PURPOSE
Children with average-risk medulloblastoma (MB) experience survival rates of ≥ 80% at the expense of adverse consequences of treatment. Efforts to mitigate these effects include deintensification of craniospinal irradiation (CSI) dose and volume.
METHODS
ACNS0331 (ClinicalTrials.gov identifier: NCT00085735) randomly assigned patients age 3-21 years with average-risk MB to receive posterior fossa radiation therapy (PFRT) or involved field radiation therapy (IFRT) following CSI. Young children (3-7 years) were also randomly assigned to receive standard-dose CSI (SDCSI; 23.4 Gy) or low-dose CSI (LDCSI; 18 Gy). Post hoc molecular classification and mutational analysis contextualized outcomes according to known biologic subgroups (Wingless, Sonic Hedgehog, group 3, and group 4) and genetic biomarkers. Neurocognitive changes and ototoxicity were monitored over time.
RESULTS
Five hundred forty-nine patients were enrolled on study, of which 464 were eligible and evaluable to compare PFRT versus IFRT and 226 for SDCSI versus LDCSI. The five-year event-free survival (EFS) was 82.5% (95% CI, 77.2 to 87.8) and 80.5% (95% CI, 75.2 to 85.8) for the IFRT and PFRT regimens, respectively, and 71.4% (95% CI, 62.8 to 80) and 82.9% (95% CI, 75.6 to 90.2) for the LDCSI and SDCSI regimens, respectively. IFRT was not inferior to PFRT (hazard ratio, 0.97; 94% upper CI, 1.32). LDCSI was inferior to SDCSI (hazard ratio, 1.67%; 80% upper CI, 2.10). Improved EFS was observed in patients with Sonic Hedgehog MB who were randomly assigned to the IFRT arm (P = .018). Patients with group 4 MB receiving LDCSI exhibited inferior EFS (P = .047). Children receiving SDCSI exhibited greater late declines in IQ (estimate = 5.87; P = .021).
CONCLUSION
Reducing the radiation boost volume in average-risk MB is safe and does not compromise survival. Reducing CSI dose in young children with average-risk MB results in inferior outcomes, possibly in a subgroup-dependent manner, but is associated with better neurocognitive outcome. Molecularly informed patient selection warrants further exploration for children with MB to be considered for late-effect sparing approaches.
INTRODUCTION
Medulloblastoma (MB) is an embryonal cerebellar tumor of childhood with propensity to disseminate along the neuroaxis. Children > 2 years who have had gross total resection of their tumor and no evidence of CNS dissemination at diagnosis (average risk) are conventionally treated with 23.4 Gy of craniospinal irradiation (CSI) with concomitant vincristine, 32.4 Gy posterior fossa radiation therapy (PFRT), followed by adjuvant cis-platinum–based chemotherapy. The expected 5-year event-free survival (EFS) for children with average-risk disease with this strategy is approximately 80%-85%.1
CONTEXT
Key Objective
The prognosis of average-risk medulloblastoma has improved with multimodality therapy, but adverse effects of radiation therapy on patient outcomes can be devastating. In this phase III trial, we sought to de-intensify the craniospinal irradiation (CSI) dose in younger children and reduce the volume of the boost in all children receiving treatment for this disease. Neurocognitive and audiologic outcomes were measured. Post hoc molecular classifications were performed on patient tumor samples.
Knowledge Generated
It is safe to reduce the volume of the radiotherapy boost in these children. A reduction in CSI dose in young children is associated with unacceptable rate of failure. Outcomes appear dependent on molecular subgroups. Low-dose CSI reduces the degree of late decline in patient intelligence quotient.
Relevance
Reduced, involved field volume boost radiation therapy is the standard of care for children with average-risk medulloblastoma. Efforts to reduce treatment intensity may require molecularly informed patient selection.
Standard treatment is associated with negative impact on cognitive, auditory, and endocrine functions.2-4 Delaying, reducing, or hyperfractionating radiation therapy (RT) represents strategies to mitigate its toxicity.5-11 Several series suggested that reduced radiation boost volumes may reduce late effects without compromising disease control.12-14 ACNS0331 was a phase III study undertaken in patients with average-risk MB to determine if a reduction in RT boost volume in all children and CSI dose in younger children accompanied by chemotherapy could maintain favorable outcomes while minimizing late toxicity.
The WHO currently recognizes at least four distinct molecular subgroups of MB (ie, Wingless [WNT], Sonic Hedgehog [SHH], group 3, and group 4)15 that exhibit divergent genetics, demographics, clinical behaviors, and prognoses.16-19 Using conventional genomic profiling, the outcomes of ACNS0331 were contextualized by molecular subgroup.
METHODS
Key Trial Eligibility Criteria
Patients with average-risk MB age 3-21 years at diagnosis were eligible. Anaplastic histology was excluded.1 Patients had no dissemination and ≤ 1.5 cm2 of residual tumor after surgery. Patients had adequate kidney, liver, and bone marrow function and good performance status. Treatment must have begun within 31 days of definitive surgery.
Local Institutional Review Board approval of the trial was required before a site could enroll patients. All patients and/or their parents or legal guardians were required to provide written informed consent for participation in the primary study and other ancillary research. The trial was conducted according to the ethical principles described in the Declaration of Helsinki.
Radiation Therapy
Patients age 3-7 years underwent two treatment random assignments. The first was to standard-dose CSI (SDCSI) of 23.4 Gy or low-dose CSI (LDCSI) of 18 Gy. All patients were randomly assigned to receive PFRT or involved field radiation therapy (IFRT) to a cumulative dose of 54 Gy. Radiation planning details are described in Protocol 1 (online only). Proton therapy was allowed as part or for the entirety of radiation treatment. Radiation treatment plans and portal films were reviewed by the principal investigator, retrospectively.
Chemotherapy
Patients received weekly vincristine with radiotherapy. Maintenance chemotherapy began four weeks after completing chemoradiotherapy with nine cycles of alternating regimens with a cycle schedule of AABAABAAB. Cycle A consisted of cisplatin, CCNU (lomustine), and vincristine. Cycle B consisted of cyclophosphamide and vincristine. Drug administration instructions, schedule, and modifications for toxicity are given in Protocol 1. Audiologic and renal function was monitored throughout cisplatin treatment, and dose was reduced, postponed, or discontinued in the presence of severe ototoxicity or nephrotoxicity.
Staging and Pathology Review
Central radiology review of cranial and spinal magnetic resonance imaging was performed after study entry. A finding of excess residual disease or dissemination on review was recorded as unevaluable. Cases of anaplastic MB determined by central pathology review were recorded as unevaluable. Eligible but unevaluable patients are excluded in the primary end point analysis.
Molecular Classification and Whole-Exome Sequencing
Molecular analyses were conducted under an NCI-approved Protocol ACNS16B1-Q (Protocol 2, online only). Data were generated from fresh-frozen and formalin-fixed paraffin-embedded tissue samples. Samples were analyzed using Illumina Infinium Methylation EPIC BeadChip arrays in accordance with the manufacturer's instructions. Beta values representing the proportion of methylated cytosine present at each CpG site were calculated using the Minfi R package (version 1.36.0). CNS tumor entity and MB subgroup predictions were determined using a DNA methylation–based classification approach20 (MolecularNeuropathology21 version 11b4). Predictions were further evaluated by implementing an ExtraTrees classifier (scikit-learn version 0.20.3) trained on a reference data set composed of 2,801 CNS tumors. The resulting group 3 and group 4 subgroup assignments were further evaluated by implementing another ExtraTrees classifier trained on a reference data set of 740 previously classified group 3 and group 4 tumors.22 Copy number variation analysis from methylation array data was performed using the Conumee Bioconductor package (version 1.20). Genomic segments with a log2 ratio outside one median absolute deviation from the median were considered for manual curation. Additionally, commonly altered genes in MB were considered for focal amplifications or deletions.
For tumor samples with sufficient genomic DNA following methylation array analysis, whole-exome sequencing libraries were generated using the TruSeq DNA Exome kit specific for the Illumina HiSeq instrument, followed by exome enrichment. All next-generation sequencing data were processed with an unpaired analysis pipeline using MuTect2. The resulting mutations affecting known MB-associated genes were then curated manually using Integrative Genomics Viewer (Broad Institute, Boston, MA; University of California, San Diego, CA) to ensure consistent mutation calling.
Follow-Up Assessments
Patients received physical examinations and CNS magnetic resonance imaging and had bloodwork to monitor hematologic, liver, and kidney functions every 3 months for 1 year, every 6 months for 3 years, and annually for 10 years after enrollment. Conventional pure-tone audiometry (0.25-8 kHz) was required before starting treatment and before each chemotherapy cycle. Audiometric data were graded by the International Society of Pediatric Oncology Ototoxicity Scale.
Neurocognitive assessments were collected three times over a 6-year period (approximately 9, 30, and 60 months postdiagnosis) as part of either this trial or companion study ALTE0C71. Intellectual outcomes included selected subtests from age-appropriate versions of the standardized and norm-referenced Wechsler Intelligence Scales23 (Data Supplement, online only). Vocabulary and Block Design subtests, which are thought to place demands on very distinct regions of a child's brain, were used to calculate estimated IQ on the basis of Sattler's24 recommendations. This short-form combination correlates highly with Full Scale IQ and can be used with all three Wechsler IQ tests. In addition, Coding and Symbol Search subtests were used to calculate the Processing Speed Index. Type of insurance (eg, public v private) was a proxy for socioeconomic status.
Statistical Considerations
The primary end point is EFS calculated from the date of study entry to date of disease progression, recurrence, second malignant neoplasm (SMN), or death from any cause, whichever occurred first or to the date of last follow-up. Overall survival (OS) was calculated from date of study entry to date of death from any cause or to the date of last follow-up. EFS and OS were estimated using the Kaplan-Meier method. Trial design was noninferiority for both random assignments. The trial was designed to detect with 94% power a 10% reduction in 5-year EFS (corresponding to a hazard ratio [HR] of 1.6) for the IFRT versus PFRT comparison and with 80% power a 10% reduction in 5-year EFS (HR of 1.6) for LDCSI versus SDCSI at 0.2 type I error rate. Outcomes are reported as survival probability and a 95% CI. The log-rank test was used to compare outcome distributions by treatment group among MB subgroups. Median follow-up was estimated using the reverse Kaplan-Meier method. Gray's test was used to compare the cumulative incidence of local failure (and distant failure) among treatment groups. Competing risks included distant failure, second malignancy, or death before progression in analyses related to local failure; and local failure, second malignancy, or death before progression in analyses related to distant failure. Analyses of outcome by molecular subgroup were performed post hoc since consensus molecular subgroups were established long after initiation of the trial.25
Multivariable logistic regression was used to explore associations between dichotomized audiology outcomes (no hearing loss [HL] v any HL) and a predetermined set of covariates, which included radiation treatment arm along with age at diagnosis, sex, molecular subgroup, and cumulative cisplatin dose. Ordinal logistic regression was used to examine associations between International Society of Pediatric Oncology grade HL (none [grade 0] v mild [grades 1 and 2] v severe [grades 3 and 4]) and the same set of covariates. General linear models were built to explore the relationship between neurocognitive outcomes and random assignment groups at each timepoint, and for change over time, after adjusting for sex, type of insurance, and age at diagnosis.
RESULTS
Patient Characteristics
Patients were enrolled from April 30, 2004-January 6, 2014. Of 549 patients enrolled, 36 were deemed ineligible by study chair review because of late start of protocol therapy (n = 24), histology other than MB (n = 5), inadequate organ function requirements (n = 3), or combinations of these or other causes (n = 4) (Fig 1). Forty-two of these 513 eligible patients had excess residual disease or dissemination by central radiology review, and seven had anaplasia on histopathologic review. This left 464 eligible and evaluable patients with no dissemination, residual disease, or anaplasia available for the primary analysis of the PFRT versus IFRT random assignment, and 226 young patients (3-7 years) for the comparison of SDCSI versus LDCSI (Fig 1). The median follow-up for patients without events was 9.3 years (interquartile range, 6.8-10.5). Baseline characteristics of sex, race, and age were balanced between randomly assigned groups (Table 1).
FIG 1.

CONSORT diagram. NOTE. All patients received weekly vincristine (six doses) during radiation phase of therapy. aBoth random assignments occurred at the time of study enrollment. bPatients age 3-7 years randomly assigned to the reduced-dose (18 Gy) craniospinal radiation were given an additional dose of 5.4 Gy to the posterior fossa (18 Gy + 5.4 Gy = total 23.4 Gy) before the final boost dose (cumulative 54 Gy). IFRT, involved field radiation therapy; LDCSI, low-dose craniospinal irradiation; PFRT, posterior fossa radiation therapy; SDCSI, standard-dose craniospinal irradiation.
TABLE 1.
Characteristics of Eligible, Evaluable Patients

The majority of RT plans were appropriate (61%) or had minor deviations (22%). Only 7% had major deviations, 7% were unevaluable (missing data), 2% received no RT, and 1% had no review. Forty-two patients received proton therapy (< 10%) for the boost only (n = 12) or CSI and the boost (n = 30).
Sufficient tumor tissue was available to complete methylation classification for 380 of 464 eligible and evaluable patients (82%): 64 WNT (16.8%), 66 SHH (17.4%), 76 group 3 (20.0%), 156 group 4 (41.1%), six non-MB (1.6%), and 12 inconclusive (3.2%) (Table 1; Figs 2A and 2B; Data Supplement). There were no statistically significant differences in the distributions of sex, age, treatment group, or outcome among patients who were and were not profiled (380 v 84; data not shown). Significant differences in the distributions of age and sex by subgroup were observed (P < .001 for both; Table 1). Tumor-only whole-exome sequencing data were generated for 272 patients (75.1% of molecularly confirmed MBs). Recurrently mutated genes and copy number alterations were summarized by molecular subgroup (Fig 2C; Data Supplement).
FIG 2.

Molecular classification of patients in ACNS0331. (A) Pie charts summarizing the molecular classification results for eligible and evaluable patients (molecular cohort) profiled by DNA methylation array. (B) t-SNE plot summarizing molecular subgroup distribution of tumors profiled by DNA methylation array. (C) Oncoprint summarizing recurrently mutated genes and chromosomal events by molecular subgroup (NGS cohort). CSI, craniospinal irradiation; IFRT, involved field radiation therapy; LDCSI, low-dose craniospinal irradiation; MB, medulloblastoma; NGS, next-generation sequencing; PFRT, posterior fossa radiation therapy; SDCSI, standard-dose craniospinal irradiation; SHH, Sonic Hedgehog; SNV, single nucleotide variant; t-SNE, t-distributed stochastic neighbor embedding; WNT, Wingless.
Patient Outcomes
Among 464 eligible and evaluable cases, 5-year estimates of EFS and OS were 81.4% (95% CI, 77.7 to 85.1) and 84.9% (95% CI, 81.4 to 88.4), respectively. The estimated HR for comparing EFS between IFRT and PFRT was 0.97, and its one-sided 94% upper confidence limit was 1.35. Since this confidence limit is lower than 1.6, the prespecified noninferiority boundary, IFRT, was deemed to be noninferior compared with PFRT in EFS. The conclusion is consistent with OS. The 5-year EFS estimate was 82.5% (95% CI, 77.2 to 87.8) with IFRT compared with 80.5% (95% CI, 75.2 to 85.8) with PFRT (P = .44; Fig 3A). The 5-year OS estimate was 84.6% (95% CI, 79.7 to 89.5) with IFRT compared with 85.2% (95% CI, 80.5 to 89.9) with PFRT (P = .44; Fig 3B).
FIG 3.
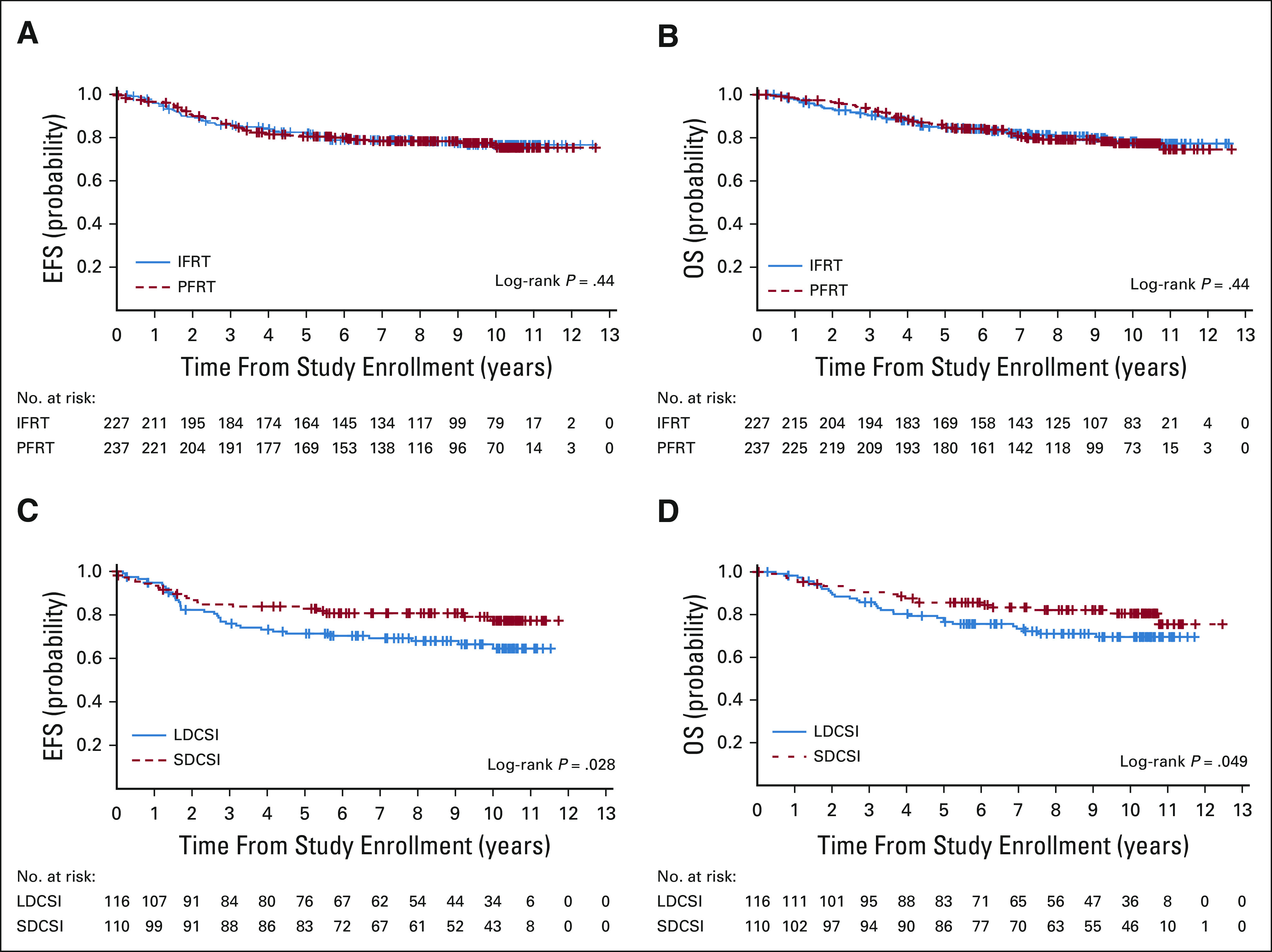
Outcomes by random assignment arm. (A and B) EFS and OS of all eligible and evaluable patients by RT group. (C and D) EFS and OS of all eligible and evaluable patients by CSI group. CSI, craniospinal irradiation; EFS, event-free survival; IFRT, involved field radiation therapy; LDCSI, low-dose craniospinal irradiation; OS, overall survival; PFRT, posterior fossa radiation therapy; RT, radiation therapy; SDCSI, standard-dose craniospinal irradiation.
The estimated HR for comparing EFS between LDCSI and SDCSI was 1.67, and its one-sided 80% upper confidence limit of the HR was 2.10. Since this confidence limit is larger than 1.6, LDCSI was deemed to be inferior compared with SDCSI in EFS. The conclusion is consistent with OS. Children receiving LDCSI had an EFS estimate of 71.4% (95% CI, 62.8 to 80) compared with 82.9% (95% CI, 75.6 to 90.2) with SDCSI (P = .028; Fig 3C). The 5-year OS estimate was 77.5% (95% CI, 69.7 to 85.3) with LDCSI compared with 85.6% (95% CI, 78.7 to 92.5) ±3.5% with SDCSI (P = .049; Fig 3D). When all 513 eligible (including unevaluable) patients were analyzed, the differences in EFS and OS were no longer statistically significant (Data Supplement). Anaplastic pathology or radiographic findings of excess residual disease or dissemination on central review (unevaluable cases) were associated with worse EFS and OS (Data Supplement).
Outcomes by Molecular Subgroup
Of 362 patients with molecularly confirmed MB, 5-year EFS differed significantly by molecular subgroup: WNT 93.3% (95% CI, 86.8 to 99.8), SHH 82.6% (95% CI, 72.6 to 92.6), group 3 63.3% (95% CI, 52.1 to 74.5), and group 4 86.7% (95% CI, 81.0 to 92.4) (P < .0001 for both EFS and OS; Figs 4A and 4B). The distribution of CSI and RT random assignments was balanced within subgroups (Figs 4C and 4D).
FIG 4.

Outcomes by molecular subgroup. (A and B) EFS and OS of all molecularly classified patients by subgroup. (C and D) Distribution of patients by trial random assignment according to molecular subgroup (CSI and RT groups, respectively). CSI, craniospinal irradiation; EFS, event-free survival; IFRT, involved field radiation therapy; LDCSI, low-dose craniospinal irradiation; OS, overall survival; PFRT, posterior fossa radiation therapy; RT, radiation therapy; SDCSI, standard-dose craniospinal irradiation; SHH, Sonic Hedgehog; WNT, Wingless.
There was no significant difference in EFS by RT group (IFRT v PFRT) among the WNT, group 3, or group 4 subgroups (Figs 5A, 5C, and 5D). By contrast, SHH subgroup patients treated on the IFRT arm had improved EFS compared with the PFRT arm (5-year estimates: 90.7% (95% CI, 80.1 to 100) for IFRT v 74.9% (95% CI, 58.8 to 91.0) for PFRT, P = .018; Fig 5B). Three of 34 (8.8%) SHH subgroup patients treated on the IFRT arm had events (three relapsed or progressive disease and no SMN), compared with 11 of 32 (34.4%) treated on the PFRT arm (seven relapsed or progressive disease and four SMN).
FIG 5.

Random assignment outcomes by molecular subgroup. (A-D) EFS for each molecular subgroup stratified by RT group of WNT, SHH, group 3, and group 4, respectively. (E-H) EFS for each molecular subgroup stratified by CSI group. CSI, craniospinal irradiation; EFS, event-free survival; IFRT, involved field radiation therapy; LDCSI, low-dose craniospinal irradiation; PFRT, posterior fossa radiation therapy; RT, radiation therapy; SDCSI, standard-dose craniospinal irradiation; SHH, Sonic Hedgehog; WNT, Wingless.
Among young children age 3-7 years, no significant difference in EFS by CSI group (SDCSI v LDCSI) was observed among the WNT, SHH, or group 3 subgroups (Figs 5E-5G). By contrast, young group 4 subgroup patients treated on the LDCSI arm had worse EFS compared with the SDCSI arm (5-year estimates: 77.2% [95% CI, 63.5 to 90.9] for LDCSI v 97.1% [95% CI, 91.4 to 100] for SDCSI, P = .047; Fig 5H).
Additional clinically relevant genetic biomarkers were investigated in a subgroup-specific manner and are summarized in the Data Supplement. Seven patients were observed to have TP53 alterations (focal deletion or mutation), and they had a significantly worse EFS (5-year estimates: 14.3% [95% CI, 0 to 32.7] v 84.7% [95% CI, 80.0 to 89.4], P < .001; Data Supplement). Five of these were SHH subgroup, all of whom had events (four relapsed or progressive disease and one SMN) and died (Data Supplement). Among young children with group 4 tumors treated with LDCSI, outcomes were most inferior in those exhibiting balanced chromosomes 11 and 17 (Data Supplement), although additional stratification according to these chromosomal alterations resulted in small patient groups.
Patterns of Failure
The majority of first recurrences (65 of 82) involved the neuroaxis in isolation (41.5%) or combined with a posterior fossa recurrence (37.8%). Isolated posterior fossa recurrences represented 18.3% of failures. One additional patient had posterior fossa and distant non-CNS recurrence (1.2%), and for one patient, failure pattern was not available. The cumulative incidence of local failure did not differ by radiation boost volume (P = .21; 5-year estimates: 10.1% [95% CI, 6.1 to 14.1] for IFRT v 7.6% [95% CI, 4.1 to 11.0] for PFRT; Fig 6A). None of the patients receiving the IFRT boost experienced posterior fossa failures outside the limited boost volume. The cumulative incidence of isolated neuroaxis failure did not differ by CSI dose (P = .34; 5-year estimates: 12.5% (95% CI, 6.3 to 18.6) for LDSCI v 8.7% (95% CI, 3.2 to 14.1) for SDCSI; Fig 6B). Patterns of failure varied by molecular subgroup with local failure predominant in WNT and SHH and neuroaxis failure predominant in group 3 and group 4 subgroups (Gray's test, local, P = .035; local plus distant, P = .003; and distant, P = .002; Fig 6C; Data Supplement).
FIG 6.

Pattern of failure by random assignment arm. (A) Cumulative incidence of local failure (local and local plus distant) by RT group. (B) Cumulative incidence of isolated distant failure by CSI group (3-7 years). (C) Pattern of failure by molecular subgroup. CSI, craniospinal irradiation; IFRT, involved field radiation therapy; LDCSI, low-dose craniospinal irradiation; PFRT, posterior fossa radiation therapy; RT, radiation therapy; SDCSI, standard-dose craniospinal irradiation; SHH, Sonic Hedgehog; WNT, Wingless.
Sixteen eligible and 14 eligible or evaluable patients developed SMNs, with one patient experiencing three (Data Supplement). SMNs occurred in two WNT, four SHH, 4 (n = 3 evaluable) group 3, 4 (n = 3 evaluable) group 4, and 2 unclassified patients at a median of 5.8 years from study enrollment, with range (1.9-10.0) years. Observed SMNs included glioma or glioblastoma (n = 10), acute myelogenous leukemia or myelodysplastic syndrome (n = 5), and others (n = 3).
Treatment Effects
Patients receiving SDCSI had a higher incidence of grade 3 or greater thrombocytopenia, elevation of liver transaminases, and peripheral sensory neuropathy (Data Supplement). Patients receiving PFRT and LDCSI had a lower incidence of peripheral motor neuropathy and anorexia (Data Supplement).
Of the 513 eligible patients, 148 (29%) patients had evaluable baseline and off-treatment audiograms available. 11 patients were excluded because of pretherapy HL. One hundred eleven (81%) patients had at least a mild HL (grade > 0) at the end of treatment, and 33 (24.1%) had severe HL (grade ≥ 3). In a multivariable logistic regression model, age at diagnosis was the only significant predictor of HL. Younger age was significantly associated with developing any HL (P = .003; odds ratio = 1.21; 95% CI, 1.07 to 1.37) and more severe HL (P = .002; odds ratio = 1.17; 95% CI, 1.06 to 1.28). After adjusting for other variables, radiation treatment groups were not associated with HL (Data Supplement).
Neurocognitive assessments were obtained from 356 (76.7%) eligible and evaluable participants across three timepoints. Patients with relapsed or progressive disease or SMN were excluded from analyses of neurocognitive end points if these data were obtained following these events. The majority of patients (n = 207; 58.2%) were receiving private or military-sponsored insurance at the time of diagnosis. The first assessment (T1) was administered to 85.7% (324 or 378) of patients at 4-15 months postdiagnosis. The second (T2) was completed for 52.4% (174 of 332) of patients, at 27-48 months. The final assessment (T3) was completed for 43.4% (129 of 297) of patients, at 49-72 months. Enrolled participants who contributed data (65.5% male and 82.6% White) averaged 9 years old at diagnosis (SD = 4.0). At T1, estimated IQ scores (mean = 96.0; SD = 15.0) for the entire sample were within the average range, but processing speed scores averaged a full standard deviation below the mean (mean = 85.7; SD = 14.6). After controlling for covariates, 3- to 7-year-old children who received SDCSI exhibited significantly greater declines in IQ between T1 and T2 (estimate = 7.34; P = .02), although this difference did not reach significance between T1 and T3 (estimate = 5.65; P = .12) (Fig 7A), possibly because of sample size at T3. Moreover, processing speed scores for younger participants did not differ between groups at any time (Fig 7B). Few significant differences were attributable to boost random assignment after controlling for CSI group and covariates (Fig 7C). Although participants randomly assigned to receive IFRT had significantly higher IQ scores at T2 than those receiving PFRT (estimate = 6.04; P = .01), this difference did not remain significant at T3 (estimate = 2.92; P = .34). Of importance, patients ≥ 8 years at diagnosis exhibited average estimated IQ scores at all three timepoints (T1 = 96.8; T2 = 97.3; T3 = 98.6), although processing speed scores remained weak (T1 = 81.9; T2 = 80.3; T3 = 84.4). Consistent with prior data, younger age at diagnosis was a significant predictor of worse intellectual functioning in models including CSI group, RT group, sex, and insurance status (estimate T1-T2 = 0.77, P = .004; T1-T3 = 1.11, P = .0005).
FIG 7.
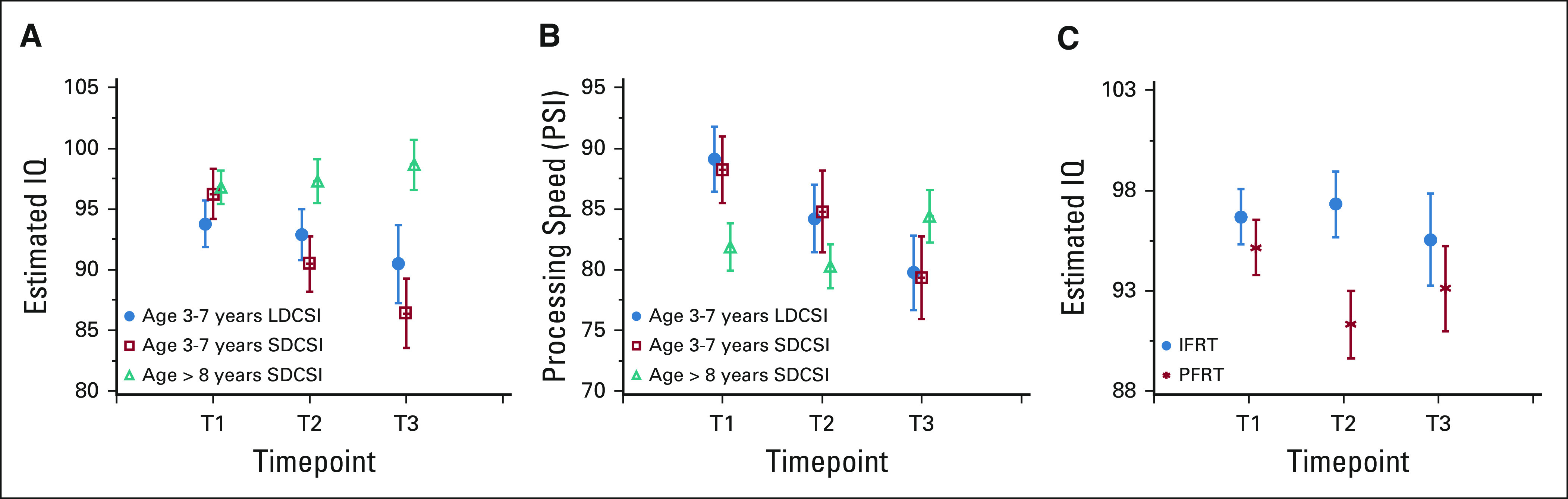
Neurocognitive outcomes by trial random assignment. (A) Decrease of IQ from T1 to T2 is 7.34 units more (P = .02) in SDCSI group compared with LDCSI group in patients between 3 and 7 years old on the basis of the GLM, which controls sex, age at diagnosis, and insurance status. Decrease of IQ from T1 to T3 is not significantly different between LDCSI and SDCSI groups in patients between 3 and 7 years old on the basis of the GLM, which controls sex, age at diagnosis, and insurance status (T1 to T3 estimate = 5.65, P = .12). (B) Decrease of PSI from T1 to T2 is not significantly different between LDCSI and SDCSI groups in patients between 3 and 7 years old on the basis of the GLM, which controls sex, age at diagnosis, and insurance status (T1 to T2 estimate = −2.35, P = .65; T1 to T3 estimate = −5.75, P = .25). (C) At T2, IQ of reduced boost group is 6.04 units more (P = .01) than PF boost group on the basis of the GLM, which controls sex, age at diagnosis, and insurance status. CSI, craniospinal irradiation; GLM, generalized linear model; IFRT, involved field radiation therapy; IQ, intelligence quotient; LDCSI, low-dose craniospinal irradiation; PFRT, posterior fossa radiation therapy; RT, radiation therapy; SDCSI, standard-dose craniospinal irradiation.
DISCUSSION
In this phase III noninferiority trial of children with average-risk MB, a reduction in the boost volume from the posterior fossa to a limited tumor bed boost was not associated with worse EFS or OS. Unfortunately, the attempt to reduce the radiation dose delivered to the craniospinal axis (LDCSI) in children age 3-7 years was associated with worse EFS and OS.
The relatively high (8%) incidence of incorrect institutional staging or pathology on our central review raises concern. Going forward, Children's Oncology Group intends to mandate preregistration review of radiology and molecular diagnostics for trials of patients with CNS tumors.
Smaller RT boost volumes did not affect patterns of failure. There was no increase in isolated posterior fossa failures with IFRT. None of the posterior fossa recurrences occurred outside the boost volume in patients who received IFRT. There was no significant increase in isolated neuroaxis failures in younger patients treated with LDCSI.
SMNs remain a problem in survivors of MB. In this study, 16 patients developed second cancers, mostly high-grade glioma seen as early as 1.9 years after study entry, which were often fatal. The incidence of SMN could actually be higher as not all relapses were pathologically confirmed.
Consensus molecular subgroups of MB were officially recognized in 2012,25 during the final 2 years of ACNS0331 enrollment. As such, all biologic analyses were performed post hoc and restricted to patient tumor DNA extracted from FFPE slides without available patient-matched germline material. Nonetheless, we confidently determined molecular subgroup status, genome-wide copy number alterations, and inferred somatic mutations in the majority of eligible and evaluable patients, enabling contextualization of trial outcomes according to current MB biology. Expectedly, WNT subgroup patients had favorable outcomes, and group 3 subgroup patients the worst, consistent with numerous retrospective studies.16-19
Inferior outcomes in the LDCSI group were driven primarily by group 4 patients, the predominant subgroup in this age group. The number of patients in the favorable WNT group is small (n = 64, seven in the LDCSI arm), but there is no suggestion that exploring a de-intensification strategy in this population is unwarranted. Multiple trials evaluating LDCSI (15-18 Gy) in WNT subgroup patients are ongoing (ClinicalTrials.gov identifier: NCT01878617, NCT02724579, and NCT02066220). Unexpectedly, SHH subgroup patients receiving PFRT experienced worse EFS. Of SHH subgroup patients who experienced events, SMNs were only seen in those receiving the larger boost volume. Lack of available germline DNA prohibited investigation into whether or not these patients harbored pathogenic germline variants characteristic of known cancer predisposition syndromes. It is conceivable that the larger volume radiated to higher doses in these patients was a contributing factor to the development of SMNs. As we contemplate future clinical trials for MB, understanding treatment response to different interventions according to molecular subgroup will allow for a more personalized approach to care.
A key aim of treatment de-intensification was to reduce late effects. Importantly, there was significantly less decline in IQ in younger children with LDCSI compared with those who received SDCSI. Smaller RT boost volumes did not affect long-term IQ. Children age 8 years and older at diagnosis treated with SDCSI exhibited no declines in IQ following treatment. This represents a significant departure from most prior outcomes associated with craniospinal therapy and suggests that modern radiotherapy techniques may be associated with substantial reduction in global cognitive morbidity for older children. Future investigations will select patients for treatment de-escalation driven by molecular subgroups that will minimize late cognitive effects while maintaining high EFS. Of note, a more comprehensive assessment of socioeconomic status would also be of benefit in future trials. The impact of RT on hearing was not significant in either volume or dose random assignment. This may be due to the deleterious effects of cisplatin chemotherapy following RT. Chemotherapy treatments continued at full dose unless signs of hearing impairment were present during subsequent chemotherapy cycles.
In conclusion, a dose reduction in CSI for young children with average-risk MB resulted in an unacceptably inferior EFS and OS that was predominantly driven by a single MB subgroup. By contrast, a reduction in radiation boost volume was safe and effective for all patients. Real-time central review of diagnostic imaging and pathology is critical for the success of any study seeking to lessen treatment intensity. Future trials for average-risk MB warrant stratification according to molecular subgroup to safely improve outcomes without compromising patient survival.
ACKNOWLEDGMENT
We are indebted to the Children's Oncology Group Biorepository for their assistance with this project. From St Jude, we explicitly acknowledge the exceptional services provided by The Diagnostic Biomarkers Shared Resource in the Department of Pathology and The Hartwell Center for Biotechnology.
Jeff M. Michalski
Stock and Other Ownership Interests: ViewRay
Consulting or Advisory Role: Mevion Medical Systems, Boston Scientific, Merck Sharp & Dohme, Blue Earth Diagnostics
Research Funding: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Boston Scientific, Merck Sharp & Dohme
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Kristina K. Hardy
Employment: Bayer
Honoraria: Bayer
Speakers' Bureau: Bayer
Travel, Accommodations, Expenses: Bayer
Stephanie M. Perkins
Consulting or Advisory Role: Mevion Medical Systems
Thomas E. Merchant
Travel, Accommodations, Expenses: Philips Healthcare
Nancy J. Tarbell
Consulting or Advisory Role: Mevion Medical Systems, Advanced Onco
Patents, Royalties, Other Intellectual Property: Spouse is an editor for UpToDate
Maryam Fouladi
Research Funding: PTC Therapeutics, Bayer Schering Pharma
Roger J. Packer
Honoraria: Novartis
Consulting or Advisory Role: Novartis, AstraZeneca
Amar Gajjar
Consulting or Advisory Role: Roche/Genentech, QED Therapeutics
Research Funding: Genentech, Kazia Therapeutics
No other potential conflicts of interest were reported.
DISCLAIMER
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data and had the final responsibility to submit for publication.
SUPPORT
Supported by NCTN Operations Center Grant U10CA180886, COG Chairs Grant U10CA098543, NCTN Statistics & Data Center U10CA098413 and U10CA180899, QARC U10CA29511, IROC U24CA180803, COG Biospecimen Bank Grant U24CA196173, St Baldrick's Foundation (PAN), The Brain Tumor Charity (PAN), American Lebanese Syrian Associated Charities (PAN), and St Jude Children's Research Hospital (PAN).
CLINICAL TRIAL INFORMATION
J.M.M. and P.A.N. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Jeff M. Michalski, Anna J. Janss, L. Gilbert Vezina, Leanne M. Embry, Patricia L. Cullen, Scott L. Pomeroy, Thomas E. Merchant,Thomas J. Fitzgerald, Nancy J. Tarbell, Ian F. Pollack, Roger J. Packer, Amar Gajjar
Administrative support: Jeff M. Michalski
Financial support: Paul A. Northcott
Provision of study materials or patients: Jeff M. Michalski, Patricia L. Cullen,Karin M. Muraszko, Ian F. Pollack, Roger J. Packer, Amar Gajjar
Collection and assembly of data: Jeff M. Michalski, Anna J. Janss, L. Gilbert Vezina, Kyle S. Smith, Peter C. Burger, Leanne M. Embry, Patricia L. Cullen, Kristina K. Hardy, Scott L. Pomeroy, Johnnie K. Bass, Stephanie M. Perkins, Thomas E. Merchant, Paul D. Colte, Thomas J. Fitzgerald, Timothy N. Booth, Jennifer Hadley, Rahul Kumar, Roger J. Packer, Amar Gajjar, Paul A. Northcott
Data analysis and interpretation: Jeff M. Michalski, Anna J. Janss, L. Gilbert Vezina, Kyle S. Smith, Catherine A. Billups, Peter C. Burger, Leanne M. Embry, Patricia L. Cullen, Kristina K. Hardy, Scott L. Pomeroy, Johnnie K. Bass, Thomas E. Merchant, Paul D. Colte, Thomas J. Fitzgerald, Joel M. Cherlow, Karin M. Muraszko, Rahul Kumar, Yuanyuan Han, Nancy J. Tarbell, Maryam Fouladi, Roger J. Packer, Yimei Li, Amar Gajjar, Paul A. Northcott
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Children's Oncology Group Phase III Trial of Reduced-Dose and Reduced-Volume Radiotherapy With Chemotherapy for Newly Diagnosed Average-Risk Medulloblastoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jeff M. Michalski
Stock and Other Ownership Interests: ViewRay
Consulting or Advisory Role: Mevion Medical Systems, Boston Scientific, Merck Sharp & Dohme, Blue Earth Diagnostics
Research Funding: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Boston Scientific, Merck Sharp & Dohme
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Kristina K. Hardy
Employment: Bayer
Honoraria: Bayer
Speakers' Bureau: Bayer
Travel, Accommodations, Expenses: Bayer
Stephanie M. Perkins
Consulting or Advisory Role: Mevion Medical Systems
Thomas E. Merchant
Travel, Accommodations, Expenses: Philips Healthcare
Nancy J. Tarbell
Consulting or Advisory Role: Mevion Medical Systems, Advanced Onco
Patents, Royalties, Other Intellectual Property: Spouse is an editor for UpToDate
Maryam Fouladi
Research Funding: PTC Therapeutics, Bayer Schering Pharma
Roger J. Packer
Honoraria: Novartis
Consulting or Advisory Role: Novartis, AstraZeneca
Amar Gajjar
Consulting or Advisory Role: Roche/Genentech, QED Therapeutics
Research Funding: Genentech, Kazia Therapeutics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Packer RJ, Gajjar A, Vezina G, et al. : Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol 24:4202-4208, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Silber JH, Radcliffe J, Peckham V, et al. : Whole-brain irradiation and decline in intelligence: The influence of dose and age on IQ score. J Clin Oncol 10:1390-1396, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Radcliffe J, Packer RJ, Atkins TE, et al. : Three- and four-year cognitive outcome in children with noncortical brain tumors treated with whole-brain radiotherapy. Ann Neurol 32:551-554, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Sklar CA, Constine LS: Chronic neuroendocrinological sequelae of radiation therapy. Int J Radiat Oncol Biol Phys 31:1113-1121, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Thomas PR, Deutsch M, Kepner JL, et al. : Low-stage medulloblastoma: Final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol 18:3004-3011, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Packer RJ, Goldwein J, Nicholson HS, et al. : Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group study. J Clin Oncol 17:2127-2136, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Ris MD, Packer R, Goldwein J, et al. : Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: A Children's Cancer Group study. J Clin Oncol 19:3470-3476, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Sutton LN, Elterman R, et al. : Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg 81:690-698, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Packer RJ, Boyett JM, Janss AJ, et al. : Growth hormone replacement therapy in children with medulloblastoma: Use and effect on tumor control. J Clin Oncol 19:480-487, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Carrie C, Kieffer V, Figarella-Branger D, et al. : Exclusive hyper-fractionated radiotherapy and reduced boost volume for standard-risk medulloblastoma: Pooled analysis of the two French multicentric studies MSFOP98 and MSFOP 2007 and correlation with molecular subgroups. Int J Radiat Oncol Biol Phys 108:1204-1217, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Lannering B, Rutkowski S, Doz F, et al. : Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: Results from the randomized multicenter HIT-SIOP PNET 4 trial. J Clin Oncol 30:3187-3193, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Merchant TE, Happersett L, Finlay JL, et al. : Preliminary results of conformal radiation therapy for medulloblastoma. Neuro Oncol 1:177-187, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolden SL, Dunkel IJ, Souweidane MM, et al. : Patterns of failure using a conformal radiation therapy tumor bed boost for medulloblastoma. J Clin Oncol 21:3079-3083, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Huang E, Teh BS, Strother DR, et al. : Intensity-modulated radiation therapy for pediatric medulloblastoma: Early report on the reduction of ototoxicity. Int J Radiat Oncol Biol Phys 52:599-605, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Perry A, Reifenberger G, et al. : The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol 131:803-820, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Northcott PA, Korshunov A, Witt H, et al. : Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29:1408-1414, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kool M, Korshunov A, Remke M, et al. : Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, group 3, and group 4 medulloblastomas. Acta Neuropathol 123:473-484, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho YJ, Tsherniak A, Tamayo P, et al. : Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol 29:1424-1430, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson MC, Fuller C, Hogg TL, et al. : Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 24:1924-1931, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Capper D, Jones DTW, Sill M, et al. : DNA methylation-based classification of central nervous system tumours. Nature 555:469-474, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MolecularNeuroPathology: www.molecularneuropathology.org
- 22.Northcott PA, Buchhalter I, Morrissy AS, et al. : The whole-genome landscape of medulloblastoma subtypes. Nature 547:311-317, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Embry L, Annett RD, Kunin-Batson A, et al. : Implementation of multi-site neurocognitive assessments within a pediatric cooperative group: Can it be done? Pediatr Blood Cancer 59:536-539, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Sattler JM: Resource Guide to Accompany Assessment of Children: Cognitive Foundations (ed 5). San Diego, CA, J.M. Sattler, 2008 [Google Scholar]
- 25.Taylor MD, Northcott PA, Korshunov A, et al. : Molecular subgroups of medulloblastoma: The current consensus. Acta Neuropathol 123:465-472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


