Background
Prevention of complications to reduce morbidity and mortality, and improve patient satisfaction is of paramount importance to plastic surgeons. This study aimed to evaluate the predictive risk factors for complications and to validate a novel risk assessment model, using artificial intelligence.
METHODS
A retrospective review of esthetic surgery procedures performed by the author between 2015 and 2020 was conducted. The Pearson correlation test was used to analyze the risk factors and complications. Differences in the mean risk scores among the three risk groups were tested using one-way analysis of variance. Risk scoring was validated using a machine learning process with a support vector machine in a Google Colaboratory environment.
RESULTS
Of the 372 patients, 28 (7.5%) experienced complications. The Pearson correlation coefficients between the risk score and body mass index (BMI: 0.99), age (0.97), and Caprini score of 5 or more (0.98) were statistically significant (P < 0.01). The correlations between the risk scores and sex (−0.16, P = 0.58), smoking habit (−0.16, P = 0.58), or combined procedures (−0.16, P = 0.58) were not significant. Necrosis was significantly correlated with dehiscence (0.92, P = 0.003) and seroma (0.77, P = 0.041). The accuracy of the predictive model was 100% for the training sample and 97.3% for the test sample.
CONCLUSIONS
Body mass index, age, and the Caprini score were risk factors for complications following esthetic surgery. The proposed risk assessment system is a valid tool for improving eligibility and preventing complications.
INTRODUCTION
Although several studies have identified risk factors associated with complications in cosmetic surgery, research on predicting the risk of complications to systematically reduce their occurrence and improve patient safety is limited.1–4 As complications could also lead to increased medical costs related to reoperations and decrease patient satisfaction, reducing the risk of complications and the associated costs is the primary goal of plastic surgeons.5–7 Therefore, the current challenge is to accurately predict the risk of the most frequent complications to reduce morbidity and mortality, improve eligibility, and provide recommendations to reduce the patient’s risk before surgery.
To my knowledge, to date, a real-time risk-assessment system assisted by artificial intelligence (AI) to stratify and predict the risk of complications in esthetic surgeries has not been reported. This study presents an automated risk-assessment system to evaluate the risk of complications after esthetic surgery. The purpose of this study was to evaluate the predictive risk factors for complications and to validate the novel risk-assessment system using machine learning.
PATIENTS AND METHODS
A retrospective analysis was conducted involving 372 patients operated on by the author between 2015 and 2020 to ensure consistency of results for internal validation. The Pearson correlation test was used for analysis of risk factors and complications. The differences in mean risk scores among the three risk groups were evaluated using one-way analysis of variance.
Method Search Strategy
The 19 most frequent plastic surgery procedures, which represented 98% of the cosmetic surgeries reported in 2019 according to the International Society of Aesthetic Plastic Surgery statistics (n = 10.607.227), were selected. The most frequent surgeries included breast augmentation, liposuction, blepharoplasty, abdominoplasty, rhinoplasty, breast lift, fat grafting (face), breast reduction, facelift, buttock augmentation (implants and fat transfer), gynecomastia surgery, ear surgery, neck lift, brow lift, upper arm lift, labiaplasty, facial bone contouring, thigh lift, lower body lift, and buttock lift (Table 1).8
Table 1.
Types of Procedures and Relative Percentages Based on the Total Number of Cases (n = 10,607,227) Reported by ISAPS8
| Type of Procedure | Percentage of Total Cases (%) |
|---|---|
| Breast augmentation | 17.6 |
| Liposuction | 16.3 |
| Eyelid surgery | 10.4 |
| Abdominoplasty | 8.4 |
| Rhinoplasty | 6.9 |
| Breast lift | 6.7 |
| Fat grafting (face) | 5.1 |
| Breast reduction | 5.0 |
| Facelift | 3.8 |
| Buttock augmentation (implants and fat transfer) | 3.3 |
| Gynecomastia | 2.5 |
| Ear surgery | 2.5 |
| Neck lift | 2.1 |
| Brow lift | 2.1 |
| Upper arm lift | 1.3 |
| Labiaplasty (excluding vaginal rejuvenation) | 1.3 |
| Facial bone contouring | 0.9 |
| Thigh lift | 0.8 |
| Lower body lift | 0.8 |
| Buttock lift | 0.4 |
Inclusion and Exclusion Criteria
Independent risk factors and complications following the above-listed procedures were searched in the PubMed and Google Scholar databases. For the analysis of risk factors and the relative risk (RR) of complications after esthetic surgeries, systematic reviews and meta-analyses, multicentric analyses, and evidence-based research studies classified as type 1 and 2, with more than 1000 cases within the last five years, were included. Case reports, small case series, and evidence-based research studies classified as type 4 or 5 were excluded.
The 12 identified papers that fulfilled the inclusion criteria and exhibited a statistically significant (P < 0.05) positive correlation between risk factors and complications are summarized in Table 2. The incidence of independent factors for each complication was analyzed, and a RR indicator or weighted coefficient (f) was assigned to each risk factor to stratify the risk of complications. Subsequently, a weight addition method with relevant risk factors (w) was used to create a risk scale from 1 to 2.5. Minimal risk was assigned the number 1 to obtain a minimum multiplication factor because every surgery involves some risk that is different from 0. Risk scoring is a weighted sum method assisted by a narrow AI, mathematically expressed by the following formula:
Table 2.
Summary of Evidence for Statistically Significant (P < 0.05) Positive Correlations between Risk Factors and Complications
| Reference | Summary | Diabetes | Smoking Habit | Age ≥ 40 Years | BMI | Combined Procedures | Male Gender |
|---|---|---|---|---|---|---|---|
| Winocour et al7 | A prospective cohort of 129,007 patients over a 5-year period using data from the CosmetAssure database | VTE (RR 1.02) | VTE (RR 1.06), major complications (RR 1.21) | VTE (RR 2.4) | |||
| Theocharidis et al9 | A systematic review and meta-analysis of 53 studies | Cutaneous necrosis (OR 4.78, RR 0.83); DWH (OR 2.80, RR 0.74); major complications (OR 2.36, RR 0.70) | |||||
| Hillam et al10 | Data on 13,984 patients from the 2009 to 2014 American College of Surgeons National Surgical Quality Improvement Program | Any complication (RR 0.63) | |||||
| Keyes et al11 | A retrospective analysis of 6,388,744 patients over a 10-year period from the IBQAP database | VTE (OR 0.67) | VTE (OR 0.43) | ||||
| Kaoutzanis et al35 | A prospective cohort of patients who underwent liposuction between 2008 and 2013 from the CosmetAssure database | VTE (RR 1.02); major complications (RR 1.01) | VTE (RR 1.06); major complications (RR 1.05) | VTE (RR 5.65); major complications (RR 4.81); SSI (RR 2.41) | |||
| Kaoutzanis et al12 | Five-year prospective cohort review of 129,007 patients from the CosmetAssure database | Hematoma (RR 1.01) | Hematoma (RR 1.35) | ||||
| Layliev et al3 | Five-year prospective cohort review of 129,007 patients from the CosmetAssure database | Any complication (RR 2.05) | Hematoma (RR 3.38) | ||||
| Gupta et al15 | Five-year prospective cohort review of 73,608 cases from the CosmetAssure database | Major complications (RR 1.01); Hematoma (RR 1.01); SSI (RR 1.02) | SSI (RR 1.09) | ||||
| Winocour et al16,36 | 25,478 abdominoplasties over a 5-year period from the CosmetAssure database | Major complications (RR 1.4) | Major complications (RR 1.3) | Major complications (RR 1.5) | Major complications (RR 1.8) | ||
| Bamba et al16 | Five-year prospective cohort review of 129,007 patients from the CosmetAssure database | SSI (RR 1.05), any complication (RR 1.31) | |||||
| Kaoutzanis17 | Five-year prospective cohort review of 129,007 patients from the CosmetAssure database | SSI (RR 1.58) | SSI (RR 1.61) | SSI (RR 1.01) | SSI (RR 1.07) | SSI (RR 1.88) | |
| Pluvy et al25 | Systematic review of 60 randomized, controlled observational studies | Cutaneous necrosis (RR 0.76); DWH (RR 0.71); Epidermolysis (RR 0.92); SSI (RR 0.70) | |||||
DWH, delayed wound healing; SSI, surgical site infection; PE, pulmonary embolism.
where W1,2,M are the risk factors, and f is the weighted coefficient of each factor.20
The author classified the three risk groups according to their risk scores, as low risk (1 to <1.2 points), moderate risk (≥1.2 to <1.4 points), and high risk (≥1.4 points) of complications.
Predictive Model
Descriptive and inferential statistics were performed using R studio and Google Colaboratory software to analyze the correlation among variables and to validate the assessment method (See Shiny app, which shows the dataset and relationships among age, body mass index (BMI), and the Caprini score. https://bukret.shinyapps.io/AiraShinnyApp/).
The scoring classification model was validated using a machine learning process in the Colaboratory environment using a support vector machine from the sklearn library (scikit-learn). The algorithm included the following risk factors that were selected based on their relative importance, using the Pearson correlation test (>0.50) and statistical significance (P < 0.01): age, BMI, and the Caprini score. Smoking habit was included in the scoring based on current recommendations and literature.9,10,21–25
The dataset was split into training (0.80) and testing (0.20) samples, with an aleatory seed of 100 cases. Patients with missing data were excluded from the dataset. The measurements used to assess model performance were as follows: accuracy score, precision (positive predictive value), recall (sensitivity), and harmonic mean of the precision and recall (f1-score, or Sørensen–Dice coefficient).26,27 The complete process of data analysis is illustrated in Figure 1.
Fig. 1.
Data analysis validated with a machine learning process using a support vector machine in a Google Colaboratory environment. Note the dataset deployment in the Shiny app.
All procedures performed in this study were in accordance with the Declaration of Helsinki. All patient information was disidentified and retrospective.
RESULTS
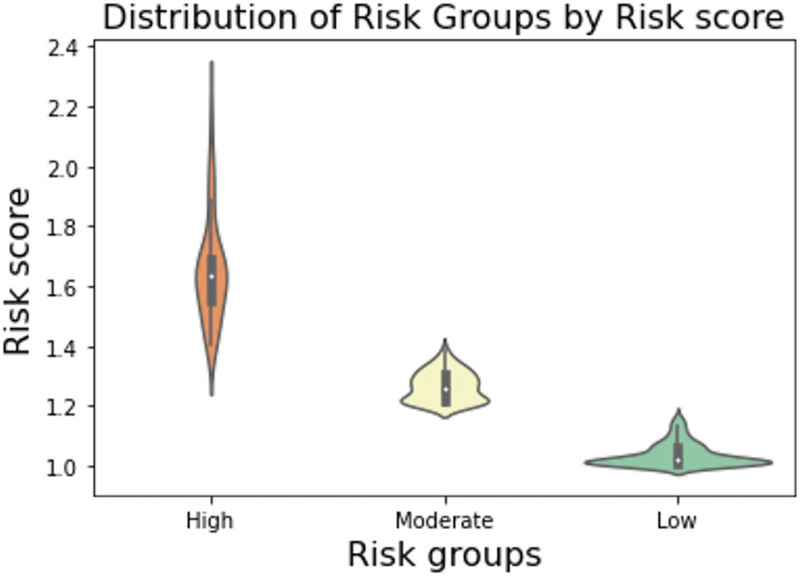
Complications were noted in 28 of the 372 patients (7.5%). The observed complications were seroma (n = 11, 3.0%), dehiscence (n = 9, 2.4%), hematoma (n = 3, 0.8%), necrosis (n = 3, 0.8%), skin incision site infection (n = 1, 0.3%), and pulmonary embolism (n = 1, 0.3%) (Table 3). The frequency of cases, complications, odds ratios (ORs), and the mean risk score in each risk group were as follows: low risk, 215 cases (9 complications, mean = 1.04, OR = 0.04, 99% confidence interval [CI] = 1.045–1.035); moderate risk, 113 cases (9 complications, mean = 1.27, OR = 0.09, 99% CI = 1.277–1.263); and high risk, 44 cases (10 complications, mean = 1.67, OR = 0.29, 99% CI = 1.673–1.627). The relative difference in complications was 6.7-fold greater in the high-risk group (RR, 0.29) and two-fold higher in the moderate-risk group (RR, 0.09) than in the low-risk group (RR, 0.04) (Table 4).
Table 3.
Incidence of Complications and Relative Percentages Based on the Total Number of Cases (n = 372)
| Complication | Frequency | Incidence (%) | OR | RR | Mean |
|---|---|---|---|---|---|
| Hematoma | 3 | 0.8 | 0.011 | 0.008 | 0.008 |
| Necrosis | 3 | 0.8 | 0.011 | 0.008 | 0.008 |
| SSI | 1 | 0.3 | 0.004 | 0.003 | 0.003 |
| Seroma | 11 | 3.0 | 0.042 | 0.030 | 0.030 |
| Dehiscence | 9 | 2.4 | 0.034 | 0.025 | 0.024 |
| PE | 1 | 0.3 | 0.004 | 0.003 | 0.003 |
SSI, surgical site infection; PE, pulmonary embolism.
Table 4.
Frequency of Cases, Complications, and ORs per Risk Group Based on the Total Number of Cases (n = 372)
| Risk Group | Cases | Complications | Mean | OR | SD | 99% CI | RR | ||
|---|---|---|---|---|---|---|---|---|---|
| Low | 215 | 9 | 1.04 | 0.04 | 0.04 | 0.005 | 1.035 | 1.045 | 0.04 |
| Moderate | 113 | 9 | 1.27 | 0.09 | 0.05 | 0.007 | 1.263 | 1.277 | 0.09 |
| High | 44 | 10 | 1.65 | 0.29 | 0.17 | 0.023 | 1.627 | 1.673 | 0.29 |
SD, standard deviation.
The difference between the means of the risk groups tested with analysis of variance was as follows: f-value = 1461.2, P-value = 4.54828884e-176 (Fig. 2). The accuracy score for prediction with the support vector machine was 100% in the training sample and 97.3% in the test sample. The positive predictive value of the method was 0.98, sensitivity showed a weighted average of 0.97, and the f1-score showed a weighted average of 0.97 (Table 5).
Fig. 2.
Difference between means of the risk groups tested using one-way analysis of variance (f-value = 1461.2, P = 4.54828884e-176).
Table 5.
Model Performance per Risk Group
| Precision | Recall | f1 Score | Support | |
|---|---|---|---|---|
| Low | 1.00 | 0.83 | 0.91 | 6 |
| Moderate | 1.00 | 0.98 | 0.99 | 48 |
| High | 0.91 | 1.00 | 0.95 | 21 |
| Accuracy | 0.97 | 75 | ||
| Weighted avg | 0.98 | 0.97 | 0.97 | 75 |
Avg, average; f1-score, harmonic mean of the precision and recall; precision, positive predictive value; recall, sensitivity.
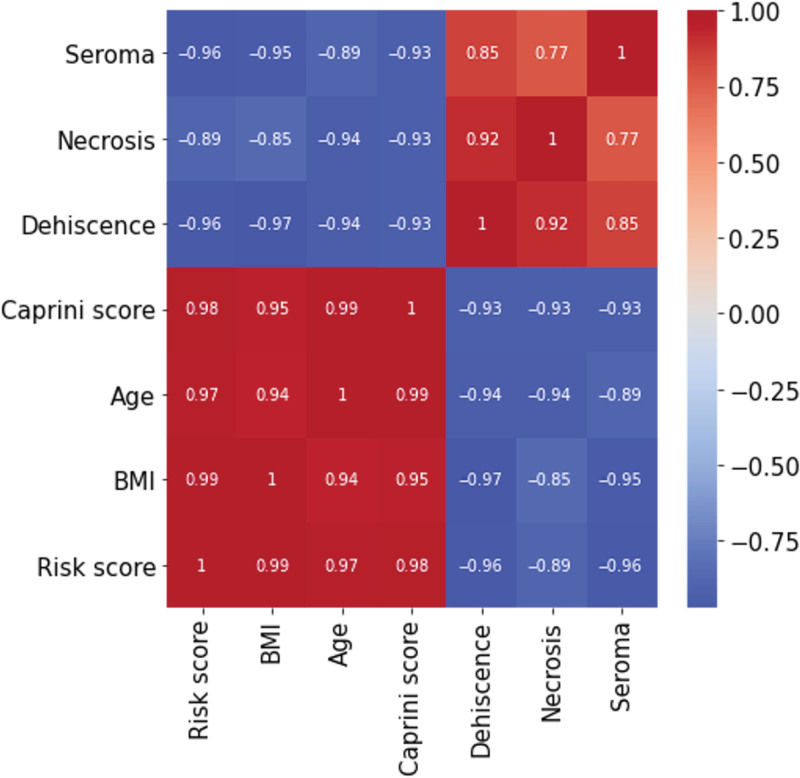
The Pearson correlation coefficients between the risk score and BMI (0.99), age (0.97), and Caprini score of 5 or more (0.98) were statistically significant (P < 0.001). However, smoking was not found to be significantly correlated with the risk score (−0.17, P = 0.58), neither did any of the complications exhibit significant correlations. Necrosis was significantly correlated with dehiscence (0.92, P = 0.003) and seroma (0.77, P = 0.041). Conversely, the correlation between necrosis and other complications was not statistically significant (P > 0.1). Additionally, dehiscence was significantly correlated with seroma (0.85, P = 0.014) (Fig. 3). A low Pearson correlation was observed between the risk score and necrosis (0.12, P = 0.69), dehiscence (−0.06, P = 0.84), hematoma (−0.24, P = 0.43), seroma (−0.06, P = 0.84), and venous thrombosis/pulmonary embolism (VT/PE; −0.06, P = 0.83). Furthermore, the correlation was not statistically significant between the risk score and male gender (0.50, P = 0.08), combined procedures (−0.10, P = 0.74), and diabetes (−0.032, P = 0.88).
Fig. 3.
Correlation analysis of the risk score, main risk factors, and complications (Pearson test > 0.5, P ≤ 0.05).
DISCUSSION
One of the main findings in this study was a significant correlation (P < 0.01) of the risk scores with age (0.97), BMI (0.99), and Caprini score of 5 or more (0.98). This is the reason for the inclusion of the 2005 Caprini scoring system in the risk assessment model, in addition to the evidence in literature, and its universal acceptance for prophylaxis of venous thromboembolism.28 Habitual smoking, length of surgery, and other comorbidities included in the Caprini score were assessed in the predictive scoring. This was because of their established relationships with deep vein thrombosis and pulmonary embolism, which have been extensively addressed in the literature.11,29–34 However, no significant correlation was found between the risk scores and smoking habit (−0.13, P = 0.57) or any other complication (P > 0.05). Nevertheless, beyond the evidence, and in accordance with current recommendations, it is reasonable to include smoking in the scoring until the relationship between this factor and surgical complications is fully established. This will eventually affect the score positively, augmenting the sensitivity of the method (weighted average, 0.97) (Table 5).9,10,25,28 Interestingly, necrosis showed a significant correlation with dehiscence (0.92, P = 0.003) and seroma (0.77, P = 0.041).
Previous studies have reported that better quality data regarding patient safety and risk are required to establish evidence-based guidelines to reduce morbidity and mortality in patients undergoing cosmetic surgery.6,22,28,35 Furthermore, in a systematic review in 2019, Morzycki et al suggested the need for reporting standards for adverse events in plastic surgery literature.6 In 2011, the task force of the American Society of Plastic Surgeons provided recommendations for improving patient safety and classifying high-risk patients according to the following criteria: BMI more than 35 kg/m2, operative times more than 6 h, lipoaspirate volumes more than 3 L, and combined procedures.21 More recently Rohrich, Mendez, and Afrooz22 classified patients at high-risk as those with a BMI more than 30 kg/m2, operative times more than 4 h, lipoaspirate volumes more than 3 L, and undergoing combined procedures. They also recognized that a majority of previous studies failed to identify specific risk factors for adverse outcomes in the outpatient setting.
To date, most studies have demonstrated an association between complications and some independent risk factors after frequent plastic surgeries, especially reconstructive surgery, but the effects of combined factors on the risk of complications remain unclear. Moreover, the relationships of some factors, such as smoking or gender, with the occurrence of complications have not been fully elucidated. Kaoutzanis et al reported that men exhibited similar overall major complication rates as women (2.1% versus 2.1%, P = 0.97), but when specific complications were analyzed, men exhibited higher hematoma rates and a lower incidence of surgical site infection.12,13 In this study, no significant correlation was found between risk score and male gender (0.50, P = 0.08) or any complications.
Pluvy et al, in a systematic review with meta-analysis, revealed that smoking significantly increased the risk of cutaneous necrosis, delayed wound healing (OR = 2.5; [95% CI = 1.49–4.08]; P < 0.001), and additional surgical site infections (OR = 2.3; [95% CI = 1.51–3.54]; P < 0.001).25 In a meta-analysis, Theocharidis et al reported that tobacco use significantly increased the total number of postoperative complications following abdominoplasty (OR = 5.43; 95% CI = 2.92–10.10), breast reduction (OR = 2.36; 95% CI = 1.64–3.39), and breast reconstruction (OR = 1.91; 95% CI = 1.69–2.17).9 Hillam et al analyzed 13,503 cases and reported that smoking was an independent risk factor for major surgical site infections following esthetic surgery (0.6% versus 0.5%, P = 0.04). However, overall, major complications were similar between smokers and nonsmokers (2.0% versus 1.9%, P = 0.57).10
In 2017, Layliev et al revealed that age equal to or greater than 40 years was an independent risk factor for developing complications after rhinoplasties. On multivariate logistic regression analysis, combined procedures were found to be the only independent risk factor for the development of hematomas (RR = 3.38; P = 0.03) and pulmonary complications (RR = 7; P = 0.03) but was not a risk factor for infectious complications (RR = 1.10; P = 0.90) (Tables 3–5).13 Gupta et al analyzed the incidence and risk factors for major complications after breast surgery in 73,608 cases, and age was the only significant predictor of hematomas (RR = 1.01; P < 0.01). Increasing age (RR = 1.02; P = 0.03) and BMI (RR = 1.09; P < 0.01) were risk factors for infection.14 In 2016, the same authors reported that hematoma (1.1%) and infection (0.3%) were the most common major complications after a facelift. Other independent risk factors were male gender, BMI of 25 or more, and combined procedures.15 In a multivariate analysis, Winocour et al demonstrated that significant risk factors (P < 0.05) included male gender (RR = 1.8), age greater than or equal to 55 years (1.4), BMI ≥ 30 kg/m2 (1.3), multiple procedures (1.5), and procedural performance at a hospital or surgical center versus performance in an office-based surgical suite (1.6).36 The same group analyzed the incidence of complications after liposuction; independent predictors of major complications included combined procedures (RR = 4.81), age (1.01), BMI (1.05), and procedures performed in hospitals (1.36). They concluded that combined procedures, especially in obese or older individuals, could significantly increase complication rates.35
Bamba et al conducted a multivariate analysis, and patients with diabetes had significantly more complications than nondiabetics (3.1% versus 1.9%, P < 0.01); diabetes was an independent risk factor for any complication (RR = 1.31; P = 0.03) and infection (RR = 1.70, P < 0.01).16 Kaoutzanis et al in a multivariate analysis found that independent predictors of surgical site infection included age (RR = 1.01), female gender (1.86), BMI (1.07), smoking (1.61), diabetes (1.58), hospital or ambulatory surgical center procedures (1.39), trunk/extremity procedures (2.42), and combined procedures (1.88).17 The same group evaluated the incidence of hematomas in 129,007 patients, and 1,180 (0.91%) had a major hematoma. They concluded that major hematomas were the most common complication following esthetic surgery. Male patients and those undergoing breast or combined procedures have a significantly higher risk of developing hematomas. On multivariate analysis, independent predictors of hematoma included age (RR = 1.01), male gender (1.98), procedures performed in a hospital rather than an office-based setting (1.68), combined procedures (1.35), and breast procedures rather than body/extremity and face procedures (1.81).12
Kaoutzanis et al reported that independent predictors of major complications in men included BMI (RR 1.05), hospital or ambulatory surgical center procedures (RR 3.47), and combined procedures (RR 2.56).23 In 2009, Momeni observed that obesity (BMI > 30 kg/m2) was associated with a significant increase in major complications after abdominoplasty (20.8% versus 9.7%; P < 0.05).37 Keyes et al conducted a retrospective analysis of 6,388,744 patients over 10 years by using data from the IBQAP database and found that age (OR = 0.67, P < 0.01) and BMI (OR = 0.43, P < 0.01) were independent risk factors for venous thromboembolism (VTE).11
The practice of individualized VTE risk stratification and specific utilization of the 2005 Caprini score are explicitly advocated by the American Society of Plastic Surgeons and the American Association of Plastic Surgeons.3–5,7,21 In a prospective study of 1000 patients, Swanson detected nine deep venous thromboses (0.9%) from days 1 to 35 after surgery.29 Winnocurt et al reported a 0.09% venous thromboembolism rate; multivariate logistic regression demonstrated the following significant risk factors (P < 0.05) for VTE: body procedures (RR = 13.47), combined procedures (2.4), increased BMI (1.06), and increased age (1.02).7
For this study, a weighted risk-assessment scoring validated using a support vector classification machine learning tool was developed, which could classify patients into three levels of risk of complications. Notably, the relative difference in complications was 6.7-fold higher in the high-risk group (RR 0.29) and two-fold higher in the moderate-risk group (RR 0.09) than in the low-risk group (RR 0.04) (Table 4). This novel system is effective and easy to use in daily practice to automatically assess risk factors in patients by implementing narrow AI, which is also useful for analyzing systematically collected data to improve patient safety and satisfaction.
To my knowledge, this is the first AI-assisted risk-assessment system to prevent complications in cosmetic surgery. Systematically assessing risk factors could strategically improve eligibility, surgical planning, patient education, and safety by preventing adverse outcomes, modifying risk factors before surgery, and selecting the appropriate surgical setting for each patient.19,38–40
The limitations of this study are the size of the case series and retrospective design. Additionally, patient recommendations and preoperative measures to reduce operative risks were not analyzed or discussed because they largely exceed the scope of this communication. Finally, it is necessary to highlight that AI-based assessments should not replace traditional clinical history and anamnesis but should augment evaluations of patient eligibility and be used to reduce human error and bias when collecting and analyzing patient data.
CONCLUSIONS
BMI, age, and the Caprini score were independent predictors of complications following esthetic surgery. The proposed risk-assessment system is a valid tool to improve patient eligibility by identifying patients aged between 16 and 67 years at a higher risk of complications. This scoring system can guide plastic surgeons in the decision-making process while reducing risks and costs. However, further studies are required for external validation of this system.
ACKNOWLEDGMENT
The author thanks Editage (www.editage.com) for English language editing.
Footnotes
Published online 27 July 2021.
Disclosure: The author has no financial interest in relation to the content of this manuscript. The study did not receive any financial support.
REFERENCES
- 1.Fray JD, Salibian AA, Choi M, et al. Putting together the pieces: development and validation of a risk-assessment model for nipple-sparing mastectomy. Plast Reconstr Surg. 2020;145:273e–283e. [DOI] [PubMed] [Google Scholar]
- 2.Pannucci CJ, MacDonald JK, Ariyan S, et al. Benefits and risks of prophylaxis for deep venous thrombosis and pulmonary embolus in plastic surgery: a systematic review and meta-analysis of controlled trials and consensus conference. Plast Reconstr Surg. 2016;137:709–730. [DOI] [PubMed] [Google Scholar]
- 3.Murphy RX, Jr, Alderman A, Gutowski K, et al. Evidence-based practices for thromboembolism prevention: summary of the ASPS venous thromboembolism task force report. Plast Reconstr Surg. 2012;130:168e–175e. [DOI] [PubMed] [Google Scholar]
- 4.Pannucci CJ, Barta RJ, Portschy PR, et al. Assessment of postoperative venous thromboembolism risk in plastic surgery patients using the 2005 and 2010 Caprini Risk score. Plast Reconstr Surg. 2012;130:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pannucci CJ. Venous thromboembolism in aesthetic surgery: risk optimization in the preoperative, intraoperative, and postoperative settings. Aesthet Surg J. 2019;39:209–219. [DOI] [PubMed] [Google Scholar]
- 6.Morzycki AD, Hudson AS, Samargandi OA, et al. Reporting adverse events in plastic surgery: a systematic review of randomized controlled trials. Plast Reconstr Surg. 2019;143:199e–208e. [DOI] [PubMed] [Google Scholar]
- 7.Winocour J, Gupta V, Kaoutzanis C, et al. Venous thromboembolism in the cosmetic patient: analysis of 129,007 patients. Aesthet Surg J. 2017;37:337–349. [DOI] [PubMed] [Google Scholar]
- 8.International Society of Aesthetic Plastic Surgery. 2019 plastic surgery statistics report. 2020. Available at https://www.isaps.org/wp-content/uploads/2020/12/Global-Survey-2019.pdf. Accessed March 10, 2021.
- 9.Theocharidis V, Katsaros I, Sgouromallis E, et al. Current evidence on the role of smoking in plastic surgery elective procedures: a systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2018;71:624–636. [DOI] [PubMed] [Google Scholar]
- 10.Hillam JS, Borsting EA, Chim JH, et al. Smoking as a risk factor for breast reduction: an analysis of 13,503 cases. J Plast Reconstr Aesthet Surg. 2017;70:734–740. [DOI] [PubMed] [Google Scholar]
- 11.Keyes GR, Singer R, Iverson RE, et al. Incidence and predictors of venous thromboembolism in abdominoplasty. Aesthet Surg J. 2018;38:162–173. [DOI] [PubMed] [Google Scholar]
- 12.Kaoutzanis C, Winocour J, Gupta V, et al. Incidence and risk factors for major hematomas in aesthetic surgery: analysis of 129,007 patients. Aesthet Surg J. 2017;37:1175–1185. [DOI] [PubMed] [Google Scholar]
- 13.Layliev J, Gupta V, Kaoutzanis C, et al. Incidence and preoperative risk factors for major complications in aesthetic rhinoplasty: analysis of 4978 patients. Aesthet Surg J. 2017;37:757–767. [DOI] [PubMed] [Google Scholar]
- 14.Gupta V, Yeslev M, Winocour J, et al. Aesthetic breast surgery and concomitant procedures: incidence and risk factors for major complications in 73,608 cases. Aesthet Surg J. 2017;37:515–527. [DOI] [PubMed] [Google Scholar]
- 15.Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1–13. [DOI] [PubMed] [Google Scholar]
- 16.Bamba R, Gupta V, Shack RB, et al. Evaluation of diabetes mellitus as a risk factor for major complications in patients undergoing aesthetic surgery. Aesthet Surg J. 2016;36:598–608. [DOI] [PubMed] [Google Scholar]
- 17.Kaoutzanis C, Gupta V, Winocour J, et al. Incidence and risk factors for major surgical site infections in aesthetic surgery: analysis of 129,007 patients. Aesthet Surg J. 2017;37:89–99. [DOI] [PubMed] [Google Scholar]
- 18.Momeni A, Heier M, Bannasch H, et al. Complications in abdominoplasty: a risk factor analysis. J Plast Reconstr Aesthet Surg. 2009;62:1250–1254. [DOI] [PubMed] [Google Scholar]
- 19.Horton JB, Reece EM, Broughton G, II, et al. Patient safety in the office-based setting. Plast Reconstr Surg. 2006;117:61e–80e. [DOI] [PubMed] [Google Scholar]
- 20.Zadeh LA. Optimality and non-scalar-valued performance criteria. IEEE Trans Autom Control. 1963;AC-8:1. [Google Scholar]
- 21.American Society of Plastic Surgeons; ASPS Patient Safety Committee. Pathways to preventing adverse events in ambulatory surgery in 2011. 2011. Available at https://www.plasticsurgery.org/Documents/Health-Policy/Patient-Safety/patient-safety-2011-adverse-events-ambulatory-surgery.pdf. Accessed March 10, 2021.
- 22.Rohrich RJ, Mendez BM, Afrooz PN. An update on the safety and efficacy of outpatient plastic surgery: a review of 26,032 consecutive cases. Plast Reconstr Surg. 2018;141:902–908. [DOI] [PubMed] [Google Scholar]
- 23.Kaoutzanis C, Winocour J, Yeslev M, et al. Aesthetic surgical procedures in men: major complications and associated risk factors. Aesthet Surg J. 2018;38:429–441. [DOI] [PubMed] [Google Scholar]
- 24.Kaoutzanis C, Winocour J, Gupta V, et al. The effect of smoking in the cosmetic surgery population: analysis of 129,007 patients. Aesthet Surg J. 2019;39:109–119. [DOI] [PubMed] [Google Scholar]
- 25.Pluvy I, Panouillères M, Garrido I, et al. Smoking and plastic surgery, part II. Clinical implications: a systematic review with meta-analysis. Ann Chir Plast Esthet. 2015;60:e15–e49. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Kongelige Danske Videnskabernes Selskab. 1948;5:1–34. [Google Scholar]
- 27.Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- 28.Rohrich RJ, Savetsky IL, Avashia YJ. Assessing cosmetic surgery safety: the evolving data. Plast Reconstr Surg Glob Open. 2020;8:e2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swanson E. Prospective study of Doppler ultrasound surveillance for deep venous thromboses in 1000 plastic surgery outpatients. Plast Reconstr Surg. 2020;145:85–96. [DOI] [PubMed] [Google Scholar]
- 30.Caprini JA. Thrombosis risk assessment as a guide to quality patient care. Dis Mon. 2005;51:70–78. [DOI] [PubMed] [Google Scholar]
- 31.Caprini JA, Arcelus JI, Reyna JJ. Effective risk stratification of surgical and nonsurgical patients for venous thromboembolic disease. Semin Hematol. 2001;38(2 suppl 5):12–19. [DOI] [PubMed] [Google Scholar]
- 32.Cronin MA, Demgler N, Krauss ES, et al. Completion of the update Caprini risk assessment model (2013 version). Clin Appl Thromb Haemost. 2019;25:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatef DA, Trussler AP, Kenkel JM. Procedural risk for venous thromboembolism in abdominal contouring surgery: a systematic review of the literature. Plast Reconstr Surg. 2010;125:352–362. [DOI] [PubMed] [Google Scholar]
- 34.Haeck PC, Swanson JA, Schechter LS, et al. ; ASPS Patient Safety Committee. Evidence-based patient safety advisory: blood dyscrasias. Plast Reconstr Surg. 2009;124(4 suppl):82S–95S. [DOI] [PubMed] [Google Scholar]
- 35.Kaoutzanis C, Gupta V, Winocour J, et al. Cosmetic liposuction: preoperative risk factors, major complication rates, and safety of combined procedures. Aesthet Surg J. 2017;37:680–694. [DOI] [PubMed] [Google Scholar]
- 36.Winocour J, Gupta V, Ramirez JR, et al. Abdominoplasty: risk factors, complication rates, and safety of combined procedures. Plast Reconstr Surg. 2015;136:597e–606e. [DOI] [PubMed] [Google Scholar]
- 37.Gupta V, Parikh R, Nguyen L, et al. Is office-based surgery safe? Comparing outcomes of 183,914 aesthetic surgical procedures across different types of accredited facilities. Aesthet Surg J. 2017;37:226–235. [DOI] [PubMed] [Google Scholar]
- 38.Keyes GR, Singer R, Iverson RE, et al. Mortality in outpatient surgery. Plast Reconstr Surg. 2008;122:245–250. [DOI] [PubMed] [Google Scholar]
- 39.Byrd HS, Barton FE, Orenstein HH, et al. Safety and efficacy in an accredited outpatient plastic surgery facility: a review of 5316 consecutive cases. Plast Reconstr Surg. 2003;112:636–641. [DOI] [PubMed] [Google Scholar]
- 40.Iverson RE; ASPS Task Force on Patient Safety in Office-Based Surgery Facilities. Patient safety in office-based surgery facilities: I. Procedures in the office-based surgery setting. Plast Reconstr Surg. 2002;110:1337–1342. [DOI] [PubMed] [Google Scholar]