PURPOSE
Effective therapies are needed for the treatment of patients with human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer (MBC) with brain metastases. A trastuzumab radioisotope has been shown to localize in brain metastases of patients with HER2-positive MBC, and intracranial xenograft models have demonstrated a dose-dependent response to trastuzumab.
METHODS
In the phase II PATRICIA study (ClinicalTrials.gov identifier: NCT02536339), patients with HER2-positive MBC with CNS metastases and CNS progression despite prior radiotherapy received pertuzumab plus high-dose trastuzumab (6 mg/kg weekly) until CNS or systemic disease progression or unacceptable toxicity. The primary end point was confirmed objective response rate (ORR) in the CNS per Response Assessment in Neuro-Oncology Brain Metastases criteria. Secondary end points included duration of response, clinical benefit rate (complete response plus partial response plus stable disease ≥ 4 or ≥ 6 months) in the CNS, and safety.
RESULTS
Thirty-nine patients were treated for a median (range) of 4.5 (0.3-37.3) months at clinical cutoff. Thirty-seven patients discontinued treatment, most commonly because of CNS progression (n = 27); two remained on treatment. CNS ORR was 11% (95% CI, 3 to 25), with four partial responses (median duration of response, 4.6 months). Clinical benefit rate at 4 months and 6 months was 68% and 51%, respectively. Two patients permanently discontinued study treatment because of adverse events (left ventricular dysfunction [treatment-related] and seizure, both grade 3). No grade 5 adverse events were reported. No new safety signals emerged with either agent.
CONCLUSION
Although the CNS ORR was modest, 68% of patients experienced clinical benefit, and two patients had ongoing stable intracranial and extracranial disease for > 2 years. High-dose trastuzumab for HER2-positive CNS metastases may warrant further study.
INTRODUCTION
Approximately 40%-50% of patients with human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer (MBC) develop brain metastases.1-3 Before approval of tucatinib by the US Food and Drug Administration in April 2020, no systemic therapy had been indicated for treatment of brain metastases in this population, prompting the search for effective and tolerable systemic therapies.
CONTEXT
Key Objective
Development of CNS metastasis is common in patients with human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer (MBC), but systemic therapies effective against brain metastases are limited. The phase II PATRICIA study examined pertuzumab plus high-dose trastuzumab (6 mg/kg weekly) in patients with HER2-positive MBC with CNS metastases that had progressed following radiotherapy.
Knowledge Generated
Although the overall response rate in the CNS was limited, 68% of patients treated with pertuzumab plus high-dose trastuzumab experienced clinical benefit in the CNS. No new safety signals were observed.
Relevance
Despite prior assumptions that antibody treatments are unable to penetrate the CNS, we observed that pertuzumab plus high-dose trastuzumab may have clinical activity against progressive CNS metastases in some patients with HER2-positive MBC. Future studies should further investigate antibody-based regimens, including high-dose trastuzumab in combination with chemotherapy or other targeted therapies, to optimize the treatment of CNS disease.
Trastuzumab improves survival in early-stage and metastatic HER2-positive breast cancer.4 Despite assumptions that monoclonal antibodies do not cross the blood-brain barrier, a radioisotope form of trastuzumab, 89zirconium-trastuzumab (89Zr-trastuzumab), has been shown to localize in brain metastases of patients with HER2-positive MBC,5 and increased concentrations of trastuzumab have been detected in the CSF of patients with HER2-positive MBC following whole-brain radiotherapy.6 Additionally, data from a retrospective database analysis7 and the observational registHER study1 suggest that trastuzumab is associated with longer survival in patients with HER2-positive MBC presenting with CNS metastases.
Pertuzumab is a humanized monoclonal antibody targeting HER2.8 In an exploratory analysis of the phase III CLEOPATRA study, patients with HER2-positive MBC who received first-line pertuzumab with trastuzumab plus docetaxel experienced a delay in CNS disease onset relative to those administered trastuzumab plus docetaxel alone.9
Isolated CNS progression in the setting of controlled extracranial disease is common in patients with HER2-positive MBC. This suggests that subtherapeutic drug concentrations in the brain may be a driver of CNS progression rather than intrinsic tumor resistance.10 Other phase I or II studies have explored escalating or pulsatile doses of HER2-targeted tyrosine kinase inhibitors (TKIs) in an attempt to drive higher concentrations of drug into the brain and improve efficacy.11,12 With respect to monoclonal antibodies, in a preclinical model of HER2-positive breast cancer brain metastasis, up to three-times the dose of trastuzumab shown to be effective in mammary tumor grafts was needed to achieve similar responses in brain tumor grafts,13 suggesting that increased trastuzumab doses may improve CNS activity. Importantly, when administered at doses 2- or 3-times higher than the standard dose for HER2-positive MBC, trastuzumab was not associated with increased cardiotoxicity or adverse events (AEs), most likely because extracranial HER2 receptors become saturated at lower trastuzumab doses.14,15
Given these preclinical and clinical findings, the phase II PATRICIA study examined pertuzumab plus high-dose trastuzumab (6 mg/kg weekly) in patients with HER2-positive MBC presenting with progressive brain metastases. We hypothesized that (1) high-dose trastuzumab would confer CNS efficacy in patients who previously progressed on standard-dose trastuzumab and (2) no increase in cardiotoxicity would be observed. This report summarizes results from the primary efficacy analysis of PATRICIA.
METHODS
Study Design
PATRICIA (ClinicalTrials.gov identifier: NCT02536339) was an open-label, single-arm, phase II study conducted at 16 sites in the United States (Appendix Fig A1, online only). Study participants received intravenous pertuzumab (840 mg loading dose, 420 mg every 3 weeks thereafter) and high-dose intravenous trastuzumab (6 mg/kg once weekly) until CNS or systemic disease progression, unacceptable toxicity, withdrawal, or study termination. Dose reductions were not permitted. If pertuzumab was discontinued because of a treatment-related AE, patients were allowed to continue high-dose trastuzumab. However, if high-dose trastuzumab was discontinued because of an AE, pertuzumab was also discontinued. Patients who discontinued study treatment were followed until disease progression, and all patients were followed for 12 months after the last treatment visit for survival.
To optimize the ability to detect any incremental benefit provided by pertuzumab plus high-dose trastuzumab, no changes were allowed to existing treatment regimens for systemic disease, except for patients receiving trastuzumab emtansine (T-DM1) or lapatinib. These patients were instructed to discontinue T-DM1 or lapatinib 3 weeks and 1 week, respectively, before initiating study therapy.
PATRICIA was conducted in compliance with the Declaration of Helsinki, International Conference on Harmonization Guidelines for Good Clinical Practice, and applicable national and local regulatory requirements. The study protocol was approved by the Independent Ethics Committee or Institutional Review Board at each site. All patients provided written informed consent.
Patients
Eligible participants were adults (≥ 18 years) with confirmed HER2-positive MBC presenting with documented CNS progression, despite prior radiotherapy (stereotactic radiosurgery and/or whole-brain radiotherapy), and stable extracranial disease. Additional inclusion criteria included completion of radiotherapy > 60 days before study entry, presence of ≥ 1 measurable CNS metastasis (≥ 10 mm per Response Assessment in Neuro-Oncology Brain Metastases [RANO-BM] criteria),16 Eastern Cooperative Oncology Group performance status score of 0-1, left ventricular ejection fraction (LVEF) ≥ 50%, and adequate hematologic and organ function.
Exclusion criteria included presence of leptomeningeal disease, symptomatic pulmonary disease, history of intolerance (grade ≥ 3) or hypersensitivity to study treatment, significant cardiac disease, or active infection. Concurrent use of nonapproved or investigational treatments (≤ 21 days before enrollment) or anthracyclines was also prohibited.
Outcomes
The primary efficacy end point was objective response rate (ORR) in the CNS, defined as the proportion of patients with confirmed complete response (CR) or partial response (PR) per RANO-BM criteria (Appendix Table A1, online only).16 Brain responses were measured by magnetic resonance imaging at weeks 6, 12, 20, and 28, and every 12 weeks thereafter until progressive disease (PD). Secondary efficacy end points included duration of response (time from first documented CR or PR to PD or death) and clinical benefit rate (CBR; CR plus PR plus stable disease [SD] of ≥ 4 or ≥ 6 months) in the CNS. Duration of clinical benefit in the CNS (time from first documented CR or PR or first treatment dose for patients with SD to PD or death) was also determined. Extracranial responses were measured using computed tomography, magnetic resonance imaging, or positron-emission tomography computed tomography at weeks 8 and 16, and every 12 weeks thereafter until PD, and were assessed per RECIST version 1.1.17 AEs were coded per Medical Dictionary for Regulatory Activities version 21.1 and graded per Common Terminology Criteria for Adverse Events version 4.0. LVEF was assessed at screening, at 6 weeks, at 12 weeks, and then every 3 months during the treatment period and every 6 months during survival follow-up (12 months after the treatment discontinuation visit). Incidence of congestive heart failure (CHF) was also recorded. A preplanned interim analysis required study suspension if ≥ 2 of the first 15 patients experienced study treatment-related CHF. Pharmacokinetics of both study treatments were evaluated at week 1 (predose and within 30 minutes of completion of both infusions), week 4 (predose), week 10 (predose), and week 16 (predose and within 30 minutes of completion of both infusions).
Statistical Analyses
A total of forty patients were targeted for enrollment. Sample size was based on an assumed CNS ORR of 20%, which would result in a 95% CI of 8.4 to 36.9 in 35 efficacy-evaluable patients. The 95% CIs for ORR and CBR were calculated using the Clopper-Pearson exact method. DOR and duration of clinical benefit were estimated using the Kaplan-Meier approach, with 95% CIs for the median time-to-event calculated using the Brookmeyer-Crowley method. Patients who did not experience PD or death were censored at the last date they were known to be progression-free. AEs were summarized using descriptive statistics.
The efficacy-evaluable population comprised all treated patients who had ≥ 1 post-treatment CNS tumor assessment or who died without a follow-up tumor assessment within 30 days of the last dose of study treatment. The safety population included all patients who received any dose of study treatment. The pharmacokinetic-evaluable population included all patients who received any dose of study treatment and had ≥ 1 pharmacokinetic assessment.
The clinical cutoff date was May 1, 2019. The study is ongoing, with two patients remaining on treatment.
RESULTS
Patients
A total of forty patients were enrolled between December 15, 2015, and May 18, 2017 (Table 1). Median age was 48 years. In total, 48% (19/40) of patients were hormone receptor–positive at the time of initial breast cancer diagnosis, and 40% (16/40) were using corticosteroids at screening. Twenty-eight percent (11/40) of patients continued concomitant nonstudy systemic treatments during the study, most commonly aromatase inhibitor (n = 4), capecitabine (n = 3), or palbociclib (n = 3).
TABLE 1.
Baseline Demographics and Disease Characteristics

Thirty-nine patients received any dose of study treatment; one patient withdrew before receiving any treatment. Median (range) treatment duration was 4.8 (0.7-29.9) months for pertuzumab and 4.5 (0.5-37.4) months for trastuzumab. Patients received a median (range) of 7.0 (1-51) doses of pertuzumab and 20.0 (2-145) doses of trastuzumab. By clinical cutoff, 95% (37/39) of patients had discontinued study treatment, with 5% (2/39) still on treatment. Patients discontinued treatment because of CNS progression (n = 27), symptomatic deterioration (n = 4), withdrawal (n = 3), change in LVEF (n = 1), death (n = 1), and protocol deviation (n = 1). In total, 15% (6/40) of patients remained on study at clinical cutoff. The median (range) study duration was 16.6 (0.8-37.2) months, and the most common reason for study discontinuation was death (59% [20/34]). Causes of death were PD (n = 16), other with cancer being a contributing factor (n = 3), and unknown (n = 1). The safety-evaluable population comprised 39 patients, as one patient did not receive study treatment. The efficacy-evaluable population included 37 patients; two patients from the safety-evaluable population were excluded because they did not have any postbaseline assessments because of withdrawal (n = 1) and treatment discontinuation owing to symptomatic deterioration (n = 1).
Efficacy
The CNS ORR per RANO-BM criteria was 11% (4/37; 95% CI, 3 to 25), with four confirmed PRs (Table 2). DOR in these four patients was 3.2 (censored), 3.3, 4.6, and 5.6 months. DOR was censored in one patient by treatment discontinuation because of a protocol deviation (use of disallowed concomitant therapy); the other three patients experienced PD. CBR in the CNS at 4 months (CR plus PR plus SD ≥ 4 months) was 68% (25/37) (Fig 1), with a median (range) duration of 6.6 (3.2-36.8) months (Fig 2). All patients who derived clinical benefit in the CNS had stable or better extracranial disease. CBR in the CNS at 6 months (CR plus PR plus SD ≥ 6 months) was 51% (19/37), with a median (range) duration of 9.2 (3.2-36.8) months (Fig 1). One patient had ongoing SD for > 2 years, and another had ongoing SD for > 3 years. The largest percentage change in the sum of target lesion diameters is summarized in Figure 3. In a subgroup analysis of CBR at 4 months by prior HER2-targeted treatments, including prior trastuzumab, T-DM1, lapatinib, or neratinib, patients derived clinical benefit regardless of the prior treatment received (Appendix Table A2, online only).
TABLE 2.
Efficacy Within the CNS per RANO-BM Criteria

FIG 1.
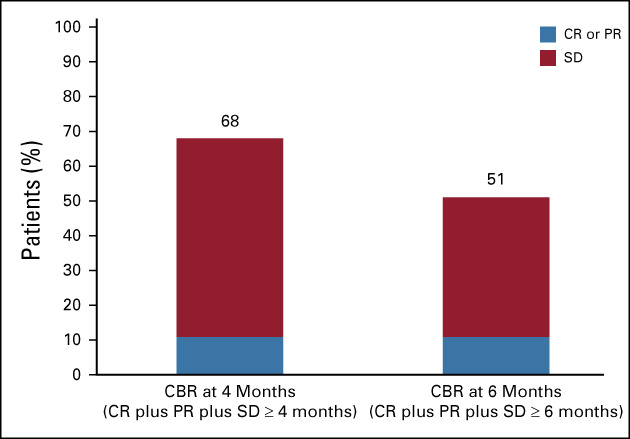
CBR in the CNS at 4 months (n = 25) and 6 months (n = 19). CBR, clinical benefit rate; CR, complete response; PR, partial response; SD, stable disease.
FIG 2.
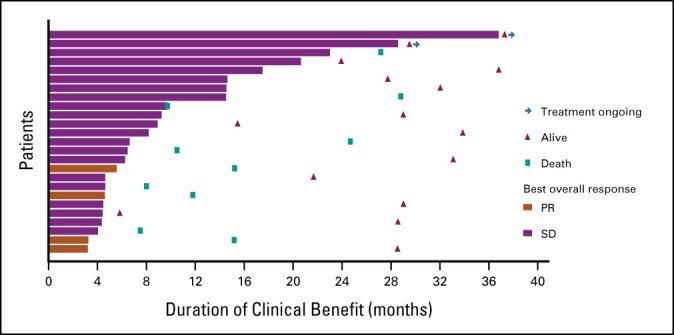
Duration of clinical benefit in the CNS at 4 monthsa (n = 25). aPatients with confirmed CR, PR, or SD ≥ 4 months. CR, complete response; PR, partial response; SD, stable disease.
FIG 3.
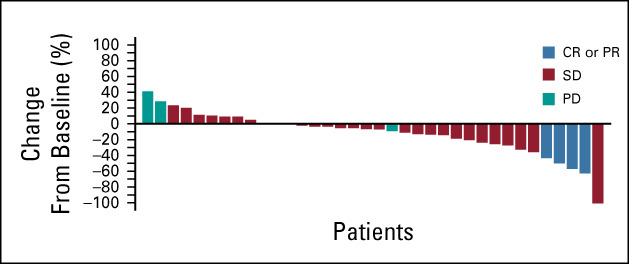
Largest percentage change in total sum of CNS target lesion diameters (n = 37). One patient without a postbaseline assessment was omitted from this analysis because of death within 30 days of last treatment, but was included in the efficacy-evaluable population. One patient was with an unconfirmed PR and was considered to have a confirmed best response of SD in the study. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Safety
In a preplanned interim safety analysis (n = 15), no patients experienced treatment-related CHF; thus, the criteria to suspend the study because of CHF-related events was not met. Overall, 97% (38/39) of patients reported ≥ 1 AE, most commonly diarrhea (n = 23), fatigue (n = 17), and nausea (n = 12). All diarrhea episodes were grade 1 or 2, and none led to treatment discontinuation. In total, 44% (17/39) of patients reported ≥ 1 grade 3 (n = 14) or grade 4 (n = 3) AE (Table 3). No grade 5 AEs were reported. Thirty (77%) patients had ≥ 1 treatment-related AE, most commonly diarrhea (n = 16) and fatigue (n = 11) (Table 3). Two patients experienced a total of three treatment-related grade 3 AEs (left ventricular dysfunction, n = 1; asthenia and fatigue, n = 1). One patient experienced treatment-related grade 4 hypertension. Only one patient experienced a treatment-related cardiac AE (grade 3 left ventricular dysfunction in a patient with prior cardiac history). The patient subsequently discontinued both study drugs; one other patient in the study also discontinued because of an AE (grade 3 seizure, not considered to be treatment-related).
TABLE 3.
Safety Summary

Serious AEs were reported in 7 (18%) patients. Of these, one patient experienced three serious AEs (grade 3 seizure, grade 3 hydrocephalus, and grade 2 headache); the other six patients each experienced a single serious AE (seizure, n = 3; gastroenteritis viral, n = 1; hypertension, n = 1; and parainfluenza virus infection, n = 1). Hypertension (grade 4) was the only serious AE considered related to study treatment. No new safety signals emerged with either study treatment.
Pharmacokinetics
All treated patients were included in the pharmacokinetic-evaluable population (n = 39). The mean observed maximum concentration (Cmax) of pertuzumab at steady-state (Cmax,ss; week 16; dose 6) was 226 ± 56.2 μg/mL, and the mean observed trough concentration (Cmin) was 102 ± 41.6 μg/mL. For trastuzumab, mean observed Cmin increased over time and appeared to approach steady-state by week 10. Mean Cmax,ss and Cmin,ss for high-dose trastuzumab at steady-state (week 16; dose 6) were 394 ± 94.1 and 306 ± 90.2 μg/mL, respectively (Appendix Table A3, online only).
DISCUSSION
In the phase II PATRICIA study, we evaluated the safety and efficacy of pertuzumab plus high-dose trastuzumab (6 mg/kg intravenous once weekly) in patients with progressive brain metastases despite prior radiotherapy. Although the observed CNS ORR per RANO-BM criteria was only 11%, CBR in the CNS was 68% at 4 months and 51% at 6 months, with two patients having SD for > 2 years. Clinical benefit was also observed in patients with prior exposure to T-DM1, lapatinib, and/or neratinib. Notably, extracranial disease responses were stable or better in patients with clinical benefit in the CNS. To our knowledge, this is the first report of a monoclonal antibody (trastuzumab) conferring CNS objective responses and clinical benefit following an increase in the systemic dose of a drug on which patients had previously progressed.
The observed efficacy signal is supported mechanistically by prior data demonstrating accumulation of 89Zr-trastuzumab in brain metastases of patients with HER2-positive MBC5 and preclinical data suggesting a dose-dependent response to trastuzumab in the CNS.13 Thus, despite their size, monoclonal antibodies have the potential to enter the CNS compartment, but the extent to which they do so is likely determined by the integrity of the blood-brain barrier. Published studies describe disruption of the blood-brain barrier by tumor metastasis that increases blood-brain barrier permeability.18,19 The fact that monoclonal antibodies can access the CNS is further supported by multiple reports suggesting CNS activity of T-DM1, an antibody-drug conjugate composed of trastuzumab and DM1.20,21
As expected, steady-state serum trastuzumab exposure was greater with the high-dose trastuzumab regimen (6 mg/kg weekly) relative to the approved regimen (4 mg/kg loading dose, 2 mg/kg weekly thereafter). Observed Cmax,ss and Cmin,ss in PATRICIA were 394 ± 94.1 and 306 ± 90.2 μg/mL, respectively, versus model-predicted Cmax,ss and Cmin,ss of 109 and 66.1 μg/mL, respectively, for the approved regimen.4 In the phase III APHINITY study, trastuzumab was administered at the same maintenance dose as in PATRICIA (6 mg/kg), but on a 3-weekly versus weekly schedule. As expected, observed Cmax,ss and Cmin,ss in PATRICIA were higher than model-predicted exposures in APHINITY (225.5 ± 80 and 67.7 ± 32 μg/mL, respectively).22 Observed Cmax,ss and Cmin,ss values for pertuzumab in PATRICIA (226 ± 56.2 and 102 ± 41.6 μg/mL, respectively) were also comparable to model-predicted Cmax,ss and Cmin,ss values (184.9 [range, 124-260] and 52.1 [range, 16-97] μg/mL, respectively) derived from a population pharmacokinetic model informed by patients with solid tumors, including HER2-positive MBC.23
Importantly, the pertuzumab and high-dose trastuzumab combination did not lead to the emergence of new safety signals.4,8 No increase in the incidence of cardiotoxicity was observed with high-dose trastuzumab, supporting results of earlier studies that explored higher doses and more intensive dosing of trastuzumab versus the approved regimen.14,15
When PATRICIA was being designed, phase II data on the use of lapatinib in treating patients with progressive HER2-positive brain metastases were available; however, CNS data for neratinib or tucatinib were not. In the first phase II study of patients with HER2-positive progressive brain metastases treated with single-agent lapatinib, the CNS ORR was only 3%, with a CBR at 4 months of 18%.24 In a subsequent phase II study of lapatinib monotherapy, the CNS ORR was 6%, and 15% of patients were alive and progression-free at 4 months.25 After the planning stages of PATRICIA, neratinib was evaluated as monotherapy in the phase II Translational Breast Cancer Research Consortium 022 study and in combination with capecitabine in the phase III NALA study, and tucatinib was explored in combination with trastuzumab in a phase Ib study and trastuzumab plus capecitabine in the HER2 CLIMB study. In Translational Breast Cancer Research Consortium 022, neratinib was associated with a CNS ORR of 8% and a CBR at 4 months of approximately 20%.26 In NALA, neratinib plus capecitabine led to a delay in the time to intervention for symptomatic CNS disease versus treatment with lapatinib plus capecitabine (overall cumulative incidence 22.8% v 29.2%; P = .043).27 In the phase Ib tucatinib plus trastuzumab study, intracranial responses were observed in 12% of patients receiving twice-daily tucatinib 300 mg plus trastuzumab and 6% of patients receiving once-daily tucatinib 750 mg plus trastuzumab; CBRs at 4 months were 35% and 53%, respectively.12 In HER2CLIMB, tucatinib plus trastuzumab and capecitabine significantly increased CNS ORR (47.3% v 20.0%; P = .03) and reduced the risk of CNS progression or death by 68% versus trastuzumab plus capecitabine alone.28
In general, use of HER2-targeted TKIs combined with chemotherapy results in higher CNS ORRs relative to TKIs alone.25,28-30 However, patients in PATRICIA were not permitted to switch chemotherapy upon study entry; only a minority (28%) were using nonstudy systemic treatment for MBC and none of the patients with a confirmed PR received concurrent chemotherapy while on study. Thus, we believe that prior studies examining single-agent TKIs are more appropriate for placing the clinical efficacy of pertuzumab plus high-dose trastuzumab into context. Although one must be cautious with cross-trial comparisons, given possible differences in patient populations, especially as the PATRICIA study required stable extracranial disease at study baseline, the pertuzumab and high-dose trastuzumab combination yielded a CNS ORR and CBR rate at 4 months that compare favorably with the commercially available HER2-targeted TKIs when given without chemotherapy. Furthermore, although the HER2 TKI studies enrolled both patients with and without active extracranial disease, the CNS remained the most common site of disease progression.25,26
In summary, although the CNS response rate observed in PATRICIA was modest (11%), the majority (68%) of patients experienced clinical benefit, and two patients had ongoing stable intracranial and extracranial disease for > 2 years. Importantly, no new safety signals were observed. These data suggest that (1) pertuzumab plus high-dose trastuzumab may have clinical utility in some patients with HER2-positive MBC and progressive CNS metastases and (2) there is potential to further optimize the dose and schedule of monoclonal antibodies to combat CNS disease. Future studies may be warranted to clarify whether additional CNS gains could be made by combining high-dose trastuzumab with chemotherapy and/or other targeted agents.
ACKNOWLEDGMENT
The authors would like to thank all of the investigators and patients involved in the PATRICIA study. Editorial and medical writing support for this manuscript was provided by Tiffany DeSimone, PhD, of Ashfield Ashfield MedComms, an Ashfield Health Company, and was funded by F. Hoffmann-La Roche/Genentech (South San Francisco, CA).
Appendix
FIG A1.
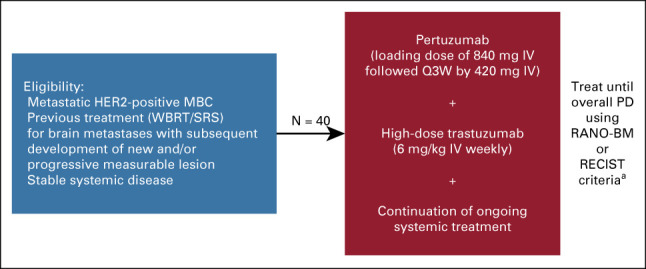
Study design. Primary efficacy end point: ORR in the CNS. Secondary efficacy end points: DOR in the CNS, CBR in the CNS, PFS (CNS), PFS (CNS or non-CNS), PFS (non-CNS), and OS. Safety end point: Safety of pertuzumab and trastuzumab for the treatment of HER2-positive MBC with CNS progression postradiotherapy. aFollowing a protocol update in October 2018, patients were followed up for 12 months post-treatment. CBR, clinical benefit rate; DOR, duration of response; HER2, human epidermal growth factor receptor 2; IV, intravenous; MBC, metastatic breast cancer; ORR, objective response rate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; Q3W, once every 3 weeks; RANO-BM, Response Assessment in Neuro-Oncology Brain Metastases; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
TABLE A1.
RANO-BM Criteria
TABLE A2.
Clinical Benefit at 4 Months by Prior HER2-Targeted Treatment Group

TABLE A3.
Summary of Serum Concentrations of High-Dose Trastuzumab (6 mg/kg) Administered Weekly

Nancy U. Lin
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, California Institute for Regenerative Medicine (CIRM), Denali Therapeutics
Research Funding: Genentech, Pfizer, Seattle Genetics, Merck, Novartis
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Mark Pegram
Consulting or Advisory Role: Genentech/Roche, Pfizer, Seattle Genetics, Puma, Novartis
Solmaz Sahebjam
Consulting or Advisory Role: Merck, Boehringer Ingelheim
Research Funding: Bristol Myers Squibb, Brooklyn ImmunoTherapeutics, Merck
Travel, Accommodations, Expenses: Lilly
Nuhad Ibrahim
Consulting or Advisory Role: Ipsen, Immunomedics
Research Funding: Nektar Therapeutics
Anita Fung
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Anna Cheng
Employment: Genentech
Stock and Other Ownership Interests: Roche
Alan Nicholas
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Whitney Kirschbrown
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Priya Kumthekar
Consulting or Advisory Role: Orbus Therapeutics, Janssen, ElevateBio
Research Funding: Angiochem
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in poster format at the 2019 San Antonio Breast Cancer Symposium (P1-18-03), San Antonio, TX, December 8-11, 2019.
SUPPORT
The PATRICIA study was sponsored by F. Hoffmann-La Roche/Genentech.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Nancy U. Lin, Mark Pegram, Anita Fung, Alan Nicholas
Provision of study materials or patients: Mark Pegram, Solmaz Sahebjam, Nuhad Ibrahim
Collection and assembly of data: Nancy U. Lin, Mark Pegram, Solmaz Sahebjam, Nuhad Ibrahim, Anita Fung, Anna Cheng, Alan Nicholas, Whitney Kirschbrown
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pertuzumab Plus High-Dose Trastuzumab in Patients With Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Nancy U. Lin
Consulting or Advisory Role: Seattle Genetics, Puma Biotechnology, Daiichi Sankyo, California Institute for Regenerative Medicine (CIRM), Denali Therapeutics
Research Funding: Genentech, Pfizer, Seattle Genetics, Merck, Novartis
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, Royalties, Jones & Bartlett
Mark Pegram
Consulting or Advisory Role: Genentech/Roche, Pfizer, Seattle Genetics, Puma, Novartis
Solmaz Sahebjam
Consulting or Advisory Role: Merck, Boehringer Ingelheim
Research Funding: Bristol Myers Squibb, Brooklyn ImmunoTherapeutics, Merck
Travel, Accommodations, Expenses: Lilly
Nuhad Ibrahim
Consulting or Advisory Role: Ipsen, Immunomedics
Research Funding: Nektar Therapeutics
Anita Fung
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Anna Cheng
Employment: Genentech
Stock and Other Ownership Interests: Roche
Alan Nicholas
Employment: Genentech
Stock and Other Ownership Interests: Genentech
Whitney Kirschbrown
Employment: Genentech/Roche
Stock and Other Ownership Interests: Genentech/Roche
Priya Kumthekar
Consulting or Advisory Role: Orbus Therapeutics, Janssen, ElevateBio
Research Funding: Angiochem
No other potential conflicts of interest were reported.
REFERENCES
- 1.Brufsky AM Mayer M Rugo HS, et al. : Central nervous system metastases in patients with HER2-positive metastatic breast cancer: Incidence, treatment, and survival in patients from registHER. Clin Cancer Res 17:4834-4843, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Pestalozzi BC Holmes E de Azambuja E, et al. : CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: A retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol 14:244-248, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Olson EM Najita JS Sohl J, et al. : Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast 22:525-531, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trastuzumab (HERCEPTIN) Prescribing Information. South San Francisco, CA, Genentech, 2018 [Google Scholar]
- 5.Dijkers EC Oude Munnink TH Kosterink JG, et al. : Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther 87:586-592, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Stemmler HJ Schmitt M Willems A, et al. : Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs 18:23-28, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Dawood S Broglio K Esteva FJ, et al. : Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 19:1242-1248, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Pertuzumab (PERJETA) Prescribing Information. South San Francisco, CA, Genentech, 2020 [Google Scholar]
- 9.Swain SM Baselga J Miles D, et al. : Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: Results from the randomized phase III study CLEOPATRA. Ann Oncol 25:1116-1121, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendell JC Domchek SM Burstein HJ, et al. : Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97:2972-2977, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Morikawa A de Stanchina E Pentsova E, et al. : Phase I study of intermittent high-dose lapatinib alternating with capecitabine for HER2-positive breast cancer patients with central nervous system metastases. Clin Cancer Res 25:3784-3792, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metzger O Leone JP Li T, et al. : Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann Oncol 31:1231-1239, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Lewis Phillips GD Nishimura MC Lacap JA, et al. : Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res Treat 164:581-591, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel CL Cobleigh MA Tripathy D, et al. : Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719-726, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Leyland-Jones B Colomer R Trudeau ME, et al. : Intensive loading dose of trastuzumab achieves higher-than-steady-state serum concentrations and is well tolerated. J Clin Oncol 28:960-966, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Lin NU Lee EQ Aoyama H, et al. : Response Assessment in Neuro-Oncology (RANO) group: Response assessment criteria for brain metastases: Proposal from the RANO group. Lancet Oncol 16:e270-e278, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA Therasse P Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Bart J Groen HJ Hendrikse NH, et al. : The blood-brain barrier and oncology: New insights into function and modulation. Cancer Treat Rev 26:449-462, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Régina A Demeule M Laplante A, et al. : Multidrug resistance in brain tumors: Roles of the blood-brain barrier. Cancer Metastasis Rev 20:13-25, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Fabi A Alesini D Valle E, et al. : T-DM1 and brain metastases: Clinical outcome in HER2-positive metastatic breast cancer. Breast 41:137-143, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Montemurro F Delaloge S Barrios CH, et al. : Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol 31:1350-1358, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Kirschbrown WP Wang B Nijem I, et al. : Pharmacokinetic and exposure-response analysis of pertuzumab in patients with HER2-positive metastatic gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol 84:539-550, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg A Quartino A Li J, et al. : Population pharmacokinetic and covariate analysis of pertuzumab, a HER2-targeted monoclonal antibody, and evaluation of a fixed, non-weight-based dose in patients with a variety of solid tumors. Cancer Chemother Pharmacol 74:819-829, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Lin NU Carey LA Liu MC, et al. : Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 26:1993-1999, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin NU Diéras V Paul D, et al. : Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res 15:1452-1459, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Freedman RA Gelman RS Wefel JS, et al. : Translational Breast Cancer Research Consortium (TBCRC) 022: A phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol 34:945-952, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saura C Oliveira M Feng Y, et al. : Neratinib + capecitabine versus lapatinib + capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. J Clin Oncol 37, 2019. (suppl 15; abstr 1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin NU Murthy R Anders C, et al. : Tucatinib vs placebo added to trastuzumab and capecitabine for patients with previously treated HER2+ metastatic breast cancer with brain metastases (HER2CLIMB). J Clin Oncol 38, 2020. (suppl 15; abstr 1005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachelot T Romieu G Campone M, et al. : Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol 14:64-71, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Freedman RA Gelman RS Anders CK, et al. : Translational Breast Cancer Research Consortium: TBCRC 022: A phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol 37:1081-1089, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]



