PURPOSE
Effective treatment options are limited for patients with advanced (metastatic or unresectable) melanoma who progress after immune checkpoint inhibitors and targeted therapies. Adoptive cell therapy using tumor-infiltrating lymphocytes has demonstrated efficacy in advanced melanoma. Lifileucel is an autologous, centrally manufactured tumor-infiltrating lymphocyte product.
METHODS
We conducted a phase II open-label, single-arm, multicenter study in patients with advanced melanoma who had been previously treated with checkpoint inhibitor(s) and BRAF ± MEK targeted agents. Lifileucel was produced from harvested tumor specimens in central Good Manufacturing Practice facilities using a streamlined 22-day process. Patients received a nonmyeloablative lymphodepletion regimen, a single infusion of lifileucel, and up to six doses of high-dose interleukin-2. The primary end point was investigator-assessed objective response rate (ORR) per RECIST, version 1.1.
RESULTS
Sixty-six patients received a mean of 3.3 prior therapies (anti–programmed death 1 [PD-1] or programmed death ligand 1 [PD-L1]: 100%; anticytotoxic T-lymphocyte-associated protein-4: 80%; BRAF ± MEK inhibitor: 23%). The ORR was 36% (95% CI, 25 to 49), with two complete responses and 22 partial responses. Disease control rate was 80% (95% CI, 69 to 89). Median duration of response was not reached after 18.7-month median study follow-up (range, 0.2-34.1 months). In the primary refractory to anti–PD-1 or PD-L1 therapy subset, the ORR and disease control rate were 41% (95% CI, 26 to 57) and 81% (95% CI, 66 to 91), respectively. Safety profile was consistent with known adverse events associated with nonmyeloablative lymphodepletion and interleukin-2.
CONCLUSION
Lifileucel demonstrated durable responses and addresses a major unmet need in patients with metastatic melanoma with limited treatment options after approved therapy, including the primary refractory to anti–PD-1 or PD-L1 therapy subset.
INTRODUCTION
The treatment of advanced (unresectable or metastatic) melanoma with immune checkpoint inhibitors (ICI) and targeted oncogenic pathway inhibition with BRAF and MEK inhibitors has improved patient outcomes.1-7 Forty percent to 65% of patients with advanced melanoma have primary resistance to ICI.8-11 Of those with initial disease control, 30%-40% develop acquired resistance.8,12 Approximately 15% to 20% of BRAF V600 mutation-positive patients fail to respond to targeted therapy initially,13 and only 22% remain progression-free at 3 years.14 Although primary resistance is lower in patients treated with programmed death 1 (PD-1) blocking antibody plus anticytotoxic T-lymphocyte–associated protein 4 (CTLA-4) therapy, 36% of patients discontinue therapy because of treatment-emergent adverse events (TEAEs), with 88% developing immune-related adverse events (irAEs), many of these being persistent.10 Patients progressing after anti–PD-1 therapy, anti–PD-1 plus anti–CTLA-4 therapy, and targeted agents have limited options.15-17 Only 4%-10% of these patients have objective responses to chemotherapy, with a limited median overall survival (OS) of 7 months.15,16,18,19 There are no treatment options with approval based on data from patients with advanced melanoma who have progressed after one line of ICI therapy (for BRAF wild-type tumors), or two lines of therapy (for BRAF V600 mutation-positive tumors). In addition, patients recurring with advanced melanoma after adjuvant anti–PD-1 therapy for high-risk disease represent an emerging unmet need.20-22
CONTEXT
Key Objective
This study evaluated the efficacy and safety of lifileucel, a one-time, autologous tumor-infiltrating lymphocyte (TIL) product, in patients with metastatic melanoma who had progressed on standard immune checkpoint inhibitors (ICI) and targeted therapies (if applicable), who otherwise have limited treatment options. Notably, chemotherapy post-ICI shows poor response rates (4%-10%).
Knowledge Generated
Sixty-six patients received lifileucel infusion with > 1 × 109 TIL cells. Lifileucel was efficacious with an objective response rate of 36%, and a median duration of response that is not reached at 18.7-month median study follow-up.
Relevance
Lifileucel represents a significant improvement in the treatment of advanced melanoma, particularly in the post-ICI patient population, which is an expanding population. The study contributes to the advancement in TIL therapy through a centrally standardized manufacturing approach for autologous TIL, allowing broader patient access.
Adoptive cell therapy with tumor-infiltrating lymphocytes (TIL) offers a potential therapeutic option for metastatic melanoma, although it has not been studied extensively in the ICI era.23-25 TIL are enriched with polyclonal T cells with diverse antigen specificity.26 Extraction of a fragment of tumor followed by ex vivo expansion removes TIL from the hostile tumor microenvironment and reduces the immunosuppressive effects of intratumoral regulatory T cells. Expansion of TIL ex vivo rejuvenates the cells, yielding billions of such cells to be infused back into the patient. Melanoma is characterized by a high mutational burden27 and highly individualized neoantigens.28 A cellular therapy product that can address the broad nature of neoantigens and the unique array from each patient would lead to the possibility of a tailored response. Lifileucel (LN-144) is an autologous TIL therapy that uses tumor-tissue T cells capable of recognizing tumor antigens and being expanded ex vivo while maintaining the heterogeneous repertoire of T cells, using a centralized manufacturing process. We report the safety and efficacy of lifileucel, a one-time cellular therapy, in patients with advanced melanoma who have progressed on ICI and BRAF inhibitors (if BRAF V600 mutation-positive).
METHODS
Trial Conduct
The study was approved by the institutional review board at each site and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonization. All patients provided written informed consent. The study was designed and sponsored by Iovance Biotherapeutics, Inc. All authors discussed, analyzed, and interpreted the results, and vouch for the accuracy and completeness of the data analyses and adherence to the Protocol (online only). All authors contributed to this study and the writing of the manuscript. Professional medical writing or editorial assistance was paid for by the sponsor.
Patients and Study Design
The parent study (ClinicalTrials.gov identifier: NCT02360579) consisted of multiple cohorts (Data Supplement, online only). Cohort 2 data are reported here. Patients were enrolled from April 2017 to January 2019 at 26 sites (see the Data Supplement for the investigator list).
Patients had unresectable or metastatic melanoma (stage IIIC or IV) with confirmed radiologic progression. Patients must have progressed following one or more prior systemic therapies including a PD-1–blocking antibody and if BRAF V600 mutation-positive, a BRAF ± MEK inhibitor. Key eligibility criteria are detailed in the Data Supplement. Patients with a history of irAEs were eligible, as outlined in the Data Supplement.
At least one resectable lesion (or aggregate of lesions) measuring a minimum of 1.5 cm in diameter postresection was required. Resected tumor was processed in a protocol-specified manner and shipped to a Good Manufacturing Practice facility in the provided tumor procurement kit. The optimized manufacturing conditions involved a centralized 22-day process, resulting in a cryopreserved product (Data Supplement). Lifileucel (LN-144) was shipped to the clinical sites after meeting prespecified release criteria. Patients received a nonmyeloablative lymphodepleting (NMA-LD) regimen with cyclophosphamide (60 mg/kg) once daily for 2 days followed by fludarabine (25 mg/m2) once daily for 5 days. A single infusion of lifileucel (1 × 109 – 150 × 109 cells) was thawed and administered after approximately 24 hours from the last dose of fludarabine. A short course of bolus interleukin (IL)-2 (600,000 IU/kg) was infused every 8-12 hours for up to six doses, starting within 3-24 hours of completing lifileucel infusion.
End Points and Assessments
The primary objective was to evaluate the efficacy of a single infusion of lifileucel in patients with unresectable or metastatic melanoma using investigator-assessed objective response rate (ORR) by RECIST v1.1.29 Secondary end points included duration of response (DOR), disease control rate (DCR), OS, and safety. Efficacy assessments started at week 6. Subsequent efficacy, adverse event (AE), and serious AE (SAE) assessment schedules are outlined in the Data Supplement.
Statistical Analysis
The analyses for efficacy and safety were conducted on the full analysis set (FAS), defined as patients from cohort 2 who received lifileucel that met manufacturer's specifications, including a cell dose 1 × 109 – 150 × 109. The planned sample size was 60 based on estimation of ORR using the maximum half-width of the two-sided 95% CI of < 13.2% when ORR is expected to be 20%-50%. This was considered meaningful, assuming that the historical response rate of similar patients after chemotherapy is 10%.15,30 The FAS consisted of 66 patients because of rapid enrollment.
The ORR was analyzed as a binomial proportion with two-sided 95% CI estimated based on the Clopper-Pearson exact method. Time-to-event efficacy end points were estimated using the Kaplan-Meier product limit method, and two-sided corresponding 95% CIs were based on log-log transformation. Safety data were reported descriptively. All statistical analyses were conducted using the Statistical Analysis System (SAS) version 9.4.
RESULTS
Patients and Treatment
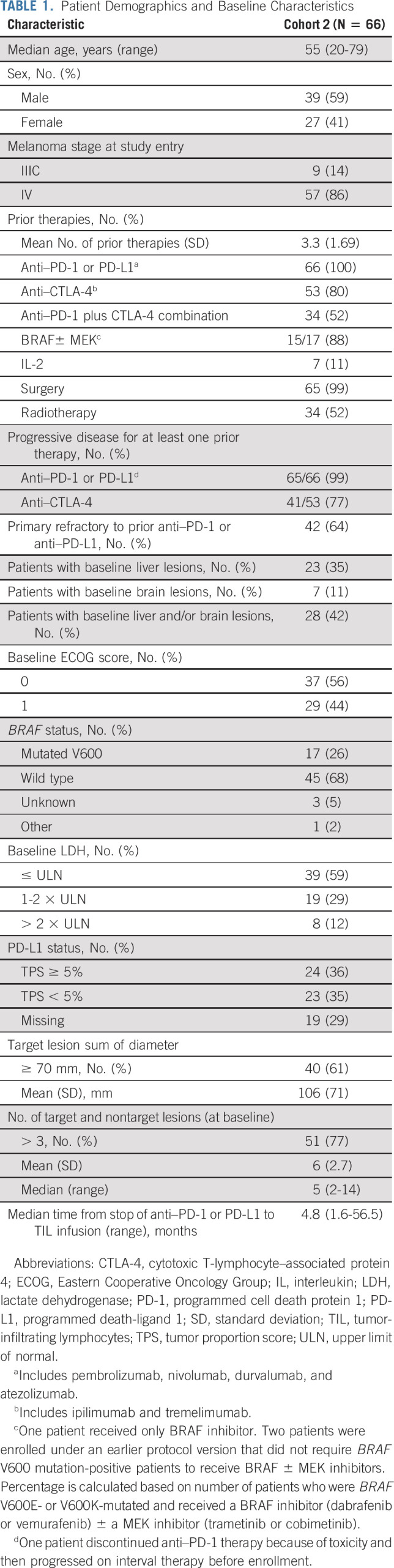
Seventy-eight patients underwent tumor resection. Sixty-six patients received lifileucel (LN-144) infusion with > 1 × 109 but < 150 × 109 TIL cells and comprised the FAS. Three patients either did not receive TIL or received < 1 × 109 TIL cells, whereas nine patients could not be treated because of other causes (Data Supplement). Table 1 details the demographics and baseline characteristics. Patients had received a mean of 3.3 lines of prior therapies (range, 1-9 lines). All patients had received prior anti–PD-1 or anti–programmed death ligand 1 (PD-L1) therapy, and 53 (80%) had received prior anti–CTLA-4 therapy. Fifty-two percent of the patients had received concurrent CTLA-4 plus PD-1 blockade. Notably, 99% had progressed on prior anti–PD-1 or PD-L1 therapy, and 77% had progressed on prior anti–CTLA-4 therapy. Overall, 42 patients (64%) had a best response of progressive disease to initial anti–PD-1 or PD-L1 therapy (primary refractory subset). Of the 17 patients who were BRAF V600 mutation-positive, 88% had received BRAF ± MEK inhibitors. Forty patients (61%) had a baseline target lesion sum of diameters (SOD) ≥ 70 mm, 51 (77%) patients had more than three target and nontarget lesions at baseline, and 27 (41%) had baseline lactate dehydrogenase levels higher than institutional upper limit of normal. Overall, patients had a high tumor burden at baseline (mean SOD for the target lesions: 106 mm); 28 patients (42%) had liver and/or brain lesions at baseline.
TABLE 1.
Patient Demographics and Baseline Characteristics
The harvested tumor was collected from a variety of sites, such as skin, lymph nodes, liver, lung, peritoneum, musculoskeletal sites, breast, and other organs. The median number of cyclophosphamide and fludarabine doses were 2 (range, 1-2) and 5 (range, 2-5), respectively. The mean number of TIL cells infused was 27.3 × 109 (range, 1.2 × 109 to 99.5 × 109). The median number of IL-2 doses administered was 5.5 (range, 1-6).
Efficacy
Sixty-six patients received a lifileucel infusion of ≥ 1 × 109 TIL cells. At the data cutoff of April 23, 2020 (median follow-up of 18.7 months [range, 0.2-34.1 months]), the investigator-assessed ORR was 36% (95% CI, 25 to 49) and the DCR was 80% (95% CI, 69 to 89) (Table 2), with 2 (3%) complete responses (CRs), 22 (33%) partial responses (PRs), and 29 (44%) patients showing stable disease (SD). Sixty-two patients (94%) had a baseline and at least one postbaseline radiologic assessment. Of the four patients in the FAS who did not undergo postbaseline assessment, three had died of disease, and one received an additional line of systemic therapy; all were considered as not evaluable for best overall response. Of the evaluable patients, 50 (81%) had a reduction in tumor burden (Fig 1A). Data Supplement details the percentage change in target SOD from baseline over time in patients who achieved a confirmed response. Response to lifileucel was observed regardless of age, prior anti–CTLA-4 use, BRAF mutation status, PD-L1 status as measured by tumor proportion score, baseline Eastern Cooperative Oncology Group performance status, tumor burden (assessed by lactate dehydrogenase elevation above upper limit of normal and target lesion SOD at baseline), presence of liver and/or brain lesions at baseline, and timing of prior PD-1 therapy (Fig 1B).
TABLE 2.
Efficacy Outcomes by Investigator Assessment
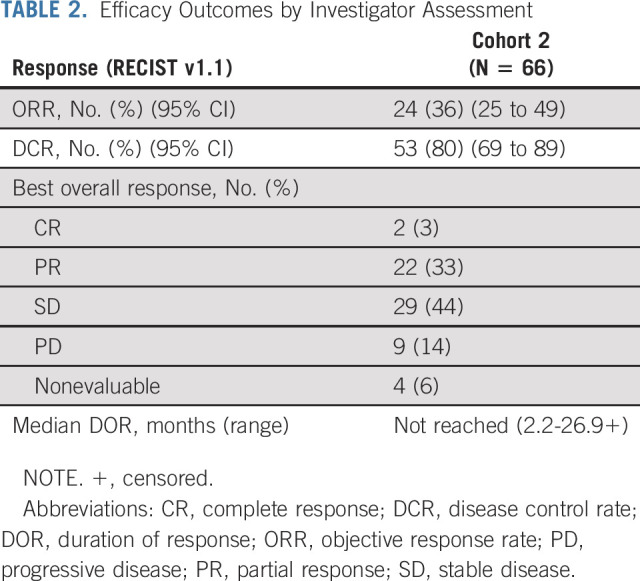
FIG 1.
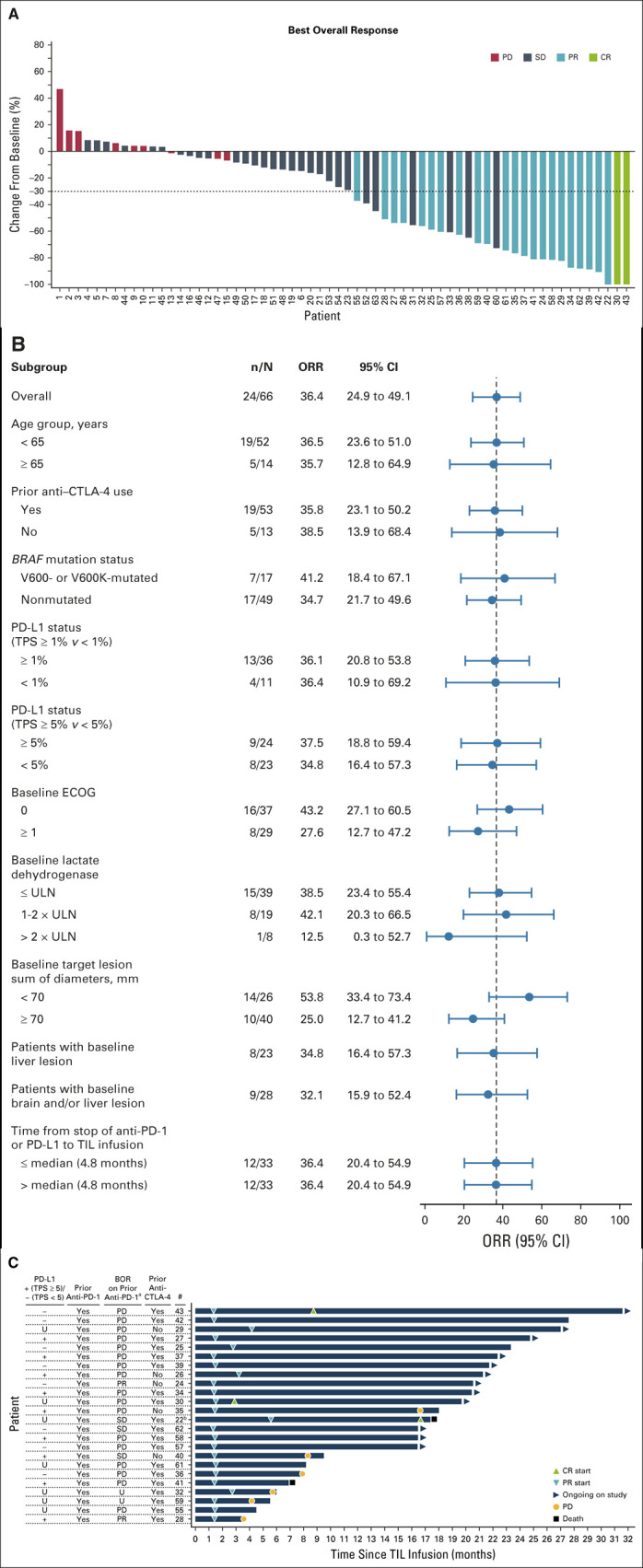
Change in tumor burden of target lesions, response by subgroup, and response assessment in individual patients. (A) Waterfall plot depicting BOR as assessed by investigator and the best change from baseline in the SOD of the target lesions (per RECIST v1.1 criteria) in the FAS. A change of −100% from baseline is presented for CR assessment that includes lymph node lesions that resolved to < 10 mm. The horizontal dashed line indicates a 30% reduction in the tumor burden in the target lesions. Twelve patients had an increase in the SOD of the target lesions, whereas 50 patients had a decrease in the SOD of the target lesions. Thirty patients (two CR, 22 PR, and six SD) had > 30% reduction in the SOD of the target lesions. Three patients had no post-TIL assessments because of early death. One patient had no post-TIL assessment because of start of new anticancer therapy before day 42.
Median time from lifileucel infusion to best response was 1.4 months (range, 1.3-8.7 months). Time to response for individual patients is illustrated in Figure 1C; 19 of 24 patients achieved response by the time of first planned assessment (6 weeks after lifileucel infusion). Only 25% of patients had progressed after achieving a response. The median DOR has not been reached (95% CI, 11.8 months to not reached) (Fig 2A) with a 1-year DOR of 69% (95% CI, 46 to 84). The median OS was 17.4 months (95% CI, 11.0 to not reached; Fig 2B). Of the patients who had SD and PR or CR, 38% and 92% patients, respectively, had an OS ≥ 1 year. Progression-free survival for the FAS is shown in the Data Supplement, and duration of SD in individual patients is outlined in the Data Supplement.
FIG 2.
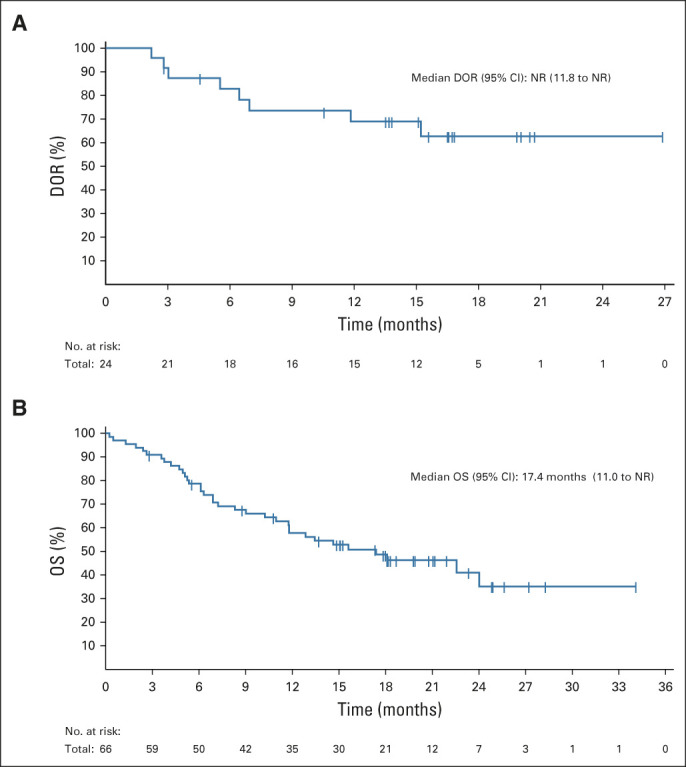
(A) The Kaplan-Meier curve for DOR in confirmed responders who achieved a PR or better. The DOR is measured from the time point at which the initial measurement criteria are met for a PR or CR, whichever occurred first, until the first date that PD or death occurred. (B) The Kaplan-Meier curve for OS in the full analysis set. OS was defined as the time (in months) from the start date of lifileucel infusion to death because of any cause. Patients who were alive at the time of data cutoff had their event times censored on the last date of their known survival status. The median OS was 17.4 months (95% CI, 11.0 to NR), with 1-year OS of 58% (95% CI, 45 to 69). CR, complete response; DOR, duration of response; NR, not reached; OS, overall survival; PD, progressive disease; PR, partial response.
An efficacy analysis was performed for the primary-refractory subset (42 patients primary refractory to anti–PD-1 or PD-L1 therapy). The ORR was 41% (95% CI, 26 to 57), with 2 CRs (5%) and 15 PRs (36%), and the DCR was 81% (95% CI, 66 to 91). Seventeen (41%) of these patients had SD, and five (12%) had progressive disease; three patients were nonevaluable. Median DOR was not reached for this subpopulation.
Thirty-four (52%) patients received anti–PD-1 plus anti–CTLA-4 combination therapy, either as frontline (n = 15, 23%), or after failing frontline therapy (n = 19, 29%). The ORRs for lifileucel in these two subsets were 33% (5/15) and 32% (6/19), respectively. The ORRs for lifileucel in patients with primary resistance (n = 17) or acquired resistance (n = 11) to anti–PD-1 plus anti–CTLA-4 combination therapy were 35% (6/17) and 27% (3/11), respectively. Details of these patients who responded to lifileucel are outlined in the Data Supplement.
Exploratory analyses of product-specific characteristics, including levels of phenotypic markers of T-cell lineage, memory subset, youth, activation or exhaustion, or trafficking (Data Supplement), did not demonstrate association with response. Tumor burden reductions were seen across the continuum of cell doses (Data Supplement).
Safety
All patients experienced at least one TEAE, with the most common (≥ 30%) grade 3 or 4 TEAEs being thrombocytopenia (82%), anemia (56%), febrile neutropenia (55%), neutropenia (39%), hypophosphatemia (35%), leukopenia (35%), and lymphopenia (32%) (Table 3), consistent with the toxicity profile of NMA-LD and IL-2. Fatal TEAEs occurred in two patients—1 death was because of intra-abdominal tumor hemorrhage reported as possibly related to TIL, and one was because of acute respiratory failure assessed as not related to TIL by the investigator. The incidence of TEAEs, including grade 3 or 4 TEAEs, decreased rapidly over time (Fig 3) with no lifileucel-related SAEs reported after 6 months, and no recurrence of irAEs related to prior ICI. Tumor harvest AEs related to surgery are outlined in the Data Supplement.
TABLE 3.
TEAEs Occurring in ≥ 20% of Patients
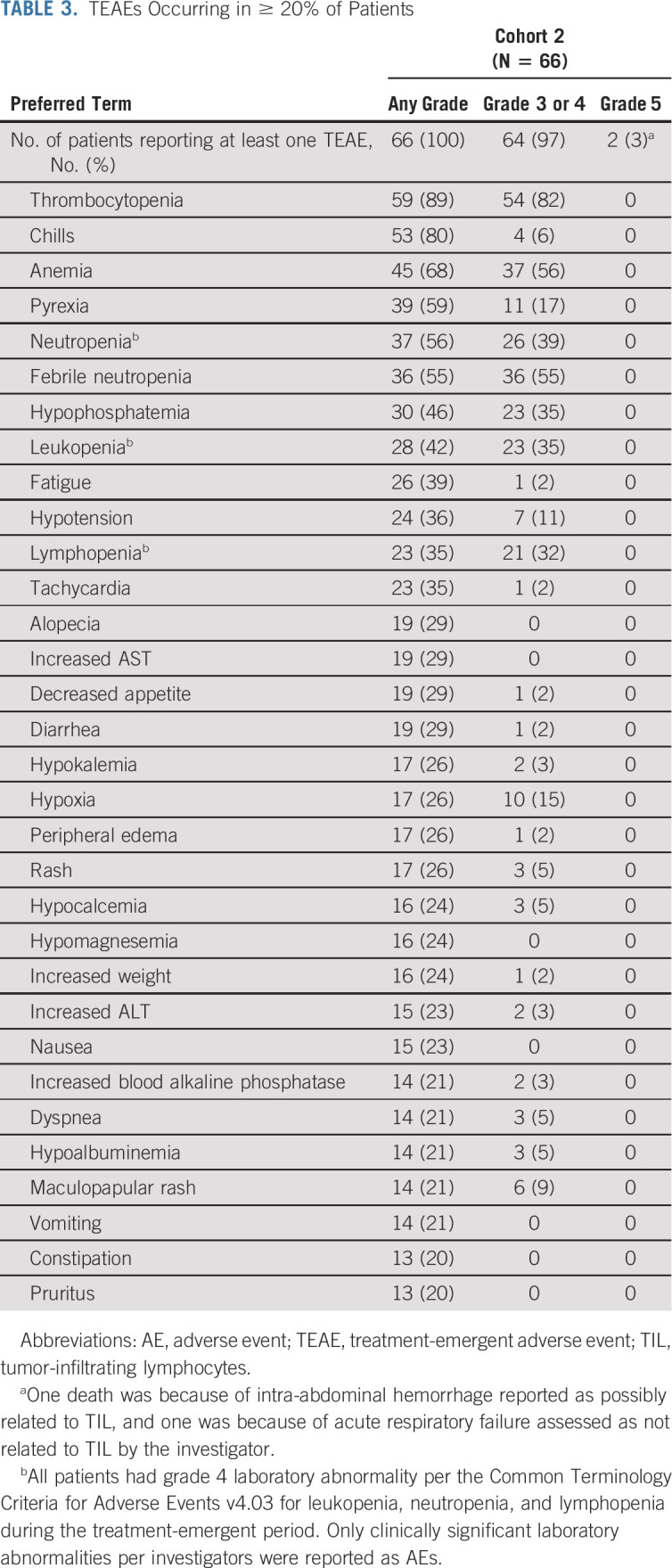
FIG 3.
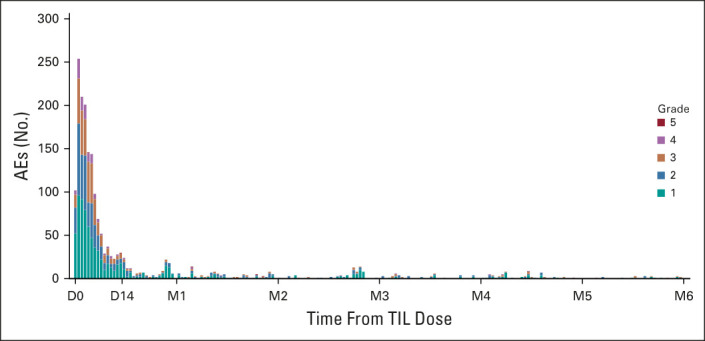
AEs over time. The distribution of onset of AEs starting from lifileucel infusion until 6 months postinfusion is shown. A TEAE was defined as any AE with onset after start of lifileucel through day 30 postinfusion. All occurrences of AEs were counted if a patient experienced a new onset of the same AE at different timepoints. If multiple records were reported on the electronic case report form because of toxicity grade decrease of the same AE that had not resolved, then the event was counted once with the highest grade reported. Overall, 24 AEs were reported post month 6 until data cutoff date, which are not shown in the histogram. No SAEs related to lifileucel were reported post month 6. AE, adverse event; D, day; M, month; SAE, serious adverse event; TEAE, treatment-emergent adverse event; TIL, tumor-infiltrating lymphocytes.
DISCUSSION
Treatment options for patients with advanced melanoma who progress after treatment with ICI and BRAF ± MEK inhibitors are limited. Cytotoxic chemotherapy has shown poor response rates,15,16,18,19 with a limited median OS of 7 months.15 Many of the patients in our study had exhausted all approved therapy (mean lines of prior therapy, 3.3). The encouraging antitumor activity of lifileucel observed in our study addresses a major unmet need in patients with advanced melanoma after progression on ICI, and targeted agents if indicated.
Lifileucel, a one-time cellular therapy, represents a significant improvement in the treatment of advanced melanoma, particularly the current post-ICI patient population. First, lifileucel demonstrated an ORR of 36%, meeting the study primary end point in a patient population that had failed frontline anti–PD-1 therapy, the current standard of care. This is noteworthy because prior TIL therapy studies were conducted in the pre-ICI era, or enrolled a very small population of patients who had received prior anti–PD-1 therapy.23-25,31 A previous cohort 2 analysis has demonstrated a high concordance rate of 89.4% between the Independent Review Committee–assessed and investigator-assessed ORR.32 Second, at a median 18.7-month follow-up, the median DOR has not been reached, emphasizing the durability of lifileucel responses in a heavily pretreated post-ICI patient population with a high baseline tumor burden. Third, the efficacy of lifileucel was equivalent agnostic of PD-L1 status, BRAF mutation status, or prior anti–CTLA-4 therapy. Lifileucel was efficacious in the subset of patients who were primarily refractory to anti–PD-1/PD-L1 therapy, demonstrating an ORR of 41% and a DCR of 81% in this subgroup. Furthermore, lifileucel demonstrated similar ORR in patients who received anti–PD-1 plus anti–CTLA-4 combination as a frontline therapy (33%) or after failing frontline therapy (32%).
TIL recognize multiple tumor-specific neoantigens,33 which may be required for response in solid tumors with high mutational burden. Removal from the hostile microenvironment and ex vivo expansion enable TIL to evade a broad array of immunosuppressive mechanisms. Indeed, both downregulation of PD-1 expression and restored functionality were reported for ex vivo expanded TIL.34,35 By contrast, ICI target only a limited number of pathways in situ. Additionally, in vitro culture results in large-scale expansion of TIL, potentially increasing the number of tumor-specific T cells available for tumor targeting after adoptive transfer. The T cells comprising the TIL product are recovered directly from the tumor tissue, a site enriched for T-cell clones that are able to recognize patient-specific tumor antigens.36,37 As a result, a polyclonal product is obtained that has the potential to target multiple relevant antigens, addressing (1) the ability to identify the unique spectrum of patient-specific tumor antigens38; (2) the heterogeneous nature of solid tumors39; and (3) immune escape through antigen loss.40 Finally, substantial fractions of TIL-derived T cells were shown to persist for at least 6 weeks,41 consistent with the memory phenotype of the majority of the T cells comprising the product.35 These varied mechanisms and TIL properties likely contribute to the antitumor efficacy of lifileucel.
The tumors were harvested with minimal surgical morbidity, although 58% were extranodal or nonskin/subcutaneous lesions. A small subset of enrolled patients (12%) could not be treated because of progression, death, or other causes.
TEAEs occurred during or immediately after NMA-LD or IL-2 administration and were generally transient, with no new lifileucel-associated SAEs reported after 6 months. Although patients were hospitalized for NMA-LD and IL-2 administration, lifileucel is a one-time cellular therapy with durable responses, as demonstrated by an ongoing response in 50% of responders at a median follow-up of 18.7 months. In addition, the safety profile indicates that lifileucel is a viable option for patients who are not eligible for ICI because of prior significant irAEs, as it is not associated with recrudescence of irAEs.
Single-center studies conducted at NCI23,31 have been important in laying the groundwork for TIL therapy in patients with advanced melanoma but were limited to a few centers with dedicated on-site cell therapy facilities. Although lifileucel centralized manufacturing required shipping of the tumor samples, TIL could be manufactured in 96% of patients. The present multicenter study constitutes a significant advance by successfully demonstrating the feasibility of a centrally standardized manufacturing approach for TIL therapy, which allows for broadened patient access, whereas cryopreservation of lifileucel provides flexibility in treatment scheduling in the real-world clinical setting.
In summary, lifileucel, a first-in-class centrally manufactured autologous TIL cell therapy, was efficacious and demonstrated durable responses in heavily pretreated patients and represents a potential new standard of care for patients with advanced melanoma following failure of ICI and targeted therapy. Patients with advanced melanoma who have failed anti–PD-1 therapy (and BRAF ± MEK inhibitors if BRAF V600 mutation-positive), irrespective of baseline tumor characteristics, should be considered for the one-time lifileucel therapy as second-line therapy (third-line if BRAF V600 mutation positive) if they have performance status and organ function adequate for administration of lymphodepleting chemotherapy and a shortened course of IL-2. The US Food and Drug Administration has granted lifileucel a Regenerative Medicine Advanced Therapy designation, Orphan Drug designation, and a Fast Track designation for advanced melanoma. Based on these encouraging results, an additional cohort has been fully enrolled, using Independent Review Committee–assessed ORR for registration purposes. Given the favorable risk-benefit profile of lifileucel, its role earlier in the disease course and in combination with ICI is being investigated in melanoma, as well as additional studies in other metastatic solid malignancies.
Amod A. Sarnaik
Honoraria: Physicans' Education Resource
Consulting or Advisory Role: B4CC, Iovance Biotherapeutics, Guidepoint Global, Defined Health, Gerson Lehrman Group
Research Funding: Provectus, Genentech, Iovance Biotherapeutics
Patents, Royalties, Other Intellectual Property: Compositions and methods for improving tumor-infiltrating lymphocytes for adoptive cell therapy, filed March 20, 2014 US Patent Application No. 61/955,970 and second Application No. 61/973,002
Travel, Accommodations, Expenses: Iovance Biotherapeutics
Omid Hamid
Honoraria: Bristol Myers Squibb, Novartis, Sanofi/Regeneron, Pfizer
Consulting or Advisory Role: Amgen, Novartis, Roche, Bristol Myers Squibb, Merck, Aduro Biotech, Akeso Biopharma, BeiGene, Genentech, GlaxoSmithKline, Immunocore, Incyte, Janssen, NextCure, Regeneron, Sanofi, Seattle Genetics, Tempus, Zelluna, BioAtla, Idera, Pfizer
Speakers' Bureau: Bristol Myers Squibb, Novartis, Sanofi/Regeneron, Pfizer
Research Funding: Bristol Myers Squibb, Genentech, Immunocore, Incyte, Merck, Merck Serono, Novartis, Pfizer, Roche, Amgen, CytomX Therapeutics, Iovance Biotherapeutics, NextCure, GlaxoSmithKline, Arcus Biosciences, Aduro Biotech, Akeso Biopharma, Exelixis, Moderna Therapeutics, Regeneron, Sanofi, Seattle Genetics, Torque, Zelluna, Bioatla, Idera
Nikhil I. Khushalani
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, Mazor Robotics, Amarin Corporation, Asensus Surgical
Honoraria: Sanofi
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Regeneron, Array BioPharma, Immunocore, Merck, Incyte, Jounce Therapeutics, Iovance Biotherapeutics, NCCN/Pfizer
Research Funding: Bristol Myers Squibb, Merck, Novartis, GlaxoSmithKline, HUYA Bioscience International, Amgen, Regeneron, Celgene, Replimune
Karl D. Lewis
Honoraria: Array BioPharma, Iovance Biotherapeutics
Consulting or Advisory Role: Array BioPharma, Merck, Roche, Regeneron, Sanofi, Iovance Biotherapeutics
Research Funding: Roche/Genentech, Merck, Array BioPharma, Incyte, Nektar, Iovance Biotherapeutics, Bristol Myers Squibb, Kartos Therapeutics, OncoSec, Regeneron, Alkermes, Neon Therapeutics, Ultimovacs, Senhwa Biosciences, Replimune, Amgen
Travel, Accommodations, Expenses: Merck, Roche/Genentech, Regeneron, Neon Therapeutics, Alkermes
Uncompensated Relationships: Roche/Genentech, Regeneron
Theresa Medina
Consulting or Advisory Role: Bristol Meyer Squibb, Iovance Biotherapeutics
Research Funding: Merck, Replimune, Bristol Myers Squibb, Iovance Biotherapeutics, Nektar, Immunocore, Checkmate Pharmaceuticals, BioAtla
Harriet M. Kluger
Consulting or Advisory Role: Nektar, Celldex, Iovance Biotherapeutics, Merck, Immunocore, Array BioPharma, ElevateBio, Instil Bio, Clinigen Group, Shionogi, Bristol Myers Squibb
Research Funding: Merck, Bristol Myers Squibb, Apexigen
Travel, Accommodations, Expenses: Apexigen
Sajeve S. Thomas
Speakers' Bureau: BMS, Merck, Genentech, Ipsen, Amgen, Pfizer, Novartis
Evidio Domingo-Musibay
Honoraria: Regeneron
Consulting or Advisory Role: Sanofi/Regeneron, Castle Biosciences
Speakers' Bureau: Sanofi/Regeneron
Research Funding: Clinigen Group, Iovance Biotherapeutics, Nektar
Anna C. Pavlick
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Regeneron, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb, Merck, Millennium, Regeneron, Replimune
Eric D. Whitman
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, Castle Biosciences, Novartis, Eisai, Pfizer
Speakers' Bureau: Bristol Myers Squibb, Merck Sharp & Dohme, Castle Biosciences, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb, Merck Sharp & Dohme, Castle Biosciences, Genentech/Roche, Amgen, TRACON Pharma, AstraZeneca/MedImmune, Provectus, Oncolys BioPharma, Iovance Biotherapeutics, Dynavax Technologies, OncoSec, Toray Industries, Array BioPharma
Patents, Royalties, Other Intellectual Property: Nerve monitoring dissection device, Lighted Polyhedral surgical retractor
Salvador Martin-Algarra
Consulting or Advisory Role: MSD Oncology, Sanofi, Regeneron, AstraZeneca
Speakers' Bureau: Bristol Myers Squibb, MSD Oncology, AstraZeneca, Novartis, Roche, Sanofi/Regeneron
Travel, Accommodations, Expenses: Pierre Fabre, Roche, MSD Oncology
Pippa Corrie
Honoraria: Novartis, Merck Sharp & Dohme, Pierre Fabre, Bristol Myers Squibb
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Incyte, Pierre Fabre, Roche, Microbiotica
Speakers' Bureau: Merck Sharp & Dohme, Novartis, Bristol Myers Squibb
Research Funding: MSD, Bristol Myers Squibb, Novartis, Array BioPharma, Celgene, Halozyme, Iovance Biotherapeutics, Lilly
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck Sharp & Dohme
Brendan D. Curti
Honoraria: Clinigen Group, Nektar
Consulting or Advisory Role: Merck
Research Funding: Bristol Myers Squibb, Galectin Therapeutics, Clinigen Group
Patents, Royalties, Other Intellectual Property: Biomarkers for OX40 response
Travel, Accommodations, Expenses: Agonox
Jose Lutzky
Consulting or Advisory Role: Castle Biosciences, Iovance Biotherapeutics, Replimune, Regeneron
Research Funding: Bristol Myers Squibb, Novartis, Iovance Biotherapeutics, Immunocore, Regeneron, Replimune, Vyriad
Jeffrey S. Weber
Stock and Other Ownership Interests: CytomX Therapeutics, Biond Biologics, Protean Biodiagnostics, Neximmune
Honoraria: Bristol Myers Squibb, Merck, Genentech, AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Amgen, Roche, Celldex, CytomX Therapeutics, Novartis, Sellas Life Sciences, WindMIL, Takeda, Moderna Therapeutics, Jounce Therapeutics, Kirin Pharmaceuticals, Regeneron, Idera, Oncosec
Consulting or Advisory Role: Celldex, Bristol Myers Squibb, Merck, Genentech, Roche, Amgen, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo, CytomX Therapeutics, Novartis, Sellas Life Sciences, WindMIL, Jounce Therapeutics, Moderna Therapeutics, Kirin Pharmaceuticals, Protean Biodiagnostics, Idera, Oncosec
Research Funding: Bristol Myers Squibb, Merck, GlaxoSmithKline, Genentech, Astellas Pharma, Incyte, Roche, Novartis, NextCure, Moderna Therapeutics
Patents, Royalties, Other Intellectual Property: Named on a patent submitted by Moffitt Cancer Center for an IPILIMUMAB biomarker, named on a patent from Biodesix for a PD-1 antibody biomarker, named on a patent for 41BB induced TIL by Moffitt Cancer Center
Travel, Accommodations, Expenses: Bristol Myers Squibb, GlaxoSmithKline, Roche, Celldex, Amgen, Merck, AstraZeneca, Genentech, Novartis
James M. G. Larkin
Honoraria: Eisai, Bristol Myers Squibb, MSD, GlaxoSmithKline, Pfizer, Novartis, Roche/Genentech, Pierre Fabre, EUSA Pharma, Achilles Therapeutics, AstraZeneca, Boston Biomedical, Ipsen, Imugene, Incyte, iOncologi, Merck Serono, Nektar, Vitaccess, Kymab, Secarna
Consulting or Advisory Role: Eisai, Bristol Myers Squibb, MSD, GlaxoSmithKline, Pfizer, Novartis, Roche/Genentech, Pierre Fabre, EUSA Pharma, Achilles Therapeutics, AstraZeneca, Boston Biomedical, Ipsen, Imugene, Incyte, iOncologi, Merck Serono, Nektar, Vitaccess, Secarna, Kymab
Research Funding: Pfizer, Novartis, MSD, Bristol Myers Squibb, Achilles Therapeutics, Roche, Nektar, Covance, Immunocore, AVEO
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pfizer, Novartis, Roche/Genentech, AstraZeneca, Boston Biomedical, Incyte, GlaxoSmithKline, Pierre Fabre, Merck Serono
Wen Shi
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Travel, Accommodations, Expenses: Iovance Biotherapeutics
Toshimi Takamura
Employment: Iovance Biotherapeutics Inc
Madan Jagasia
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Consulting or Advisory Role: Kadmon
Harry Qin
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Xiao Wu
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Travel, Accommodations, Expenses: Iovance Biotherapeutics
Cecile Chartier
Employment: Iovance Biotherapeutics
Leadership: Iovance Biotherapeutics
Patents, Royalties, Other Intellectual Property: PCT/US2019/059598 for Expansion of TILs Utilizing AKT Pathway Inhibitors. PCT/US2019/012729 for Processes for Generating TIL Products Enriched for Tumor Antigen-specific T-cells. PCT/US2019/029286 for Gene Editing of Tumor Infiltrating Lymphocytes and Uses of Same in Immunotherapy. PCT/US2019/065892 for Methods of Expanding Tumor Infiltrating Lymphocytes Using Engineered Cytokine Receptor Pairs and Uses Thereof. PCT/US2019/012733 for Processes for Generating TIL Products Enriched for Tumor Antigen-Specific T-Cells. PCT/US2020/013095 for System and Methods for Monitoring Adoptive Cell Therapy Clonality and Persistence. PCT/US2020/063767 for Processes for the Production of Tumor Infiltrating Lymphocytes (TILs) and Methods of Using the Same. PCT/US2020/057135 for Gene Editing of Tumor Infiltrating Lymphocytes and Uses of Same in Immunotherapy. One patent application is nonpublic, for which Iovance declines to furnish any information
Travel, Accommodations, Expenses: Iovance Biotherapeutics
Friedrich Graf Finckenstein
Employment: Adverum, Iovance Biotherapeutics, Roche/Genentech
Leadership: Iovance Biotherapeutics
Stock and Other Ownership Interests: Roche/Genentech, Bristol Myers Squibb, Johnson & Johnson, Iovance Biotherapeutics, Adverum
Travel, Accommodations, Expenses: Iovance Biotherapeutics, Roche/Genentech
Maria Fardis
Employment: Iovance Biotherapeutics, Acerta Pharma
Leadership: Iovance Biotherapeutics, Acerta Pharma
Stock and Other Ownership Interests: Iovance Biotherapeutics, Acerta Pharma, Gilead Sciences, AbbVie, Kartos Therapeutics
Patents, Royalties, Other Intellectual Property: Iovance Biotherapeutics, Acerta Pharma
Travel, Accommodations, Expenses: Iovance Biotherapeutics, Acerta Pharma
John M. Kirkwood
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Iovance Biotherapeutics, Elsevier, Amgen, Checkmate Pharmaceuticals, Harbour BioMed, Istari Oncology, OncoSec, Scopus BioPharma, Pfizer
Speakers' Bureau: Bristol Myers Squibb
Research Funding: Amgen, Bristol Myers Squibb, Castle Biosciences, Checkmate Pharmaceuticals, Immunocore, Iovance Biotherapeutics, Novartis, Merck
Jason A. Chesney
Research Funding: Amgen, Replimune, Iovance Biotherapeutics, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: University of Louisville US Patents
No other potential conflicts of interest were reported.
See accompanying editorial on page 2640
PRIOR PRESENTATION
Presented in part at the annual meeting of the Society for Immunotherapy of Cancer (SITC), November 6-10, 2019, National Harbor, MD; annual meeting of the Society of Melanoma Research (SMR), November 20-23, 2019, Salt Lake City, UT; and the ASCO 2020 Virtual Annual Meeting, May 29-June 1, 2020.
SUPPORT
Supported by Iovance Biotherapeutics Inc. NIH grant that was funded during the clinical trial 5K23CA178083-03, A.A.S.
Iovance Biotherapeutics sponsored the C-144-01 trial, provided the trial drugs, and collaborated with the authors on the trial design and on the collection, analysis, and interpretation of the data. Medical writing support, funded by Iovance Biotherapeutics (with specific direction from authors), was provided by Swati Ghatpande, PhD, of Second City Science (Vaniam Group LLC).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Amod A. Sarnaik, Harriet M. Kluger, Eric D. Whitman, Wen Shi, Xiao Wu, Friedrich Graf Finckenstein, Maria Fardis, Jason A. Chesney
Provision of study materials or patients: Nikhil I. Khushalani, Karl D. Lewis, Theresa Medina, Evidio Domingo-Musibay, Anna C. Pavlick, Salvador Martin-Algarra, Pippa Corrie, Brendan D. Curti, Jose Lutzky, Jeffrey S. Weber, James M. G. Larkin, Cecile Chartier
Collection and assembly of data: Amod A. Sarnaik, Omid Hamid, Nikhil I. Khushalani, Karl D. Lewis, Theresa Medina, Harriet M. Kluger, Sajeve S. Thomas, Evidio Domingo-Musibay, Anna C. Pavlick, Eric D. Whitman, Salvador Martin-Algarra, Pippa Corrie, Brendan D. Curti, Judit Oláh, Jose Lutzky, Jeffrey S. Weber, Wen Shi, Toshimi Takamura, Xiao Wu, Cecile Chartier, John M. Kirkwood, Jason A. Chesney
Data analysis and interpretation: Amod A. Sarnaik, Omid Hamid, Nikhil I. Khushalani, Harriet M. Kluger, Sajeve S. Thomas, Evidio Domingo-Musibay, Eric D. Whitman, Salvador Martin-Algarra, Pippa Corrie, Brendan D. Curti, James M. G. Larkin, Wen Shi, Madan Jagasia, Harry Qin, Xiao Wu, Cecile Chartier, Friedrich Graf Finckenstein, Maria Fardis, John M. Kirkwood, Jason A. Chesney
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Lifileucel, a Tumor-Infiltrating Lymphocyte Therapy, in Metastatic Melanoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Amod A. Sarnaik
Honoraria: Physicans' Education Resource
Consulting or Advisory Role: B4CC, Iovance Biotherapeutics, Guidepoint Global, Defined Health, Gerson Lehrman Group
Research Funding: Provectus, Genentech, Iovance Biotherapeutics
Patents, Royalties, Other Intellectual Property: Compositions and methods for improving tumor-infiltrating lymphocytes for adoptive cell therapy, filed March 20, 2014 US Patent Application No. 61/955,970 and second Application No. 61/973,002
Travel, Accommodations, Expenses: Iovance Biotherapeutics
Omid Hamid
Honoraria: Bristol Myers Squibb, Novartis, Sanofi/Regeneron, Pfizer
Consulting or Advisory Role: Amgen, Novartis, Roche, Bristol Myers Squibb, Merck, Aduro Biotech, Akeso Biopharma, BeiGene, Genentech, GlaxoSmithKline, Immunocore, Incyte, Janssen, NextCure, Regeneron, Sanofi, Seattle Genetics, Tempus, Zelluna, BioAtla, Idera, Pfizer
Speakers' Bureau: Bristol Myers Squibb, Novartis, Sanofi/Regeneron, Pfizer
Research Funding: Bristol Myers Squibb, Genentech, Immunocore, Incyte, Merck, Merck Serono, Novartis, Pfizer, Roche, Amgen, CytomX Therapeutics, Iovance Biotherapeutics, NextCure, GlaxoSmithKline, Arcus Biosciences, Aduro Biotech, Akeso Biopharma, Exelixis, Moderna Therapeutics, Regeneron, Sanofi, Seattle Genetics, Torque, Zelluna, Bioatla, Idera
Nikhil I. Khushalani
Stock and Other Ownership Interests: Bellicum Pharmaceuticals, Mazor Robotics, Amarin Corporation, Asensus Surgical
Honoraria: Sanofi
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca, Regeneron, Array BioPharma, Immunocore, Merck, Incyte, Jounce Therapeutics, Iovance Biotherapeutics, NCCN/Pfizer
Research Funding: Bristol Myers Squibb, Merck, Novartis, GlaxoSmithKline, HUYA Bioscience International, Amgen, Regeneron, Celgene, Replimune
Karl D. Lewis
Honoraria: Array BioPharma, Iovance Biotherapeutics
Consulting or Advisory Role: Array BioPharma, Merck, Roche, Regeneron, Sanofi, Iovance Biotherapeutics
Research Funding: Roche/Genentech, Merck, Array BioPharma, Incyte, Nektar, Iovance Biotherapeutics, Bristol Myers Squibb, Kartos Therapeutics, OncoSec, Regeneron, Alkermes, Neon Therapeutics, Ultimovacs, Senhwa Biosciences, Replimune, Amgen
Travel, Accommodations, Expenses: Merck, Roche/Genentech, Regeneron, Neon Therapeutics, Alkermes
Uncompensated Relationships: Roche/Genentech, Regeneron
Theresa Medina
Consulting or Advisory Role: Bristol Meyer Squibb, Iovance Biotherapeutics
Research Funding: Merck, Replimune, Bristol Myers Squibb, Iovance Biotherapeutics, Nektar, Immunocore, Checkmate Pharmaceuticals, BioAtla
Harriet M. Kluger
Consulting or Advisory Role: Nektar, Celldex, Iovance Biotherapeutics, Merck, Immunocore, Array BioPharma, ElevateBio, Instil Bio, Clinigen Group, Shionogi, Bristol Myers Squibb
Research Funding: Merck, Bristol Myers Squibb, Apexigen
Travel, Accommodations, Expenses: Apexigen
Sajeve S. Thomas
Speakers' Bureau: BMS, Merck, Genentech, Ipsen, Amgen, Pfizer, Novartis
Evidio Domingo-Musibay
Honoraria: Regeneron
Consulting or Advisory Role: Sanofi/Regeneron, Castle Biosciences
Speakers' Bureau: Sanofi/Regeneron
Research Funding: Clinigen Group, Iovance Biotherapeutics, Nektar
Anna C. Pavlick
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Regeneron, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb, Merck, Millennium, Regeneron, Replimune
Eric D. Whitman
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol Myers Squibb, Castle Biosciences, Novartis, Eisai, Pfizer
Speakers' Bureau: Bristol Myers Squibb, Merck Sharp & Dohme, Castle Biosciences, Sanofi/Regeneron
Research Funding: Bristol Myers Squibb, Merck Sharp & Dohme, Castle Biosciences, Genentech/Roche, Amgen, TRACON Pharma, AstraZeneca/MedImmune, Provectus, Oncolys BioPharma, Iovance Biotherapeutics, Dynavax Technologies, OncoSec, Toray Industries, Array BioPharma
Patents, Royalties, Other Intellectual Property: Nerve monitoring dissection device, Lighted Polyhedral surgical retractor
Salvador Martin-Algarra
Consulting or Advisory Role: MSD Oncology, Sanofi, Regeneron, AstraZeneca
Speakers' Bureau: Bristol Myers Squibb, MSD Oncology, AstraZeneca, Novartis, Roche, Sanofi/Regeneron
Travel, Accommodations, Expenses: Pierre Fabre, Roche, MSD Oncology
Pippa Corrie
Honoraria: Novartis, Merck Sharp & Dohme, Pierre Fabre, Bristol Myers Squibb
Consulting or Advisory Role: Novartis, Bristol Myers Squibb, Merck Sharp & Dohme, Incyte, Pierre Fabre, Roche, Microbiotica
Speakers' Bureau: Merck Sharp & Dohme, Novartis, Bristol Myers Squibb
Research Funding: MSD, Bristol Myers Squibb, Novartis, Array BioPharma, Celgene, Halozyme, Iovance Biotherapeutics, Lilly
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck Sharp & Dohme
Brendan D. Curti
Honoraria: Clinigen Group, Nektar
Consulting or Advisory Role: Merck
Research Funding: Bristol Myers Squibb, Galectin Therapeutics, Clinigen Group
Patents, Royalties, Other Intellectual Property: Biomarkers for OX40 response
Travel, Accommodations, Expenses: Agonox
Jose Lutzky
Consulting or Advisory Role: Castle Biosciences, Iovance Biotherapeutics, Replimune, Regeneron
Research Funding: Bristol Myers Squibb, Novartis, Iovance Biotherapeutics, Immunocore, Regeneron, Replimune, Vyriad
Jeffrey S. Weber
Stock and Other Ownership Interests: CytomX Therapeutics, Biond Biologics, Protean Biodiagnostics, Neximmune
Honoraria: Bristol Myers Squibb, Merck, Genentech, AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Amgen, Roche, Celldex, CytomX Therapeutics, Novartis, Sellas Life Sciences, WindMIL, Takeda, Moderna Therapeutics, Jounce Therapeutics, Kirin Pharmaceuticals, Regeneron, Idera, Oncosec
Consulting or Advisory Role: Celldex, Bristol Myers Squibb, Merck, Genentech, Roche, Amgen, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo, CytomX Therapeutics, Novartis, Sellas Life Sciences, WindMIL, Jounce Therapeutics, Moderna Therapeutics, Kirin Pharmaceuticals, Protean Biodiagnostics, Idera, Oncosec
Research Funding: Bristol Myers Squibb, Merck, GlaxoSmithKline, Genentech, Astellas Pharma, Incyte, Roche, Novartis, NextCure, Moderna Therapeutics
Patents, Royalties, Other Intellectual Property: Named on a patent submitted by Moffitt Cancer Center for an IPILIMUMAB biomarker, named on a patent from Biodesix for a PD-1 antibody biomarker, named on a patent for 41BB induced TIL by Moffitt Cancer Center
Travel, Accommodations, Expenses: Bristol Myers Squibb, GlaxoSmithKline, Roche, Celldex, Amgen, Merck, AstraZeneca, Genentech, Novartis
James M. G. Larkin
Honoraria: Eisai, Bristol Myers Squibb, MSD, GlaxoSmithKline, Pfizer, Novartis, Roche/Genentech, Pierre Fabre, EUSA Pharma, Achilles Therapeutics, AstraZeneca, Boston Biomedical, Ipsen, Imugene, Incyte, iOncologi, Merck Serono, Nektar, Vitaccess, Kymab, Secarna
Consulting or Advisory Role: Eisai, Bristol Myers Squibb, MSD, GlaxoSmithKline, Pfizer, Novartis, Roche/Genentech, Pierre Fabre, EUSA Pharma, Achilles Therapeutics, AstraZeneca, Boston Biomedical, Ipsen, Imugene, Incyte, iOncologi, Merck Serono, Nektar, Vitaccess, Secarna, Kymab
Research Funding: Pfizer, Novartis, MSD, Bristol Myers Squibb, Achilles Therapeutics, Roche, Nektar, Covance, Immunocore, AVEO
Travel, Accommodations, Expenses: Bristol Myers Squibb, Pfizer, Novartis, Roche/Genentech, AstraZeneca, Boston Biomedical, Incyte, GlaxoSmithKline, Pierre Fabre, Merck Serono
Wen Shi
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Travel, Accommodations, Expenses: Iovance Biotherapeutics
Toshimi Takamura
Employment: Iovance Biotherapeutics Inc
Madan Jagasia
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Consulting or Advisory Role: Kadmon
Harry Qin
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Xiao Wu
Employment: Iovance Biotherapeutics
Stock and Other Ownership Interests: Iovance Biotherapeutics
Travel, Accommodations, Expenses: Iovance Biotherapeutics
Cecile Chartier
Employment: Iovance Biotherapeutics
Leadership: Iovance Biotherapeutics
Patents, Royalties, Other Intellectual Property: PCT/US2019/059598 for Expansion of TILs Utilizing AKT Pathway Inhibitors. PCT/US2019/012729 for Processes for Generating TIL Products Enriched for Tumor Antigen-specific T-cells. PCT/US2019/029286 for Gene Editing of Tumor Infiltrating Lymphocytes and Uses of Same in Immunotherapy. PCT/US2019/065892 for Methods of Expanding Tumor Infiltrating Lymphocytes Using Engineered Cytokine Receptor Pairs and Uses Thereof. PCT/US2019/012733 for Processes for Generating TIL Products Enriched for Tumor Antigen-Specific T-Cells. PCT/US2020/013095 for System and Methods for Monitoring Adoptive Cell Therapy Clonality and Persistence. PCT/US2020/063767 for Processes for the Production of Tumor Infiltrating Lymphocytes (TILs) and Methods of Using the Same. PCT/US2020/057135 for Gene Editing of Tumor Infiltrating Lymphocytes and Uses of Same in Immunotherapy. One patent application is nonpublic, for which Iovance declines to furnish any information
Travel, Accommodations, Expenses: Iovance Biotherapeutics
Friedrich Graf Finckenstein
Employment: Adverum, Iovance Biotherapeutics, Roche/Genentech
Leadership: Iovance Biotherapeutics
Stock and Other Ownership Interests: Roche/Genentech, Bristol Myers Squibb, Johnson & Johnson, Iovance Biotherapeutics, Adverum
Travel, Accommodations, Expenses: Iovance Biotherapeutics, Roche/Genentech
Maria Fardis
Employment: Iovance Biotherapeutics, Acerta Pharma
Leadership: Iovance Biotherapeutics, Acerta Pharma
Stock and Other Ownership Interests: Iovance Biotherapeutics, Acerta Pharma, Gilead Sciences, AbbVie, Kartos Therapeutics
Patents, Royalties, Other Intellectual Property: Iovance Biotherapeutics, Acerta Pharma
Travel, Accommodations, Expenses: Iovance Biotherapeutics, Acerta Pharma
John M. Kirkwood
Consulting or Advisory Role: Bristol Myers Squibb, Novartis, Iovance Biotherapeutics, Elsevier, Amgen, Checkmate Pharmaceuticals, Harbour BioMed, Istari Oncology, OncoSec, Scopus BioPharma, Pfizer
Speakers' Bureau: Bristol Myers Squibb
Research Funding: Amgen, Bristol Myers Squibb, Castle Biosciences, Checkmate Pharmaceuticals, Immunocore, Iovance Biotherapeutics, Novartis, Merck
Jason A. Chesney
Research Funding: Amgen, Replimune, Iovance Biotherapeutics, Bristol Myers Squibb
Patents, Royalties, Other Intellectual Property: University of Louisville US Patents
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hodi FS O'Day SJ McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711-723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robert C Ribas A Schachter J, et al. : Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol 20:1239-1251, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Larkin J Chiarion-Sileni V Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB Hauschild A Robert C, et al. : Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507-2516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C Grob JJ Stroyakovskiy D, et al. : Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med 381:626-636, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Ascierto PA Dummer R Gogas HJ, et al. : Update on tolerability and overall survival in COLUMBUS: Landmark analysis of a randomised phase 3 trial of encorafenib plus binimetinib vs vemurafenib or encorafenib in patients with BRAF V600-mutant melanoma. Eur J Cancer 126:33-44, 2020 [DOI] [PubMed] [Google Scholar]
- 7.McArthur GA Johnson DB Larkin J, et al. : 5-year survival update of cobimetinib plus vemurafenib BRAF V600 mutation-positive advanced melanoma: Final analysis of the coBRIM study. Presented at the 16th International Congress of the Society for Melanoma Research, Salt Lake City, UT, November 20-23, 2019
- 8.Mooradian MJ, Sullivan RJ: What to do when anti-PD-1 therapy fails in patients with melanoma. Oncology (Williston Park) 33:141-148, 2019 [PubMed] [Google Scholar]
- 9.Gide TN Wilmott JS Scolyer RA, et al. : Primary and acquired resistance to immune checkpoint inhibitors in metastatic melanoma. Clin Cancer Res 24:1260-1270, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Larkin J Chiarion-Sileni V Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD Chiarion-Sileni V Gonzalez R, et al. : Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377:1345-1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamid O Robert C Daud A, et al. : Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol 30:582-588, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czarnecka AM Bartnik E Fiedorowicz M, et al. : Targeted therapy in melanoma and mechanisms of resistance. Int J Mol Sci 21:4576, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long GV Flaherty KT Stroyakovskiy D, et al. : Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann Oncol 28:1631-1639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldinger SM Lo S Hassel JC, et al. : The utility of chemotherapy after immunotherapy failure in metastatic melanoma: A multicenter case series. J Clin Oncol 36, 2018. (suppl; abstr e21588) [Google Scholar]
- 16.Weichenthal M Ugurel S Leiter UM, et al. : Salvage therapy after failure from anti-PD-1 single agent treatment: A study by the German ADOReg melanoma registry. J Clin Oncol 37, 2018. (suppl; abstr 9505) [Google Scholar]
- 17.Buchbinder EI Dutcher JP Daniels GA, et al. : Therapy with high-dose Interleukin-2 (HD IL-2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer 7:49, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larkin J Minor D D'Angelo S, et al. : Overall survival in patients with advanced melanoma who received nivolumab versus investigator's choice chemotherapy in CheckMate 037: A randomized, controlled, open-label phase III trial. J Clin Oncol 36:383-390, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribas A Puzanov I Dummer R, et al. : Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol 16:908-918, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eggermont AMM Blank CU Mandala M, et al. : Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378:1789-1801, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Weber J Mandala M Del Vecchio M, et al. : Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824-1835, 2017 [DOI] [PubMed] [Google Scholar]
- 22.Weber JS Del Vecchio M Mandala M, et al. : Adjuvant nivolumab (NIVO) versus ipilimumab (IPI) in resected stage III/IV melanoma: 3-year efficacy and biomarker results from the phase 3 CheckMate 238 trial. Ann Oncol 30:v533-v563, 2019 [Google Scholar]
- 23.Rosenberg SA Yang JC Sherry RM, et al. : Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 17:4550-4557, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radvanyi LG Bernatchez C Zhang M, et al. : Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res 18:6758-6770, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellebaek E Iversen TZ Junker N, et al. : Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J Transl Med 10:169, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maeurer MJ Gollin SM Martin D, et al. : Tumor escape from immune recognition: Lethal recurrent melanoma in a patient associated with downregulation of the peptide transporter protein TAP-1 and loss of expression of the immunodominant MART-1/Melan-A antigen. J Clin Invest 98:1633-1641, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexandrov LB Nik-Zainal S Wedge DC, et al. : Signatures of mutational processes in human cancer. Nature 500:415-421, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher TN, Schreiber RD: Neoantigens in cancer immunotherapy. Science 348:69-74, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Eisenhauer EA Therasse P Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Weber JS D'Angelo SP Minor D, et al. : Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375-384, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Goff SL Dudley ME Citrin DE, et al. : Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol 34:2389-2397, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarnaik A Khushalani N Chesney J, et al. : Safety and efficacy of lifileucel (LN-144) tumor infiltrating lymphocyte therapy in metastatic melanoma patients after progression on multiple therapies—Independent review committee data update. J Immunother Cancer 8, 2020. (abstr P865) [Google Scholar]
- 33.Lu YC Yao X Crystal JS, et al. : Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin Cancer Res 20:3401-3410, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thommen DS Koelzer VH Herzig P, et al. : A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 24:994-1004, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simpson-Abelson MR Hilton F Fardis M, et al. : Iovance generation-2 tumour-infiltrating lymphocyte (TIL) product is reinvigorated during the manufacturing process. Ann Oncol 31:S645-S671, 2020. (suppl 4) [Google Scholar]
- 36.Rosenberg SA Packard BS Aebersold PM, et al. : Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 319:1676-1680, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Cohen CJ Gartner JJ Horovitz-Fried M, et al. : Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J Clin Invest 125:3981-3991, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumacher TN, Scheper W, Kvistborg P: Cancer neoantigens. Annu Rev Immunol 37:173-200, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Wolf Y Bartok O Patkar S, et al. : UVB-induced tumor heterogeneity diminishes immune response in melanoma. Cell 179:219-235.e21, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orlando EJ Han X Tribouley C, et al. : Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 24:1504-1506, 2018 [DOI] [PubMed] [Google Scholar]
- 41.Gontcharova V Suzuki S Simpson-Abelson MR, et al. : Persistence of cryopreserved tumor-infiltrating lymphocyte product lifileucel (LN-144) in C-144-01 study of advanced metastatic melanoma. Cancer Res 79, 2019. (abstr LB-069) [Google Scholar]


