Abstract
Objective
: To study the correlation between alcohol consumption and the risks of liver, esophageal squamous cell carcinoma (ESCC), and gastric cancers in China mainland by meta-analysis.
Methods
: We systematically searched electronic databases to identify the case–control studies that reported the association between alcohol consumption and the risks of liver, ESCC, and gastric cancers from January 1, 2010 to April 1, 2020. The Newcastle–Ottawa Scale (NOS) was used to evaluate literature quality, and I2 analyzes were used to evaluate the heterogeneity.
Results
: A total of 2855-related studies were retrieved. After conditional screening, we included 26 case–control studies for meta-analysis. Meta-analysis showed that alcohol consumption was associated with increased risks of liver, ESCC, and gastric cancers (total pooled odds ratio [OR], 1.83; 95% confidence interval [CI], 1.58–2.11; liver cancer OR, 1.83; 95% CI, 1.39–2.40; ESCC OR, 2.00; 95% CI, 1.66–2.40; gastric-cancer OR, 1.54; 95% CI, 1.10–2.15). Subgroup analysis results showed that the pooled ORs of volume of alcohol consumed, years of drinking, age of starting drinking, and drinking status were 1.71 (95% CI, 1.36–2.15), 1.65 (95% CI, 1.33–2.06), 1.38 (95% CI, 0.98–1.94), and 2.00 (95% CI, 1.42-2.81), respectively. Regression analysis showed that geographical region was a source of heterogeneity.
Conclusion
: Alcohol consumption increased the risks of liver cancer, ESCC, and gastric cancers in China. Volume of alcohol consumed, years of drinking, age of starting drinking, and drinking status were all significant factors for these risks.
Keywords: alcohol consumption, esophageal squamous cell carcinoma, gastric cancer, liver cancer, meta-analysis
1. Introduction
The Global Burden of Disease Study 2016 (2016) showed that alcohol consumption is one of the main risk factors for cancer death.[1] Liver cancer, stomach cancer, and esophageal cancer have always been the common causes of cancer death in China. According to Global Cancer Observatory (GLOBOCAN) 2012 data, patients in China with these 3 types of cancer accounted for nearly half of such cancer patients worldwide; liver, gastric, and esophageal cancers had standardized mortality rates of 17.1/100000, 17.5/100000, and 12.7/100000,[2] ranking as the third, second, and fourth most common causes of cancer deaths in China, respectively.[3] As these cancers have caused a heavy disease burden, it is necessary to study their risk factors and strengthen prevention. Meta-analysis of the correlation between alcohol consumption and cancer shows that heavy drinking increases the risks of liver (relative risk [RR], 2.07), esophageal (RR, 4.95), and gastric (RR, 1.21) cancers.[4] Previous studies have not adequately studied the relationship between the Chinese population and these 3 cancers. Other studies have mainly focused on the effects of heavy alcohol consumption; instead, few studies focus on volume of alcohol consumed, years of drinking, age of starting drinking, or drinking status, the relationship between drinking and cancer is studied as a relatively simple 1.
Esophageal cancer has 2 predominant histopathological subtypes: esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). There are significant molecular differences at both the genomic and epigenomic levels between ESCC and EAC and these 2 cancer types have different sets of driver genes, mutational signatures, and prognostic biomarkers, which are almost mutually exclusive.[5] Among all patients with esophageal cancer, a large proportion of them are ESCC, especially in the high-incidence area (i.e., Central and East Asia Asians). A host of studies have shown that there is a strong positive correlation between alcohol drinking and ESCC.[6–9] However, the association between drinking and EAC weakened.[10,11] Therefore, this article only focuses on ESCC.
To comprehensively assess the association between alcohol consumption and the risks of liver, ESCC, and gastric cancers in China, explore the impact of alcohol-related factors (i.e., volume of alcohol consumed, years of drinking, age of starting drinking, or drinking status) on the risk of developing these 3 cancers, and to provide more evidence with which to establish effective prevention methods, we conducted a meta-analysis of case cancer-control studies on alcohol consumption and these 3 cancers in China over the past decade.
2. Materials and methods
2.1. Literature retrieval strategies
This study followed the Standards for Preferred Reporting Items for Systematic Reviews and Meta-Analyses.[12] PICOS scheme was followed for reporting inclusion criteria. We searched PubMed, the China National Knowledge Infrastructure, the Wanfang database, and the China Hospital Knowledge Database using the Medical Subject Headings terms “(drinking OR alcohol) AND (liver cancer) AND (China OR Chinese), (drinking OR alcohol) AND (esophageal cancer) AND (China OR Chinese), (drinking OR alcohol) AND (stomach cancer OR gastric cancer) AND (China OR Chinese), (drinking OR alcohol) AND (gastrointestinal cancer OR digestive tract cancer) AND (China OR Chinese)” to identify relevant published studies in Chinese and English. The searched was performed for articles published from January 1, 2010 to April 1, 2020.
2.2. Literature inclusion criteria and exclusion criteria
Inclusion criteria were as follows:
-
1.
Chinese- or English-language study;
-
2.
research on people in mainland China;
-
3.
Case–control study;
-
4.
clear sample size, with original research data (including odds ratio [OR] value and 95% confidence interval [CI] fully provided;
-
5.
publication year from January 1, 2010 to April 1, 2020.
Exclusion criteria were as follows:
-
1.
duplicate publications or incomplete information;
-
2.
patient population who (a) did not have liver, ESCC, or gastric cancer but instead had other cancers or non-cancer diseases such as hypertension or diabetes, and (b) were non-mainland Chinese;
-
3.
non-case–control study and/or year of publication outside the match parameters; and
-
4.
OR value and 95% CI not given.
2.3. Literature quality evaluation
The quality of the literature was evaluated according to the Newcastle–Ottawa Scale (NOS). The NOS includes items on population selection (4 items, 4 points), comparability between groups (1 item, 2 points), and exposure evaluation (3 items, 3 points), for a total of 9 points. Scores of 7 to 9 points indicate high-quality studies, those of 4 to 6 points indicate moderate-quality studies, and those of 1 to 3 points indicate low-quality studies. All studies included in this meta-analysis scored greater than 7 points. Any discrepancies between authors we turned to the original literature and relevant experts.
2.4. Information extraction
We screened studies according to the inclusion and exclusion criteria, evaluated literature quality, and then extracted the data. Extracted content included author, year of publication, age of study subjects, type of cancer, case–control matching, number of cases, number of control groups, geographical region, comprehensive statistical-index OR value, and the OR value's 95% CI.
To avoid the omission and duplication of the literature included in this study, we were done separately by 2 people in the process of literature search, screening and information extraction. In addition, to control confounding factors, the effect value OR and 95% CI included in the meta-analysis were adjusted by multivariate or stratified analysis in the original articles.
2.5. Ethics
This study did not involve human beings or experimental subjects. No ethical approval is required.
2.6. Statistical analysis
We used Stata software version 15 (StataCorp, College Station, Texas, US) to perform meta-analysis. Heterogeneity among studies was assessed using I2 statistics, with I2 > 50% representing significant heterogeneity. When the heterogeneity I2 was <50% (P ≥ .05), we selected the fixed-effects model; when the heterogeneity I2 was ≥ 50% (P < .05), we selected the random-effects model. Count data were calculated by pooled OR and 95% CI. We performed subgroup analysis to evaluate the effects of variables relevant to alcohol consumption. To find the source of heterogeneity, we further performed meta-regression with covariables, such as NOS score, matching ratio, and geographical region. Egger test was used to quantitatively assess publication bias. P < .05 was considered statistically significant.
This study conducted subgroup analysis on volume of alcohol consumed, years of drinking, age of starting drinking, and drinking status. Grouping was as follows:
-
1.
units of drinking volume were converted to g/d: ≤ 40, 40–79, 80–120, or >120; (2) years of drinking (years) ≤30 or >30;
-
2.
age of starting drinking (years) was ≤30 or >30; and
-
3.
drinking status was past drinking or current drinking.
3. Results
3.1. Results of literature retrieval and screening
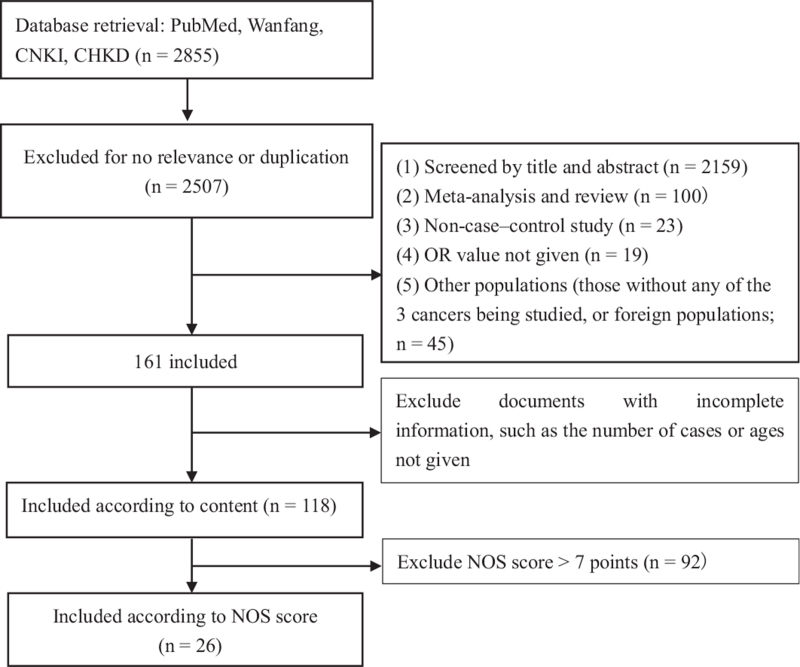
According to the above inclusion and exclusion criteria, we retrieved a total of 2855 articles. After conditional screening, we included 26 articles.[13–38] for meta-analysis, including 11 articles on liver cancer, 10 on ESCC, and 5 on gastric cancer. The literature screening process is shown in Figure 1. The total number of cases was 1643642 cases, with 87309 cases in the case group and 1556333 cases in the control group. All of the participants were age >35 years (Table 1).
Figure 1.
Flowchart of the systematic search of literature on alcohol consumption and the risks of liver, ESCC, and gastric cancers in China.
Table 1.
Case–control studies on the association between alcohol consumption and the risks of liver, ESCC, and gastric cancers in China.
| Author | Publication year | Type of cancer | Matching | Cases (M1/N1)1 | Controls (M2/N2)2 | Region | NOS score |
| Long Ji | 2010 | Liver | Other | 394/500 | 374/507 | Guangxi | 7 |
| Yujian Lan | 2012 | Liver | 1:1 | 162/200 | 162/200 | Guangxi | 7 |
| Xiaoli Wang | 2012 | Liver | 1:1 | 215/251 | 215/251 | Guangdong | 7 |
| Xiangui Tong | 2013 | ESCC | Other | 133/164 | 266/328 | Anhui | 7 |
| Shuping Nie | 2012 | ESCC | Other | 426/612 | 547/770 | Jiangsu | 8 |
| Dandan Chen | 2013 | Liver | Other | 237/323 | 256/443 | Henan | 7 |
| Haifeng Yu | 2014 | Liver | 1:1 | 90/104 | 90/104 | Guangdong | 7 |
| Yuefen Zhou | 2018 | Gastric | 1:1 | 148/210 | 148/210 | Zhejiang | 8 |
| Ming Wu | 2011 | ESCC | Other | 1191/1520 | 2916/3879 | Jiangsu | 7 |
| Xiaorong Yang | 2017 | ESCC | 1.3:1 | 921/1353 | 432/1961 | Jiangsu | 7 |
| Huabin Wu | 2012 | Liver | 1:1 | 956/1254 | 956/1254 | Jiangsu | 9 |
| Qing Zhu | 2019 | Gastric | Other | 166/215 | 330/430 | Gansu | 7 |
| Zhongpei Xie | 2013 | ESCC | Other | 114/196 | 127/201 | Guiyue | 7 |
| Sa Tang | 2014 | ESCC | Other | 3030/3759 | 3447/5196 | Henan | 7 |
| Xueke Zhao | 2014 | Liver | Other | 602/762 | 458/798 | Guizhou | 7 |
| Shasha Chen | 2013 | Liver | Other | 98/120 | 156/199 | Guizhou | 7 |
| Jianxue Duan | 2018 | Liver | Other | 268/330 | 353/464 | Chongqing | 8 |
| Yan Li | 2016 | Gastric | 1: 1 | 36/71 | 36/71 | Jiangsu | 7 |
| Jianli Hu | 2010 | ESCC | 1: 1.5-2 | 283/283 | 538/538 | Beijing | 7 |
| Fansong Meng | 2019 | ESCC | Other | 203/278 | 406/556 | Shandong | 7 |
| Ying Liu | 2014 | Liver | 1: 1 | 812/1007 | 488/1007 | Hebei | 7 |
| Hong Lu | 2012 | ESCC | Other | 310/400 | 565/752 | Gansu | 7 |
| Qingping Xue | 2015 | Gastric | 1: 1 | 224/308 | 224/308 | Sichuan | 7 |
| Shaoyi Lin | 2011 | Liver | 1: 1 | 303/388 | 303/388 | Fujian | 7 |
| Jia Wang | 2012 | Gastric | 1: 1 | 347/476 | 347/476 | Jiangsu | 7 |
| Weihong Gan | 2011 | ESCC | 1: 2 | 81/97 | 162/194 | Shanghai | 7 |
1: M1—Number of male in the case group; N1—Total number of cases; 2: M2—Number of male in the control group; N2—Total number of controls.
3.2. Meta-analysis of the literature on the relationship between alcohol consumption and the risks of liver, esophageal squamous cell carcinoma, and gastric cancers
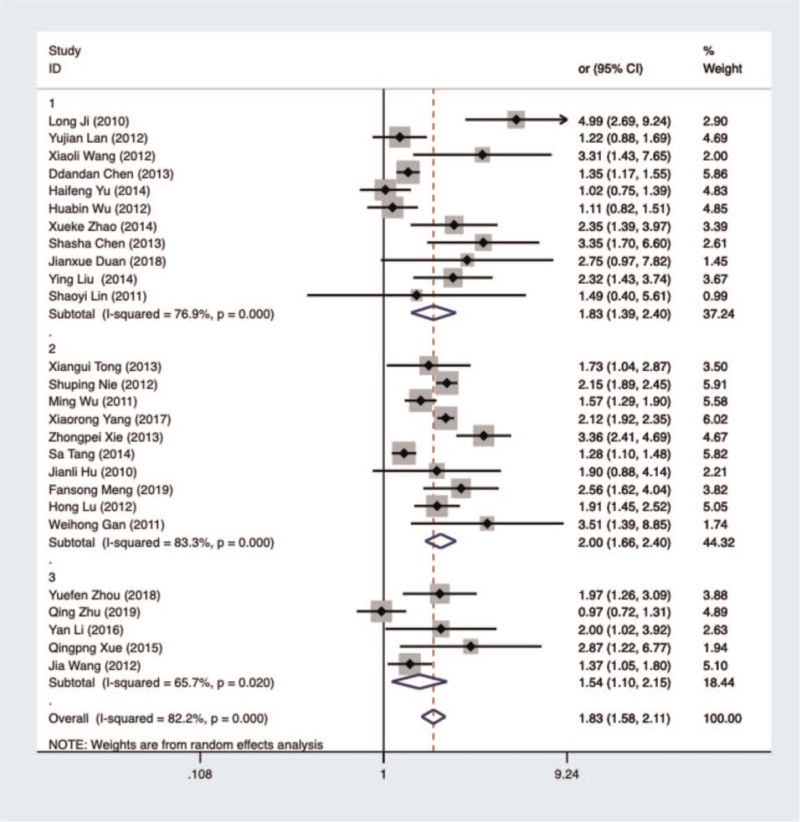
Meta-analysis results showed that alcohol consumption was associated with increased risks of liver, ESCC, and gastric cancers (total pooled OR, 1.83 [95% CI, 1.58–2.11]; liver cancer OR, 1.83 [95% CI, 1.39–2.40]; ESCC OR, 2.00 [95% CI, 1.66–2.40]; gastric-cancer OR, 1.54 [95% CI, 1.10–2.15]). The difference between drinking and the 3 cancers types was statistically significant (P < .05). A forest plot of the relationship between alcohol consumption and liver, ESCC, and gastric cancers is shown in Figure 2.
Figure 2.
Forest plot of analyzed literature on the relationship between alcohol consumption and liver, ESCC, and gastric cancers in China. 1—liver cancer; 2—ESCC; 3—gastric cancer.
3.3. Subgroup analysis of alcohol-related variables and liver, esophageal squamous cell carcinoma, and gastric cancers
We performed a subgroup analysis based on volume of alcohol consumed, years of drinking, age of starting drinking, and drinking status. The combined-effect OR values were 1.71 (95% CI, 1.36–2.15), 1.65 (95% CI, 1.33–2.06), 1.38 (95% CI, 0.98 –1.94), and 2.00 (95% CI, 1.42–2.81), respectively. The greater the daily alcohol consumption, the greater the OR value of cancer risk. Drinking <40 g daily had no statistical significance to the risk of cancer (OR, 1.50 [95% CI, 0.94–2.40]; I2 = 93.7%, P > .05). Risk was greatest when daily volume of alcohol consumed exceeded 120 g / d (OR, 2.44 [95% CI, 1.96–3.02]; I2 = 85.0%, P ≤ .001). Drinking for >30 years, beginning to drink at >30 years old, and past drinking were all statistically significant (Table 2).
Table 2.
Subgroup analysis of meta-analysis between alcohol consumption and the risks of liver, ESCC, and gastric cancers.
| Subgroup | Cases | Controls | OR | 95% CI | I2 (%) | P |
| Volume of alcohol consumption (g/d) | ||||||
| ≤40 | 4176 | 6679 | 1.50 | (0.94–2.40) | 93.7 | .091 |
| 40–79 | 449 | 845 | 1.58 | (1.09–2.28) | 78.7 | .016 |
| 80–120 | 533 | 804 | 1.96 | (1.44–2.68) | 00.1 | .000 |
| ≥120 | 686 | 854 | 2.44 | (1.96–3.02) | 85.0 | .000 |
| Total | 5844 | 9182 | 1.71 | (1.36–2.15) | 90.4 | .000 |
| Years of drinking | ||||||
| ≤30 | 1502 | 2682 | 1.26 | (0.99–1.60) | 68.0 | .056 |
| >30 | 1663 | 2281 | 2.31 | (1.60–3.34) | 90.8 | .000 |
| Total | 3165 | 4963 | 1.65 | (1.33–2.06) | 85.5 | .000 |
| Age of starting drinking | ||||||
| ≤30 yrs old | 1340 | 2221 | 1.28 | (0.79–2.08) | 97.2 | .315 |
| >30 yrs old | 508 | 691 | 1.55 | (1.02–2.36) | 91.0 | .040 |
| Total | 1848 | 2912 | 1.38 | (0.98–1.94) | 96.0 | .061 |
| Drinking status | ||||||
| Past drinking | 1151 | 2378 | 2.24 | (1.35–3.70) | 76.0 | .006 |
| Current drinking | 1574 | 3164 | 1.84 | (1.00–3.40) | 94.4 | .000 |
| Total | 2725 | 5542 | 2.00 | (1.42–2.81) | 89.8 | .000 |
3.4. Meta-regression analysis of non-research variables (Newcastle–Ottawa Scale score, matching, and geographical region)
The heterogeneity test showed that I2 = 82.2% and P ≤ .001. This large degree of heterogeneity suggested the data were original. To determine the difference between drinking and the risks of these 3 cancers, we conducted a meta-regression analysis to explore the combined-effect size of non-study variables (NOS score, matching ratio, and geographical region) on our meta-analysis of drinking and liver, ESCC, and gastric cancers. The NOS scores included in the regression analysis were all ≥7 points. Matching ratio groups were 1: 1, 1: 2, 1: 1.5–1.2, 1.3: 1, and other. The regression model showed a good fit (τ2 = 0.098), and the heterogeneous performance accounted for 91.10% of the residual variance. The meta-regression results suggested that geographical region was a source of heterogeneity (P = .03 < .05) (Table 3).
Table 3.
Meta-regression analysis of NOS score, matching ratio, and geographical region.
| Variables | OR | 95%CI | P | P a |
| NOS score | 1.06 | (0.86–1.25) | .66 | 0.95 |
| Matching | 1.04 | (0.92–1.02) | .18 | 0.43 |
| Regions | 1.03 | (1.01–1.04) | .03 | 0.04 |
Pa: adjusted P value.
3.5. Publication bias
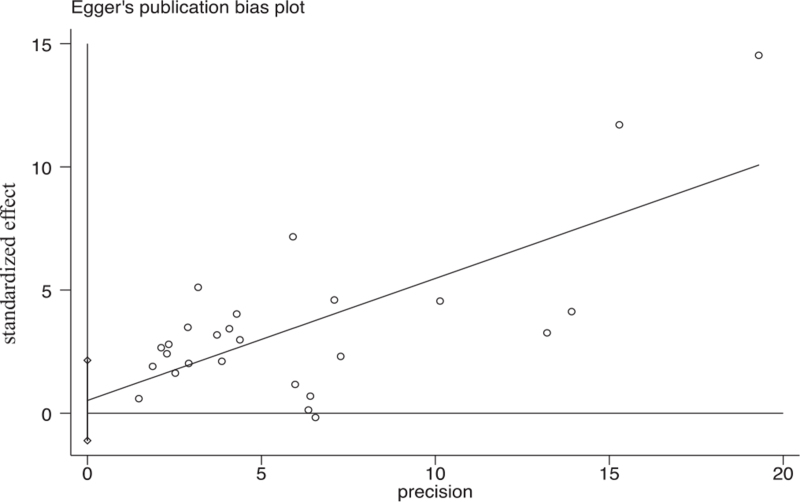
The results of Egger test indicated that there was no significant publication bias observed in the selected studies (P = .518) (Fig. 3).
Figure 3.
Results of Egger test for publication bias.
4. Discussion
This meta-analysis included 26 case–control studies on alcohol consumption and the risks of liver, ESCC, and gastric cancers in China. The results showed that drinking alcohol was a risk factor for all 3 types of cancer (pooled OR, 1.83 [95% CI, 1.58–2.11]). The forest plot in Figure 2 shows that drinking increased the risk of and was significantly related to ESCC (OR, 2.00 [95% CI, 1.66–2.40]), liver cancer (OR, 1.83 [95% CI, 1.39–2.40], and gastric cancer (OR, 1.54 [95% CI, 1.10 –2.15]). Another study found that among all types of cancer caused by alcohol consumption, liver cancer has the highest mortality rate and disease burden ratio.[39] Evidence for the relationship between alcohol consumption and gastric cancer is not clear, but excessive drinking is currently considered one of the risk factors for this cancer.[40] The carcinogenic mechanism of alcohol consumption is currently not fully understood. Acetaldehyde is the first metabolic product of ethanol, which modifies DNA by producing DNA adducts and induces oxidative stress and genetic changes in the function of alcohol-metabolizing enzymes, thereby exerting carcinogenic and mutagenic effects. It is part of the carcinogenic effect of drinking on the liver and upper digestive tract.[41]
At present, it is believed that alcohol causes hepatocellular carcinogenesis (HCC) mainly through 3 aspects. On the one hand, alcohol consumption as an inducer and promoter of liver cancer to promote the occurrence of it.[42] Long-term alcohol intake can reduce the liver's detoxification function, reduce the intake of nutrients and reduce the body's immunity. Secondly, acetaldehyde, the intermediate metabolite of alcohol, is considered to be the positive factor for the occurrence of HCC. Modern genetic studies have found that acetaldehyde induces HCC as the target of acetaldehyde dehydrogenase 2 gene (ALDH2) on chromosome 12.[43] Thirdly, heavy alcohol consumption may cause alcoholic cirrhosis, which may further develop into HCC.[44,45] Study has found that the human liver can only completely metabolize 80 mL of ethanol per day, and the consumption of more than 80 mL will increase and accumulate the concentration of acetaldehyde in the blood. Acetaldehyde has the role of carcinogenicity and gene mutation and plays a major role in the process of alcohol-related carcinogenesis.[46]
This study found a positive dose relationship between alcohol consumption and liver, ESCC, and gastric cancers. As volume of alcohol consumed daily increased, so did the risk of cancer, which was similar to the results of similar studies. There is a certain dose relationship between alcohol consumption and the incidence of esophageal cancer.[47] Alcohol intake increases the risk of esophageal cancer according to the daily ethanol intake, the type of alcoholic beverages consumed, time of abstinence, age of starting drinking, population, and differences in esophageal-cancer subtypes.[48] The relationship between alcohol consumption and cancer is still controversial, especially when consumption is light or moderate.[49] Our results showed that drinking <40 g daily was not statistically significant to the risk of cancer; the risk was greatest when volume of alcohol consumed daily was >120 g / d. This differed somewhat from the results of similar studies.[50,51] In addition, a meta-analysis of the relationship between alcohol consumption and cancer that included 222 studies found that in studies conducted only in Asian populations, the effect of low alcohol consumption on esophageal-cancer risk was statistically significant (RR, 1.49 [95% CI, 1.12–1.98]).[52] This suggests to some extent that Asians have more genetic polymorphisms encoding alcohol-metabolizing enzymes than other populations do, and therefore the amount of alcohol consumed in these populations should be controlled within an appropriate range.
Our study found that subjects who had been drinking for more than 30 years had a cancer risk OR value greater than those who had been drinking for less than 30 years, which might be related to long-term drinking and increased alcohol consumption. Studies have found that the risk of developing esophageal cancer increases along with alcohol consumption.[53] Compared with not drinking throughout life, the risk of esophageal cancer is positively correlated with the amount of alcohol consumed, and moderate and high levels of drinking are associated with an increased risk of esophageal cancer.[54] A summary analysis of 7 studies (5 case–controls and 2 cohort studies) conducted by the International Barrett and Esophageal Adenocarcinoma Federation (BEACON) found that an increase in years of drinking was associated with an increased risk of esophageal cancer.[55] Another study found that drinking frequency was positively correlated with the risks of esophageal and liver cancers. There is a positive dose–response relationship between amount of alcohol consumed and the risks of esophageal and liver cancers. People who drink ≥50 g/day on average have an increased risk of stomach cancer.[56] Our study found that the combined effects of the 3 types of cancer with an age of starting drinking ≥30 years was greater than when the age of starting drinking was <30 years. The effect of the age at which a person starts drinking on the risk of ESCC is uncertain. Studies have found that as they increase their daily alcohol intake, people who start drinking at an old age are more likely to suffer from ESCC.[57] In Castellsague et al study,[58] people who started drinking alcohol at an older age were more likely to develop esophageal cancer as their daily alcohol intake increased. However, there are also studies showing no association between age of beginning drinking and the risk of developing ESCC.[59]
This study could only search in Chinese and English databases, and no literature in other languages was collected, which may be influenced by the bias of language selection. Most of the results of heterogeneity tests indicated that there was significant heterogeneity among the studies, which might be related to the design of the studies, the variety and number of confounding factors controlled, and ethnic or regional differences. We conducted a further meta-regression analysis, the results of which showed that geographical region was the source of heterogeneity. The source areas of the literature included in this article were Shanxi, Guangxi, Guangdong, Anhui, Jiangsu, Henan, Zhejiang, Gansu, Guizhou, Chongqing, Beijing, Shandong, Sichuan, Fujian, and Shanghai, in China. Incidences and mortality rates of liver, ESCC, and gastric cancers vary from region to region,[60] which might explain the high degree of heterogeneity among the studies we analyzed. Another source of heterogeneity may be the exclusion of the earlier literature on this topic. The methods of diagnosis and treatment of cancer are changing rapidly, and the diagnostic criteria reported in the earlier literature are somewhat different from those reported in the more recent literature, as are the detection rates and survival rates. For example, simple esophagectomy is associated with a higher recurrence rate, with a low 5-year survival rate of 5 to 34 percent.[61] Recent advances in the treatment of patients requiring esophagectomy have been neoadjuvant chemoradiotherapy or chemotherapy. Randomized controlled trials have shown that neoadjuvant chemoradiotherapy or chemotherapy provides a survival benefit for both types of esophageal cancer compared to surgery alone.[62–64]
Besides, the occurrence of cancer is the result of many factors. Drinking alcohol is a known and major cause of esophageal squamous cell carcinoma, especially in countries with low incidence. However, in high-incidence areas of Asia and Africa, other risk factors may be more important, including poor diet, indoor air pollution, consumption of hot drinks, poor oral health, use of non-tap water and use of opium.[65] A case–control study in the Islamic Republic of Iran, which measured exposure to PAHs in endoscopically normal esophageal tissues from cases of oesophageal squamous cell carcinoma and controls, reported odds ratios of more than 25 for the most exposed quintile compared with the least exposed quintile.[66] According to the previous studies the important risk factors for gastric cancers are the host's genetic makeup, the characteristics of H. pylori strains, and environmental factors, notably diet.[65] Globally, hepatitis B virus infection was responsible for 33% of deaths from liver cancer, alcohol consumption for 30%, hepatitis C virus infection for 21%, and other causes for 16%, with significant variation in the underlying etiologies among regions and countries.[67]
This study showed that the combined effect of drinking in the past was greater than that of current alcohol consumption. This was consistent with the Gao Shan[68] study, which found that drinking is associated with an increased risk of liver cancer, but other studies have shown that current alcohol consumption poses a greater risk of cancer.[69] Long-term alcohol intake can reduce the liver's detoxification function and the body's nutritional intake and immunity, and then induce and accelerate liver cancer risk factors to promote the occurrence of liver cancer.[70] This suggests that the effects of drinking, whether past or current, on cancer cannot be ignored.
The strengths of this study lay in the analysis of drinking and its more related subgroup factors. To date, this study provides complete and recent evidence on the association between alcohol drinking and liver, ESCC, and gastric cancers. We were able to explore the relationship between drinking and the risks of these 3 cancers across many aspects, not limited to study on alcohol consumption. Our study results can provide a specific basis for cancer prevention methods. In addition, this analysis focused on important disease burden factors such as digestive-tract tumors and drinking in China, which has practical value for providing new ideas for the study of key tumors and their risk factors.
The limitations also exist. The occurrence and development of liver cancer, ESCC and gastric cancer are the result of a combination of factors, such as genes, smoking, dietary habits, and exposure environment. We included only case–control studies of alcohol consumption and the supporting evidence was weak. More rigorous scientific research such as prospective cohort studies are needed to continue to analyze the relationship between alcohol consumption and cancer risks. Second, men have more opportunities to drink alcohol than women, but this paper did not compare men and women alcohol consumption due to the lack of data from gender analysis in the original articles.
In conclusion, alcohol consumption increased the risks of liver cancer, ESCC, and gastric cancer in China. Volume of alcohol consumed, and years of drinking were related to all 3 types of cancer. The risk of cancer increased along with daily alcohol intake. Our results suggested that controlling alcohol consumption and reducing the years of drinking could help prevent the occurrence of liver, ESCC, and gastric cancers, and that in particular people age >30 years and populations with a high incidence of cancer should reduce their alcohol consumption.
Author contributions
Conceptualization: Baohua Wang.
Methodology: Yuting Sha.
Writing – original draft: Fengdie He.
Footnotes
Abbreviations: CI = confidence interval, EAC = esophageal adenocarcinoma, ESCC = esophageal squamous cell carcinoma, HCC = hepatocellular carcinogenesis, NOS = Newcastle–Ottawa Scale, OR = odds ratio, RR = relative risk.
How to cite this article: He F, Sha Y, Wang B. Relationship between alcohol consumption and the risks of liver cancer, esophageal cancer, and gastric cancer in China: meta-analysis based on case–control studies. Medicine. 2021;100:33(e26982).
National Key R&D Program of China (2016YFC1302603, 2016 YFC1302600).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].GBD 2016 Alcohol Collaborators. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].World Health Organization. The Global Cancer Observatory: Globocan 2020 [EB/OL]. https://gco.iarc.fr/today/data/factsheets/populations/160-china-fact-sheets.pdf. Accessed: March 22, 2021 [Google Scholar]
- [3].He J. China Cancer Registry Annual Report 2018. Beijing: People's Medical Publishing House; 2019. [Google Scholar]
- [4].Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br J Cancer 2015;112:580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lin DC, Dinh HQ, Xie JJ, et al. Identification of distinct mutational pat- terns and new driver genes in oesophageal squamous cell carcinomas and adenocarcinomas. Gut 2018;67:1769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kumagai N, Wakai T, Akazawa K, Ling Y, et al. Heavy alcohol intake is a risk factor for esophageal squamous cell carcinoma among middle-aged men: a case-control and simulation study. Mol Clin Oncol 2013;1:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lubin JH, Cook MB, Pandeya N, et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett's Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol 2012;36:306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Freedman ND, Murray LJ, Kamangar F, et al. Alcohol intake and risk of oesophageal adenocarcinoma: a pooled analysis from the BEACON consortium. Gut 2011;60:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yaegashi Y, Onoda T, Morioka S, et al. Joint effects of smoking and alcohol drinking on esophageal cancer mortality in Japanese men: findings from the Japan collaborative cohort study. Asian Pac J Cancer Prev 2014;15:1023–9. [DOI] [PubMed] [Google Scholar]
- [10].Thrift AP, Kramer JR, Richardson PA, El-Serag HB. No significant effects of smoking or alcohol consumption on risk of Barrett's esophagus. Dig Dis Sci 2014;59:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lou Z, Xing H, Li D. Alcohol consumption and the neoplastic progression in Barrett's esophagus: a systematic review and meta-analysis. PLoS One 2014;9:e105612.Doi: 10.1371/journal.pone.0105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liberati A, Altman DG, Tetzlaff J, Cynthia M, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100.Doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Long J. A case control study on risk factors of hepatocellular carcinoma in Guangxi. Cancer Prevention Treatment 2010;23:453–7. [Google Scholar]
- [14].Guangxi Medical University, Yujian L. Investigation and Analysis of Risk Factors of Primary Liver Cancer in Central Guangxi. 2015;Doi:10.7666/d.Y2876948. [Google Scholar]
- [15].Wang X, Yu X, Chengyu Zhou, et al. Analysis of risk factors of primary liver cancer in Shunde, Guangdong. J Pract Med 2012;28:3074–6. [Google Scholar]
- [16].Anhui Medical University, Tong G. A Study on Influencing Factors of Malignant Tumors of the Digestive Tract in Rural Residents of Anhui. 2015;Doi:10.7666/d.D708417. [Google Scholar]
- [17].Shandong University, Nie S. Study on the Relationship between Smoking and Drinking, Helicobacter pylori Infection and Esophageal Cancer. 2015;Doi:10.7666/d.Y2793382. [Google Scholar]
- [18].Chen D, Liu Y, Ji X, et al. A case-control study on the influencing factors of primary liver cancer. J Zhengzhou Univers 2013;48:249–53. [Google Scholar]
- [19].Yu H, Gao Y, Ye X. Investigation and analysis of risk factors of primary liver cancer in Leliu Town, Shunde District, Foshan City. Primary Health Care China 2014;28:95–8. [Google Scholar]
- [20].Zhou Y, Wu M, Xie Y, et al. A case-control study on risk factors of gastric cancer in Lishui City. Chin Med Herald 2018;15:45–50. [Google Scholar]
- [21].Wu M, Zhao J, Zhang Z, Han R, et al. Smoking and alcohol drinking increased the risk of esophageal cancer among Chinese men but not women in a high-risk population. Cancer Causes Control 2011;22:649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang X, Chen X, Zhuang M, et al. Smoking and alcohol drinking in relation to the risk of esophageal squamous cell carcinoma: a population-based case-control study in China. Sci Rep 2017;7:17249.Doi: 10.1038/s41598-017-17617-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu H, Ming H, Liu A, et al. A case-control study on influencing factors of liver cancer in areas with high tumor incidence in Jiangsu Province. Jiangsu Preventive Med 2012;23:26–8. [Google Scholar]
- [24].Lanzhou University, Zhu Q. Risk Factors for Gastric Cancer of Rural Residents in Central and Eastern Parts of Gansu Province:A Case-Control Study. 2019;37. [Google Scholar]
- [25].Xie Z, Zhou H, Teng Y, et al. A case-control study on the influencing factors of esophageal cancer among residents in coastal areas of Guangdong and Guangxi. J Guangxi Med Univers 2013;30:695–8. [Google Scholar]
- [26].Zhengzhou University, Tang S. Alcohol Flushing, Drinking and ALDH2 Gene Polymorphism with Esophageal Cancer Risk and Prognosis. 2014;16. [Google Scholar]
- [27].Zhao X, Cheng M, Zhang Q, et al. Analysis of risk factors of 762 patients with liver cancer in Guizhou. Chin J Hepatol 2014;22:33–7. [Google Scholar]
- [28].Chen S, Zhao X, Cheng M, et al. A case-control study on the risk factors of primary liver cancer in Guiyang. J Guiyang Med Coll 2013;38:01–4. [Google Scholar]
- [29].Huazhong University of Science and Technology, Hu J. Study on the association between drinking, HO-1 gene promoter microsatellite polymorphism and susceptibility to esophageal squamous cell carcinoma. 2010;30–32. [Google Scholar]
- [30].Yan L, Lu H, Cao T, et al. Investigation and analysis of risk factors for gastric cancer in young adults. Chinese Med Guide 2016;14:14–5. 17. [Google Scholar]
- [31].Huazhong University of Science and Technology, Hu J. Study on the Association between Drinking, HO-1 Gene Promoter Microsatellite Polymorphism and Susceptibility to Esophageal Squamous Cell Carcinoma. 2010;30–32. [Google Scholar]
- [32].Meng F, Zhang N, Ma H, et al. A case-control study on risk factors of esophageal cancer among rural residents in some districts and counties of Jinan City. Chin J Cancer Prevention Treatment 2019;26:609–12. [Google Scholar]
- [33].Ying L, Zhao T, Xu Y, et al. A case-control study on the susceptibility factors of primary liver cancer in Hebei Province. Chin J Coal Industry Med 2014;17:2009–12. [Google Scholar]
- [34].Hong Lu, Wang P, Wu J. A case-control study on risk factors of esophageal cancer in Wuwei City. Modern Preventive Med 2012;39:3486–7. 3490. [Google Scholar]
- [35].Xue Q, Pan X, Li S, et al. Analysis on the relationship between gastric cancer and living habits and behavior influencing factors in Sichuan Province. Modern Preventive Med 2015;42:1257–60. [Google Scholar]
- [36].Lin S, Hu Z. Conditional logistic regression analysis of the relationship between smoking, drinking and liver cancer. Straits J Preventive Med 2011;17:15–7. [Google Scholar]
- [37].Wang J, Li P, Fu G, et al. A case-control study on environmental factors of gastric cancer among Han residents in Nanjing. Chinese Public Health 2012;28:1137–9. [Google Scholar]
- [38].Hongwei Gan, Gao X, Zhou J, et al. A case-control study on risk factors of esophageal cancer in Jinshan District, Shanghai. Occupation Health 2011;27:58–9. [Google Scholar]
- [39].Rehm J, Shield K. Alcohol use and cancers of the gastrointestinal tract. Epidemiology and preventive implications. Front Oncol 2020;10:403.Doi: 10.3389/fonc.2020.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang P, Xiao F, Gong B, Liu F. Alcohol drinking and gastric cancer risk: a meta-analysis of observational studies. Oncotarget 2017;8:99013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Seitz HK, Stickel F. Molecular mechanisms of alcohol- mediated carcinogenesis. Nat Rev Cancer 2007;7:599–612. [DOI] [PubMed] [Google Scholar]
- [42].Yu MW, Hsu FC, Sheen IS, et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in a-symptomatic hepatitis B virus. Am J Epidemiol 1997;145:1039–47. [DOI] [PubMed] [Google Scholar]
- [43].Ding J, Wu J, Gao C. Aldehyde dehydrogenase 2 gene polymorphisms, drinking habits and susceptibility to liver cancer. Oncology 2010;24:309–10. [Google Scholar]
- [44].Zimmerlin L, Donnenberg VS, Rubin JP, et al. Mesenchymal markers on human adipose stem /progenitor cells. Cytometry A 2013;83:134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–7. [DOI] [PubMed] [Google Scholar]
- [46].Posch IG, Seitz HK. Alcohol and cancer. Alcohol 2004;39:155–65. [DOI] [PubMed] [Google Scholar]
- [47].Pan Y, Zhang L, Pan E, et al. A case-control study on risk factors of early esophageal cancer among residents in Huaian city. Jiangsu J Prev Med 2017;28:515–7. [Google Scholar]
- [48].Peng Q, Chen H, Huo JR. Alcohol consumption and corresponding factors: a novel perspective on the risk factors of esophageal cancer. Oncol Lett 2016;11:3231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cao Y, Willett WC, Rimm EB, Stampfer MJ, Giovannucci EL. Light to moderate intake of alcohol, drinking patterns, and risk of cancer: results from two prospective US cohort studies. Brit Med J 2015;351:h4238.Doi: 10.1136/bmj.h4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Choi YJ, Lee DH, Han KD, et al. The relationship between drinking alcohol and esophageal, gastric or colorectal cancer: a nation-wide population-based cohort study of South Korea. PLoS One 2017;12:e0185778.Doi: 10.1371/journal.pone.0185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Park H, Shin SK, Joo I, Song DS, Jang JW, Park JW. Systematic review with meta-analysis: low-level alcohol consumption and the risk of liver cancer. Gut Liver 2020;Doi: 10.5009/gnl19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bagnardi V, Rota M, Botteri E, Scotti##L, et al. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol 2013;24:301–8. [DOI] [PubMed] [Google Scholar]
- [53].Kumagai N, Wakai T, Akazawa K, et al. Heavy alcohol intake is a risk factor for esophageal squamous cell carcinoma among middle-aged men: a case-control and simulation study. Mol Clin Oncol 2013;1:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Islami F, Fedirko V, Tramacere I, et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: a systematic review and meta-analysis. Int J Cancer 2011;129:2473e84.Doi: 10.1002/ijc.25885. [DOI] [PubMed] [Google Scholar]
- [55].Lubin JH, Cook MB, Pandeya N, et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett's Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol 2012;36:306e16.Doi: 10.1016/j.canep.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhao J, Wu M, Kim CH, et al. Jiangsu four cancers study: a large case-control study of lung, liver, stomach and esophageal cancers in Jiangsu Province, China. Eur J Cancer Prev 2017;26:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Qiao P, Chen H, Huo J. Alcohol consumption and corresponding factors: a novel perspective on the risk factors of esophageal cancer. Oncol Lett 2016;11:3231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Castellsagué X, Muñoz N, De Stefani E, et al. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer 1999;82:657–64. [DOI] [PubMed] [Google Scholar]
- [59].Zambon P, Talamini R, La Vecchia C, et al. Smoking, type of alcoholic beverage and squamous-cell oesophageal cancer in northern Italy. Int J Cancer 2000;86:144–9. [DOI] [PubMed] [Google Scholar]
- [60].Sun K, Zheng R, Zhang S, et al. Report of cancer epidemiology in China, 2015. China Cancer 2019;28:01–11. [DOI] [PubMed] [Google Scholar]
- [61].Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499–509. [DOI] [PubMed] [Google Scholar]
- [62].Morgan MA, Lewis WG, Casbard A, et al. Stage-for-stage comparison of definitive chemoradiotherapy, surgery alone and neoadjuvant chemotherapy for oesophageal carcinoma. Br J Surg 2009;96:1300–7. [DOI] [PubMed] [Google Scholar]
- [63].Van Hagen P, Hulshof MC, van Lan-schot JJ, et al. Preoperative chemoradio-therapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- [64].Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wild CP, Weiderpass E, Stewart BW. World Cancer Report: Cancer Research for Cancer Prevention http://publications.iarc.fr/586http://publications.iarc.fr/586. Accessed March 22, 2021. [Google Scholar]
- [66].Abedi-Ardekani B, Kamangar F, Hewitt SM, et al. Polycyclic aromatic hydrocarbon exposure in oesophageal tissue and risk of oesophageal squamous cell carcinoma in north-eastern Iran. Gut 2010;59:1178–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Akinyemiju T, Abera S, Ahmed M, et al. Global burden of disease liver cancer collaboration. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Fudan University, Gao S. Epidemiological Studies of Primary Liver Cancer in Urban Shanghai. 2011;8–11. [Google Scholar]
- [69].Jia S, He L, Zeng H, et al. Association of cancer prevention awareness with esophageal cancer screening participation rates: Results from a population-based cancer screening program in rural China. Chin J Cancer Res 2019;31:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Li S, Wu J, Ding J, et al. Impact of genetic polymorphisms of glutathione S-transferaseT1, M1 on the risk of primary hepatocellular carcinoma in alcohol drinkers. Pract J Cancer 2012;19:229–30. [Google Scholar]