Abstract
This study aimed to investigate the association between serum uric acid (SUA) level and nonalcoholic fatty liver disease (NAFLD) in patients with type 2 diabetes (T2DM).
T2DM patients hospitalized in the Department of Hepatology, Yantai Qishan Hospital, between April 2012 and December 2018 were classified into the NAFLD group and the non-NAFLD group. Clinical data, glucose and lipid metabolism biomarkers, and liver and kidney function parameters were retrospectively collected.
Five hundred eighty-three T2DM patients met the inclusion and exclusion criteria; 227 patients were included in the non-NAFLD group and 356 patients were included in the NAFLD group. Multiple linear regression analyses showed that SUA was positively correlated with body mass index (P = .003), triglycerides (P = .009), aspartate aminotransferase (P = .036), and alanine aminotransferase (P = .038) and negatively correlated with estimated glomerular filtration rate (P < .001) in T2DM patients. Multivariate regression analyses demonstrated that after adjusting for confounding factors, the SUA tertile was still significantly associated with NAFLD occurrence in T2DM patients (P for trend = .008). With reference to SUA tertile I, the odds ratios for NAFLD in the SUA tertile II and tertile III groups were 1.729 (95% confidence interval [CI]: 1.086–2.753) and 2.315 (95% CI: 1.272–4.213), respectively.
The level of SUA in T2DM patients was associated with the occurrence of NAFLD. Elevated SUA was associated with a significantly increased prevalence of NAFLD. The SUA level was an independent risk factor for NAFLD occurrence in patients with T2DM.
Keywords: insulin resistance, nonalcoholic fatty liver disease, serum uric acid, type 2 diabetes
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) refers to pathological changes characterized by diffuse steatosis and lipid accumulation in hepatocytes in individuals without a history of alcohol consumption equivalent to <140 g/week for men or <70 g/week for women after eliminating other identified causes.[1] According to whether the lesion is combined with an inflammatory reaction and/or fibrosis, NAFLD could be classified into the nonalcoholic simple fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), and NASH-related cirrhosis.[2] NAFLD is a common cause of abnormal liver function and chronic liver diseases, and NAFLD affects 20% to 30% of the general population in Western developed countries.[3] Recently, NAFLD has become the second most common chronic hepatic disease in China, ranking only after hepatitis virus B, and the development of cirrhosis in NASH patients is as high as 15% to 25% within 10 to 15 years.[4] Potential risk factors for NAFLD include a high-fat, high-calorie dietary pattern; lifestyles with more sitting and less physical activity; metabolic syndrome (MS) and its main components (obesity, hypertension, dyslipidemia, and hyperglycemia); and insulin resistance (IR).[5]
Uric acid is the end metabolite of purine in the body.[6] In recent years, the incidence of hyperuricemia (HUA) has increased rapidly. HUA has been confirmed to be associated with IR, MS, and its related components, such as diabetes, hypertension, obesity, hyperlipidemia, and nonalcoholic fatty liver. Previous studies have confirmed that HUA is also one of the important components of MS, and HUA may trigger IR and maybe an independent risk factor for MS.[7,8]
Many studies have clarified that the serum uric acid (SUA) level is closely related to NAFLD. Li et al[9] confirmed that ultrasound-diagnosed NAFLD patients had obviously higher SUA levels than controls, that SUA levels were associated with NAFLD, and that elevated SUA levels were an independent risk factor for NAFLD. Petta et al[10] demonstrated that the degree of SUA elevation was independently associated with the severity of liver tissue damage in NAFLD patients. Hwang et al[11] showed that elevated SUA, even within the normal range, was also independently associated with NAFLD occurrence. A prospective observational study also found that elevated SUA levels could independently predict an increased risk for incident NAFLD.[12] In contrast, some studies have shown that the SUA level was not associated with NAFLD occurrence.[13] Thus, the association between SUA level and NAFLD is still uncertain, and few studies have been conducted on T2DM patients. Herein, we explored the relationship between SUA levels and NAFLD in T2DM patients.
2. Patients and methods
2.1. Patients
We enrolled T2DM patients who were hospitalized in the Department of Hepatology, Yantai Qishan Hospital between April 2012 and December 2018. Diet intake was based on the standard meals for all T2DM patients provided by the hospital. Patients received liver examination through color Doppler ultrasonography with a 3.5-MHz probe (Toshiba, Tokyo, Japan). T2DM was diagnosed according to the 1999 World Health Organization diagnostic criteria (14). Patients with 1 of the following conditions were excluded: 1) type 1 diabetes, gestational diabetes, or acute complications of diabetes; 2) viral hepatitis, liver fat deposition caused by autoimmune liver disease, liver cirrhosis, or other causes; 3) acute and chronic urinary tract infection, acute and chronic nephritis, primary nephropathy, diabetic nephropathy stage V, and other severe kidney diseases; 4) current oral administration of drugs that affect uric acid metabolism; 5) acute infectious diseases, congestive heart failure (New York heart association class >2), or inadequate hepatic, pulmonary, and renal function; and 6) medical history of hyperuricemia or gout.
The diagnostic criteria for NAFLD were in accordance with the 2010 Guidelines for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Diseases: no history of excessive alcohol consumption (>140 g/week for males and >70 g/week for females) and histological changes in liver biopsy consistent with the pathological diagnostic criteria for fatty liver disease. Considering that liver histology diagnosis is difficult to obtain, NAFLD was defined as liver imaging manifestation meeting the diagnostic criterion for diffuse fatty liver (the presence of at least 2 of the following 3 abnormal findings: diffuse hyperechogenicity of the liver relative to the kidneys, attenuation of the ultrasound beam, and poor visualization of intrahepatic architectural details), with no other explanation.[1,14] Patients with T2DM were classified into the NAFLD group and the non-NAFLD group. This study was approved by the Ethics Committee of Yantai Qishan Hospital, and the requirement for informed consent was waived by the Ethics Committee due to the retrospective design.
2.2. Data collection
The baseline data, including age, sex, smoking and alcohol consumption history, T2DM duration, complications (viral or autoimmune hepatitis, hypertension, tumor, primary kidney, etc), and usage of statins or antihypertensive drugs were collected from the medical records. Physical examinations were performed and clinical data were collected, including height, weight, waist circumference, waist-to-hip ratio, systolic blood pressure (SBP), and diastolic blood pressure. Venous blood samples were obtained for the detection of SUA, creatinine, triglycerides (TGs), cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, glycosylated hemoglobin A1c (HbA1c), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and glutamyl transpeptidase. Fasting plasma glucose (FPG), plasma glucose 2 h after a 75 g oral glucose load, fasting insulin (FINS), and fasting C-peptide (FC-P) were also recorded. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease-Epidemiology Collaboration formula (15). A homeostasis model was performed to evaluate IR: Homeostatic model assessment for insulin resistance (HOMA-IR) = FINS (mIU/L) × FPG (mmol/L)/22.5.
2.3. Statistical analysis
The distributions of continuous variables were analyzed by the Kolmogorov–Smirnov test. Normally distributed data are shown as the mean ± standard deviation and were compared by Student t test. Non-normally distributed data are indicated as the median (interquartile range) and were analyzed using the Mann–Whitney U test. Categorical variables are shown as frequencies and were analyzed using the chi-square test or Fisher exact test if appropriate. Pearson correlation analyses and multiple linear regression analyses (stepwise) were performed to explore the correlation between SUA level and other clinical characteristics (such as age, disease duration of T2DM, body mass index (BMI), blood pressure, glucose and lipid metabolism parameters, biochemical results of liver and kidney function, etc).
Multivariate regression analyses, including 3 calibration models, were performed to analyze factors independently associated with the occurrence of NAFLD in patients with T2DM (entered method), and the corresponding odds ratio (OR) and 95% confidence interval (CI) were calculated. Model I was adjusted for age, sex, T2DM duration, BMI, SBP, alcohol consumption history, and eGFR; Model II was adjusted for TGs, HbA1c, HOMA-IR, usage of statins, or antihypertensive drugs in addition to the factors adjusted in Model I. Model III was adjusted for FC-P, HDL-C, AST, and ALT in addition to the factors adjusted in Model II. The SUA tertiles were defined as follows: SUA tertile I: <278 μmol/L; SUA tertile II: 278–354 μmol/L; and SUA tertile III: >354 μmol/L. All analyses were performed by SPSS 20.0 (IBM, Armonk, NY, USA). A two-sided P-value <0.05 was considered significant.
3. Results
3.1. Demographic and clinical characteristics of the T2DM patients
Five hundred eighty-three patients who met the inclusion and exclusion criteria were classified into the non-NAFLD group (n = 227) and the NAFLD group (n = 356). Patients with NAFLD had a shorter T2DM duration (P < .001), lower levels of HDL-C (P < .001), and higher levels of BMI (P < .001), waist circumference (P < .001), waist-to-hip ratio (P < .001), diastolic blood pressure (P = .002), cholesterol (P = .002), TGs (P < .001), ALT (P < .001), AST (P < .001), glutamyl transpeptidase (P < .001), eGFR (P = .031), 2-h plasma glucose (P = .013), FINS (P < .001), and HOMA-IR (P < .001) than those without NAFLD. It is worth noting that the SUA level in the NAFLD group was significantly higher than that in the non-NAFLD group (342 ± 88 vs 295 ± 93 μmol/L, P < .001). The baseline data of the 2 groups are presented in Table 1.
Table 1.
Comparison of clinical baseline data between non-NAFLD group and NAFLD group.
| Characteristics | non-NAFLD (n = 227) | NAFLD (n = 356) | P |
| Age (years) | 55 ± 14 | 54 ± 16 | .427 |
| Male, n (%) | 109 (48.0%) | 163 (45.8%) | .599 |
| T2DM duration (years) | 11 (4–18) | 7 (3–13) | <.001 |
| BMI (kg/m2) | 23.8 ± 4.2 | 26.6 ± 3.7 | <.001 |
| Waist (cm) | 89.2 ± 8.3 | 96.8 ± 9.5 | <.001 |
| WHR | 0.91 ± 0.06 | 0.96 ± 0.05 | <.001 |
| SBP (mmHg) | 130 ± 18 | 132 ± 19 | .201 |
| DBP (mmHg) | 79 ± 12 | 82 ± 10 | .002 |
| Alcohol drinker, n (%) | 39 (17.2%) | 68 (19.1%) | .559 |
| TC (mmol/L) | 4.5 ± 1.3 | 4.8 ± 0.9 | .002 |
| TGs (mmol/L) | 1.18 (0.80–1.71) | 1.92 (1.37–3.21) | <.001 |
| HDL-C (mmol/L) | 1.2 ± 0.2 | 0.8 ± 0.3 | <.001 |
| LDL-C (mmol/L) | 2.7 ± 0.9 | 2.8 ± 0.8 | .173 |
| ALT (IU/L) | 15 (11–20) | 21 (16–33) | <.001 |
| AST (IU/L) | 17 (14–22) | 20 (16–29) | <.001 |
| GGT (IU/L) | 20 (14–28) | 31 (21–50) | <.001 |
| Scr (μmol/L) | 60 (49–72) | 62 (51–73) | .320 |
| SUA (μmol/L) | 295 ± 93 | 342 ± 88 | <.001 |
| eGFR (mL/min/1.73 m2) | 150.8 ± 44.7 | 159.2 ± 47.2 | .031 |
| HbA1c (%) | 9.3 ± 2.5 | 9.4 ± 2.1 | .617 |
| FPG (mmol/L) | 8.3 ± 3.3 | 8.7 ± 2.8 | .131 |
| 2hPG (mmol/L) | 12.6 ± 3.9 | 13.5 ± 4.7 | .013 |
| FINS (mIU/L) | 3.7 ± 2.6 | 5.3 ± 2.7 | <.001 |
| HOMA-IR | 1.2 ± 0.7 | 1.9 ± 0.8 | <.001 |
| FC-P (nmol/L) | 2.06 ± 1.11 | 2.13 ± 1.28 | .485 |
| Anti-hypertensive drug use | 73 (32.2%) | 132 (37.1%) | .225 |
| Statin use | 27 (11.9%) | 54 (15.2%) | .265 |
2hPG = 2-h plasma glucose, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, FC-P = fasting C-peptide, FINS = fasting insulin, FPG = fasting plasma glucose, GGT = glutamyl transpeptidase, HbA1c = glycosylated hemoglobin A1C, HDL-C = high-density lipoprotein cholesterol, HOMR-IR = homeostatic model assessment for insulin resistance, LDL-C = low-density lipoprotein cholesterol, NAFLD = nonalcoholic fatty liver disease, SBP = systolic blood pressure, Scr = creatinine, SUA = serum uric acid, T2DM = type 2 diabetes, TC = cholesterol, TGs = triglycerides, WHR = waist-to-hip ratio.
3.2. Correlation analyses between SUA and other metabolic risk factors
Pearson correlation analyses showed that the SUA level was positively correlated with BMI (P < .001), TGs (P < .001), HOMA-IR (P = .041), AST (P = .002), and ALT (P = .008) but negatively correlated with HDL-C (P = .027) and eGFR levels (P < .001). Multiple linear regression analyses (stepwise) revealed that SUA was independently and positively correlated with BMI (P = .003), TGs (P = .009), AST (P = .036), and ALT (P = .038) and independently and negatively correlated with eGFR (P < .001) in T2DM patients. The results are illustrated in Table 2.
Table 2.
Pearson correlation analyses and multiple linear regression analyses of parameters associated with SUA.
| Pearson correlation | Multiple linear regression | |||
| Variables | r | P | Standardized β | P |
| Age | 0.031 | .627 | – | – |
| Duration of T2DM | 0.082 | .271 | – | – |
| BMI | 0.347 | <.001 | 0.415 | .003 |
| SBP | 0.018 | .729 | – | – |
| DBP | 0.076 | .572 | – | – |
| TC | 0.058 | .413 | – | – |
| TGs | 0.331 | <.001 | 0.354 | .009 |
| HDL-C | −0.274 | .027 | – | – |
| LDL-C | −0.047 | .629 | – | – |
| ALT | 0.176 | .008 | 0.297 | .038 |
| AST | 0.278 | .002 | 0.329 | .036 |
| eGFR | −0.312 | <.001 | −0.415 | <.001 |
| HbA1c | −0.127 | .421 | – | – |
| FPG | −0.051 | .375 | – | – |
| HOMA-IR | 0.213 | .041 | – | – |
ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, FPG = fasting plasma glucose, HbA1c = glycosylated hemoglobin A1C, HDL-C = high-density lipoprotein cholesterol, HOMR-IR = Homeostatic model assessment for insulin resistance, LDL-C = low-density lipoprotein cholesterol, SBP = systolic blood pressure, T2DM = type 2 diabetes, TC = cholesterol, TGs = triglyceride.
3.3. Relationship between SUA tertile and NAFLD occurrence in T2DM patients
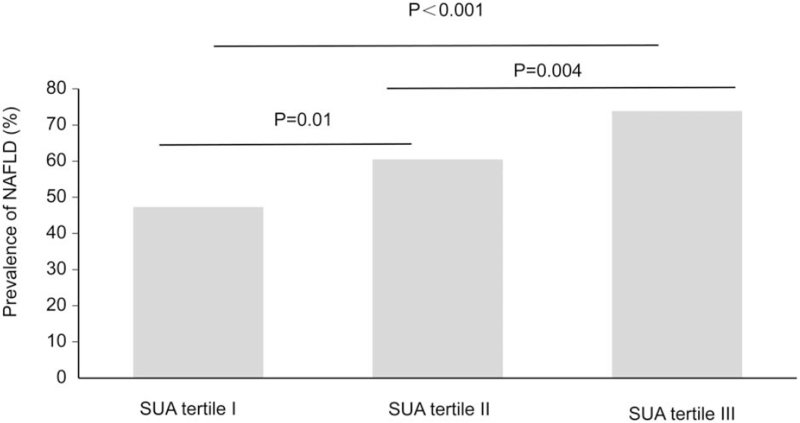
Comparisons of NAFLD occurrence in T2DM patients classified according to SUA tertile are shown in Figure 1. The results showed that the prevalence of NAFLD gradually increased as SUA levels increased in these groups; the prevalence of NAFLD was 47.3% in the tertile I group, 60.5% in the tertile II group, and 73.9% in the tertile III group. The differences between the groups were statistically significant (P for trend <.001). In addition, the proportions of T2DM patients with anti-hypertensive agents or stain use did not show a significant difference among the SUA tertile I, SUA tertile II, and SUA tertile III groups (all P values for pairwise comparisons >.05).
Figure 1.
Prevalence of NAFLD in T2DM patients according to SUA tertile. SUA tertile I: <278 μmol/L; SUA tertile II: 278–354 μmol/L; SUA tertile III: >354 μmol/L; and P for trend <.001. NAFLD = nonalcoholic fatty liver disease, SUA = serum uric acid, T2DM = type 2 diabetes.
The results from multivariate regression analyses of the association between the SUA tertile and NAFLD occurrence in patients with T2DM after adjusting for other confounding factors are shown in Table 3. The results showed that the SUA tertile was significantly associated with the prevalence of NAFLD (P for trend <0.001) after adjusting for risk factors, including age, sex, T2DM duration, BMI, SBP, alcohol consumption history, and eGFR (Model I).
Table 3.
Multivariate regression analyses of the association between SUA tertiles and occurrence of NAFLD in T2DM patients.
| OR (95% CI) | ||||
| SUA tertile I | SUA tertile II | SUA tertile III | P for trend | |
| Model I | 1 | 1.835 (1.115–3.021) | 2.663 (1.221–2.173) | |
| P | .017 | <.001 | <.001 | |
| Model II | 1 | 1.629 (1.084–2.449) | 2.571 (1.378–4.797) | |
| P | .019 | .003 | .018 | |
| Model III | 1 | 1.729 (1.086–2.753) | 2.315 (1.272–4.213) | |
| P | .021 | .006 | .008 | |
The data was presented as an OR value and a 95% CI compared to the tertile I group. T2DM without NAFLD patients were defined as 0, and T2DM with NAFLD patients were defined as 1.
Model I: Adjust for age, gender, T2DM duration, BMI, SBP, drinking history, and eGFR;
Model II: Adjust for TGs, HbA1c, HOMA-IR, usage of statin, or anti-hypertensive drugs on the basis of Model I.
Model III: Adjust for FC-P, HDL-C, AST, and ALT on the basis of Model II.
SUA tertile I: <278 μmol/L; SUA tertile II: 278–354 μmol/L; and SUA tertile III: >354 μmol/L.
CI = confidence interval, OR = odds ratio, SUA = serum uric acid.
Taking the tertile I group as the reference, the ORs for NAFLD in the tertile II group and the tertile III group were 1.835 (95% CI: 1.115–3.021) and 2.663 (95% CI: 1.221–2.173), respectively. After adjusting for TGs, HbA1c, HOMA-IR, usage of statins, or antihypertensive drugs in addition to the factors adjusted in Model I (Model II), there was a significant correlation between the SUA tertile and NAFLD occurrence (P for trend = .018). Using tertile I as a reference, the ORs for NAFLD in tertile II and tertile III were 1.629 (95% CI: 1.084–2.449) and 2.571 (95% CI: 1.378–4.797), respectively. After further adjusting for FC-P, HDL-C, AST, and ALT in addition to the factors adjusted in Model II (Model III), a significant correlation between the SUA tertile and NAFLD occurrence could still be noted (P for trend = .008). Using the tertile I group as a reference, the ORs for NAFLD in tertile II and tertile III were 1.729 (95% CI: 1.086–2.753) and 2.315 (95% CI: 1.272–4.213), respectively.
4. Discussion
Our study found that the SUA levels in T2DM patients in the NAFLD group were significantly higher than those in the non-NAFLD group (P < .001). Multiple linear regression analyses indicated that SUA was positively and independently correlated with BMI, TGs, AST, and ALT and negatively and independently correlated with eGFR in T2DM patients. Logistic regression analyses demonstrated that after adjusting for confounding factors, the SUA tertiles were still significantly associated with the occurrence of NAFLD. Using SUA tertile I as the reference, the OR for NAFLD in SUA tertile II and SUA tertile III were 1.729 (95% CI: 1.086–2.753) and 2.315 (95% CI: 1.272–4.213), respectively.
The results of our study also suggested that the SUA level was an independent risk factor for the comorbidity of NAFLD in T2DM patients, which was in line with the result of a meta-analysis.[13] Many previous studies, including cross-sectional surveys and prospective studies, have established the viewpoint that SUA levels are increased in NAFLD patients and are an independent risk factor for NAFLD occurrence. The occurrence of NAFLD in 583 T2DM patients in this study was 61.6%, which was close to the previous pooled prevalence of NAFLD in T2DM patients in a meta-analysis (59.7%, 95% CI: 54.3–64.9),[15] highlighting the unmet requirement of identifying the risk factors for incidental NAFLD. Sung et al[16] found that the SUA level was an independent predictor of NAFLD occurrence in obese children in Italy. Hayden and Tyagi[17] also found that uric acid had a strong oxidant effect in patients with MS and was associated with the occurrence of NAFLD. Lee et al[18] conducted a study in 3768 healthy Koreans and found that HUA was an independent predictor of NAFLD, independent of MS, sex, BMI, fasting blood glucose, blood lipids, and other potential risk factors. A study of 10,732 nondiabetic US adults evaluated the relationship between HUA and ultrasound-diagnosed NAFLD and found that elevated SUA was significantly associated with the occurrence and severity of NAFLD.[19] A large cohort of 5741 healthy Korean males found that 1717 subjects (29.9%) developed ultrasound-diagnosed NAFLD during the 5-year follow-up period, and the hazard ratio of individuals with SUA ≥ 7.0 mg/dL was 1.29 (1.14–1.46) compared to those with SUA < 7.0 mg/dL.[20] These previous studies not only indicated that SUA evaluation could be applied to predict current or future NAFLD occurrence but also implied that SUA might contribute to the development of NAFLD.
In our study, the results from Pearson correlation analyses showed that the SUA level was positively correlated with BMI, TGs, HOMA-IR, AST, and ALT but negatively correlated with HDL-C and eGFR levels, which was broadly in line with a previous study of Chinese T2DM patients.[21,22] Obesity, increased TGs, and impaired IR are factors generally known to be associated with an increased risk of NAFLD occurrence,[23] suggesting the possibility that the correlation between SUA and NAFLD could be mediated, at least in part, through these potential factors.
Our study demonstrated the association between increased SUA (vs SUA tertile I) and elevated NAFLD occurrence in T2DM patients, which was in broad agreement with findings from previous studies.[21,24,25] Interestingly, after adjustment for numerous potential risk factors and metabolic factors, including age, sex, T2DM duration, BMI, SBP, alcohol consumption history, eGFR, TGs, HbA1c, HOMA-IR, usage of statins or antihypertensive drugs, FC-P, HDL-C, AST, and ALT, the clear trend toward the association between a higher SUA tertile and a higher occurrence rate of NAFLD in T2DM patients could still be noted, with significant differences detected. Therefore, an independent association between SUA tertiles and NAFLD occurrence was indicated by our analysis.
This study also had some shortcomings. First, this was a single-center study with small sample size, the clinical applicability may be limited, and sex differences in the relationship between SUA tertiles and NAFLD occurrence were not further explored. Second, due to the cross-sectional study design, it was impossible to determine the causal relationship between SUA and NAFLD in T2DM patients. Therefore, the exact contribution to increased SUA levels attributed to the nature of NAFLD or obesity-related metabolic syndrome still could not be differentiated or concluded. Third, the sensitivity and specificity of liver ultrasonography for detecting mild hepatic steatosis were not as good as liver biopsy, which is considered the gold standard of NAFLD diagnosis. In addition, although the advantages of ultrasonography are its noninvasiveness and wide application, NAFLD severity was not graded in this survey, and the correlation between SUA and the severity of hepatic steatosis should be explored in future studies.
5. Conclusion
The SUA level in T2DM patients was significantly associated with NAFLD occurrence. With elevated SUA levels, the prevalence of NAFLD was obviously increased. Elevated SUA levels were an independent risk factor for NAFLD occurrence in patients with T2DM.
Author contributions
Conceptualization: Haifeng Yu, Ling Zhao, Lijuan Liu, Yanfang Li, Jing Sun, Youde Liu.
Data curation: Haifeng Yu, Ling Zhao, Lijuan Liu, Yanfang Li, Youde Liu.
Formal analysis: Lijuan Liu, Yanfang Li, Youde Liu.
Funding acquisition: Yanfang Li.
Investigation: Haifeng Yu, Ling Zhao, Lijuan Liu, Yanfang Li, Jing Sun, Youde Liu.
Methodology: Haifeng Yu, Ling Zhao, Lijuan Liu, Jing Sun, Youde Liu.
Project administration: Haifeng Yu, Lijuan Liu, Yanfang Li, Jing Sun, Youde Liu.
Resources: Jing Sun, Youde Liu.
Software: Ling Zhao, Lijuan Liu, Yanfang Li, Youde Liu.
Supervision: Ling Zhao, Lijuan Liu, Yanfang Li, Jing Sun, Youde Liu.
Validation: Lijuan Liu, Yanfang Li, Youde Liu.
Visualization: Lijuan Liu, Youde Liu.
Writing – original draft: Haifeng Yu, Youde Liu.
Writing – review & editing: Haifeng Yu, Youde Liu.
Footnotes
Abbreviations: 2hPG = 2-h plasma glucose, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, FC-P = fasting C-peptide, FINS = fasting insulin, FPG = fasting plasma glucose, GGT = glutamyl transpeptidase, HbA1c = glycosylated hemoglobin A1C, HDL-C = high-density lipoprotein cholesterol, HOMR-IR = Homeostatic model assessment for insulin resistance, HUA = huperuricemia, IR = insulin resistance, LDL-C = low-density lipoprotein cholesterol, MS = metabolic syndrome, NAFL = non-alcoholic simple fatty liver, NAFLD = nonalcoholic fatty liver disease, NASH = non-alcoholic steatohepatitis, OR = odds ratio, SBP = systolic blood pressure, Scr = creatinine, SUA = serum uric acidT2DM = type 2 diabetes, TC = cholesterol, TG = triglyceride, WHR = waist-to-hip ratio.
How to cite this article: Yu H, Zhao L, Liu L, Li Y, Sun J, Liu Y. Relationship between serum uric acid level and nonalcoholic fatty liver disease in type 2 diabetes patients. Medicine. 2021;100:33(e26946).
HY and LZ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- [1].Fan JG, Jia JD, Li YM, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010: (published in Chinese on Chinese Journal of Hepatology 2010;18:163–166). J Dig Dis 2011;12:38–44. [DOI] [PubMed] [Google Scholar]
- [2].McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol 2015;62:1148–55. [DOI] [PubMed] [Google Scholar]
- [3].Wilkins T, Tadkod A, Hepburn I, Schade RR. Nonalcoholic fatty liver disease: diagnosis and management. Am Fam Physician 2013;88:35–42. [PubMed] [Google Scholar]
- [4].Traussnigg S, Kienbacher C, Halilbasic E, et al. Challenges and management of liver cirrhosis: practical issues in the therapy of patients with cirrhosis due to NAFLD and NASH. Dig Dis 2015;33:598–607. [DOI] [PubMed] [Google Scholar]
- [5].Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62: 1 Suppl: S47–64. [DOI] [PubMed] [Google Scholar]
- [6].Jalal DI. Hyperuricemia, the kidneys, and the spectrum of associated diseases: a narrative review. Curr Med Res Opin 2016;32:1863–9. [DOI] [PubMed] [Google Scholar]
- [7].Kanbay M, Jensen T, Solak Y, et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med 2016;29:03–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cibičková Ľ, Langová K, Vaverková H, Kubíčková V, Karásek D. Correlation of uric acid levels and parameters of metabolic syndrome. Physiol Res 2017;66:481–7. [DOI] [PubMed] [Google Scholar]
- [9].Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol 2009;50:1029–34. [DOI] [PubMed] [Google Scholar]
- [10].Petta S, Cammà C, Cabibi D, Di Marco V, Craxì A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2011;34:757–66. [DOI] [PubMed] [Google Scholar]
- [11].Hwang IC, Suh SY, Suh AR, Ahn HY. The relationship between normal serum uric acid and nonalcoholic fatty liver disease. J Korean Med Sci 2011;26:386–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xu C, Yu C, Xu L, Miao M, Li Y. High serum uric acid increases the risk for nonalcoholic fatty liver disease: a prospective observational study. PLoS One 2010;5:e11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou Y, Wei F, Fan Y. High serum uric acid and risk of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Clin Biochem 2016;49:636–42. [DOI] [PubMed] [Google Scholar]
- [14].Jian-Gao F. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition. Zhonghua Gan Zang Bing Za Zhi 2010;18:163–6. [PubMed] [Google Scholar]
- [15].Dai W, Ye L, Liu A, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: a meta-analysis. Medicine (Baltimore) 2017;96:e8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sung KC, Ryan MC, Kim BS, Cho YK, Kim BI, Reaven GM. Relationships between estimates of adiposity, insulin resistance, and nonalcoholic fatty liver disease in a large group of nondiabetic Korean adults. Diabetes Care 2007;30:2113–8. [DOI] [PubMed] [Google Scholar]
- [17].Hayden MR, Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab 2004;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee YJ, Lee HR, Lee JH, Shin YH, Shim JY. Association between serum uric acid and non-alcoholic fatty liver disease in Korean adults. Clin Chem Lab Med 2010;48:175–80. [DOI] [PubMed] [Google Scholar]
- [19].Sirota JC, McFann K, Targher G, Johnson RJ, Chonchol M, Jalal DI. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: Liver ultrasound data from the National Health and Nutrition Examination Survey. Metabolism 2013;62:392–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ryu S, Chang Y, Kim SG, Cho J, Guallar E. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men. Metabolism 2011;60:860–6. [DOI] [PubMed] [Google Scholar]
- [21].Fan N, Zhang L, Xia Z, Peng L, Wang Y, Peng Y. Sex-specific association between serum uric acid and nonalcoholic fatty liver disease in type 2 diabetic patients. J Diabetes Res 2016;2016:3805372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu L, Li T, Yin J, et al. Association between serum uric acid and nonalcoholic fatty liver disease in community patients with type 2 diabetes mellitus. PeerJ 2019;7:e7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- [24].Wu SJ, Zhu GQ, Ye BZ, et al. Association between sex-specific serum uric acid and non-alcoholic fatty liver disease in Chinese adults: a large population-based study. Medicine (Baltimore) 2015;94:e802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yu XL, Shu L, Shen XM, Zhang XY, Zheng PF. Gender difference on the relationship between hyperuricemia and nonalcoholic fatty liver disease among Chinese: an observational study. Medicine (Baltimore) 2017;96:e8164. [DOI] [PMC free article] [PubMed] [Google Scholar]