Abstract
Objective:
The purpose of this study is to investigate whether aspirin improves the prognosis of breast cancer patients by meta analysis.
Methods:
Searched PubMed, EMBASE, and other databases for literature on the relationship between aspirin use and breast cancer prognosis, with the deadline of October 2019. The related results of all-cause death, breast cancer-specific death, and breast cancer recurrence/metastasis were extracted to combine the effect amount. The sensitivity analysis and published bias analysis were carried out for the included data. Stata12.0 software was used to complete all statistical analysis.
Results:
A total of 13 papers were included in the study, including 142,644 breast cancer patients. The results of meta-analysis showed that patients who took aspirin were associated with lower breast cancer-specific death (HR = 0.69, 95% CI = 0.61–0.76), all-cause death (HR = 0.78, 95% CI = 0.71–0.84), and risk of recurrence/metastasis (HR = 0.91, 95% CI: 0.82–1.00).
Conclusions:
Aspirin use may improve all-cause mortality, specific mortality, and risk of recurrence/metastasis in patients with breast cancer.
Keywords: aspirin, breast cancer, meta-analysis, survival
1. Introduction
Breast cancer (BC) is a common malignant tumor in women worldwide, affecting approximately 12% of women.[1] According to research by Ferlay et al,[2] about 2088.8 million women were diagnosed with breast cancer, which is the most common cancer among women in the world except South Africa, and more than 500,000 people die of breast cancer every year.[3] In addition, in the study of Wu et al,[4] the metastatic rate of breast cancer was 7.7%. According to reports, 5% to 10% of breast cancers were caused by genes, and 90% to 95% of the environment was determined[5] such as: use of hormonal drugs, environmental deterioration, unhealthy behavioral lifestyles, mental and psychological factors, etc.[6–8] Therefore, in the past few years, nonsteroidal anti-inflammatory drugs (NASIDs) have been applied to the treatment and prevention of breast cancer, including ibuprofen, nimesulide, celecoxib, aspirin, etc.
Aspirin has antipyretic, analgesic, and anti-inflammatory effects and the preventive effect of aspirin on colon cancer, breast cancer, and gastric cancer has also been confirmed[9,10] and several studies have found that the use of aspirin can reduce breast cancer mortality,[11] however, another part of the study proves that taking aspirin can increase breast cancer mortality.[12] Sharpe et al[13] found that aspirin can reduce the risk of breast cancer recurrence, in contrast to the results of Bens et al.[14] Therefore, the results of aspirin on reducing breast cancer mortality and breast cancer recurrence and metastasis are still controversial.
In this light, we conducted a meta-analysis of the relationship between oral aspirin and specific death, all-cause death, and recurrence in patients with breast cancer. We also hope that our work can provide evidence-based medical evidence for the treatment of breast cancer.
2. Method
2.1. Publication search
We searched the papers in the PubMed, Embase, and other databases. The search time was limited to the establishment of the database until October 30, 2019. The following keywords were retrieved: “aspirin,” “nonsteroidal anti-inflammatory drugs,” “NSAIDs,” “breast cancer.” This search was completed independently by 2 investigators.
2.2. Inclusion and exclusion of publications
Inclusion: The outcomes of the papers included breast cancer-specific death, all-cause death, and breast cancer recurrence/metastasis. The studied population intake aspirin. HR with 95% CI was used to evaluate the data. Cohort study.
Exclusion: HR with 95% CI was not used to evaluate data. The object being studied is not human. Study type is not a cohort study.
2.3. Data extraction
The following data information was extracted from all selected studies: first author, year of publication, the city where the participants are located, type of study, follow-up time, number of samples, number of people taking/not taking aspirin, outcome, HR with 95% CI. The 2 researchers cross-checked the results of the papers, and the differences that occurred during the screening process were not discussed, the third party involved in the discussion and decision.
2.4. Quality evaluation
The Newcastle-Ottawa Scale[15] was used by 2 researchers to evaluate the quality of the selected papers, and the evaluation process was completed independently. When the results of 2 independent investigators were different, the discussion would be held first, when the opinions were still inconsistent, the third investigator would conduct the quality evaluation.
2.5. Ethical statements
No ethical approval is required since this is a literature-based study.
2.6. Statistical analysis
The aggregated statistical data from the meta-analysis was HR and 95% CI. Statistical heterogeneity between studies was performed using the Q test and the value of I2 was calculated. If P ≥ .1 and I2 ≤ 50%, there was no statistical heterogeneity between the studies, and the combined effect model was used for the combined analysis. On the contrary, P < .1 and I2 > 50%, there was statistical heterogeneity between the studies, and the random effects model was used for the combined analysis. Sensitivity analysis was used to evaluate the impact of each study on the overall hazard ratio. Egger test was used to assess the trial error. STATA version 12.0 was used for us to analysis the data. Significance test level was α = 0.05.
3. Results
3.1. Basic characteristics and quality evaluation of the research
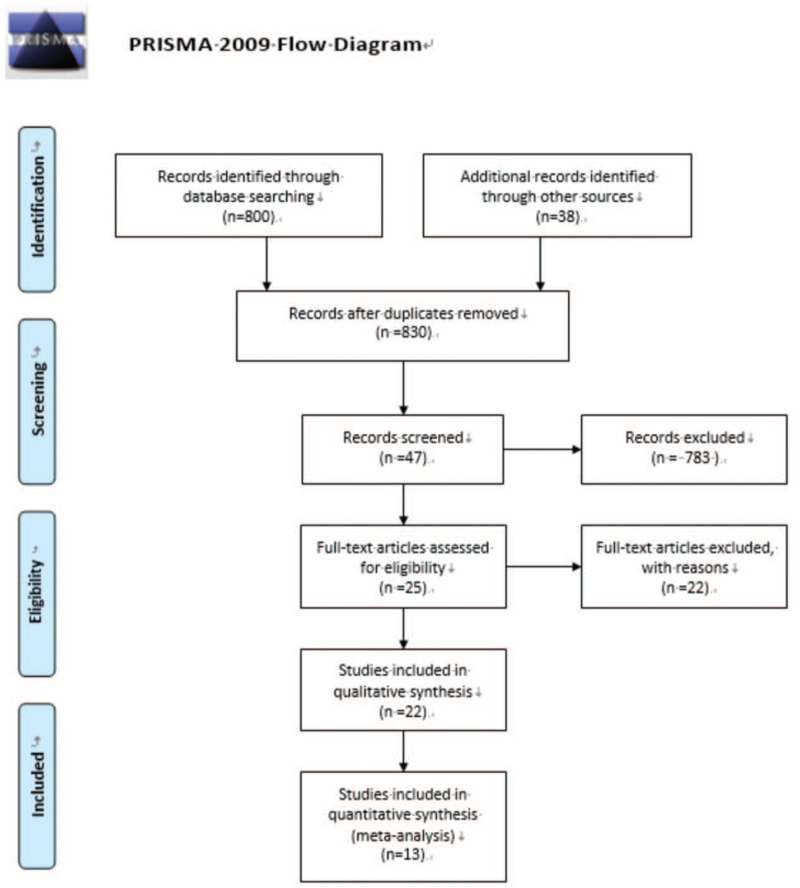
Initially, a total of 838 papers were retrieved, 590 from PubMed, 248 from Embase. After reading the abstract and the title, there were 55 papers remaining. Finally, a total of 13[11,16–27] studies were included to read the full text. A total of 142,644 research samples were included. The search process was shown in Figure 1.
Figure 1.
Retrieving flow chart.
Table 1 summarized the basic characteristics of the included studies. In all papers, 6 were conducted in America,[16–18,21–22,24] 2 were conducted in United Kingdom,[11,23] 2 were organized in Denmark,[26,27] 2 were organized in Ireland,[19,20] and other was launched in Sweden.[25] A total of 142,644 participants were included. Outcome events in 11 studies were breast cancer-specific deaths,[11,16–25] 10 studies included all-cause death outcome events,[11,16–24] and 4 studies reported recurrence/metastasis rates.[22,25–27] Document quality evaluation was showed in Table 2.
Table 1.
Basic characteristics of studies.
| Author | Year of publication | Origin | Type of study | Follow-up time | Number of samples (n) | Use aspirin/no use (n) | Outcome | Breast cancer-specific death | All-cause death | Recurrence/metastasis |
| Blair CK[16] | 2007 | America | Cohort study | 1992–2001 | 591 | 254/337 | 1,2 | 0.53 (0.30,0.93) | 0.53 (0.36,0.79) | |
| Wernli KJ[17] | 2011 | America | Cohort study | 1998–2006 | 3022 | 1059/1963 | 1,2 | 0.64 (0.37,1.37) | 0.91 (0.65,1.29) | |
| Li Y[18] | 2012 | America | Cohort study | 1996–2006 | 1024 | Not clear | 1,2 | 0.89 (0.53,.52) | 0.82 (0.54,1.24) | |
| Fraser DM[11] | 2014 | England | Cohort study | 1998–2008 | 2617 | 815/1802 | 1,2 | 0.42 (0.31,0.55) | 0.53 (0.45,0.63) | |
| Barron TI[19] | 2014 | Ireland | Cohort study | 2000–2006 | 2796 | 740/2056 | 1,2 | 0.99 (0.68, 1.45) | 1.11 (0.83, 1.50) | |
| Barron TI[20] | 2015 | Ireland | Cohort study | 2001–2012 | 4540 | 764/3776 | 1,2 | 0.98 (0.74,1.30) | 1.10 (0.90,1.33) | |
| Bradley MC[21] | 2016 | America | Cohort study | 1993–2009 | 2925 | 1274/1651 | 1,2 | 0.95 (0.68,1.31) | 0.93 0.75,1.15 | |
| Shiao J[22] | 2016 | America | Cohort study | 1998–2016 | 222 | 65/157 | 1,2,3 | 0.41 (0.20,0.83) | 0.67 (0.35,1.27) | 0.34 (0.15,0.81) |
| Mc Menamin MC[23] | 2017 | England | Cohort study | 2009–2015 | 15,140 | 2822/12,318 | 1,2 | 0.92 (0.75,1.14) | 1.21 (1.04,1.40) | |
| Wang T[24] | 2019 | America | Cohort study | 1996–2014 | 1442 | 301/1141 | 1,2 | 0.87 (0.59,1.29) | 1.21 (0.99,1.48) | |
| Frisk G[25] | 2018 | Sweden | Cohort study | 2006–2012 | 21,414 | 9582/11,832 | 1,3 | 0.99 (0.79, 1.23) | 0.97 (0.86,1.10). | |
| Cronin-Fenton DP[26] | 2016 | Denmark | Cohort study | 1996–2008 | 34,188 | 6802/27,386 | 3 | 1.0 (0.85, 1.3) | ||
| Bens A[27] | 2018 | Denmark | Cohort study | 1996–2012 | 52,723 | 5295/47,428 | 3 | 0.88 (0.69,1.13) |
Note: 1: Breast cancer-specific death; 2: all-cause death; 3: recurrence (metastasis).
Table 2.
Quality evaluation of studies.
| Selection | Comparability | Outcome | |||||||
| Representativeness of the exposed cohort | Selection of nonexposure group | Exposure confirmation | Outcome events before the start of the study | Comparability between research design and structure | assessment of outcome | was follow-up long enough for outcomes to occur | adequacy of follow-up of cohort | Total | |
| Blair CK[16] | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 |
| Wernli KJ[17] | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 |
| Li Y[18] | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 |
| Fraser DM[11] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Barron TI[19] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| Barron TI[20] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Bradley MC[21] | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 7 |
| Shiao J[22] | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Mc Menamin MC[23] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| Wang T[24] | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 6 |
| Frisk G[25] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
| Cronin-Fenton DP[26] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 |
| Bens A[27] | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 |
3.2. Breast cancer-specific death
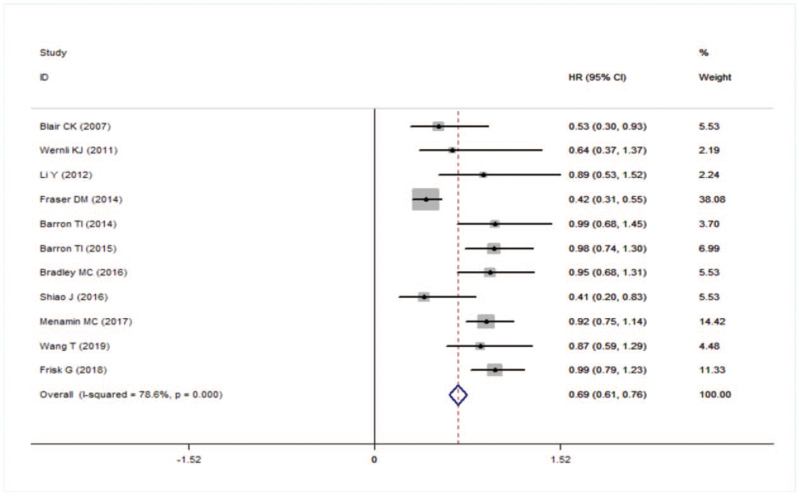
In all studies included in the meta-analysis, the use of aspirin reduced the specific death of breast cancer (HR = 0.69, 95% CI: 0.61–0.76). Higher heterogeneity between studies (I2 = 78.6%, P = .000) (Fig. 2).
Figure 2.
Forest plot of specific death.
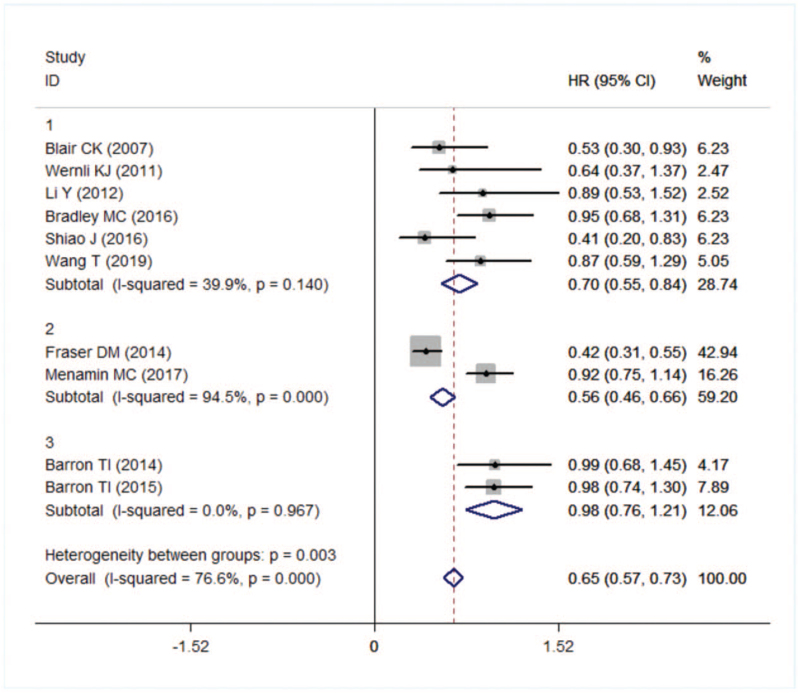
Then we conducted a subgroup analysis based on the source of the city (1 = United States, 2 = UK, 3 = Ireland). In addition, the use of aspirin in the United States and the United Kingdom reduced the risk of breast cancer-specific death by 30% and 44%, respectively (HR = 0.70, 95% CI: 0.55–0.84; HR = 0.56, 95% CI: 0.46–0.66), while the use of aspirin in Ireland was not associated with breast cancer-specific death (HR = 0.98, 95% CI: 0.76–1.21). However, heterogeneity was not found by subgroup analysis (Fig. 3).
Figure 3.
Forest plot of specific death in different country.
Sensitivity analysis showed that the study of Fraster[11] was an outlier, and the reconsolidation analysis was excluded (Fig. 4). The meta-analysis of 10 studies showed that the aspirin-specific death was 15% lower than the no used (HR = 0.85, 95% CI: 0.76–0.96), and the heterogeneity result was (I2 = 43.6%, P = .068). Egger’ test results suggested that there was no publication bias (t = 1.22, P = .255).
Figure 4.
Forest plot of all-cause death.
3.3. All-cause death
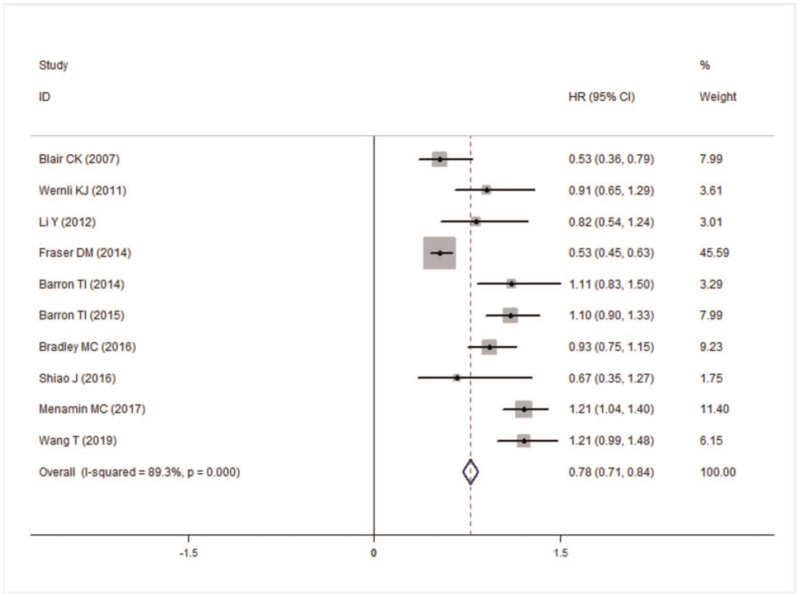
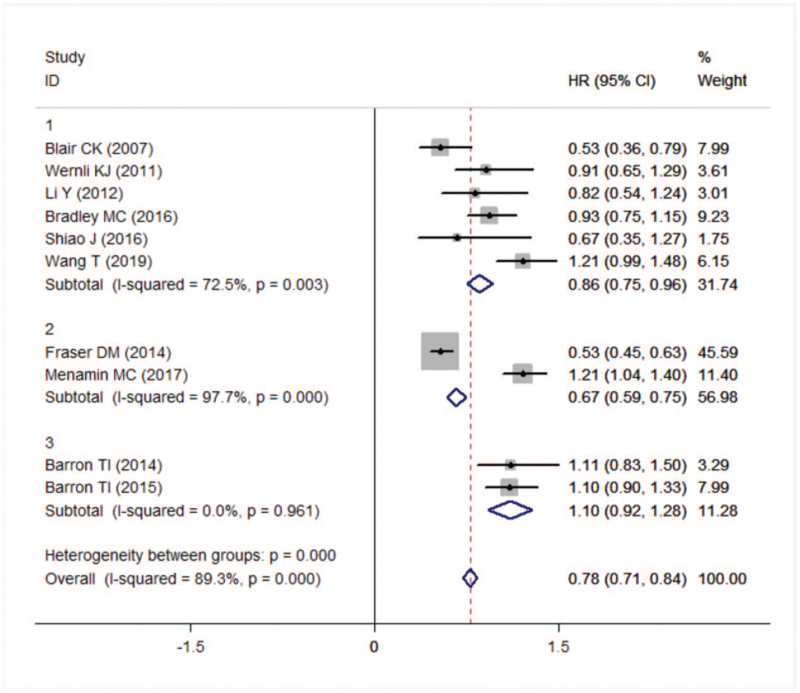
The results showed that taking aspirin could reduce the risk of all-cause death in breast cancer patients (HR = 0.78, 95% CI: 0.71–0.84). However, there was heterogeneity between the papers (I2 = 0.83, P = .000) (Fig. 4).
A subgroup analysis based on national sources revealed no statistically significant studies from Ireland (HR = 1.10, 95% CI: 0.92–1.28) while reduced all-cause mortality from studies in the United States (HR = 0.86, 95% CI: 0.75–0.96) and the United Kingdom (HR = 0.67, 95% CI: 0.59–0.75) (Fig. 5). However, heterogeneity sources were not found in subgroup analyses.
Figure 5.
Forest plot of all-cause death in different country.
The sensitivity analysis showed that all-cause death of Fraser[11] and Mc Menamin[23] studies were outliers. After remerging, the heterogeneity changes little, indicating that the results were stable. Egger test results suggested that there was no publication bias (t = 0.57, P = .582).
3.4. Recurrence/metastasis
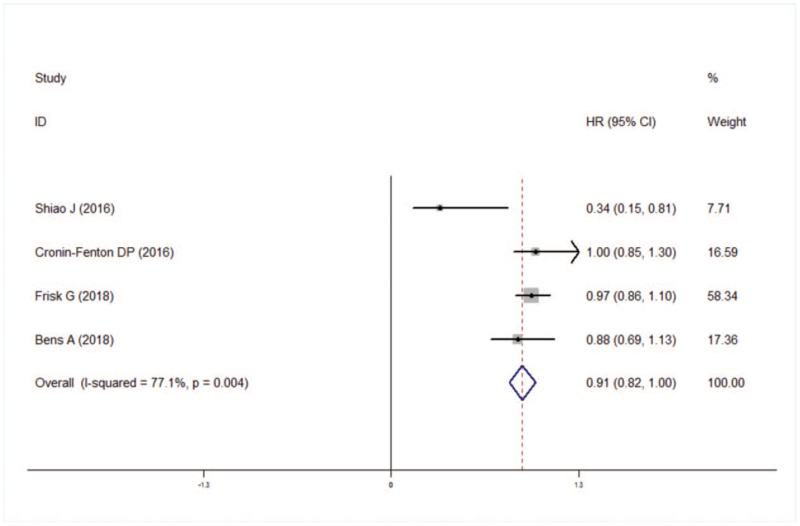
Meta-analysis showed that risk of breast cancer patients using aspirin was associated with lower risk recurrence/metastasis (HR: 0.91, 95% CI: 0.82–1.00), but there was heterogeneity between studies (I2 = 77.1%, P = .004) (Fig. 6).
Figure 6.
Forest plot of recurrence/metastasis.
Sensitivity analysis did not reveal the source of heterogeneity. Egger test results suggested that there was no publication bias (t = 2.58, P = .123).
4. Discussion
A total of 13[11,16–27] studies were selected for this meta-analysis, and they included 140,644 breast cancer patients. Our meta-analysis found that the use of aspirin reduced breast cancer-specific death by 31% and all-cause death by 22%, while risk of recurrence/metastasis by 9%. So the use of aspirin was beneficial for breast cancer patients.
Aspirin is a nonsteroidal anti-inflammatory drug that is mainly used for antipyretic and analgesic, anti-inflammatory, anti-rheumatic, and anti-platelet aggregation. In recent years, more and more studies have shown that aspirin can reduce the risk of breast cancer death and recurrence.[28] The mechanism of action of aspirin on breast cancer has also been proved by some experiments. Recent reports by Hsieh and Wang[29] demonstrate that aspirin inhibits crosstalk between 4T1 and RAW 264.7 cells and regulates M1/M2 macrophage subtypes by regulating angiogenesis and inflammatory mediator production, thereby contributing to the treatment of breast cancer. Another study found that the anti-tumor effect of aspirin mainly inhibits the activity of cyclooxygenase in the body, thereby inhibiting breast cancer cell proliferation, tumor angiogenesis, and tumor cell infiltration[30] Another mechanism of action of aspirin on breast cancer is by anti-adenocarcinoma by inhibiting NF-κB and TGF-β/SMAD-mediated signaling pathways.[31]
Subgroup analysis by country source showed that the use of aspirin reduced the risk of specific death and all-cause mortality in both the United States and the United Kingdom, while that the use of aspirin was not associated with all-cause death and specific death in breast cancer patients, in Ireland. May be due to different lifestyles and eating habits in different countries, resulting in different prognosis of breast cancer.[32] A subgroup analysis of breast cancer-specific deaths found no heterogeneity between the 2 subgroups of the United States and Ireland, but the subgroup of heterogeneities in the 2 UK studies included. The possible causes of heterogeneity are that the sample size of Mc Menamin et al[23] is much higher than that of Fraser et al, and another reason may be that the follow-up time of the 2 studies is different, Mc Menamin et al[23] The study was followed for 6 years, and the cohort of Fraser et al[11] was followed for 10 years. All-cause subgroup analysis showed heterogeneity between the 2 subgroups in the United States and the United Kingdom. The heterogeneity may be due to different sample sizes, different follow-up times, and different doses of aspirin.
Sensitivity analysis found that the research of Fraser et al[11] was the heterogeneity source of specific death. After rejection, the pooled HR was 0.85, and the 95% confidence interval was 0.76 to 0.96. The sensitivity analysis of all-cause death found that studies[11,23] were outliers. However, after one-by-one rejection, the heterogeneity did not change significantly, suggesting that the meta-analysis results were stable. The reasons for the heterogeneity in this study may be: the difference in sample size, the sample size of Shiao, Blair et al[16,22] was less than 1000, and the sample size of Barron, Mc Menamin et al[23] were greater than 4000. The follow-up time is different, the shortest was 5 years and the longest was 16 years. The dose of aspirin used by the participants is different. It has been reported that different doses of aspirin use different breast cancer survival rates.[33] Sensitivity analysis of recurrence/metastasis was a source of heterogeneity in the future. The reason for the heterogeneity was that the total sample size of Shiao et al[22] was only 222, far lower than other studies, perhaps because of the low quality of Shiao et al.[22]
Like other studies, this meta-analysis is also inadequate. The number of studies included was small, no case-control studies were selected, and no statistically selected RR/OR and 95% confidence interval studies were selected. The included studies only compare whether or not to use aspirin, and ignore the influencing factors such as the dose, frequency and course of treatment of aspirin. There are 2 papers with low quality and high risk of bias.
5. Conclusion
Overall, this meta-analysis showed that aspirin use may reduce the risk of specific death from breast cancer, all-cause mortality, and recurrence/metastasis.
Author contributions
Methodology: Jiamin Liu.
Software: Meng Yang.
Writing – original draft: Fengxian Zheng, Xiaoyong Wu.
Writing – review & editing: Aimin Liu.
Footnotes
Abbreviations: BC = breast cancer, NASIDs = nonsteroidal anti-inflammatory drugs.
How to cite this article: Liu J, Zheng F, Yang M, Wu X, Liu A. Effect of aspirin use on survival benefits of breast cancer patients: A meta-analysis. Medicine. 2021;100:33(e26870).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Liu Y, Sun J, Wu T, et al. Effects of serum from breast cancer surgery patients receiving perioperative dexmedetomidine on breast cancer cell malignancy: a prospective randomized controlled trial. Cancer Med 2019;8:7603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2018;144:1941–53. [DOI] [PubMed] [Google Scholar]
- [3].Torre LA, Siegel RL, Ward EM, et al. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- [4].Wu J, Ye J, Wu W, et al. Racial disparities in young-onset patients with colorectal, breast and testicular cancer. J Cancer 2019;10:5388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kolak A, Kamiå„Ska M, Sygit K, et al. Primary and secondary prevention of breast cancer. Ann Agric Environ Med 2006;24:549–53. [DOI] [PubMed] [Google Scholar]
- [6].Day S, Bevers TB, Anderson GL, et al. Estrogen plus progestin and breast cancer incidence and mortality in the women's health initiative observational study. Breast Diseases: A Year Book Quarterly 2013;105:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Akinyemiju TF, Genkinger JM, Farhat M, et al. Residential environment and breast cancer incidence and mortality: a systematic review and meta-analysis. BMC Cancer 2015;15:01–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Anderson KN, Schwab RB, Martinez ME. Reproductive risk factors and breast cancer subtypes: a review of the literature. Breast Cancer Res Treat 2014;144:01–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ajrouche A, De Rycke Y, Dalichampt M, et al. Reduced risk of cancer among low-dose aspirin users: data from French health care databases. Pharmacoepidemiol Drug Saf 2019;28:1258–66. [DOI] [PubMed] [Google Scholar]
- [10].Uno Y. Prevention of gastric cancer by Helicobacter pylori eradication: a review from Japan. Cancer Med 2019;8:3992–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fraser DM, Sullivan FM, Thompson AM, et al. Aspirin use and survival after the diagnosis of breast cancer: a population-based cohort study. Br J Cancer 2014;111:623–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang T, Parada H, Mcclain KM, et al. Pre-diagnostic aspirin use and mortality after breast cancer. Cancer Causes Control 2018;29:417–25. [DOI] [PubMed] [Google Scholar]
- [13].Sharpe CR, Collet JP, Mcnutt M, et al. Nested case–control study of the effects of non-steroidal anti-inflammatory drugs on breast cancer risk and stage. Br J Cancer 2000;83:112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Singla A, Kumar G, Bardia A. Personalizing cardiovascular disease prevention among breast cancer survivors. Curr Opin Cardiol 2012;27:515–24. [DOI] [PubMed] [Google Scholar]
- [15].Hartling L, Milne A, Hamm MP, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol 2013;66:982–93. [DOI] [PubMed] [Google Scholar]
- [16].Blair CK, Carol S, Anderson KE, et al. NSAID use and survival after breast cancer diagnosis in post-menopausal women. Breast Cancer Res Treat 2007;101:191–7. [DOI] [PubMed] [Google Scholar]
- [17].Wernli KJ, Hampton JM, Trentham-Dietz A, et al. Use of antidepressants and NSAIDs in relation to mortality in long-term breast cancer survivors. Pharmacoepidemiol Drug Saf 2011;20:131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li Y, Brasky TM, Nie J, et al. Use of nonsteroidal anti-inflammatory drugs and survival following breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2012;21:239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barron TI, Flahavan EM, Linda S, et al. Recent prediagnostic aspirin use, lymph node involvement, and 5-year mortality in women with stage I-III breast cancer: a nationwide population-based cohort study. Cancer Res 2014;74:4065–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barron TI, Murphy LM, Brown C, et al. De Novo post-diagnosis aspirin use and mortality in women with Stage I-III breast cancer. Cancer Epidemiol Biomarkers Prev 2015;24:898–904. [DOI] [PubMed] [Google Scholar]
- [21].Bradley MC, Black A, Freedman AN, et al. Prediagnostic aspirin use and mortality in women with stage I to III breast cancer: a cohort study in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer. 2016;122:2067–2075. [DOI] [PubMed] [Google Scholar]
- [22].Shiao J, Thomas KM, Rahimi AS, et al. Aspirin/antiplatelet agent use improves disease-free survival and reduces the risk of distant metastases in Stage II and III triple-negative breast cancer patients. Breast Cancer Res Treat 2016;161:463–71. [DOI] [PubMed] [Google Scholar]
- [23].Mc Menamin ÚC, Cardwell CR, Hughes CM, et al. Low-dose aspirin use and survival in breast cancer patients: a nationwide cohort study. Cancer Epidemiol. 2017;47:20–27. [DOI] [PubMed] [Google Scholar]
- [24].Wang T, McCullough LE, White AJ, et al. Prediagnosis aspirin use, DNA methylation, and mortality after breast cancer: a population-based study. Cancer 2019;125:3836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Frisk G, Ekberg S, Lidbrink E, et al. No association between low-dose aspirin use and breast cancer outcomes overall: a Swedish population-based study. Breast Cancer Res 2018;20:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cronin-Fenton DP, Heide-Jørgensen U, Ahern TP, et al. Low-dose aspirin, nonsteroidal anti-inflammatory drugs, selective COX-2 inhibitors and breast cancer recurrence. Epidemiology 2016;27:586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bens A, Friis S, Dehlendorff C, et al. Low-dose aspirin use and risk of contralateral breast cancer: a Danish nationwide cohort study. Prev Med 2018;116:186–93. [DOI] [PubMed] [Google Scholar]
- [28].Holmes MD, Chen WY, Li L, et al. Aspirin intake and survival after breast cancer. J Clin Oncol 2010;28:1467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hsieh CC, Wang CH. Aspirin disrupts the crosstalk of angiogenic and inflammatory cytokines between 4T1 breast cancer cells and macrophages. Mediators Inflamm 2018;2018:6380643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hugo HJ, Saunders C, Ramsay RG, et al. New Insights on COX-2 in chronic inflammation driving breast cancer growth and metastasis. J Mammary Gland Biol Neoplasia 2015;20:109–19. [DOI] [PubMed] [Google Scholar]
- [31].Myriam L, Shahinoor B, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011;20:576–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Slattery ML, Lundgreen A, Torres-Mejia G, et al. Diet and lifestyle factors modify immune/inflammation response genes to alter breast cancer risk and prognosis: The Breast Cancer Health Disparities Study. Mutat Res. 2014;770:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Holmes MD, Olsson H, Pawitan Y, et al. Aspirin intake and breast cancer survival – a nation-wide study using prospectively recorded data in Sweden. BMC Cancer 2014;14:391. [DOI] [PMC free article] [PubMed] [Google Scholar]