Abstract
To explore the predictive value of preoperative serum squamous cell carcinoma antigen (SCC-Ag) level for lymph node metastasis (LNM), particularly, in patients surgically treated for early-stage cervical squamous cell carcinoma.
We enrolled 162 patients with cervical squamous cell carcinoma stages IB to IIA following the International Federation of Gynecology and Obstetrics (FIGO) 2009 classification. The patients had previously undergone radical surgery. Correlation of the SCC-Ag level with clinicopathological features and the predictive value of SCC-Ag for LNM were analyzed.
High preoperative SCC-Ag level was correlated with FIGO stage (P = .001), tumor diameter >4 cm (P < .001), stromal infiltration (P < .001), LNM (P < .001) and lymphovascular space invasion (LVSI), (P = .045). However, it was not correlated with age, histological differentiation, parametrial involvement, and positive vaginal margin (P > .05). Univariate analysis revealed that FIGO stage (P = .015), tumor diameter (P = .044), stromal infiltration (χ2 = 10.436, P = .005), SCC-Ag ≧ 2.75 ng/mL (χ2 = 14.339, P < .001), LVSI (χ2 = 12.866, P < .001), parametrial involvement (χ2 = 13.784, P < .001) were correlated with LNM, but not with age, histological differentiation, and positive vaginal margin. Moreover, multivariate analysis demonstrated that SCC-Ag ≧2.75 ng/mL (P = .011, OR = 3.287) and LVSI (P = .009, OR = 7.559) were independent factors affecting LNM. The area under the receiver operator characteristic curve of SCC-Ag was 0.703 (P < .001), while 2.75 ng/mL was the best cutoff value for predicting LNM. The sensitivity and specificity of diagnosis were 69.4% and 65.9%, respectively.
High SCC-Ag level was revealed to be an independent risk factor for the prognosis of squamous carcinoma of the cervix before an operation. Besides, SCC-Ag (2.75 ng/mL) can be utilized as a potential marker to predict LNM in early stage cervical cancer before an operation.
Keywords: lymph node metastasis, predictive value, serum squamous cell carcinoma antigen, squamous carcinoma of the cervix
1. Introduction
Cervical cancer is among the common malignant tumors that transpire in the female reproductive system. The incidence rate of cervical cancer has increased in recent years and is notably displaying a trend of rejuvenation. Global statistics indicate that nearly 569,847 new cases and 311,365 deaths from cervical cancer were reported by 2018.[1]
Squamous cell carcinoma of cervical cancer is regarded as the most common pathological type accounting for about 80% of the cases, followed by adenocarcinoma that accounts for about 20% of the total cases.[2] Squamous cell carcinoma antigen (SCC-Ag), a specific antigen produced by squamous cell carcinoma, plays an important role in auxiliary diagnosis, prognosis, and monitoring of the recurrence of cervical squamous cell carcinoma.[2–5] Therefore, in this work, we conducted a retrospective analysis of 162 patients with early-stage cervical squamous cell carcinoma and determined the correlation of SCC-Ag level before treatment with clinicopathological features of cervical squamous cell carcinoma and the predictive value of lymph node metastasis (LNM).
2. Methods
2.1. General information
In total, 334 cases of cervical malignant tumors were collected between November 2016 and April 2019 in the department of Gynecology Oncology of Dalian Obstetrics and Gynecology Hospital affiliated to Dalian Medical University. The 334 cases were grouped as follows: 275 cases of squamous cell carcinoma (82.3%), 35 cases of adenocarcinoma (10.5%), 15 cases of adenosquamous carcinoma (4.5%), 7 cases of neuroendocrine carcinoma (2.1%), and 2 cases of adenoid basal cell carcinoma (0.6%). The inclusion criteria were as follows: International Federation of Gynecology and Obstetrics (FIGO) 2009 IB to IIA stage, pathologically confirmed squamous cell carcinoma; initial treatment for extensive hysterectomy (±bilateral adnexal resection) and pelvic lymph node dissection (laparoscopic or transabdominal surgery); and preoperative detection of serum SCC-Ag. The exclusion criteria were as follows: patients who received neoadjuvant chemotherapy or had undergone conic surgery before operation. Eventually, only 162 eligible cases were included for analysis. The clinical and pathological data of the patients are shown in Table 1. All patients signed the informed consent. The institutional review board of the Dalian Obstetrics and Gynecology Hospital approved this study.
Table 1.
Relationship between SCC-Ag level and clinicopathological features (N = 162).
| SCC-Ag (ng/mL) | ||||
| Characteristic | N (%) | Median | Range | P ∗ |
| Total | 162 (100%) | 2.35 | 0.4 to 70 | |
| Age (yr) | .598 | |||
| <45 | 26 (16.0%) | 2.2 | 0.5 to 70 | |
| ≥45 | 136 (84.0%) | 2.5 | 0.4 to 42.5 | |
| FIGO stage | .001§ | |||
| IB1 | 119 (73.5%) | 2.1 | 0.5 to 31.2 | |
| IB2 | 8 (4.9%) | 11.7 | 2.5 to 40.5 | |
| IIA1 | 33 (20.4%) | 2.1 | 0.4 to 42.5 | |
| IIA2 | 2 (1.2%) | 39.5 | 8.9 to 70 | |
| Tumor diameter | <.001 | |||
| ≤4 cm | 152 (93.8%) | 2.1 | 0.4 to 42.5 | |
| >4 cm | 10 (6.2%) | 11.7 | 2.5 to 70 | |
| Histological differentiation | .197 | |||
| G1 to 2 | 49 (30.2%) | 2.7 | 0.4 to 31.2 | |
| G3 | 113 (69.8%) | 2.0 | 0.5 to 70 | |
| Stromal infiltration | <.001§ | |||
| Superficial 1/3 | 25 (15.4%) | 1.3 | 0.5 to 13 | |
| Middle 1/3 | 49 (30.3%) | 1.8 | 0.4 to 21.7 | |
| Deep 1/3 | 88 (54.3%) | 3.55 | 0.5 to 70 | |
| Lymph node metastasis | <.001 | |||
| Negative | 126 (77.8) | 1.95 | 0.4 to 42.5 | |
| Positive | 36 (22.2%) | 4.4 | 0.8 to 70 | |
| Lymphovascular space invasion | .045 | |||
| Negative | 48 (29.6%) | 2.1 | 0.4 to 11.2 | |
| Positive | 114 (70.4%) | 2.6 | 0.5 to 70 | |
| Parametrial involvement | .052 | |||
| Negative | 153 (94.4%) | 2.3 | 0.4 to 42.5 | |
| Positive | 9 (5.6) | 8.9 | 0.8 to 70 | |
| Vaginal margin | 0.854 | |||
| Negative | 158 (97.5%) | 2.35 | 0.4 to 70 | |
| Positive | 4 (2.5%) | 5.75 | 0.7 to 40.5 | |
FIGO = International Federation of Gynecology and Obstetrics, SCC-Ag = squamous cell carcinoma antigen.
Mann–Whitney U test.
Kruskal–Wallis H test.
2.2. SCC-Ag assay method
The venous blood (3 mL) were drawn from the patients early in the morning. SCC-Ag levels were detected using chemiluminescence microparticle immunoassay (using Abbott Company Kit, with the reference value: SCC-Ag 0–1.5 ng/mL).
2.3. Statistical analyses
SPSS 25.0 software (Statistical analyses were performed using IBM SPSS for Windows,Version 25.0) was used to analyze all statistical data. Mann–Whitney or Kruskal–Wallis tests were adopted to analyze the association of SCC-Ag level with clinicopathological characteristics when the values of SCC-Ag metrological data were not normally distributed. Moreover, when assessing the relationship between LNM and clinicopathological features, count data were analyzed either by the Chi-square test or Fisher accurate test (n < 40 or theoretical frequency < 5). We used binary logistic regression analysis to analyze the independent factors affecting LNM. The receiver operator characteristic (ROC) curve was generated to establish the predictive value of SCC-Ag for LNM, whereas the optimal cutoff value was determined by the Youden index. P < .05 was considered statistically significant.
3. Results
3.1. Relationship between SCC-Ag and clinicopathological features
Here, high preoperative SCC-Ag level was associated with FIGO stage (P = .001), tumor diameter >4 cm (P < .001), stromal infiltration (P < .001), LNM (P < .001), and lymphovascular space invasion (LVSI) (P = .045), independent of age, histological differentiation, parametrial involvement, and positive vaginal margin (all P > .05) (Table 1).
3.2. Relationship between pelvic lymph node metastasis and clinicopathological features
Univariate analysis revealed that LNM was associated with FIGO stage (P = .015), tumor diameter > 4 cm (P = .044), stromal infiltration (χ2 = 10.436, P = .005), SCC-Ag ≧ 2.75 ng/mL (χ2 = 14.339, P < .001), LVSI (χ2 = 12.866, P < .001), and parametrial involvement (χ2 = 13.784, P < .001) (Table 2). Multivariate analysis revealed that SCC-Ag ≧ 2.75 ng/mL (P = .011, OR = 3.287) and LVSI (P = .009, OR = 7.559) were independent factors affecting the LNM (Table 3).
Table 2.
Univariate analysis of pelvic lymph node metastasis and clinicopathological features (N = 162).
| Lymph node metastasis | |||||
| Characteristic | N (%) | Negative (%) | Positive (%) | χ2 | P ∗ |
| Total | 162 (100) | 126 (77.8) | 36 (22.2) | ||
| Age (yr) | 1.309 | .253 | |||
| <45 | 26 (16.1) | 18 (69.2) | 8 (30.8) | ||
| ≥45 | 136 (83.9) | 108 (79.4) | 28 (20.6) | ||
| FIGO stage | .015$ | ||||
| IB1 | 119 (73.5) | 98 (82.4) | 21 (17.6) | ||
| IB2 | 8 (4.9) | 5 (62.5) | 3 (37.5) | ||
| IIA1 | 33 (20.4) | 23 (69.7) | 10 (30.3) | ||
| IIA2 | 2 (1.2) | 0 (0) | 2 (100) | ||
| Tumor diameter | .044$ | ||||
| ≤4 cm | 152 (93.8) | 121 (79.6) | 31 (20.4) | ||
| >4 cm | 10 (6.2) | 5 (50) | 5 (50) | ||
| Histological differentiation | 1.413 | .235 | |||
| G1 to 2 | 49 (30.2) | 41 (83.7) | 8 (16.3) | ||
| G3 | 113 (69.8) | 85 (75.2) | 28 (24.8) | ||
| Stromal infiltration | 10.436 | .005 | |||
| Superficial 1/3 | 25 (15.4) | 23 (92.0) | 2 (8.0) | ||
| Middle 1/3 | 49 (30.3) | 43 (87.8) | 6 (12.2) | ||
| Deep 1/3 | 88 (54.3) | 60 (68.2) | 28 (31.8) | ||
| SCC-Ag (ng/mL) | 14.339 | <.001 | |||
| <2.75 | 94 (58.0) | 83 (88.3) | 11 (11.7) | ||
| ≥2.75 | 68 (42.0) | 43 (63.2) | 25 (36.8) | ||
| Lymphovascular space invasion | 12.866 | <.001 | |||
| Negative | 48 (29.6) | 46 (95.8) | 2 (4.2) | ||
| Positive | 114 (70.4) | 80 (70.2) | 34 (29.8) | ||
| Parametrial involvement | 13.784 | <.001 | |||
| Negative | 153 (94.4) | 124 (81.0) | 29 (19.0) | ||
| Positive | 9 (5.6) | 2 (22.2) | 7 (77.8) | ||
| Vaginal residual | .214$ | ||||
| Negative | 158 (97.5) | 124 (78.5) | 34 (21.5) | ||
| Positive | 4 (2.5) | 2 (50) | 2 (50) | ||
FIGO = International Federation of Gynecology and Obstetrics, SCC-Ag = squamous cell carcinoma antigen.
Pearson χ2 test.
Fisher exact test.
Table 3.
Multivariate analysis of pelvic lymph node metastasis and clinicopathological features (N = 162).
| Lymph node metastasis | |||||
| Characteristic | N (%) | Negative (%) | Positive (%) | P § | OR |
| Total | 162 (100) | 126 (77.8) | 36 (22.2) | ||
| FIGO stage | .361 | ||||
| IB1 | 119 (73.5) | 98 (82.4) | 21 (17.6) | ||
| IB2 | 8 (4.9) | 5 (62.5) | 3 (37.5) | ||
| IIA1 | 33 (20.4) | 23 (69.7) | 10 (30.3) | ||
| IIA2 | 2 (1.2) | 0 (0) | 2 (100) | ||
| Tumor diameter | .806 | ||||
| ≤4 cm | 152 (93.8) | 121 (79.6) | 31 (20.4) | ||
| >4 cm | 10 (6.2) | 5 (50) | 5 (50) | ||
| Stromal infiltration | .186 | ||||
| Superficial 1/3 | 25 (15.4) | 23 (92.0) | 2 (8.0) | ||
| Middle 1/3 | 49 (30.3) | 43 (87.8) | 6 (12.2) | ||
| Deep 1/3 | 88 (54.3) | 60 (68.2) | 28 (31.8) | ||
| SCC-Ag (ng/mL) | .011 | 3.287 | |||
| <2.75 | 94 (58.0) | 83 (88.3) | 11 (11.7) | ||
| ≥2.75 | 68 (42.0) | 43 (63.2) | 25 (36.8) | ||
| Lymphovascular space invasion | .009 | 7.559 | |||
| Negative | 48 (29.6) | 46 (95.8) | 2 (4.2) | ||
| Positive | 114 (70.4) | 80 (70.2) | 34 (29.8) | ||
| Parametrial involvement | .055 | ||||
| Negative | 153 (94.4) | 124 (81.0) | 29 (19.0) | ||
| Positive | 9 (5.6) | 2 (22.2) | 7 (77.8) | ||
FIGO = International Federation of Gynecology and Obstetrics, SCC-Ag = squamous cell carcinoma antigen.
Binary logistic regression analysis.
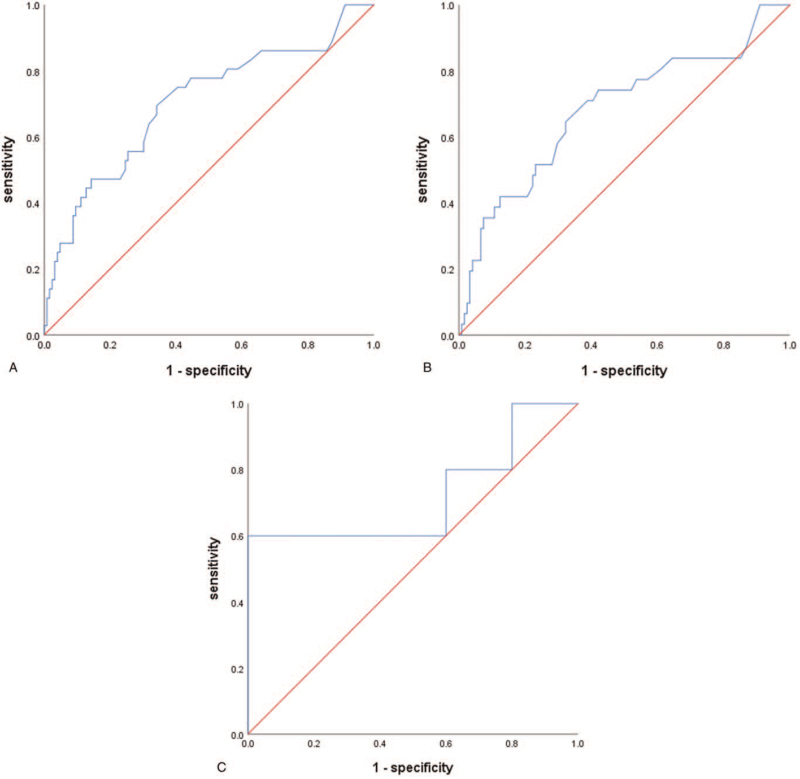
3.3. Receiver operator characteristic curve of SCC-Ag level prediction lymph node metastasis
Here, we used the ROC curve to analyze the predictive value of SCC-Ag levels on LNM for all patients (Fig. 1A). The area under curve (AUC) was 0.703 (P < .001; 95% CI, 0.599–0.807). The optimum cutoff value was determined by the Youden index. Notably, the maximum value of the index was 0.353, whereas the SCC-Ag value was 2.75 ng/mL. The sensitivity and specificity of diagnosis was 69.4% and 65.9%, respectively. The AUC of patients in group IB1 and IIA1 was 0.682 (P = .002; 95% CI, 0.568–0.796) (Fig. 1B). The cutoff of SCC-Ag was 2.75 ng/mL, with a sensitivity of 64.5% and a specificity of 67.8%. The AUC was 0.720 (P = .251) in group IB2 and IIA2 (Fig. 1C). There was no statistical difference in this group.
Figure 1.
ROC curve of SCC-Ag level prediction lymph node metastasis in the total population (A), in IB1 and IIA1 (B), in IB2 and IIA2 (C). ROC = receiver operator characteristic, SCC-Ag = squamous cell carcinoma antigen.
4. Discussion
Early-stage cervical squamous cell carcinoma predominantly occurs in patients with FIGO (2009) IB to IIA stages. The National Comprehensive Cancer Network cervical cancer treatment guidelines recommend surgical treatment for patients with IB1 or IIA1, whereas synchronous radiotherapy and chemotherapy are preferred for IB2 or IIA2 patients.[6] Compared with radiotherapy and chemotherapy, surgery efficiently restores ovarian and sexual functions and can improve the quality of life after surgery. This implicates surgery is one of the primary treatments, in particular, for young patients. The standard procedure incorporates radical hysterectomy and pelvic lymphadenectomy. Besides, LNM is regarded as an important risk factor affecting the prognosis of cervical cancer. Notably, when LNM is confirmed through postoperative pathology, supplementary radiotherapy still, must be administered postoperation. In our work, when surgery was combined with postoperative radiotherapy and chemotherapy, significantly advanced treatment-related complications occurred, however, the prognosis did not improve. Therefore, it is critically important to evaluate the risk of LNM, this will guide on the choice of treatment mode.
SCC-Ag first described by Kato and Torigoe,[7] is a widely used and reliable marker in squamous cell carcinoma. Numerous studies have found that high serum SCC-Ag level in patients with cervical squamous cell carcinoma is closely correlated with tumor diameter, FIGO stage, depth of stromal infiltration, LVSI, and LNM.[8–10] Herein, we revealed that elevated SCC-Ag levels were associated with FIGO stage (P = .001), tumor diameter > 4 cm (P < .001), depth of stromal infiltration (P < .001), LNM (P < .001), and LVSI (P = .045), this concurred with previous findings. Since larger tumor volume implies greater tumor load, thus tumor cells can produce more SCC-Ag which when released into the blood, results in elevated serum SCC-Ag levels.[11] In our study, cervical cancer patients diagnosed using cone resection surgery were excluded because they probably had reduced tumor load due to cone resection surgery which could affect the judgment of final results. Moreover, LVSI and LNM can be utilized to predict poor prognosis, and because higher SCC-Ag levels often suggest a more severe condition, the 2 parameters have a certain correlation.
LNM has been implicated as the principal factor affecting the 5-year survival rate of cervical cancer patients.[12,13] In the early stage of cervical cancer, the 5-year survival rate of positive LNM is about 50%, whereas that of nonLNM is above 90%.[14] Preoperative assessment of LNM, in most cases, relies on computerized tomography or magnetic resonance imaging examination, though minor metastases are often undetectable, resulting in a higher false-negative rate.[15] A few studies on the predictive value of preoperative SCC-Ag level for LNM in cervical squamous cell carcinoma were conducted and showed inconsistent conclusions. For instance, Lin et al[16] retrospectively analyzed the clinical data of 284 patients with stage IB and IIA cervical squamous cell carcinoma, they revealed that SCC-Ag ≧8 ng/mL was an independent risk factor for LNM before treatment. The sensitivity was 36% and the specificity was 95%. Elsewhere, Xu et al[17] assessed 192 patients with early cervical squamous cell carcinoma and reported SCC-Ag ≧ 2.35 ng/mL as an independent factor for predicting LNM (P < .001, OR = 4.825). These assessments suggest that SCC-Ag can be utilized to predict LNM in patients with cervical squamous cell carcinoma. Additionally, Lekskul et al[18] analyzed the clinical data of 231 patients with stage IB2 to IVA cervical squamous cell carcinoma, notably, they concluded that SCC-Ag levels did not predict pelvic and para-abdominal aortic LNM and were only beneficial in evaluating tumor load. The study failed to find the relationship between SCC-Ag levels and LNM, possibly because this study included the patients with cervical cancer from stage IB2 to IVA, consequently with variable tumor burden the effects of tumor size on SCC-Ag level were unavoidable. This might interfere with the analysis of SCC-Ag level to represent lymph node status since the previous studies were performed in early stage cervical cancer.
In another study[19] which conducted a meta-analysis of 17 groups consisting of 3985 patients, SCC-Ag levels were reported to be associated with LNM. Of note, 8 groups of them showed the relative risk values of LNM at elevated SCC-Ag levels, that is, between 2.3 and 40. However, there were notable differences between the cutoff values of SCC-Ag in different articles. SCC-Ag > 5 ng/mL is considered a high-risk subgroup for early cervical cancer (IB, IIA) LNM, yet radical surgery is not recommended for this category of patients. SCC-Ag > 2 ng/mL patients are 5 times more likely to receive adjuvant therapy than SCC-Ag normal patients.
Notably, the average age of patients in our study was 52.7 ± 8.5 years (30–73 years). Out of the 162 patients, 36 had pelvic LNM (22.2%), whereas 126 had no pelvic LNM (77.8%). Univariate analysis demonstrated that FIGO stage (P = .015), tumor diameter (P = .044), stromal infiltration (P = .005), SCC-Ag≧2.75 ng/mL (P < .001), LVSI (P < .001), and parametrial involvement (P < .001) were associated with LNM. The factors with statistical significance in univariate analysis were included in multivariate analysis. Using binary logistic regression analysis, we showed that the SCC-Ag level and LVSI were independent factors affecting LNM. Since LVSI requires pathological assessment after surgery, SCC-Ag is more valuable to predict LNM before the operation, because at this time, SCC-Ag is measurable.
FIGO cancer report 2018[20] suggested that maximum tumor diameter > 4 cm is a high-risk factor for LNM. However, in our study, we could not confirm the predictive value of preoperative tumor diameter for LNM. This could be attributed to the fact that we excluded patients who previously had neoadjuvant chemotherapy because of the larger tumor diameter. Consequently, the lower number of patients with stage IB2 and stage IIA2 possibly contributed to statistically insignificant differences in the analysis.
In addition, to explore the diagnostic value of SCC-Ag in LNM and determine the best predictive value, the ROC curve of SCC-Ag level prediction was plotted. The AUC value was 0.703 (P < .001) for all patients and showed a moderate predictive value (Fig. 1A). For us to exclude the effect of tumor diameter on the results, we analyzed patients with stage IB1 + IIA1 and patients with stage IB2 + IIA2, respectively. The AUC of patients in group IB1 + IIA1 was 0.682 (P = .002) and the optimal cutoff of SCC-Ag was also 2.75 ng/mL (Fig. 1B). The AUC was 0.720 (P = .251) in group IB2 and IIA2 (Fig. 1C). No statistical difference was notable in this group, and this may be attributed to the small number of patients in the group.
In conclusion, SCC-Ag can be utilized as a reliable indicator in predicting LNM for early-stage cervical cancer patients before surgery, in particular, for stage IB1 and IIA1 cervical cancer with smaller lesions. However, patients with elevated SCC-Ag levels require a combined computerized tomography, magnetic resonance imaging, or positron emission tomography/computerized tomography examination to fully assess LNM before treatment.[21,22] Thus, this will provide insights into making optimal clinical treatment decisions.
Author contributions
Conceptualization: Chenggong Zhu, Wenqing Zhang, Xiuying Wang, Jiyong Jiang.
Data curation: Chenggong Zhu, Xiuying Wang, Lanzhou Jiao, Liyan Chen.
Formal analysis: Chenggong Zhu, Xiuying Wang, Lanzhou Jiao, Liyan Chen.
Investigation: Xiuying Wang.
Methodology: Wenqing Zhang.
Software: Lanzhou Jiao.
Supervision: Wenqing Zhang, Jiyong Jiang.
Writing – original draft: Chenggong Zhu, Liyan Chen.
Writing – review & editing: Chenggong Zhu, Jiyong Jiang.
Footnotes
Abbreviations: AUC = area under curve, FIGO = International Federation of Gynecology and Obstetrics, LNM = lymph node metastasis, LVSI = lymphovascular space invasion, ROC = receiver operator characteristic, SCC-Ag = squamous cell carcinoma antigen.
How to cite this article: Zhu C, Zhang W, Wang X, Jiao L, Chen L, Jiang J. Predictive value of preoperative serum squamous cell carcinoma antigen level for lymph node metastasis in early-stage cervical squamous cell carcinoma. Medicine. 2021;100:33(e26960).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Charakorn C, Thadanipon K, Chaijindaratana S, Rattanasiri S, Numthavaj P, Thakkinstian A. The association between serum squamous cell carcinoma antigen and recurrence and survival of patients with cervical squamous cell carcinoma: a systematic review and meta-analysis. Gynecol Oncol 2018;150:190–200. [DOI] [PubMed] [Google Scholar]
- [3].Chang C, Chen J, Huang CH, Lee WY, Hsu LC, Chiang AJ. Time-dependent squamous cell carcinoma antigen in prediction of relapse and death of patients with cervical cancer. J Low Genit Tract Dis 2020;24:38–42. [DOI] [PubMed] [Google Scholar]
- [4].Han S, Cheng Z, Zhao X, Huang Y. Diagnostic value of heat shock protein 90 alpha and squamous cell carcinoma antigen in detection of cervical cancer. J Int Med Res 2019;47:5518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang Y, Cui T, Du L, et al. The correlation between the serum squamous carcinoma antigen and the prognosis of recurrent cervical squamous carcinoma. J Clin Lab Anal 2017;31:01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Koh WJ, Abu-rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019;17:64–84. [DOI] [PubMed] [Google Scholar]
- [7].Kato H, Torigoe T. Radioimmunoassay for tumor antigen of human cervical squamous cell carcinoma. Cancer 1977;40:1621–8. [DOI] [PubMed] [Google Scholar]
- [8].Xu F, Li Y, Fan L, et al. Preoperative SCC-Ag and thrombocytosis as predictive markers for pelvic lymphatic metastasis of squamous cervical cancer in early FIGO stage. J Cancer 2018;9:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Takagi K, Kougo H, Aoyagi Y, et al. Remarkably reduced tumor marker SCC levels by combined chemotherapy of paclitaxel and S-1 in two cases of advanced cervical cancer. Gan To Kagaku Ryoho 2008;35:335–7. [PubMed] [Google Scholar]
- [10].Li X, Zhou J, Huang K, et al. The predictive value of serum squamous cell carcinoma antigen in patients with cervical cancer who receive neoadjuvant chemotherapy followed by radical surgery: a single-institute study. PLoS One 2015;10:e0122361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dasari S, Wudayagiri R, Valluru L. Cervical cancer: biomarkers for diagnosis and treatment. Clin Chim Acta 2015;445:07–11. [DOI] [PubMed] [Google Scholar]
- [12].Bolger BS, Dabbas M, Lopes A, Monaghan JM. Prognostic value of preoperative squamous cell carcinoma antigen level in patients surgically treated for cervical carcinoma. Gynecol Oncol 1997;65:309–13. [DOI] [PubMed] [Google Scholar]
- [13].Huang L, Zheng M, Liu JH, et al. Risk factors and prognosis of IB-IIB cervical carcinoma with common iliac lymph node metastasis. Chin J Cancer 2010;29:431–5. [DOI] [PubMed] [Google Scholar]
- [14].Aoki Y, Sasaki M, Watanabe M, et al. High-risk group in node-positive patients with stage IB, IIA, and IIB cervical carcinoma after radical hysterectomy and postoperative pelvic irradiation. Gynecol Oncol 2000;77:305–9. [DOI] [PubMed] [Google Scholar]
- [15].Yang WT, Lam WW, Yu MY, Cheung TH, Metreweli C. Comparison of dynamic helical CT and dynamic MR imaging in the evaluation of pelvic lymph nodes in cervical carcinoma. AJR Am J Roentgenol 2000;175:759–66. [DOI] [PubMed] [Google Scholar]
- [16].Lin H, Changchien CC, Huang EY, Tseng CW, Eng HL, Huang CC. The role of pretreatment squamous cell carcinoma antigen in predicting nodal metastasis in early stage cervical cancer. Acta Obstet Gynecol Scand 2000;79:140–4. [DOI] [PubMed] [Google Scholar]
- [17].Xu D, Wang D, Wang S, Tian Y, Long Z, Ren X. Correlation between squamous cell carcinoma antigen level and the clinicopathological features of early-stage cervical squamous cell carcinoma and the predictive value of squamous cell carcinoma antigen combined with computed tomography scan for lymph node metastasis. Int J Gynecol Cancer 2017;27:1935–42. [DOI] [PubMed] [Google Scholar]
- [18].Lekskul N, Charakorn C, Lertkhachonsuk A, Rattanasiri S, Israngura Na, Ayudhya N. The level of squamous cell carcinoma antigen and lymph node metastasis in locally advanced cervical cancer. Asian Pac J Cancer Prev 2015;16:4719–22. [DOI] [PubMed] [Google Scholar]
- [19].Zhou Z, Li W, Zhang F, Hu K. The value of squamous cell carcinoma antigen (SCCa) to determine the lymph nodal metastasis in cervical cancer: a meta-analysis and literature review. PLoS One 2017;12:e0186165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri. Int J Gynaecol Obstet 2018;143: Suppl 2: 22–36. [DOI] [PubMed] [Google Scholar]
- [21].Devine C, Viswanathan C, Faria S, Marcal L, Sagebiel TL. Imaging and staging of cervical cancer. Semin Ultrasound CT MR 2019;40:280–6. [DOI] [PubMed] [Google Scholar]
- [22].Gandy N, Arshad MA, Park WE, Rockall AG, Barwick TD. FDG-PET imaging in cervical cancer. Semin Nucl Med 2019;49:461–70. [DOI] [PubMed] [Google Scholar]