PURPOSE
Tumor mutational profiling is increasingly performed in patients with advanced cancer. We determined the extent to which germline mutation profiling guides therapy selection in patients with advanced cancer.
METHODS
Patients with cancer undergoing tumor genomic profiling were prospectively consented for germline cancer predisposition gene analysis (2015-2019). In patients harboring germline likely pathogenic or pathogenic (LP/P) alterations, therapeutic actionability was classified using a precision oncology knowledge base. Patients with metastatic or recurrent cancer receiving germline genotype–directed therapy were determined.
RESULTS
Among 11,947 patients across > 50 malignancies, 17% (n = 2,037) harbored a germline LP/P variant. By oncology knowledge base classification, 9% (n = 1042) had an LP/P variant in a gene with therapeutic implications (4% level 1; 4% level 3B; < 1% level 4). BRCA1/2 variants accounted for 42% of therapeutically actionable findings, followed by CHEK2 (13%), ATM (12%), mismatch repair genes (11%), and PALB2 (5%). When limited to the 9,079 patients with metastatic or recurrent cancer, 8% (n = 710) harbored level 1 or 3B genetic findings and 3.2% (n = 289) received germline genotype–directed therapy. Germline genotype–directed therapy was received by 61% and 18% of metastatic cancer patients with level 1 and level 3B findings, respectively, and by 54% of BRCA1/2, 75% of mismatch repair, 43% of PALB2, 35% of RAD51C/D, 24% of BRIP1, and 19% of ATM carriers. Of BRCA1/2 patients receiving a poly(ADP-ribose) polymerase inhibitor, 45% (84 of 188) had tumors other than breast or ovarian cancer, wherein the drug, at time of delivery, was delivered in an investigational setting.
CONCLUSION
In a pan-cancer analysis, 8% of patients with advanced cancer harbored a germline variant with therapeutic actionability with 40% of these patients receiving germline genotype–directed treatment. Germline sequence analysis is additive to tumor sequence analysis for therapy selection and should be considered for all patients with advanced cancer.
INTRODUCTION
Tumor mutational profiling is increasingly performed in patients with advanced cancer to identify clinically actionable somatic alterations as a guide to systemic therapy selection.1-5 Recently, the National Cancer Institute's Molecular Analysis for Therapy Choice trial demonstrated the feasibility of identifying actionable somatic genetic alterations through large-scale sequencing efforts and delivering targeted treatments for underexplored advanced tumor types.6 In contrast, historically, germline genetic testing has focused more on early-stage cancers aimed at identifying cancer predisposition syndromes in patients who would benefit from risk-reducing surgery, chemoprevention, and enhanced cancer surveillance. In 2014, the first poly(ADP-ribose) polymerase inhibitor (PARP-I) was approved by the US Food and Drug Administration (FDA) for advanced ovarian cancer patients with germline BRCA1/2 alterations.7,8 Since then, additional drugs have gained approval on the basis of pathogenic germline alterations in various cancer susceptibility genes.9-19 Wider utilization of multigene germline panels and whole-exome analysis has further demonstrated that pathogenic germline alterations are quite common in patients with cancer.5,20-22 A pan-cancer analysis demonstrated that approximately 17% of patients with advanced cancer harbored a pathogenic (P) or likely pathogenic (LP) germline variant in a cancer susceptibility gene, with 55% not meeting genetic testing criteria on the basis of historical clinical guidelines.20 In response, clinical practice guidelines now incorporate germline analysis for broader cancer populations. Universal germline analysis for all patients with ovarian, pancreas, advanced prostate, and metastatic breast cancers is now endorsed by the National Comprehensive Cancer Network.23,24 Tumor testing for markers of Lynch syndrome (LS) is also recommended for all colorectal and endometrial cancers.25
CONTEXT
Key Objective
What is the impact of germline genetic testing on germline-directed therapy selection in a pan-cancer patient population?
Knowledge Generated
Among cancer patients with metastatic or recurrent cancer, 8% had a germline alteration with therapeutic actionability. Overall, 3.2% of all patients with advanced cancer received germline-directed treatment.
Relevance
Our study findings suggest that multigene germline genetic analysis should be considered for all patients with metastatic or recurrent cancer to guide treatment selection.
As many oncologists have limited training in cancer genomics, precision oncology knowledge bases (OncoKB, CIViC, and others)26-31 have been developed to better communicate the strength of evidence supporting the clinical actionability of somatic mutations. These knowledge bases stratify mutations and/or genes on the basis of the level of clinical and/or biologic data supporting their use as predictive biomarkers of drug response. For germline alterations, interpretation of variant pathogenicity for cancer risk has been well-established; however, there has been less focus on determining therapeutic actionability at the gene or gene variant level.32
Using a prospective pan-cancer cohort, the current study was designed to assess the utility of broad germline panel testing for germline-directed therapy selection and to determine the frequency with which germline genotype–directed treatment is given in patients with metastatic or recurrent cancer.
METHODS
Patient Population and Germline Genetics Analysis
Patients (N = 11,947) consented to an institutional review board–approved research protocol (ClinicalTrials.gov identifier: NCT01775072) between January 2015 and May 2019. Paired tumor-normal sequencing was performed using Memorial Sloan Kettering-IMPACT, a next-generation sequencing assay that identifies mutations, fusions, and copy number alterations in up to 468 cancer-associated genes and assesses microsatellite instability and tumor mutation burden.2 All patients provided additional consent for germline analysis in our CLIA-approved laboratory using the normal blood-derived DNA.20 Germline analysis was restricted to a subset of 76-88 genes in the Memorial Sloan Kettering-IMPACT panel (Data Supplement, online only), inclusive of all cancer-predisposing genes in the American College of Medical Genetics and Genomics guidelines.33 Likely pathogenic or pathogenic (LP/P) variants were interpreted and clinically reported as previously described; variants of unknown significance were not reported.20 Individuals with P/LP variants were offered genetic counseling.
Gene Classification on the Basis of Therapeutic Actionability
Genes with germline LP/P alterations were classified using the OncoKB knowledge base according to the level of evidence for the gene as a predictor of drug sensitivity.26 Pertinent OncoKB levels of evidence for gene classifications for this study included Level 1, an FDA-recognized biomarker predictive of response to an FDA-approved drug in this indication (tumor type–specific); Level 3B, biomarker predictive of response to an FDA-approved or investigational drug in another indication; and Level 4, compelling biologic evidence of the biomarker as being predictive of response to a drug (Data Supplement; Table 1). Level of evidence assignment for an implicated gene was based on the last OncoKB update issued on September 17, 2020 and may be different from assignment level at the time of drug delivery. One exception to the existing OncoKB classification was that patients with LS-associated LP/P germline variants (MLH1, MSH2, MSH6, PMS2, and EPCAM), who also harbored tumors exhibiting high-frequency microsatellite instability (MSI-H) and/or DNA mismatch repair deficiency (dMMR) on immunohistochemistry, were given a special classification as level 1-MSI-H, as this genotype-treatment association is based on the tumor agnostic FDA authorization of pembrolizumab for MSI-H/dMMR tumors.34,35 Patients with multiple LP/P alterations were classified according to the gene with the highest OncoKB level of evidence. Medical records of patients with level 1 or 3B alterations were reviewed to identify germline genotype–directed treatment received in a clinical or investigational setting. This analysis was limited to patients with metastatic or recurrent cancer at the time of review, where the utilization of germline genotype–directed systemic therapies is currently most pertinent.
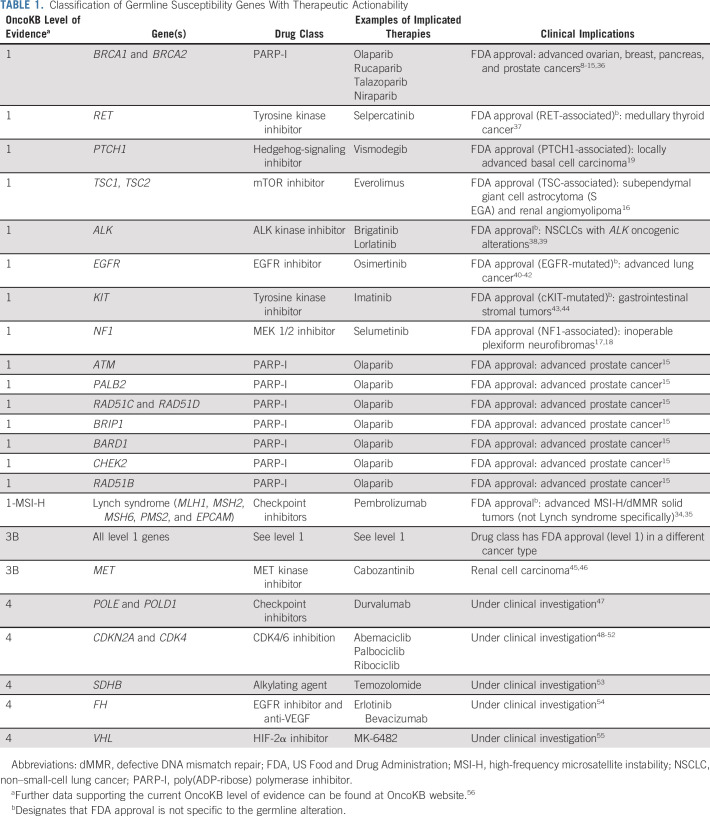
TABLE 1.
Classification of Germline Susceptibility Genes With Therapeutic Actionability
RESULTS
Cohort Characteristics and Germline Variant Detection
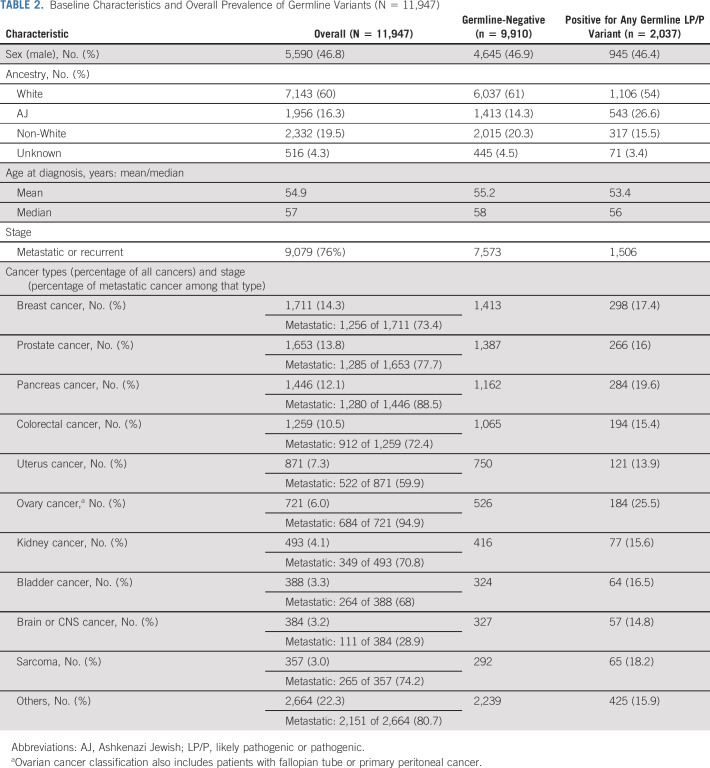
Between 2015 and 2019, 30 patients with 479 solid tumor underwent combined germline and somatic mutation analysis, with 11,947 consenting to germline analysis of cancer susceptibility genes. The most prevalent malignancies were breast (14%), prostate (14%), pancreas (12%), and colorectal cancers (11%) (Table 2). The 10 most common cancers in the germline cohort were similar to the distribution of tumor types among patients undergoing tumor analysis only, with the exception that lung cancer was under-represented in the germline analysis (Data Supplement Fig 1).
TABLE 2.
Baseline Characteristics and Overall Prevalence of Germline Variants (N = 11,947)
Among 11,947 patients, 4,593 underwent germline analysis using a 76-gene panel, whereas 7,354 had an updated 88-gene panel (Data Supplement). The LP/P germline variant prevalence was 17% (n = 2,037), similar to the variant detection rate previously reported by our group for the first 1,040 patients on this prospective protocol.20 By cancer penetrance (Data Supplement), 10% of patients harbored an LP/P variant in a high- or moderate-penetrance gene with the most frequent alterations identified in BRCA1/2 (4%) and LS-associated genes (1%).
Therapeutic Actionability of Pathogenic Germline Alterations
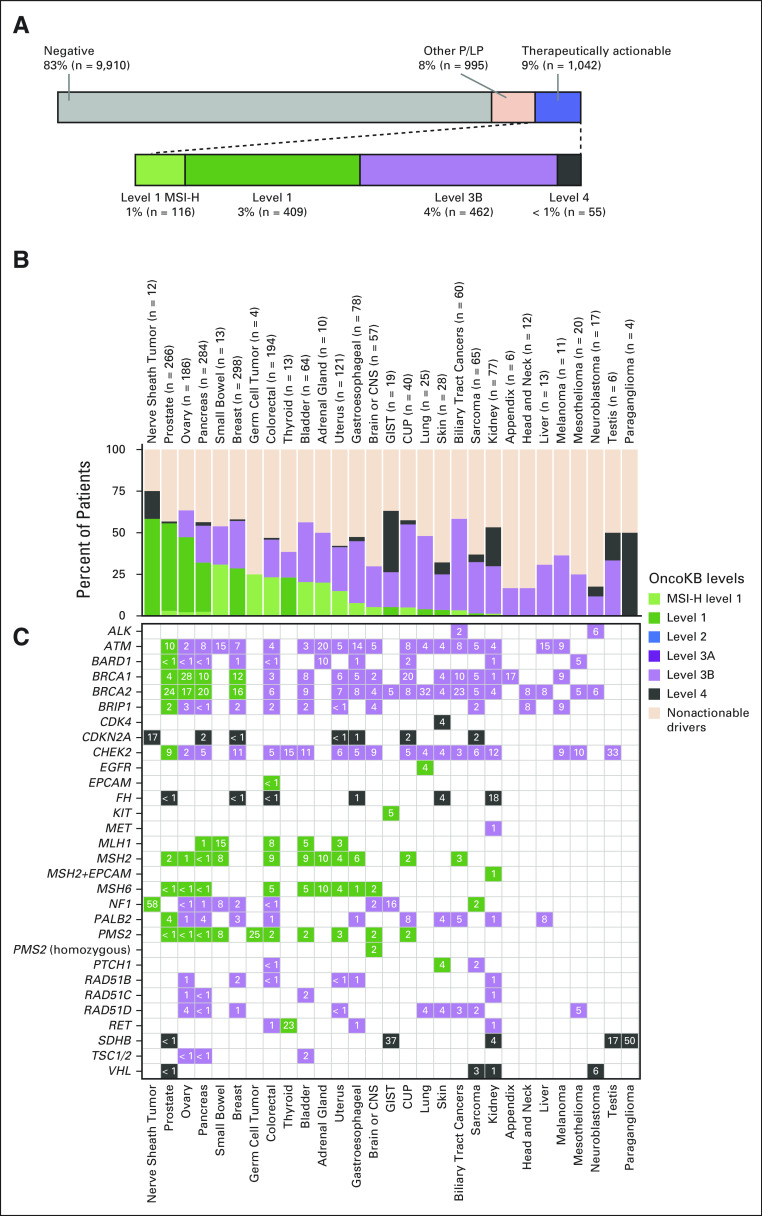
To determine how often a germline variant with therapeutic implications was detected in this pan-cancer cohort, we classified all 2,037 LP/P variants identified using the OncoKB classification system (Data Supplement). Overall, 9% of patients (n = 1,042/11,947) harbored an LP/P germline variant in a potentially therapeutically actionable gene. More specifically, 4%, 4%, and < 1% of patients had level 1 (inclusive of level 1-MSI-H), level 3B, or level 4 findings, respectively (Fig 1A). LP/P variants in BRCA1/2 were the most common therapeutically actionable germline variants (43%, n = 441) followed by CHEK2 (13%, n = 133), ATM (12%, n = 127), and PALB2 (4%, n = 47). Notably, 159 patients harbored an LP/P variant diagnostic of LS, including one patient with constitutional MMR deficiency syndrome (CMMRD). However, of these patients, only the 116 patients with corresponding MSI-H/dMMR tumors were designated as level 1-MSI-H (Fig 1A).
FIG 1.
Prevalence of germline variants with therapeutic actionability as classified by OncoKB. (A) Top panel, percent of 11,947 cancer patients with LP/P germline alterations considered therapeutically actionable by OncoKB (blue). Lower panel, breakdown of therapeutically actionable germline alterations by OncoKB level of evidence. Level 1 MSI-H (light green) indicates patients with germline LP/P alterations in the DNA mismatch repair genes whose tumors also exhibit MSI-H/dMMR. In (B and C), highest OncoKB level of evidence by cancer type and gene is shown (28 cancer types shown). (B) In the stacked bar graph, columns indicate tumor type. Number of patients with LP/P alterations per cancer type specified in labels on top x-axis. Each bar is broken down by percentage of patients harboring a germline alteration with color-indicated level of evidence or nonactionable P/LP alteration (light orange). (C) In the frequency map, rows indicate germline gene alteration present in patients and numbers indicate the percentage of patients per cancer type that harbors an alteration in each gene. CUP, cancer of unknown primary; dMMR, defective DNA mismatch repair; GIST, gastrointestinal stromal tumor; LP/P, likely pathogenic or pathogenic, MSI-H, high-frequency microsatellite instability.
The distribution of therapeutically actionable germline alterations as classified by OncoKB by tumor type is shown in Figures 1B and 1C. Of all patients harboring a germline LP/P alteration (n = 2037), the cancer types with the highest percentage of level 1 (inclusive of level 1-MSI-H) germline alterations were nerve sheath tumors followed by prostate, ovarian, pancreas, and small bowel cancers (Fig 1B). Notably, the majority of BRCA1 and BRCA2 germline variants were classified as level 1, on the basis of the recent expansion of FDA approval of PARP-I(s) to include patients with prostate15,36 and pancreas cancers.57 Since PARP-I therapy, specifically olaparib, is currently FDA-approved in only prostate cancer patients with ATM, PALB2, BRIP1, RAD51B/C/D, CHEK2, and BARD1,15 the majority of germline variants in these genes were classified by OncoKB as level 3B (Fig 1C).
We also assessed the fraction of patients who were known to have an LP/P germline variant from prior standard of care or family-directed cascade testing. Among the 1,042 patients with LP/P germline variants classified by OncoKB as having potential therapeutic actionability, 29% (n = 298) had prior knowledge of their LP/P germline alteration with 80% of these being patients with BRCA1/2 or LS. Among BRCA1/2 carriers, 75% of patients with ovarian, 69% of patients with breast, 40% of patients with pancreas, and 18% of patients with prostate cancers had prior knowledge of their LP/P genetic alteration. For ATM, PALB2, RAD51C/D, and BRIP1, only 20%, 19%, 4%, and 4% had prior knowledge of the LP/P genetic alteration, respectively.
Utilization of Germline Genotype–Directed Therapies in Clinical Practice
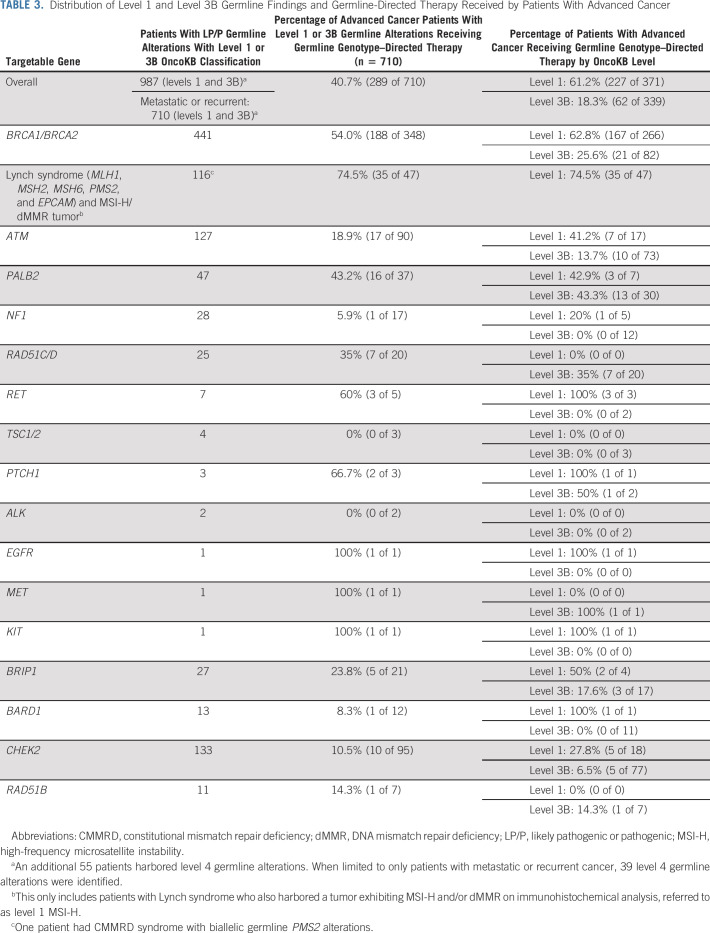
We next assessed the clinical utilization of germline genotype–directed treatment in patients with level 1 and level 3B OncoKB variants. Only patients with metastatic or recurrent cancer (stage IV) were included for this analysis, with the exception that stage IIIC ovarian cancer and inoperable nerve sheath tumors were included, as such patients require systemic therapy. Of 9,079 patients with metastatic or recurrent cancer (Table 2), 8% (n = 710) harbored a level 1 or level 3B OncoKB variant and 3.2% (n = 289) of all patients with metastatic cancer received germline-directed therapy. Of advanced cancer patients with a therapeutically actionable LP/P germline variant, 41% (n = 289) received a germline genotype–directed therapy (Table 3). As expected, germline-directed treatment was more commonly received by patients with a level 1 (61%, n = 227/371) germline alterations as opposed to patients with level 3B alterations (18%, n = 62/339) (Fig 2A; Table 3).
TABLE 3.
Distribution of Level 1 and Level 3B Germline Findings and Germline-Directed Therapy Received by Patients With Advanced Cancer
FIG 2.
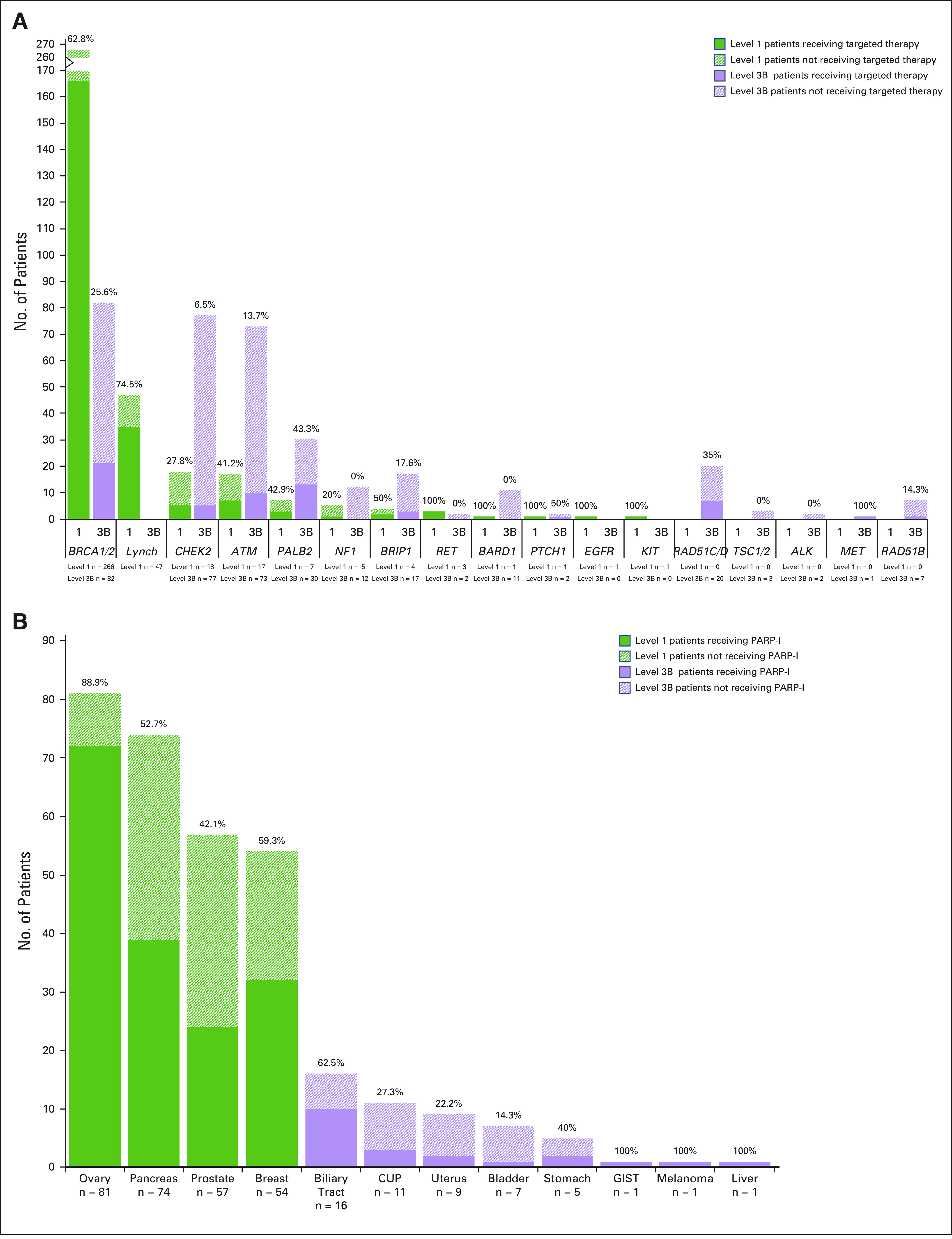
Patients with advanced cancer receiving germline genotype–directed therapy. (A) Bar graph demonstrates the 710 patients with metastatic or recurrent cancer harboring level 1 and level 3B LP/P germline variants and the percentage of these patients who received germline genotype–directed therapy by gene(s) and according to OncoKB level of evidence. (B) Bar graph demonstrates the 348 patients with advanced cancer that harbors an LP/P germline alteration in BRCA1 or BRCA2 and the percentage of these patients receiving a PARP-I by tumor type and according to the OncoKB level of evidence assigned for that tumor type. An additional 31 advanced cancer patients with LP/P BRCA1/2 germline alterations with colorectal (12), lung (5), esophagus and gastroesophageal junction (4), sarcoma (3), kidney (2), appendix (1), brain (1), neuroblastoma (1), head and neck (1), and skin (1) cancers were also identified, with none of them receiving a PARP-I. CUP, cancer of unknown primary; GIST, gastrointestinal stromal tumor; LP/P, likely pathogenic or pathogenic; PARP-I, poly(ADP-ribose) polymerase inhibitor.
Of the 188 patients receiving a PARP-I in the setting of an LP/P BRCA1/2 mutation, 55% had ovarian (n = 72) or breast (n = 32) cancer. However, 45% had other tumor types, including pancreas (n = 39), prostate (n = 24), bile duct, gastric, and other cancer types, wherein the drug, at the time of delivery, was administered in a research context. The likelihood of receiving a PARP-I was highly tumor type–dependent with 89% of ovarian, 59% of breast, 53% of pancreas, and 42% of prostate cancer patients with BRCA1/2 alterations receiving such therapy (Fig 2B). The lower frequency of PARP-Is received by patients with pancreas and prostate cancers is likely attributable to the fact that these agents had not yet been FDA-approved for these indications during the timeframe of the study.
Among patients with level 1 or 3B LP/P germline alterations in a gene involved in homologous-recombination repair (HRR), 59% (184 of 313) of level 1 and 19% (60 of 317) of level 3B patients received a PARP-I. After BRCA1/2, PARP-I for an HRR-associated germline variant was most often administered to patients with LP/P PALB2, RAD51C/D, and BRIP1 variants where 43%, 35%, and 24% of patients received a PARP-I, respectively. Although 49% of PALB2 LP/P carriers had breast or pancreas cancer, the remaining patients had other cancer types including prostate, ovary, or an unknown primary. PARP-I was received by 47% of these patients.
In patients with LS, at risk for the development of MSI-H/dMMR tumors,58 75% received immune checkpoint blockade (Fig 2A; Table 3) inclusive of one patient with CMMRD. Beyond colorectal cancer, patients with LS-associated prostate, bladder, pancreas, and ovarian cancers received immunotherapy.
Of 244 patients with cancer of unknown primary, 22 had an LP/P germline variant with therapeutic actionability including 11 BRCA1/2, 2 MMR-associated genes, 3 ATM, 3 PALB2, 2 CHEK2, and 1 BARD1 carriers. Among these patients, six (27%) received germline genotype–directed treatment. A proportion of patients with level 1 or 3B genetic alterations in RET, PTCH1, KIT, and NF1 also received a germline genotype–directed treatment (Fig 2A). In patients with LP/P variants in these genes, the targeted therapy was usually delivered for a level 1 indication (ie, selumetinib in NF1-associated neurofibroma) with some exceptions, including a colorectal cancer patient with germline PTCH1 alterations who received a hedgehog signal inhibitor.
DISCUSSION
Our study highlights the clinical utility of germline sequence analysis for therapeutic decision making in patients with advanced cancer. Specifically, among patients with metastatic or recurrent cancer, 8% harbored OncoKB level 1 or level 3B therapeutically actionable germline alterations, with an overall 3.2% of all patients with metastatic cancer receiving germline genotype–directed treatment. Importantly, we anticipate that over time, the fraction of patients receiving germline-directed treatment will increase as newer therapies are developed or as current agents are approved for additional tumor types. For example, as this study only analyzed patients sequenced before mid-2019, patients with BRCA-associated pancreatic and prostate cancer received PARP-I in a research setting. However, given the recent FDA approval of PARP-Is for advanced pancreatic and prostate cancer,15,36,57 we anticipate an increase in germline genotype–directed treatment in these cancers. In fact, the delivery of the germline genotype–directed treatments correlated with FDA regulatory approval timelines; the highest frequency of PARP-I use was observed in patients with BRCA-associated ovarian cancer (nearly 90%), the tumor type for which PARP-I therapy was first approved in 2014. As germline-directed treatments are now being evaluated in early-stage cancers (ClinicalTrials.gov.identifier: NCT03499353, NCT02032823, etc), an increasing number of patients with cancer will receive genotype-directed therapies on the basis of the identification of potentially actionable germline alterations.
Our study also demonstrates that germline analysis may identify novel, previously unrecognized, genomically directed treatment opportunities for patients with advanced cancer. For example, although PALB2 germline alterations are associated with increased susceptibility to pancreas and breast cancers, 47% of patients with LP/P PALB2 germline variants who received a PARP-I had other cancers including prostate, ovary, and cancer of unknown primary. As more than half of patients with LP/P germline variants do not meet standard clinical criteria for genetic testing,20 patients with advanced cancer may inadvertently be excluded from receiving germline-directed treatments if they are not evaluated for germline alterations. Indeed, in our group, > 80% of PALB2 carriers had no prior knowledge of their heritable and potentially actionable genetic alteration.
As the identification of a pathogenic germline alteration, even if unexpected, may have important treatment implications for patients with advanced cancer, a multigene approach to genetic analysis with incorporation of at least level 1 and 3B therapeutically actionable genes in this patient population seems reasonable. Further research into level 4 genes will be necessary to define the benefit of testing for such genes in patients with advanced cancer in need of systemic therapies. Although herein we focus on direct germline analysis, if tumor-only sequencing is performed, an alternative approach may be to perform reflex germline analysis of any tumor finding with potential germline relevance.
To our knowledge, our study is the first to use a precision oncology evidence-based knowledge base, OncoKB, and apply it in a systematic manner to categorize the therapeutic actionability of genes with germline alterations. There were unique challenges to this. We included germline alterations in the DNA MMR genes, diagnostic of LS, as having targeted therapeutic actionability if, and only if, the tumor also exhibited an MSI-H/dMMR phenotype. The FDA has approved pembrolizumab for tumors with evidence of MSI-H or dMMR, and the presence of LS predicts for the development of such tumors, but not all cancers in patients with LP/P variants in LS genes are MSI-H tumors.58 The special designation of variants such as level 1-MSI-H emphasizes that if a germline MMR gene alteration is identified in a patient with advanced cancer, tumor testing for MSI/dMMR must be undertaken before checkpoint inhibitor administration.
The distinction between genotype and phenotype in patients with Lynch syndrome also underscores the importance of integrating tumor and germline genomic information to fully understand the clinical implications of pathogenic germline variants. Further research is necessary to determine whether a germline variant alone is sufficient to induce response to germline genotype–directed treatments or if the tumor is driven by pathways unrelated to the variant, as suggested by the absence of biallelic inactivation and/or associated mutational signature. This is especially important for those genes involved in HRR, which are one of the most frequent germline findings in patients with cancer. Our study did not evaluate treatment response, inclusive of possible somatic genomic biomarkers of response; however, such studies are currently being conducted in specific cancer types and were previously assessed by our group in BRCA-associated tumors.59
Importantly, the predictive role of germline alterations in certain HRR genes remains an area of controversy. Although response to PARP-Is in BRCA-associated cancers has been demonstrated across many different cancer types, the efficacy of PARP-Is may be more modest in patients harboring other HRR gene alterations. For example, although both CHEK2 and ATM alterations received a level 1 designation in metastatic prostate cancer because of the FDA approval of olaparib15 and, thus, a level 3B designation in other cancer types, PARP-I response because of ATM and CHEK2 alterations was not observed in other studies of patients with advanced prostate and breast cancer.60,61 This highlights the need for precision OncoKBs to start to formally incorporate genes with germline alterations into their classification schemas with careful ongoing reassessment of evidence level assignments on the basis of research findings in specific cancer types.
This study has certain limitations. Although the sample size was large and included patients with a broad spectrum of cancer types, patients with lung cancer were under-represented. If 15% of our ascertainment were lung cancer cases, on the basis of our somatic profiling of > 30,000 tumors (Data Supplement Fig 1A), and the prevalence of germline alterations in lung cancer is approximately 8%,62 one may conservatively estimate that one half (approximately 4%) of these patients would have had OncoKB level 3B germline alterations. In this case, the prevalence of a germline variant with targeted therapeutic actionability would decrease in the entire somatic testing cohort from 9% to just over 8%. As expected, on the basis of population frequencies, the majority (53%) of germline alterations with therapeutic actionability were either in BRCA1/2 or in the Lynch syndrome genes, suggesting that the impact of these results may be most pertinent to patients with these two more common cancer predisposition syndromes. As some patients in the cohort received treatment outside of our institution, it is possible that more patients actually received germline-directed treatment. Previously identified predictive associations of chemotherapy response with certain germline alterations were not considered in the total, such as platinum response in BRCA1/2-positive patients or in other genes associated with DNA damage repair pathways.63 Although this study focused on patients with advanced cancer who usually receive multiple lines of therapy, germline-directed chemotherapy selection may be more pertinent in patients with early-stage cancer.
We demonstrate that germline genetic analysis has important implications for the management of patients with cancer beyond the risk reduction strategies most relevant to early-stage patients. With the increasing number of genes with germline alterations predictive of drug response and the proliferation of tumor agnostic basket trials assessing germline genotype–directed treatments, the tumor agnostic evaluation of patients with metastatic or recurrent cancer for potentially actionable germline alterations should be considered. On the basis of our study findings, using a multigene panel that incorporates BRCA1/2 and other HRR genes, as well as the MMR genes, appears a reasonable undertaking for patients with metastatic or recurrent cancers. Future endeavors including standardized classification of the increasing number of germline alterations with therapeutic actionability and the impact of germline-directed therapies in patients with early-stage cancer are needed.
Zsofia K. Stadler
Consulting or Advisory Role: Allergan (I), Genentech/Roche (I), Regeneron (I), Optos (I), Adverum (I), Novartis (I), Regenxbio (I), Gyroscope (I), Neurogene (I)
Debyani Chakravarty
Consulting or Advisory Role: Medendi Medical Travel
Christopher J. Fong
Stock and Other Ownership Interests: Various biotech ETFs
Patents, Royalties, Other Intellectual Property: Ultra-Wideband Radar System for Animals Patent September 11, 2018, Patent issuer and number US20150181840A1, Monitoring Treatment of Peripheral Artery Disease (PAD) using Diffuse Optical Imaging, Imaging interfaces for full finger and full hand optical tomography, Systems and methods for dynamic imaging of tissue using digital optical tomography.
Karen Cadoo
Honoraria: OncLive
Consulting or Advisory Role: GlaxoSmithKline/Tesaro, AstraZeneca, MSD, GlaxoSmithKline, MJH Life Sciences
Research Funding: AstraZeneca, Syndax, MSD
Ying Liu
Research Funding: AstraZeneca, Tesaro/GSK
Maria I. Carlo
Consulting or Advisory Role: Pfizer
Other Relationship: Prostate Cancer Foundation, Robert Wood Johnson Foundation
Alicia Latham
Other Relationship: Conquer Cancer Foundation
Ritika Kundra
Stock and Other Ownership Interests: Pfizer
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche, AbbVie, Clovis Oncology, AstraZeneca
Nadeem Abu-Rustum
Honoraria: Prime Oncology
Research Funding: Stryker/Novadaq, Grail
Travel, Accommodations, Expenses: Prime Oncology
Anna Varghese
Consulting or Advisory Role: Roche
Research Funding: Lilly, Verastem, BioMed Valley Discoveries, Bristol Myers Squibb, Silenseed, Illumina
Travel, Accommodations, Expenses: Roche
Eileen M. O'Reilly
Consulting or Advisory Role: Merck, Agios, AstraZeneca, Bayer, BeiGene, Berry Genomics, Celgene, CytomX Therapeutics, Debiopharm Group, Eisai, Exelixis/Ipsen, Flatiron Health, Incyte, Janssen, LAM Therapeutics, Lilly, Loxo, Genentech/Roche, Minapharma, QED Therapeutics, RedHill Biopharma, Sillajen, SOBI, Yiviva, Autem Medical, Gilead Sciences, Ipsen, Silenseed, TheraBionic, twoXAR, Vector Health
Research Funding: AstraZeneca/MedImmune, Acta Biologica, Bristol Myers Squibb, Celgene, Genentech, Halozyme, MabVax, Roche, Silenseed
Michael Morris
Consulting or Advisory Role: Bayer, Endocyte, Advanced Accelerator Applications, ORIC Pharmaceuticals, Johnson & Johnson, Curium Pharma, Athenex
Research Funding: Bayer, Sanofi, Endocyte, Progenics, Corcept Therapeutics, Roche/Genentech, Janssen
Travel, Accommodations, Expenses: Endocyte, Fujifilm
Wassim Abida
Honoraria: CARET, Roche, Medscape, Aptitude Health
Consulting or Advisory Role: Clovis Oncology, Janssen, MORE Health, ORIC Pharmaceuticals, Daiichi Sankyo
Research Funding: AstraZeneca, Zenith Epigenetics, Clovis Oncology, GlaxoSmithKline, ORIC Pharmaceuticals, Epizyme
Travel, Accommodations, Expenses: GlaxoSmithKline, Clovis Oncology, ORIC Pharmaceuticals
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicans' Education Resource, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, Peerview
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Millennium, BerGenBio, MORE Health, Lilly, Verastem, AbbVie, 14ner Oncology/Elevation Oncology, Remedica, Archer, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Melendi, Repare Therapeutics
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
Ahmet Zehir
Stock and Other Ownership Interests: Arcus Biosciences, Mirati Therapeutics
Honoraria: Illumina
Marc Ladanyi
Consulting or Advisory Role: Bristol Myers Squibb, Bayer
Research Funding: Loxo, Helsinn Therapeutics, Merus NV, Elevation Oncology
David Solit
Stock and Other Ownership Interests: Loxo, Scorpion Therapeutics, Vividion Therapeutics, Fore Therapeutics
Consulting or Advisory Role: Pfizer, Illumina, Lilly, QED Therapeutics, BridgeBio Pharma, Scorpion Therapeutics, Vividion Therapeutics, Syros Pharmaceuticals
Michael F. Berger
Research Funding: Grail
Patents, Royalties, Other Intellectual Property: Provisional patent pending for “Systems and Methods for Detecting Cancer via cfDNA Screening”
Luis A. Diaz
Leadership: Personal Genome Diagnostics, Jounce Therapeutics
Stock and Other Ownership Interests: PapGene Inc, Personal Genome Diagnostics, Jounce Therapeutics, Zydecom, Thrive Earlier Detection Corp, Neophore, Amgen, Four Paws, Seer, Kinnate Biopharma
Consulting or Advisory Role: Merck, Personal Genome Diagnostics, Zydecom, Neophore, Innovatus Capital Partners, Four Paws, Seer, Kinnate Biopharma
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: US-2010041048-A1—Circulating Mutant DNA to Assess Tumor Dynamics, US-2015344970-A1—Personalized Tumor Biomarkers, WO-2010118016-A2—Digital quantification of DNA methylation, US-2005202465-A1—Thymidylate synthase gene and metastasis, US-2014227271-A1—Somatic mutations in atrx in brain cancer, WO-2012094401-A2—Genes frequently altered in pancreatic neuroendocrine tumors, US-2013323167-A1—Detecting and treating solid tumors through selective disruption of tumor vasculature, EP-2912468-B1—Papanicolaou test for ovarian and endometrial cancers, US-9976184-B2—Mutations in pancreatic neoplasms, US-2017267760-A1—Checkpoint Blockade and Microsatellite Instability, US-2018171413-A1—Head and neck squamous cell carcinoma assays, US-2018086832-A1—HLA-restricted epitopes encoded by somatically mutated genes, US-2018258490-A1—Assaying ovarian cyst fluid, US-2016208340-A1—TERT Promoter Mutations in Urothelial Neoplasia, US-2015252415-A1—Arid1b and neuroblastoma, WO-2018071796-A2—Compositions and methods for identifying functional anti-tumor T cell responses, EP-3322824-A1—Detection of tumor-derived DNA in CSF, US-2016273049-A1—Systems and methods for analyzing nucleic acid, US-2018135044-A1—Non-unique barcodes in a genotyping assay, US-2017016075-A1—Neoantigen analysis
Travel, Accommodations, Expenses: Merck
Mark Robson
Consulting or Advisory Role: Change HealthCare
Research Funding: AstraZeneca, Pfizer, Merck
Other Relationship: Research to Practice, Clinical Care Options, Physicans' Education Resource, Invitae, Pfizer
Uncompensated Relationships: Merck, Pfizer, Daiichi Sankyo, Epic Sciences
Open Payments Link: https://openpaymentsdata.cms.gov/physician/612669/summary
No other potential conflicts of interest were reported.
DISCLAIMER
This is a US Government work. There are no restrictions on its use.
PRIOR PRESENTATION
Presented orally, in part, at the American Society of Clinical Oncology Annual Meeting, May 29-June2, 2020 (abstr 1500).
SUPPORT
Supported in part by the Marie-Josée and Henry R. Kravis Center for Molecular Oncology at Memorial Sloan Kettering; the Precision, Interception and Prevention Program at Memorial Sloan Kettering; the Robert and Kate Niehaus Center for Inherited Cancer Genomics at Memorial Sloan Kettering; the Romeo Milio Lynch Syndrome Foundation; and the National Institutes of Health National Cancer Institute Cancer Center Support Grant P30 CA008748.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Zsofia K. Stadler, Anna Maio, Debyani Chakravarty, Jesse Galle, Carol Aghajanian, Michael Walsh, David B. Solit, Luis A. Diaz, Kenneth Offit, Mark E. Robson
Administrative support: Jesse Galle, Carol Aghajanian, David B. Solit
Provision of study materials or patients: Zsofia K. Stadler, Margaret Sheehan, Erin Salo-Mullen, Kenneth Offit, Eileen M. O'Reilly, David B. Solit, Carol Aghajanian
Collection and assembly of data: Zsofia K. Stadler, Anna Maio, Debyani Chakravarty, Yelena Kemel, Margaret Sheehan, Erin Salo-Mullen, Kaitlyn Tkachuk, Christopher J. Fong, Bastien Nguyen, Amanda Erakky, Ying Liu, Maria I. Carlo, Alicia Latham, Jesse Galle, Anna Varghese, Eileen M. O'Reilly, Michael Morris, Wassim Abida, Alexander Drilon, Gowtham Jayakumaran, Ahmet Zehir, Marc Ladanyi, David B. Solit, Michael F. Berger, Kenneth Offit, Mark E. Robson
Data analysis and interpretation: Zsofia K. Stadler, Anna Maio, Debyani Chakravarty, Yelena Kemel, Margaret Sheehan, Karen Cadoo, Maria I. Carlo, Hongxin Zhang, Ritika Kundra, Shaleigh Smith, Nadeem Abu-Rustum, Anna Varghese, Eileen M. O'Reilly, Michael Morris, Michael Walsh, Alexander Drilon, Gowtham Jayakumaran, Ahmet Zehir, Ozge Ceyhan-Birsoy, David B. Solit, Nikolaus Schultz, Michael F. Berger, Diana Mandelker, Luis A. Diaz, Kenneth Offit, Mark E. Robson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Therapeutic Implications of Germline Testing in Patients with Advanced Cancers
The following represents disclosure information provided by the authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Zsofia K. Stadler
Consulting or Advisory Role: Allergan (I), Genentech/Roche (I), Regeneron (I), Optos (I), Adverum (I), Novartis (I), Regenxbio (I), Gyroscope (I), Neurogene (I)
Debyani Chakravarty
Consulting or Advisory Role: Medendi Medical Travel
Christopher J. Fong
Stock and Other Ownership Interests: Various biotech ETFs
Patents, Royalties, Other Intellectual Property: Ultra-Wideband Radar System for Animals Patent September 11, 2018, Patent issuer and number US20150181840A1, Monitoring Treatment of Peripheral Artery Disease (PAD) using Diffuse Optical Imaging, Imaging interfaces for full finger and full hand optical tomography, Systems and methods for dynamic imaging of tissue using digital optical tomography.
Karen Cadoo
Honoraria: OncLive
Consulting or Advisory Role: GlaxoSmithKline/Tesaro, AstraZeneca, MSD, GlaxoSmithKline, MJH Life Sciences
Research Funding: AstraZeneca, Syndax, MSD
Ying Liu
Research Funding: AstraZeneca, Tesaro/GSK
Maria I. Carlo
Consulting or Advisory Role: Pfizer
Other Relationship: Prostate Cancer Foundation, Robert Wood Johnson Foundation
Alicia Latham
Other Relationship: Conquer Cancer Foundation
Ritika Kundra
Stock and Other Ownership Interests: Pfizer
Carol Aghajanian
Consulting or Advisory Role: Mersana, Eisai, Roche, AbbVie, AstraZeneca/Merck, Roche/Genentech, Repare Therapeutics
Research Funding: Genentech/Roche, AbbVie, Clovis Oncology, AstraZeneca
Nadeem Abu-Rustum
Honoraria: Prime Oncology
Research Funding: Stryker/Novadaq, Grail
Travel, Accommodations, Expenses: Prime Oncology
Anna Varghese
Consulting or Advisory Role: Roche
Research Funding: Lilly, Verastem, BioMed Valley Discoveries, Bristol Myers Squibb, Silenseed, Illumina
Travel, Accommodations, Expenses: Roche
Eileen M. O'Reilly
Consulting or Advisory Role: Merck, Agios, AstraZeneca, Bayer, BeiGene, Berry Genomics, Celgene, CytomX Therapeutics, Debiopharm Group, Eisai, Exelixis/Ipsen, Flatiron Health, Incyte, Janssen, LAM Therapeutics, Lilly, Loxo, Genentech/Roche, Minapharma, QED Therapeutics, RedHill Biopharma, Sillajen, SOBI, Yiviva, Autem Medical, Gilead Sciences, Ipsen, Silenseed, TheraBionic, twoXAR, Vector Health
Research Funding: AstraZeneca/MedImmune, Acta Biologica, Bristol Myers Squibb, Celgene, Genentech, Halozyme, MabVax, Roche, Silenseed
Michael Morris
Consulting or Advisory Role: Bayer, Endocyte, Advanced Accelerator Applications, ORIC Pharmaceuticals, Johnson & Johnson, Curium Pharma, Athenex
Research Funding: Bayer, Sanofi, Endocyte, Progenics, Corcept Therapeutics, Roche/Genentech, Janssen
Travel, Accommodations, Expenses: Endocyte, Fujifilm
Wassim Abida
Honoraria: CARET, Roche, Medscape, Aptitude Health
Consulting or Advisory Role: Clovis Oncology, Janssen, MORE Health, ORIC Pharmaceuticals, Daiichi Sankyo
Research Funding: AstraZeneca, Zenith Epigenetics, Clovis Oncology, GlaxoSmithKline, ORIC Pharmaceuticals, Epizyme
Travel, Accommodations, Expenses: GlaxoSmithKline, Clovis Oncology, ORIC Pharmaceuticals
Alexander Drilon
Honoraria: Medscape, OncLive, PeerVoice, Physicans' Education Resource, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, Peerview
Consulting or Advisory Role: Ignyta, Loxo, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Millennium, BerGenBio, MORE Health, Lilly, Verastem, AbbVie, 14ner Oncology/Elevation Oncology, Remedica, Archer, Monopteros Therapeutics, Novartis, EMD Serono/Merck, Melendi, Repare Therapeutics
Research Funding: Foundation Medicine
Patents, Royalties, Other Intellectual Property: Wolters Kluwer (Royalties for Pocket Oncology)
Other Relationship: Merck, GlaxoSmithKline, Teva, Taiho Pharmaceutical, Pfizer, PharmaMar, Puma Biotechnology
Ahmet Zehir
Stock and Other Ownership Interests: Arcus Biosciences, Mirati Therapeutics
Honoraria: Illumina
Marc Ladanyi
Consulting or Advisory Role: Bristol Myers Squibb, Bayer
Research Funding: Loxo, Helsinn Therapeutics, Merus NV, Elevation Oncology
David Solit
Stock and Other Ownership Interests: Loxo, Scorpion Therapeutics, Vividion Therapeutics, Fore Therapeutics
Consulting or Advisory Role: Pfizer, Illumina, Lilly, QED Therapeutics, BridgeBio Pharma, Scorpion Therapeutics, Vividion Therapeutics, Syros Pharmaceuticals
Michael F. Berger
Research Funding: Grail
Patents, Royalties, Other Intellectual Property: Provisional patent pending for “Systems and Methods for Detecting Cancer via cfDNA Screening”
Luis A. Diaz
Leadership: Personal Genome Diagnostics, Jounce Therapeutics
Stock and Other Ownership Interests: PapGene Inc, Personal Genome Diagnostics, Jounce Therapeutics, Zydecom, Thrive Earlier Detection Corp, Neophore, Amgen, Four Paws, Seer, Kinnate Biopharma
Consulting or Advisory Role: Merck, Personal Genome Diagnostics, Zydecom, Neophore, Innovatus Capital Partners, Four Paws, Seer, Kinnate Biopharma
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: US-2010041048-A1—Circulating Mutant DNA to Assess Tumor Dynamics, US-2015344970-A1—Personalized Tumor Biomarkers, WO-2010118016-A2—Digital quantification of DNA methylation, US-2005202465-A1—Thymidylate synthase gene and metastasis, US-2014227271-A1—Somatic mutations in atrx in brain cancer, WO-2012094401-A2—Genes frequently altered in pancreatic neuroendocrine tumors, US-2013323167-A1—Detecting and treating solid tumors through selective disruption of tumor vasculature, EP-2912468-B1—Papanicolaou test for ovarian and endometrial cancers, US-9976184-B2—Mutations in pancreatic neoplasms, US-2017267760-A1—Checkpoint Blockade and Microsatellite Instability, US-2018171413-A1—Head and neck squamous cell carcinoma assays, US-2018086832-A1—HLA-restricted epitopes encoded by somatically mutated genes, US-2018258490-A1—Assaying ovarian cyst fluid, US-2016208340-A1—TERT Promoter Mutations in Urothelial Neoplasia, US-2015252415-A1—Arid1b and neuroblastoma, WO-2018071796-A2—Compositions and methods for identifying functional anti-tumor T cell responses, EP-3322824-A1—Detection of tumor-derived DNA in CSF, US-2016273049-A1—Systems and methods for analyzing nucleic acid, US-2018135044-A1—Non-unique barcodes in a genotyping assay, US-2017016075-A1—Neoantigen analysis
Travel, Accommodations, Expenses: Merck
Mark Robson
Consulting or Advisory Role: Change HealthCare
Research Funding: AstraZeneca, Pfizer, Merck
Other Relationship: Research to Practice, Clinical Care Options, Physicans' Education Resource, Invitae, Pfizer
Uncompensated Relationships: Merck, Pfizer, Daiichi Sankyo, Epic Sciences
Open Payments Link: https://openpaymentsdata.cms.gov/physician/612669/summary
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sholl LM, Do K, Shivdasani P, et al. : Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 1:e87062, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zehir A, Benayed R, Shah RH, et al. : Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheler JJ, Janku F, Naing A, et al. : Cancer therapy directed by comprehensive genomic profiling: A single center study. Cancer Res 76:3690-3701, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Hyman DM, Taylor BS, Baselga J: Implementing genome-driven oncology. Cell 168:584-599, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson DR, Wu YM, Lonigro RJ, et al. : Integrative clinical genomics of metastatic cancer. Nature 548:297-303, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty KT, Gray RJ, Chen AP, et al. : Molecular landscape and actionable alterations in a genomically guided cancer clinical trial: National cancer institute molecular analysis for therapy choice (NCI-MATCH). J Clin Oncol 38:3883-3894, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledermann J, Harter P, Gourley C, et al. : Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 15:852-861, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. : Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33:244-250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robson M, Im S-A, Senkus E, et al. : Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med 377:523-533, 2017 [DOI] [PubMed] [Google Scholar]
- 10.Litton JK, Rugo HS, Ettl J, et al. : Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 379:753-763, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner NC, Telli ML, Rugo HS, et al. : A phase II study of talazoparib after platinum or cytotoxic nonplatinum regimens in patients with advanced breast cancer and germline mutations (ABRAZO). Clin Cancer Res 25:2717-2724, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Mirza MR, Monk BJ, Herrstedt J, et al. : Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154-2164, 2016 [DOI] [PubMed] [Google Scholar]
- 13.González-Martín A, Pothuri B, Vergote I, et al. : Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Moore K, Colombo N, Scambia G, et al. : Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018 [DOI] [PubMed] [Google Scholar]
- 15.de Bono J, Mateo J, Fizazi K, et al. : Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med 382:2091-2102, 2020 [DOI] [PubMed] [Google Scholar]
- 16.Franz DN, Belousova E, Sparagana S, et al. : Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381:125-132, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Dombi E, Baldwin A, Marcus LJ, et al. : Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N Engl J Med 375:2550-2560, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross AM, Wolters PL, Dombi E, et al. : Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 382:1430-1442, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. : Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med 366:2180-2188, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandelker D, Zhang L, Kemel Y, et al. : Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA 318:825-835, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Walsh MF, Wu G, et al. : Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 373:2336-2346, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons DW, Roy A, Yang Y, et al. : Diagnostic yield of clinical tumor and germline whole-exome sequencing for children with solid tumors. JAMA Oncol 2:616-624, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daly MB, Pilarski R, Yurgelun MB, et al. : NCCN guidelines insights: Genetic/familial high-risk assessment: Breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw 18:380-391, 2020 [DOI] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network : NCCN guidelines version 2.2021: Prostate cancer. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf [DOI] [PubMed] [Google Scholar]
- 25.National Comprehensive Cancer Network : NCCN guidelines version 1.2021: Genetic/familial high-risk assessment: Colorectal. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf [Google Scholar]
- 26.Chakravarty D, Gao J, Phillips SM, et al. : OncoKB: A precision oncology knowledge base. JCO Precis Oncol 10.1200/PO.17.00011 [epub ahead of print on May 16, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffith M, Spies NC, Krysiak K, et al. : CIViC is a community knowledgebase for expert crowdsourcing the clinical interpretation of variants in cancer. Nat Genet 49:170-174, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamborero D, Rubio-Perez C, Deu-Pons J, et al. : Cancer Genome Interpreter annotates the biological and clinical relevance of tumor alterations. Genome Med 10:25, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patterson SE, Liu R, Statz CM, et al. : The clinical trial landscape in oncology and connectivity of somatic mutational profiles to targeted therapies. Hum Genomics 10:4, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang L, Fernandes H, Zia H, et al. : The cancer precision medicine knowledge base for structured clinical-grade mutations and interpretations. J Am Med Inform Assoc 24:513-519, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner AH, Walsh B, Mayfield G, et al. : A harmonized meta-knowledgebase of clinical interpretations of somatic genomic variants in cancer. Nat Genet 52:448-457, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards S, Aziz N, Bale S, et al. : Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405-424, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalia SS, Adelman K, Bale SJ, et al. : Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): A policy statement of the American College of Medical Genetics and Genomics. Genet Med 19:249-255, 2017 [DOI] [PubMed] [Google Scholar]
- 34.Le DT, Durham JN, Smith KN, et al. : Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357:409-413, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le DT, Uram JN, Wang H, et al. : PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509-2520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abida W, Patnaik A, Campbell D, et al. : Rucaparib in men with metastatic castration-resistant prostate cancer harboring a or gene alteration. J Clin Oncol 38, 3763-3772, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirth LJ, Sherman E, Drilon A, et al. : Registrational results of LOXO-292 in patients with RET-altered thyroid cancers. Ann Oncol 30:v933, 2019 [Google Scholar]
- 38.Solomon BJ, Besse B, Bauer TM, et al. : Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol 19:1654-1667, 2018 [DOI] [PubMed] [Google Scholar]
- 39.Kim D-W, Tiseo M, Ahn MJ, et al. : Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: A randomized, multicenter phase II trial. J Clin Oncol 35:2490-2498, 2017 [DOI] [PubMed] [Google Scholar]
- 40.Jänne PA, Yang JCH, Kim DW, et al. : AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 372:1689-1699, 2015 [DOI] [PubMed] [Google Scholar]
- 41.Wu Y-L, Ahn M-J, Garassino MC, et al. : CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: Data from a randomized phase III trial (AURA3). J Clin Oncol 36:2702-2709, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Soria J-C, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Demetri GD, von Mehren M, Blanke CD, et al. : Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472-480, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Heinrich MC, Owzar K, Corless CL, et al. : Correlation of kinase genotype and clinical outcome in the North American intergroup phase III trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 study by cancer and leukemia group B and southwest oncology group. J Clin Oncol 26:5360-5367, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17:917-927, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Grassi P, Verzoni E, Ratta R, et al. : Cabozantinib in the treatment of advanced renal cell carcinoma: Design, development, and potential place in the therapy. Drug Des Devel Ther 10:2167-2172, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Won Kim T; Asan Medical Center : Durvalumab for MSI-H or POLE Mutated Metastatic Colorectal Cancer. Bethesda, MD, U.S. National Library of Medicine [Google Scholar]
- 48.VanArsdale T, Boshoff C, Arndt KT, et al. : Molecular pathways: Targeting the cyclin D-CDK4/6 Axis for cancer treatment. Clin Cancer Res 21:2905-2910, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Young RJ, Waldeck K, Martin C, et al. : Loss of CDKN2A expression is a frequent event in primary invasive melanoma and correlates with sensitivity to the CDK4/6 inhibitor PD0332991 in melanoma cell lines. Pigment Cell Melanoma Res 27:590-600, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Gong X, Litchfield LM, Webster Y, et al. : Genomic aberrations that activate D-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor abemaciclib. Cancer Cell 32:761-776.e6, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Elvin JA, Gay LM, Ort R, et al. : Clinical benefit in response to palbociclib treatment in refractory uterine leiomyosarcomas with a common alteration. Oncologist 22:416-421, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dickson MA, Tap WD, Keohan ML, et al. : Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol 31:2024, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hadoux J, Favier J, Scoazec JY, et al. : SDHB mutations are associated with response to temozolomide in patients with metastatic pheochromocytoma or paraganglioma. Int J Cancer 135:2711-2720, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Srinivasan R, Gurram S, Al Harthy M, et al. : Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J Clin Oncol 38:5004, 2020 [Google Scholar]
- 55.Jonasch E, Donskov F, Iliopoulos O, et al. : Phase II study of the oral HIF-2α inhibitor MK-6482 for Von Hippel-Lindau disease–associated renal cell carcinoma. J Clin Oncol 38:5003, 2020 [Google Scholar]
- 56.OncoKB : www.oncokb.org
- 57.Golan T, Hammel P, Reni M, et al. : Maintenance olaparib for germline -mutated metastatic pancreatic cancer. N Engl J Med 381:317-327, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Latham A, Srinivasan P, Kemel Y, et al. : Microsatellite instability is associated with the presence of Lynch syndrome pan-cancer. J Clin Oncol 37:286-295, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jonsson P, Bandlamudi C, Cheng ML, et al. : Tumour lineage shapes BRCA-mediated phenotypes. Nature 571:576-579, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abida W, Campbell D, Patnaik A, et al. : Non-BRCA DNA damage repair gene alterations and response to the PARP inhibitor rucaparib in metastatic castration-resistant prostate cancer: Analysis from the phase II TRITON2 study. Clin Cancer Res 26:2487-2496, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tung NM, Robson ME, Ventz S, et al. : TBCRC 048: Phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 38:4274-4282, 2020 [DOI] [PubMed] [Google Scholar]
- 62.Mukherjee S, Zauderer MG, Ravichandran V, et al. Frequency of actionable cancer predisposing germline mutations in patients with lung cancers. J Clin Oncol 36:1504, 2018 [Google Scholar]
- 63.Park W, Chen J, Chou JF, et al. : Genomic methods identify homologous recombination deficiency in pancreas adenocarcinoma and optimize treatment selection. Clin Cancer Res 26:3239-3247, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]