggest that altered network efficiency may participate in a cascade of events from excessive synaptic elimination to brain dysconnectivity that underlies psychosis development.
Intriguingly, the reduced efficiency is primarily driven by changes in the DMN, a brain system that is activated during rest but deactivated during attention-demanding tasks (Buckner et al., 2008). A large body of work has shown that patients with psychosis have attenuated DMN deactivation during active tasks but enhanced within-DMN connections during resting state (Meyer-Lindenberg et al., 2001; Pomarol-Clotet et al., 2008; Satterthwaite et al., 2015; Whitfield-Gabrieli et al., 2009), which may relate to exaggerated internally-focused thoughts and self-reference during rest and failure in suppression of these thoughts during task (Whitfield-Gabrieli and Ford, 2012). These abnormalities have also been shown in subjects both at CHR (Falkenberg et al., 2015) and at genetic high risk (Whitfield-Gabrieli et al., 2009), suggesting a neuro-biological trait that exists even before the onset of psychosis. Here, our results extend prior findings by showing a critical association between deficits in DMN efficiency and the development of psychosis. It has been argued that the DMN dysfunction may be a consequence of diminished top–down regulation by the frontoparietal cognitive control network (Satterthwaite et al., 2015; Whitfield-Gabrieli and Ford, 2012), a neural mechanism that may as well lead to changes in DMN efficiency.
This study also found that conversion to psychosis is associated with a progressive increase in node diversity across all systems in the brain, which is negatively associated with change rate of cortical thickness in the prefrontal cortex and global efficiency. These results are in parallel with prior work revealing increased node diversity in patients with schizophrenia (Lynall et al., 2010), suggesting that the overall connectivity pattern becomes increasingly unstable and heterogeneous as psychosis develops, a phenomenon that may share the same underlying neural mechanisms with changes in cortical thickness and network efficiency. While such alteration was originally detected across all examined networks in the brain, the more stringent analysis after excluding the outlier showed that this change mainly occurred within the frontoparietal, default-mode, salience, subcortical and visual networks (Table S1). Since these networks are critical for high-order cognitive functioning in humans (Seeley et al., 2007; Spreng et al., 2010; Zanto and Gazzaley, 2013) and have been consistently reported to be implicated in schizophrenia (Baker et al., 2014; Cole et al., 2014; White et al., 2010; Whitfield-Gabrieli et al., 2009), the increased heterogeneity in cognitive networks may relate to disability of maintaining coherent and logical thoughts and difficulty in sustaining goal-directed attention in psychotic patients.
Several limitations of this study need to be clearly acknowledged. First, given the very small sample size of converters, the results reported in this paper must be considered preliminary, but merit further replication tests in larger cohorts. In particular, as we sought to exclude potential outliers in the sample, this in turn, decreased the sample size and further reduced statistical power. Despite the fact that some of the findings became no longer significant after outlier exclusion, they did, however, still show a relatively large effect size, suggesting that the results reported in this study are not simply driven by a single outlier. Second, the results reported in this study may to certain degree influenced by the preselected brain atlas. In particular, in a supplementary analysis we tested the robustness of the findings using a different node definition based on the Power atlas (Power et al., 2011). This analysis showed that while the reported effects for differential change in network diversity were robust across both atlases, the change rate of network efficiency was no longer significant when using Power atlas (see Supplementary materials), suggesting that this finding may depend on the coarseness of brain atlas when sample size is small, or may be compromised by the increase of total number of nodes in the network. Third, the follow-up scans for converters were acquired after the point of conversion. As a consequence, our study cannot be interpreted as isolating changes that occur prior to onset of psychosis. However, given the fact that progressive changes in network measures are associated with baseline cognitive ability, these changes are unlikely to be a secondary phenomenon. Fourth, medication effects may confound our findings and cannot be ruled out from our sample. Although examination of network measures separately by medication status cannot be done given the extremely small number of converters on medication, the reported results are unlikely to be solely caused by medications considering that converters and non-converters did not show significant differences in antipsychotic dosages at either baseline or follow-up. Fifth, while we have argued that some of the networks might particularly drive the global effect, we note that inter-network connectivity may contribute to such global changes to a certain degree as well. Sixth, while the primary analysis used “normalized change rates” to account for the different inter-scan intervals across subjects, we also ran the analysis on the absolute change in connectivity between baseline and follow-up. The results still showed a significant group effect on network diversity; however, the effect for network efficiency became no longer significant (see Supplementary materials), suggesting that changes in network efficiency may be more time dependent.
To sum up, individuals at CHR who converted to psychosis show progressive decreases in network efficiency and increases in network diversity. These findings provide preliminary evidence for longitudinal reconfiguration of resting-state brain networks during the development of psychosis. Further work is encouraged to replicate these findings in larger samples and to investigate the predictive power of these findings for psychosis in independent cohorts.
Supplementary Material
Acknowledgements
This work was supported by the Brain and Behavior Research Foundation and NARSAD Young Investigator Grant (No. 27068) to Dr. Cao, by National Institutes of Health (NIH) grants U01 MH081902 to Dr. Cannon, P50 MH066286 to Dr. Bearden, U01 MH081857 to Dr. Cornblatt, U01 MH82022 to Dr. Woods, U01 MH066134 to Dr. Addington, U01 MH081944 to Dr. Cadenhead, R01 U01 MH066069 to Dr. Perkins, R01 MH076989 to Dr. Mathalon, U01 MH081928 to Dr. Seidman, and U01 MH081988 to Dr. Walker.
Role of funding source
The funding body plays no role in the entire work.
Footnotes
Note: Larry Seidman passed away tragically before submission of this manuscript. His colleagues wish to honor his contributions to the work posthumously.
Conflicts of interest
Dr. Cannon has served as a consultant for Boehringer-Ingelheim Pharmaceuticals and Lundbeck A/S. The other authors report no conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.schres.2019.01.017.
References
- Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Addington JA, Cannon TD, 2012. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr. Res 142 (1–3), 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Olvet D, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Tsuang MT, van Erp TG, Walker EF, Hamann S, Woods SW, Qiu M, Cannon TD, 2015. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiat 72 (9), 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, Ongur D, 2014. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiat 71 (2), 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC, 1994. Synaptic plasticity: LTP and LTD. Curr. Opin. Neurobiol 4 (3), 389–399. [DOI] [PubMed] [Google Scholar]
- Benedict R, 1997. Brief Visuospatial Memory Test - Revised Psychological Assessment Resource, Odessa. [Google Scholar]
- Brandt J, Benedict R, 1998. Hopkins Verbal Learning Test-Revised (HVLT-R) Psychological Assessment Resources, Odessa. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL, 2008. The brain’s default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M, 2007. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci 30 (5), 211–219. [DOI] [PubMed] [Google Scholar]
- Cannon TD, 2015. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn. Sci 19 (12), 744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TG, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R, North American Prodrome Longitudinal Study C, 2015. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol. Psychiatry 77 (2), 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, Heinssen R, Jeffries CD, Mathalon DH, McGlashan TH, Perkins DO, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Kattan MW, 2016. An individualized risk calculator for research in prodromal psychosis. Am. J. Psychiatry 173 (10), 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Plichta MM, Schafer A, Haddad L, Grimm O, Schneider M, Esslinger C, Kirsch P, Meyer-Lindenberg A, Tost H, 2014. Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. NeuroImage 84, 888–900. [DOI] [PubMed] [Google Scholar]
- Cao H, Bertolino A, Walter H, Schneider M, Schafer A, Taurisano P, Blasi G, Haddad L, Grimm O, Otto K, Dixson L, Erk S, Mohnke S, Heinz A, Romanczuk-Seiferth N, Muhleisen TW, Mattheisen M, Witt SH, Cichon S, Noethen M, Rietschel M, Tost H, Meyer-Lindenberg A, 2016. Altered functional subnetwork during emotional face processing: a potential intermediate phenotype for schizophrenia. JAMA Psychiat 73 (6), 598–605. [DOI] [PubMed] [Google Scholar]
- Cao H, Harneit A, Walter H, Erk S, Braun U, Moessnang C, Geiger LS, Zang Z, Mohnke S, Heinz A, Romanczuk-Seiferth N, Muhleisen T, Mattheisen M, Witt SH, Cichon S, Nothen MM, Rietschel M, Meyer-Lindenberg A, Tost H, 2017. The 5-HTTLPR polymorphism affects network-based functional connectivity in the visual-limbic system in healthy adults. Neuropsychopharmacology 43 (2), 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, McEwen SC, Forsyth JK, Gee DG, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt BA, Carrion RE, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Thermenos H, Tsuang MT, van Erp TGM, Walker EF, Hamann S, Anticevic A, Woods SW, Cannon TD, 2018. Toward leveraging human connectomic data in large consortia: generalizability of fMRI-based brain graphs across sites, sessions, and paradigms. Cereb. Cortex 10.1093/cercor/bhy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Repovs G, Anticevic A, 2014. The frontoparietal control system: a central role in mental health. Neuroscientist 20 (6), 652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Marques TR, Taylor H, Chaddock C, Dell’Acqua F, Reinders AA, Mondelli V, DiForti M, Simmons A, David AS, Kapur S, Pariante CM, Murray RM, Dazzan P, 2017. Connectomic correlates of response to treatment in first-episode psychosis. Brain 140 (Pt 2), 487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson N, Xiao X, McDonald M, Higham DJ, Morris BJ, Pratt JA, 2014. Sustained NMDA receptor hypofunction induces compromised neural systems integration and schizophrenia-like alterations in functional brain networks. Cereb. Cortex 24 (2), 452–464. [DOI] [PubMed] [Google Scholar]
- Falkenberg I, Chaddock C, Murray RM, McDonald C, Modinos G, Bramon E, Walshe M, Broome M, McGuire P, Allen P, 2015. Failure to deactivate medial prefrontal cortex in people at high risk for psychosis. Eur. Psychiatry 30 (5), 633–640. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P).Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Ganella EP, Bartholomeusz CF, Seguin C, Whittle S, Bousman C, Phassouliotis C, Everall I, Pantelis C, Zalesky A, 2016. Functional brain networks in treatment-resistant schizophrenia. Schizophr. Res 184, 73–81. [DOI] [PubMed] [Google Scholar]
- Leow A, Ajilore O, Zhan L, Arienzo D, GadElkarim J, Zhang A, Moody T, Van Horn J, Feusner J, Kumar A, Thompson P, Altshuler L, 2013. Impaired inter-hemispheric integration in bipolar disorder revealed with brain network analyses. Biol. Psychiatry 73 (2), 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM, 2014. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr. Bull 40 (2), 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T, 2008. Disrupted small-world networks in schizophrenia. Brain 131 (Pt 4), 945–961. [DOI] [PubMed] [Google Scholar]
- Lo CY, Su TW, Huang CC, Hung CC, Chen WL, Lan TH, Lin CP, Bullmore ET, 2015. Randomization and resilience of brain functional networks as systems-level endophenotypes of schizophrenia. Proc. Natl. Acad. Sci. U. S. A 112 (29), 9123–9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E, 2010. Functional connectivity and brain networks in schizophrenia. J. Neurosci 30 (28), 9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L, 2001. Instrument for the assessment of prodromal symptoms and states. In: Miller T, Mednick SA, McGlashan TH, Libiger J, Johannessen JO (Eds.), Early Intervention in Psychotic Disorders Springer Netherlands, Dordrecht, pp. 135–149. [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF, 2001. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am. J. Psychiatry 158 (11), 1809–1817. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK, 2003. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 361 (9354), 281–288. [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, Cebamanos JM, McKenna PJ, 2008. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol. Med 38 (8), 1185–1193. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE, 2011. Functional network organization of the human brain. Neuron 72 (4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Vandekar SN, Wolf DH, Bassett DS, Ruparel K, Shehzad Z, Craddock RC, Shinohara RT, Moore TM, Gennatas ED, Jackson C, Roalf DR, Milham MP, Calkins ME, Hakonarson H, Gur RC, Gur RE, 2015. Connectome-wide network analysis of youth with Psychosis-Spectrum symptoms. Mol. Psychiatry 20 (12), 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27 (9), 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Shapiro DI, Stone WS, Woodberry KA, Ronzio A, Cornblatt BA, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Mathalon DH, McGlashan TH, Perkins DO, Tsuang MT, Walker EF, Woods SW, 2016. Association of neurocognition with transition to psychosis: baseline functioning in the second phase of the North American Prodrome Longitudinal Study. JAMA Psychiat 73 (12), 1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, Clementz BA, Lerman-Sinkoff DB, Hill SK, Barch DM, 2017. Transdiagnostic associations between functional brain network integrity and cognition. JAMA Psychiat 74 (6), 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL, 2010. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 53 (1), 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TGM, Thompson PM, Toga AW, Cannon TD, Pantelis C, 2009. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr. Res 108 (1–3), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C, 2009. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch. Gen. Psychiatry 66 (4), 366–376. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15 (1), 273–289. [DOI] [PubMed] [Google Scholar]
- Wang L, Metzak PD, Honer WG, Woodward TS, 2010. Impaired efficiency of functional networks underlying episodic memory-for-context in schizophrenia. J. Neurosci 30 (39), 13171–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Su TP, Zhou Y, Chou KH, Chen IY, Jiang T, Lin CP, 2012. Anatomical in-sights into disrupted small-world networks in schizophrenia. NeuroImage 59 (2), 1085–1093. [DOI] [PubMed] [Google Scholar]
- White TP, Joseph V, Francis ST, Liddle PF, 2010. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr. Res 123 (2–3), 105–115. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM, 2012. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ, 2009. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A 106 (4), 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Tian L, Wang Q, Zhao Q, Yue W, Yan J, Liu B, Zhang D, 2015. Compromised small-world efficiency of structural brain networks in schizophrenic patients and their unaffected parents. Neurosci. Bull 31 (3), 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Sui J, Rachakonda S, He H, Pearlson G, Calhoun VD, 2011. Altered small-world brain networks in temporal lobe in patients with schizophrenia performing an auditory oddball task. Front. Syst. Neurosci 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A, 2013. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn. Sci 17 (12), 602–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhuo C, Liu F, Qin W, Xu L, Yu C, 2016. Distinct disruptions of resting-state functional brain networks in familial and sporadic schizophrenia. Sci. Rep 6, 23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PC, Kahn RS, van Engeland H, Durston S, 2012. Progressive structural brain changes during development of psychosis. Schizophr. Bull 38 (3), 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


