Abstract
Background:
Elevated homocysteine (Hcy) levels showed increasing significance as the predisposing factor for the pathogenesis of atherosclerotic sequelae, including cardiovascular mortality, coronary artery disease, and stroke. There is increasing evidence linking plasma Hcy levels and heart failure (HF). The association between the elevated level of plasma Hcy and HF was examined by meta-analysis and systematic review in this study.
Methods:
The PubMed and ScienceDirect databases until April 2020 were utilized to collect previous literature on plasma Hcy levels and the potential relation to HF. The pooled effects were evaluated depending on standardized mean differences (SMDs) with 95% confidence intervals (CIs), and the calculation was performed using Stata 12 software. Potential sources of heterogeneity were assessed with subgroup analysis and sensitivity analysis.
Results:
A total of 12 research projects including 5506 subjects were selected. For pooled effect, the results confirmed that patients with HF had higher Hcy levels than the control subjects (SMD,1.148 and 95%CI, [0.715, 1.581]). Based on the classification of New York Heart Association (NYHA), the Hcy levels for the group of NYHA I or II (SMD, 1.484 and 95% CI, [0.442, 2.527]) and the group of NYHA III or IV (SMD, 3.361 and 95% CI, [1.902, 4.820]) were significantly increased compared to controls, while the increase was more intensive for the group of NYHA III or IV. Subgroup analyses revealed similar results.
Conclusion:
Our meta-analysis identified that plasma Hcy levels were significantly elevated in HF patients compared to control subjects, which is positively related to the advancement of NYHA class.
Keywords: hcy, heart failure, homocysteine, meta-analysis, review
Highlights
To evaluate high homocysteine levels in heart failure by conducting a meta-analysis.
Elevated homocysteine levels are significantly associated with heart failure.
Homocysteine levels were with a progressive increase for increasing New York Heart Association class.
1. Introduction
As the byproduct in the conversion of methionine to cysteine, homocysteine (Hcy) is a sulfhydryl-containing amino acid. It does not occur in the diet but is the derivative of dietary methionine due to its demethylation, becoming an essential intermediate for normal mammalian metabolism of methionine.[1] Some studies reported that moderately elevated total Hcy levels showed increasing significance for the pathogenesis of atherosclerotic sequelae, including cardiovascular mortality, coronary artery disease, and stroke.[2] The potential mechanisms of adverse effects of Hcy include endothelial dysfunction and death, oxidative stress increasing, inflammation, collagen metabolism altering, and pro-atherothrombotic.[3]
Among the fatal diseases, the global prevalence of heart failure (HF) is placed in the highest rank, and prevention of chronic HF is still an unsolved problem. In recent years, various similar studies have suggested that the elevation of plasma Hcy levels is associated with HF[4–6] and the possible role of hyper-homocysteinemia in the complex damage mechanisms of HF has also the reason for concern. However, the sample sizes of these studies are rather small. Moreover, no systematic review with a quantitative synthesis of the plasma Hcy level of HF patients compared to controls has been reported yet. Therefore, a comprehensive and critical meta-analysis of previous studies was designed and conducted to clarify evidence-based conclusions concerning the significance of Hcy level for HF.
2. Methods
2.1. Search strategy
A systematic search was conducted by 2 investigators (NJ and JH) independently through the PubMed and ScienceDirect databases until April 2020. To locate all relevant publications which documented the association of plasma levels of Hcy in HF, medical subject headings or free text words were checked with the following rule of keywords
“Homocysteine” (or “Hcy”) plus “heart failure.” The searching strategy also covered the references of the selected articles to find out additional works which were neglected in the database search. The searching strategy also required formal publication of the selected works and the availability of full text.
2.2. Inclusion and exclusion criteria
The literature searching and review were independently conducted by 2 investigators (XZ and XC). Firstly, the screening on titles and abstracts could confirm the relevance and decide the selection for some studies, and nonetheless, the inclusion or exclusion of some other studies might not be decided at this step, which would be forwarded to full-text screening. And the decisions for the inclusion of those studies were checked by 2 investigators (LH and JH), respectively. Once discrepancy in opinions occurred between the 2 investigators, the third investigator (NJ) independently checked the involved study to make the final decision.
The content of the selected literature was examined. The criteria for inclusion stated: (1) cross-sectional or case-control or cohort studies in humans; (2) focus on the significance of plasma Hcy levels for HF; (3) written in English and approved by standard peer review; and (4) provides a considerable amount of valid data on Hcy levels for both HF-positive group and HF-negative controls. The exclusion criteria stated: (1) studies with inadequate relevance; (2) review articles or case reports; (3) animal studies; and (4) failure to provide data on Hcy levels for either HF-positive group or HF-negative controls.[7]
2.3. Data extraction and quality assessment
According to the inclusion and exclusion criteria, data extraction from the included studies was conducted independently by 2 investigators (YC and JH). Specifically, the data about the following items were collected: first author, year of publication, nationality, quantity of participants, and plasma Hcy level. In case of discrepant information, 2 investigators discussed each case in detail to decide how to process it. Newcastle–Ottawa Scale (NOS) was used to evaluate the included studies. Two authors (LH and NJ) conducted the quality assessment independently.[8]
2.4. Statistical analysis
Stata version 12 was used as the calculation tool for the analytical process, while statistical significance was set to be P < .05. The standardized mean differences (SMDs) and 95% confidence intervals (CIs) were elicited for comparison. The model of random effects or fixed-effects model was used to evaluate the pooled SMD with or without heterogeneity, respectively. The statistical estimation of heterogeneity was achieved with I2 tests. Studies with I2 value higher than 50% was considered to have high heterogeneity and the model of random effects was applied; otherwise, the fixed-effects model was used.[9]
As the potential source of heterogeneity, the correlations between plasma Hcy levels and other variables involved in the selected studies were assessed with subgroup analysis. Moreover, for the variation caused by any individual study, sensitivity analysis was also conducted. Publication bias was identified with Begg test and the visualization using funnel plots.[8]
2.5. Ethics and dissemination
Since this is a protocol for meta-analysis, all data in this study come from published studies and do not involve patients, so ethical approval is not required. The findings of this research will be disseminated in peer-reviewed journals or conference presentations.
3. Results
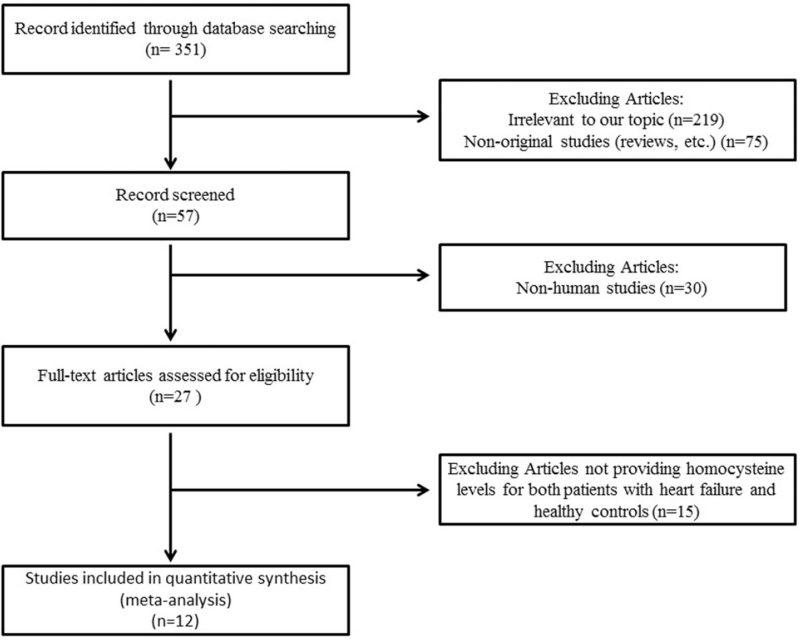
A flow diagram of the data search and study selection is presented in Figure 1. A total of 351 records were identified in the initial search according to the searching strategy as stated in the previous section. Depending on the above-described criteria of evaluation, 12 validated articles were included in the meta-analysis.[2,5,6,10–18] The main characteristics of the included studies were shown in Table 1. No additional studies were identified through our hand search of references from published studies.
Figure 1.
Flow diagram of the literature search.
Table 1.
Characteristics of subjects in included studies.
| Author | Country | HF number | HF homocysteine concentration | Controls number | Controls homocysteine concentration | NOS score |
| Herrmann et al(2005) | Germany | 95 | 17.1 ± 20.0 | 18 | 9.6 ± 2.6 | 6 |
| Gibelin et al (2006) | France | 159 | 15.8 ± 6.9 | 119 | 10.9 ± 3.2 | 8 |
| Raeder et al (2006) | Norway | 29 | 24.1 ± 2.0 | 85 | 17.9 ± 1.0 | 6 |
| May et al (2007) | USA | 621 | 15.6 ± 7.0 | 2500 | 14.6 ± 6.5 | 6 |
| Okuyan et al (2010) | Turkey | 68 | 16.9 ± 5.27 | 40 | 10.15 ± 3.49 | 8 |
| Topcuoglu et al (2010) | Turkey | 31 | 20.2 ± 9.15 | 38 | 13.6 ± 7.56 | 8 |
| Agoston-Coldea et al (2011) | Romania | 65 | 18.9 ± 10.0 | 79 | 14.1 ± 5.2 | 7 |
| Makarewicz-Wujec and Kozlowska-Wojciechowska (2011) | Poland | 55 | 13.6 ± 4.3 | 55 | 10.3 ± 2.6 | 7 |
| Hjelm et al (2014) | Sweden | 138 | 20.13 ± 7.8 | 564 | 17.17 ± 6.91 | 7 |
| Fournier et al (2015) | France | 134 | 18.4 ± 7.83 | 50 | 12.8 ± 3.14 | 7 |
| Strauss et al (2017) | Poland | 55 | 14.9 ± 6.8 | 348 | 12.45 ± 5.16 | 8 |
| El-Amrousy et al (2018) | Egypt | 80 | 11.15 ± 1.96 | 80 | 6.69 ± 0.97 | 8 |
HF = heart failure, NOS = Newcastle–Ottawa Scale.
3.1. Plasma Hcy levels and HF
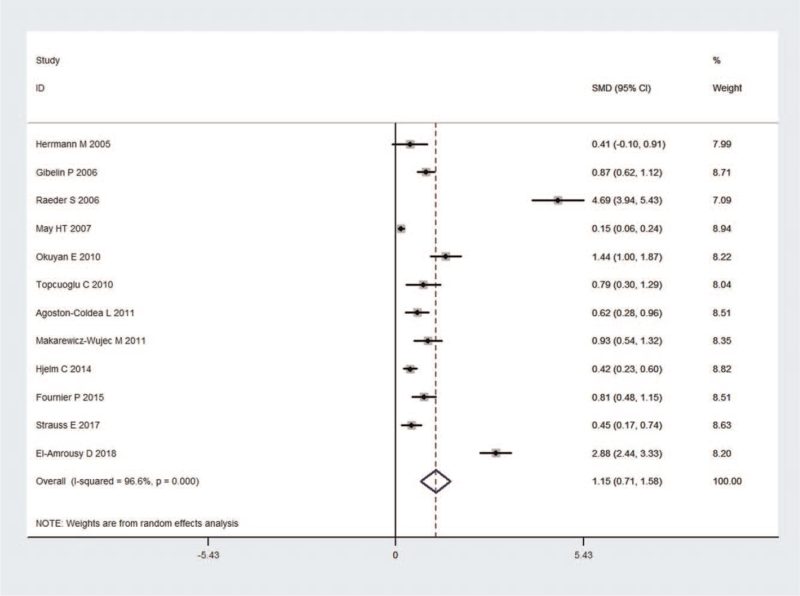
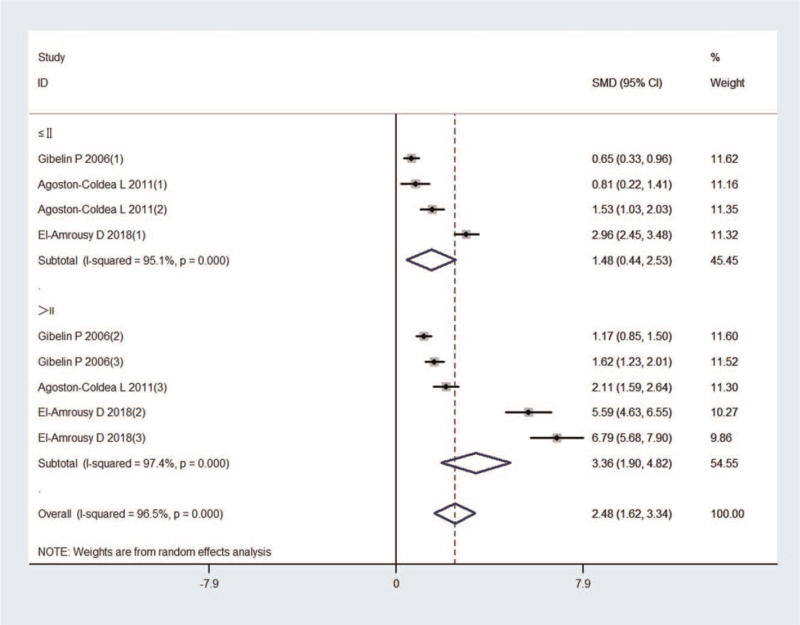
Twelve studies incorporating 5506 subjects were identified in the comparison of Hcy concentration between HF patients and control subjects. First, the heterogeneity was evaluated for the selected studies, and highly significant heterogeneity was identified (I2 = 96.6%; P < .001); thus, a random effect model was selected to estimate the pooled effect size. The results indicated that HF patients showed significantly higher Hcy levels than the control subjects (SMD, 1.148 and 95% CI, [0.715, 1.581]) (Fig. 2). Besides, according to the classification of New York Heart Association (NYHA), the Hcy levels of NYHA I or II groups (SMD, 1.484 and 95% CI, [0.442, 2.527]) in 3 studies were significantly elevated compared to controls. The Hcy levels of NYHA III or IV groups (SMD, 3.361 and 95% CI, [1.902, 4.820]) in 3 studies were also significantly increased compared to controls, and the increases were more intensive than those in the NYHA I or II groups (Fig. 3).
Figure 2.
Forest plot of studies in homocysteine levels for subjects with HF vs control subjects. The combined SMD and 95% CIs were calculated using the random-effects model. CIs = confidence intervals, HF = heart failure, SMDs = standardized mean differences.
Figure 3.
Forest plot of studies in homocysteine levels for subjects with HF vs control subjects by subgroups of New York Heart Association classes. HF = heart failure.
3.2. Subgroup analysis
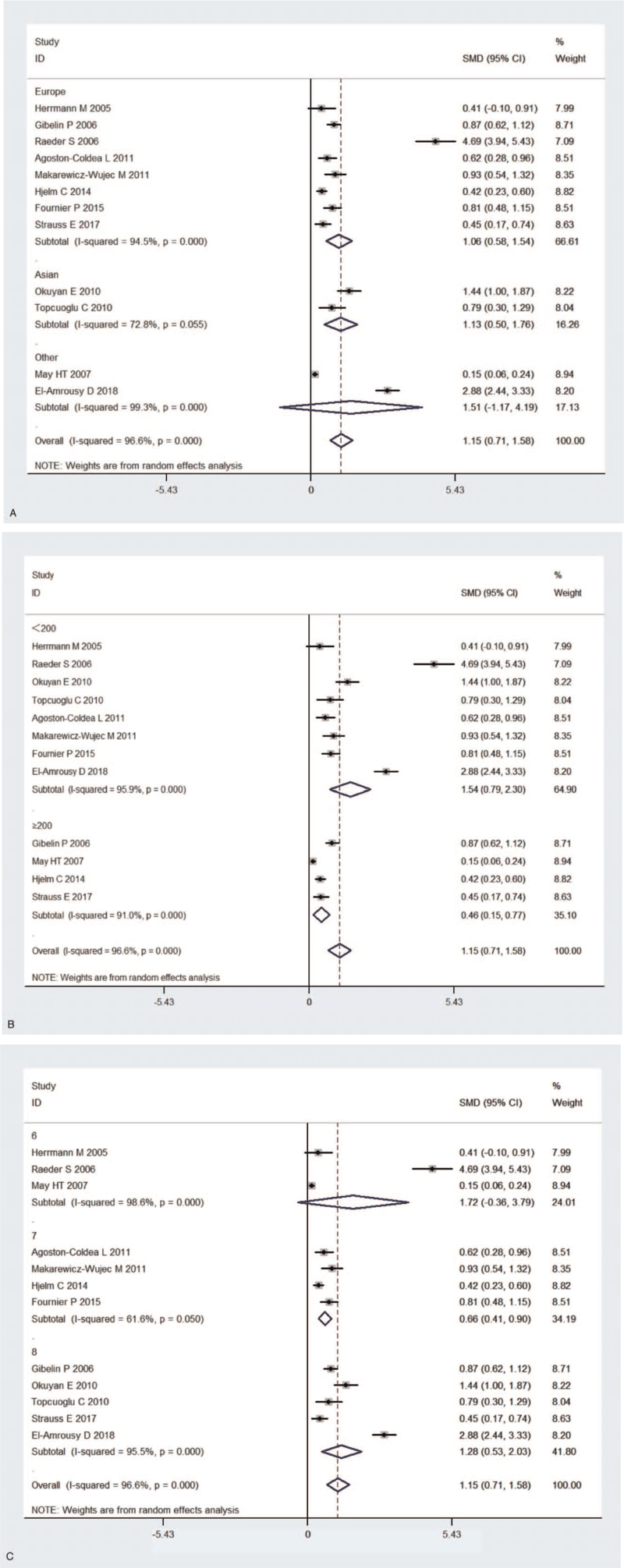
Heterogeneity among the studies was identified by subgroup analysis disaggregated by geographic location, quality assessment, and sample size. Subgroup analysis stratified by geographic location found that HF patients had significantly elevated Hcy levels compared to control subjects in Europe and Asia (Europe: SMD, 1.062 and 95% CI, [0.579, 1.545]; Asia: SMD, 1.127 and 95% CI, [0.496, 1.757]), but not in other location (SMD, 1.509 and 95% CI, [−1.169, 4.187]) (Fig. 4A). In addition, subgroup analysis stratified by sample size proved that HF patients had elevated Hcy levels compared to the control subjects (sample size <200:SMD, 1.541 and 95% CI, [0.786, 2.295]; sample size ≥200:SMD, 0.460 and 95% CI, [0.151, 0.770]) (Fig. 4B). Furthermore, the subgroup analysis stratified by NOS scores also found plasma Hcy levels were significantly increased in NOS 7 and NOS 8(NOS = 7: SMD, 0.659 and 95% CI, [0.415, 0.903]; NOS = 8: SMD, 1.279 and 95% CI, [0.528, 2.030]), but not in NOS 6(NOS = 6: SMD, 1.716 and 95% CI, [−0.357, 3.789]) (Fig. 4C). Table 2 showed the summary for subgroup analyses.
Figure 4.
(A) Subgroup analysis of studies in levels of homocysteine for subjects with HF vs control subjects. Stratified by geographical location (A). Stratified by subgroups of sample size (B). Stratified by NOS scores (C). (B) Subgroup analysis of studies in levels of homocysteine for subjects with HF vs control subjects. Stratified by geographical location (A). Stratified by subgroups of sample size (B). Stratified by NOS scores (C). (C) Subgroup analysis of studies in levels of homocysteine for subjects with HF vs control subjects. Stratified by geographical location (A). Stratified by subgroups of sample size (B). Stratified by NOS scores (C). HF = heart failure, NOS = Newcastle–Ottawa Scale.
Table 2.
Subgroup analyses of homocysteine levels and HF.
| Test of SMD = 0 | Heterogeneity | |||||
| Subgroup | Number of studies | SMD (95% CI) | Z | p for Z | I 2 | P for I2 |
| Geographical location | ||||||
| Europe | 8 | 1.062 (0.579, 1.545) | 4.31 | <.001 | 94.5% | <.001 |
| Asia | 2 | 1.127 (0.496, 1.757) | 3.50 | <.001 | 72.8% | .055 |
| Other | 2 | 1.509 (−1.169, 4.187) | 1.10 | .269 | 99.3% | <.001 |
| Sample size | ||||||
| ≥200 | 4 | 0.460 (0.151, 0.770) | 2.92 | .004 | 91.0% | <.001 |
| <200 | 8 | 1.541 (0.786, 2.295) | 4.00 | <.001 | 95.9% | <.001 |
| NOS | ||||||
| 6 | 3 | 1.716 (−0.357, 3.789) | 1.62 | .105 | 98.6% | <.001 |
| 7 | 4 | 0.659 (0.415, 0.903) | 5.28 | <.001 | 61.6% | .050 |
| 8 | 5 | 1.279 (0.528, 2.030) | 3.34 | .001 | 97.0% | <.001 |
CIs = confidence intervals, HF = heart failure, SMDs = standardized mean differences.
3.3. Sensitivity analysis and publication bias
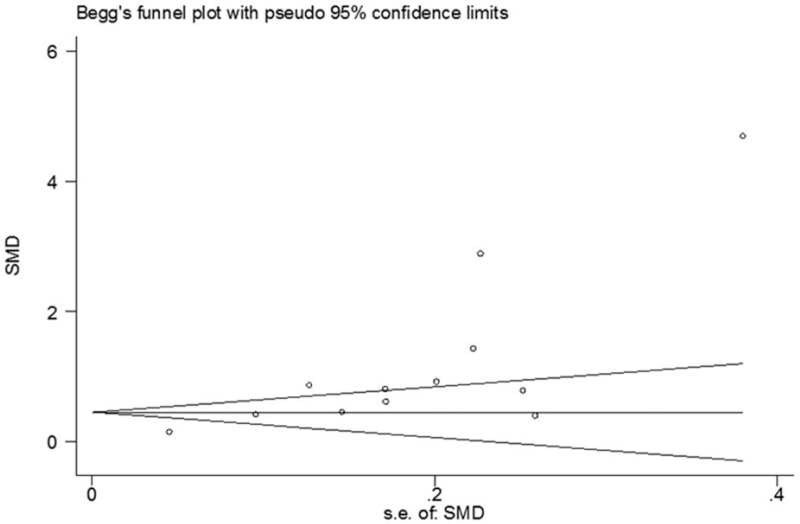
The sensitivity analysis showed that none of the studies could exert an exceptional influence on the pooled effect (Table 3). In addition, the funnel plot had obvious asymmetry, and consistently Begg test indicated there was potential publication bias (Fig. 5).
Table 3.
The heterogeneity of the included studies through sensitivity analysis.
| Excluded studies | SMD (95% CI) | I 2 | P value |
| Herrmann et al (2005) | 1.214 (0.755, 1.674) | 97.0 | <.001 |
| Gibelin et al (2006) | 1.180 (0.702, 1.659) | 96.8% | <.001 |
| Raeder et al (2006) | 0.872 (0.512,1.231) | 95.1% | <.001 |
| May et al (2007) | 1.253 (0.763,1.743) | 95.5% | <.001 |
| Okuyan et al (2010) | 1.121 (0.672, 1.571) | 96.8% | <.001 |
| Topcuoglu et al (2010) | 1.181 (0.722, 1.640) | 96.9% | <.001 |
| Agoston-Coldea et al (2011) | 1.201 (0.731, 1.671) | 96.9% | <.001 |
| Makarewicz-Wujec and Kozlowska-Wojciechowska (2011) | 1.170 (0.709, 1.1.632) | 96.9% | <.001 |
| Hjelm et al (2014) | 1.231 (0.716, 1.746) | 96.9% | <.001 |
| Fournier et al (2015) | 1.183 (0.715, 1.651) | 96.9% | <.001 |
| Strauss et al (2017) | 1.219 (0.741, 1.697) | 97.0% | <.001 |
| El-Amrousy et al (2018) | 0.970 (0.596, 1.344) | 95.3% | <.001 |
CIs = confidence intervals, SMDs = standardized mean differences.
Figure 5.
Funnel plot for studies in homocysteine levels for subjects with HF vs control subjects. HF = heart failure.
4. Discussion
In this meta-analysis including a total of 12 eligible articles, with a combined patient population of 5506 participants, we assessed the significance of plasma Hcy levels for HF. According to the current database, this is the first meta-analysis on this topic. To minimize potential errors, PubMed and ScienceDirect databases were searched thoroughly, as well as all of the items in reference lists of those relevant studies. The finding confirmed plasma Hcy levels were significantly higher in HF patients than the levels in control subjects, with a positive relationship to the advancement of NYHA class, which supports the proposition that Hcy levels differ between HF patients and control subjects. Some studies reported strong relationships between Hcy levels and the indices reflecting various aspects of clinical severity of HF.[10,11,14] According to El-Amrousyet al, plasma Hcy levels, such as plasma highly sensitive cardiac troponin T, had a remarkable prognostic value for congestive HF while the levels were significantly correlated with clinical and echocardiographic data, the severity of HF, and adverse outcomes.[18] As found by previous studies, a gradual increase in Hcy levels across NYHA classes have been observed in our study. We speculate using Hcy to reflect aspects of clinical severity of HF is potential biological parameter. Subgroup analysis showed higher plasma Hcy levels in HF patients than the levels of controls in Asia and Europe; however, plasma Hcy levels for those groups in other locations did not differ significantly. This discrepancy may be attributable to the limited scope of the selected studies.
Research also indicated a positive relationship between moderately increased Hcy levels and the increased risks of atherosclerotic vascular disease, cardiovascular disease, cerebrovascular, and peripheral artery diseases.[19,20] The molecular mechanisms contributing to the significance of plasma Hcy levels for HF remain unclear. Research indicated that Hcy was identified as an independent predisposing condition for atherosclerosis.[21] Atherosclerosis is the most common pathological process underlying the pathogenesis of cardiovascular diseases, including HF.[19] Thus, elevated Hcy levels could cause HF by inducing myocardial ischemia, promoting coronary atherosclerosis, or disturbing the endothelium coronary blood flow.[22,23] Secondly, some studies have shown that elevated Hcy levels play key roles in cardiac remodeling, which is a known precursor of HF, by resulting in significantly increased myocyte size, the proliferation of mast cells, cardiac fibrosis, and activation of matrix metalloproteinases.[24,25] Thirdly, Hcy had also been reported to cause an increase in oxygen stress, a factor known to promote myocardial dysfunction, and a decrease in endothelial function, which enhances HF events.[26–28] At last, research has indicated that the diet of patients with HF seems to differ from the diet of patients without HF. Patients with HF had significantly reduced folate acid and dietary fiber intake compared to the patients without HF, which probably resulted from the low consumption of fruit and vegetables.[29] Hence, it seems understandable that HF is associated with high Hcy concentrations as a result of dietary folic acid deficiencies. Moreover, some studies reported that the potential beneficial effect of folic acid on endothelial functions was independent of the Hcy levels.[30,31] Thus, we speculated that the folic acid deficiency may increase the risk of HF through reduced endothelial function.
According to the current databases, this is the first meta-analysis to evaluate the significance of Hcy levels for HF patients. The sensitivity analysis showed the variation caused by any single study was insignificant. However, our study did have some limitations. First, there was significant heterogeneity because the studies used diverse methodologies; thus, conclusions should be drawn with caution. Secondly, the funnel plot was asymmetrical, indicating potential publication bias due to the studies supporting insignificance might not be published or not being available for the meta-analysis. Also, conference proceedings were not included so we could have missed some minor unpublished studies. In addition, throughout the included studies, the measurement for Hcy levels in plasma is not standardized, and various assays were used in previous studies. As a result, a detection bias cannot be completely excluded in our analysis.
5. Conclusion
In conclusion, this meta-analysis and systematic review confirmed that plasma Hcy levels and HF have a close-knit and significant association. Analyses demonstrated that patients with HF had a higher Hcy level than healthy controls, with a positive relationship to the advancement of NYHA class. Meanwhile, further in-depth studies are required to verify the results and assess the underlying mechanism of how Hcy participates in the progress of HF.
Author contributions
Data curation: Jun Hong, Xuechen Zhao.
Methodology: Yujiao Chen, Yuquan Xie.
Software: Jianan Hu.
Writing – original draft: Nake Jin.
Writing – review & editing: Lei Huang, Xin Cong, Jun Pu.
Footnotes
Abbreviations: CIs = confidence intervals, Hcy = homocysteine, HF = heart failure, NOS = Newcastle–Ottawa Scale, NYHA = New York Heart Association, SMDs = standardized mean differences.
How to cite this article: Jin N, Huang L, Hong J, Zhao X, Chen Y, Hu J, Cong X, Xie Y, Pu J. Elevated homocysteine levels in patients with heart failure: a systematic review and meta-analysis. Medicine. 2021;100:33(e26875).
This work was supported by the Ningbo Medical Science and Technology Project No. 2019Y26
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Finkelstein JD, Martin JJ. Homocysteine. Int J Biochem Cell Biol 2000;32:385–9. doi:. [DOI] [PubMed] [Google Scholar]
- [2].May HT, Alharethi R, Anderson JL, et al. Homocysteine levels are associated with increased risk of congestive heart failure in patients with and without coronary artery disease. Cardiology 2007;107:178–84. doi: 10.1159/000095344. [DOI] [PubMed] [Google Scholar]
- [3].Vizzardi E, Bonadei I, Zanini G, et al. Homocysteine and heart failure: an overview. Recent Pat Cardiovasc Drug Discov 2009;4:15–21. doi:. [DOI] [PubMed] [Google Scholar]
- [4].Strauss E, Supinski W, Radziemski A, Oszkinis G, Pawlak AL, Gluszek J. Is hyperhomocysteinemia a causal factor for heart failure? The impact of the functional variants of MTHFR and PON1 on ischemic and non-ischemic etiology. Int J Cardiol 2017;228:37–44. doi: 10.1016/j.ijcard.2016.11.213. [DOI] [PubMed] [Google Scholar]
- [5].Okuyan E, Uslu A, Cakar MA, et al. Homocysteine levels in patients with heart failure with preserved ejection fraction. Cardiology 2010;117:21–7. doi: 10.1159/000320106. [DOI] [PubMed] [Google Scholar]
- [6].Fournier P, Fourcade J, Roncalli J, Salvayre R, Galinier M, Causse E. Homocysteine in chronic heart failure. Clin Lab 2015;61:1137–45. doi:. [DOI] [PubMed] [Google Scholar]
- [7].Yu X, Huang L, Zhao J, et al. The relationship between serum zinc level and heart failure: a meta-analysis. Biomed Res Int 2018;2018:2739014.doi: 10.1155/2018/2739014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liu B, Cai ZQ, Zhou YM. Deficient zinc levels and myocardial infarction: association between deficient zinc levels and myocardial infarction: a meta-analysis. Biol Trace Elem Res 2015;165:41–50. doi: 10.1007/s12011-015-0244-4. [DOI] [PubMed] [Google Scholar]
- [9].Chen A, Li G, Liu Y. Association between copper levels and myocardial infarction: a meta-analysis. Inhal Toxicol 2015;27:237–46. doi: 10.3109/08958378.2015.1030480. [DOI] [PubMed] [Google Scholar]
- [10].Herrmann M, Kindermann I, Muller S, et al. Relationship of plasma homocysteine with the severity of chronic heart failure. Clin Chem 2005;51:1512–5. doi: 10.1373/clinchem.2005.049841. [DOI] [PubMed] [Google Scholar]
- [11].Gibelin P, Serre S, Candito M, Houcher B, Berthier F, Baudouy M. Prognostic value of homocysteinemia in patients with congestive heart failure. Clin Chem Lab Med 2006;44:813–6. doi: 10.1515/cclm.2006.138. [DOI] [PubMed] [Google Scholar]
- [12].Raeder S, Landaas S, Laake K, Lyberg T, Engedal K. Homocysteine measurements in geriatric patients. Scand J Clin Lab Invest 2006;66:309–15. doi: 10.1080/00365510600615972. [DOI] [PubMed] [Google Scholar]
- [13].Topcuoglu C, Yilmaz FM, Sahin D, et al. Total-and lipid-associated sialic acid in serum and thrombocytes in patients with chronic heart failure. Clin Biochem 2010;43:447–9. doi: 10.1016/j.clinbiochem.2009.11.019. [DOI] [PubMed] [Google Scholar]
- [14].Agoston-Coldea L, Mocan T, Gatfosse M, Lupu S, Dumitrascu DL. Plasma homocysteine and the severity of heart failure in patients with previous myocardial infarction. Cardiol J 2011;18:55–62. doi:. [PubMed] [Google Scholar]
- [15].Makarewicz-Wujec M, Kozlowska-Wojciechowska M. Nutrient intake and serum level of gamma-glutamyltransferase, MCP-1 and homocysteine in early stages of heart failure. Clin Nutr 2011;30:73–8. doi: 10.1016/j.clnu.2010.07.008. [DOI] [PubMed] [Google Scholar]
- [16].Hjelm C, Brostrom A, Dahl A, Johansson B, Fredrikson M, Stromberg A. Factors associated with increased risk for dementia in individuals age 80 years or older with congestive heart failure. J Cardiovasc Nurs 2014;29:82–90. doi: 10.1097/JCN.0b013e318275543d. [DOI] [PubMed] [Google Scholar]
- [17].Luo JL, Chien KL, Hsu HC, et al. Association between plasma homocysteine concentration and the risk of all-cause death in adults with diastolic dysfunction in a community: a 13-year cohort study. Medicine (Baltimore) 2017;96:e6716.doi: 10.1097/md.0000000000006716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].El-Amrousy D, Hassan S, Hodeib H. Prognostic value of homocysteine and highly sensitive cardiac troponin T in children with acute heart failure. J Saudi Heart Assoc 2018;30:198–204. doi: 10.1016/j.jsha.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J 2015;14:06.doi: 10.1186/1475-2891-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shenoy V, Mehendale V, Prabhu K, Shetty R, Rao P. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J Clin Biochem 2014;29:339–44. doi: 10.1007/s12291-013-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pang X, Liu J, Zhao J, et al. Homocysteine induces the expression of C-reactive protein via NMDAr-ROS-MAPK-NF-kappaB signal pathway in rat vascular smooth muscle cells. Atherosclerosis 2014;236:73–81. doi: 10.1016/j.atherosclerosis.2014.06.021. [DOI] [PubMed] [Google Scholar]
- [22].Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med 1998;338:1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- [23].Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG. Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation 1999;100:1161–8. doi: 10.1161/01.cir.100.11.1161. [DOI] [PubMed] [Google Scholar]
- [24].Joseph J, Joseph L, Shekhawat NS, et al. Hyperhomocysteinemia leads to pathological ventricular hypertrophy in normotensive rats. Am J Physiol Heart Circ Physiol 2003;285:H679–86. doi: 10.1152/ajpheart.00145.2003. [DOI] [PubMed] [Google Scholar]
- [25].Miller A, Mujumdar V, Palmer L, Bower JD, Tyagi SC. Reversal of endocardial endothelial dysfunction by folic acid in homocysteinemic hypertensive rats. Am J Hypertens 2002;15:157–63. doi: 10.1016/s0895-7061(01)02286-5. [DOI] [PubMed] [Google Scholar]
- [26].Vasan RS, Beiser A, D’Agostino RB, et al. Plasma homocysteine and risk for congestive heart failure in adults without prior myocardial infarction. JAMA 2003;289:1251–7. doi: 10.1001/jama.289.10.1251. [DOI] [PubMed] [Google Scholar]
- [27].Jiang X, Yang F, Tan H, et al. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol 2005;25:2515–21. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest 1996;98:05–7. doi: 10.1172/JCI118776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Makarewicz-Wujec M, Kozlowska-Wojciechowska M, Sygnowska E, Waskiewicz A. Does heart failure determine the nutrition of patients? Kardiol Pol 2014;72:56–63. doi: 10.5603/KP.a2013.0184. [DOI] [PubMed] [Google Scholar]
- [30].Stanhewicz AE, Kenney WL. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr Rev 2017;75:61–70. doi: 10.1093/nutrit/nuw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Doshi SN, McDowell IF, Moat SJ, et al. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation 2002;105:22–6. doi: 10.1161/hc0102.101388. [DOI] [PubMed] [Google Scholar]