Abstract
Aim
Delirium is a common presenting symptom among older patients. Patients who presented with delirium may have a higher morbidity and mortality rate due to older age, other comorbidities, and atypical COVID-19 presentation. Currently, the evidence supporting delirium as one of the predictors of poor outcome of COVID-19 is still insufficient. This study aims to explore the potential association between delirium and poor outcomes from COVID-19.
Methods
We systematically searched the PubMed and Google Scholar databases using specific keywords related to our aims until January 30th, 2021. All articles published on COVID-19 and delirium were retrieved. The quality of the study was assessed using the Newcastle Ottawa Scale (NOS) tool for observational studies and Joanna Briggs Institute (JBI) Critical Appraisal Tools for case-series studies. Statistical analysis was done using Review Manager 5.4 software.
Results
Our meta-analysis of 20 studies showed that delirium symptoms on admission was associated with poor outcomes from COVID-19 [OR 2.36 (95% CI 1.80–3.09), p < 0.00001, I2 = 76%, random-effect models] and its subgroup which consist of severe COVID-19 [OR 3.89 (95% CI 1.72–8.75), p = 0.001, I2 = 91%, random-effect models], and mortality from COVID-19 [OR 1.90 (95% CI 1.55–2.33), p < 0.00001, I2 = 36%, random-effect models]. Meta-regression showed that the association was influenced by age (p = 0.005).
Conclusions
Our study suggests delirium as an important marker to identify patients at higher risk for developing poor COVID-19 outcomes. The physicians should add delirium as one of the common presenting symptoms of COVID-19 in older populations.
Keywords: Coronavirus disease 2019, COVID-19, Delirium, Confusion, Neurologic symptoms
1. Introduction
Since March 2020, Coronavirus disease (COVID-19) has been declared a pandemic by the World Health Organization (WHO). Initially, the novel virus was called the 2019 novel Coronavirus (2019-nCoV), which officially changed into severe acute respiratory syndrome coronavirus 2 by the WHO. The number of confirmed and death cases of COVID-19 is increasing in several countries and has over-capacitated the hospital capacity. As of January 30, 2021, COVID-19 has caused 2,2182,867 deaths globally (World Health Organization, 2020). Previous meta-analysis studies have demonstrated several comorbidities (Putri et al., 2021; Hariyanto and Kurniawan, 2021; Hariyanto et al., 2021a, 2021b, 2021c) and laboratory markers (Hariyanto et al., 2020a; Ivan Hariyanto and Kurniawan, 2020) which are associated with severe COVID-19 and mortality.
The manifestations of COVID-19 are non-specific and may appear as an asymptomatic disease to fatal pneumonia resulting in death (Kwenandar et al., 2020; Sheleme et al., 2020; Hariyanto et al., 2021d). Studies have reported atypical presentations of COVID-19 which may impede early recognition and management of COVID-19 (D'Adamo et al., 2020). A study from Wuhan, China reported neurological symptoms in 36.4% patients and were more frequent in severe COVID-19 patients showing atypical symptoms (Mao et al., 2020). Delirium, defined as an acute neuropsychiatric syndrome of altered level of consciousness or cognitive disturbances, presents in 11–12% of COVID-19 patients (Ticinesi et al., 2020; Garcez et al., 2020). Some patients may suffer from delirium during the clinical course of COVID-19, but it is also known to be a common presenting symptom in older patients with severe disease, thus complicates the diagnosis and management of COVID-19 (Kennedy et al., 2020; Suffoletto et al., 2013a). Patients who presented with delirium may have a higher morbidity and mortality rate due to older age, other comorbidities (e.g. dementia, epilepsy), atypical COVID-19 presentation, and worse gas exchange at the moment of admission (Ticinesi et al., 2020). Age-related changes in the immune system may reduce the ability to fight against newly encountered antigens in elderly (Aiello et al., 2019). Moreover, they usually suffer from dementia and other medical conditions with chronic inflammatory states that contribute to the increased risk of developing severe COVID-19 (Czick et al., 2020; Bianchetti et al., 2020; Hariyanto et al., 2020b, 2020c). Increased levels of blood urea nitrogen (BUN), C-reactive protein (CRP) and pro-inflammatory cytokines in delirium patients are also associated with worse COVID-19 outcome (Cheng et al., 2020; Leisman et al., 2020). There is currently insufficient evidence supporting delirium as one of the predictors of poor outcome of COVID-19. The ability to predict severe COVID-19 may help clinicians to accelerate decisions and referrals. This study aims to explore the potential association between delirium and poor outcomes from COVID-19 infection.
2. Materials and methods
2.1. Eligibility criteria
This is a systematic review and meta-analysis study. The IRB approval was not applicable for this type of article because this study did not involve human and animal subjects directly. We did not adopt a registered pre‐specified protocol. Included articles in this study are selected as potentially fulfilling the entry criteria: comply the PICO framework (P: COVID-19 patients; I: patients presenting with delirium on hospital admission; C: patients who were admitted into the hospital without delirium symptoms; O: poor outcomes from COVID-19 which consist of severe COVID-19 and mortality), type of study was a randomized control trial, cohort, clinical trial, case-cohort, and cross-over design, and if the full-text article was available. The exclusion criteria are articles other than original research (e.g., review articles, letters, or commentaries); case reports; articles reported other than in English language; articles focusing on the populations of young age (below 18 years old) and women during their pregnancy.
2.2. Search strategy and study selection
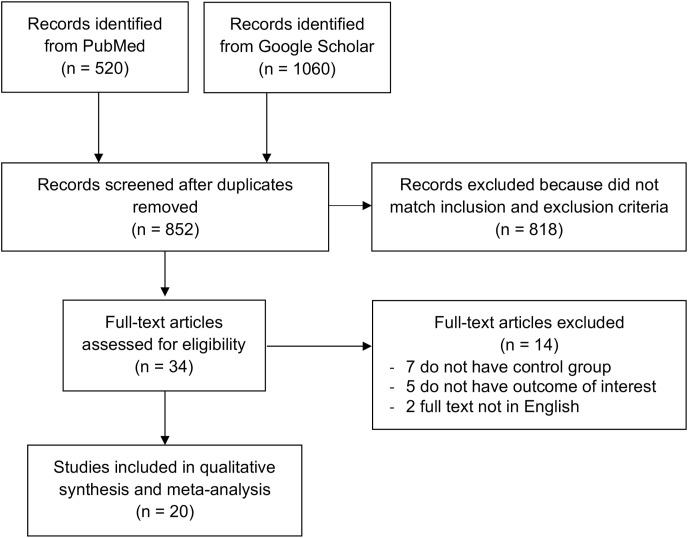
The papers were searched systemically and obtained from PubMed and Google Scholar. Search terms used include “delirium” OR “confusion” OR “acute confusional state” OR “acute mental change” AND “SARS-CoV-2” OR “coronavirus disease 2019″ OR “COVID-19″ in a time range from 2019 until the present time (January 30th, 2021) with English-language restriction. Studies evaluating the delirium symptoms in patients with COVID-19, with a valid each outcome of interest definition, were included in this study. Potential eligible articles searching was done by analyzing the papers cited by authors of all identified studies. The search strategy was presented in the PRISMA diagram (Moher et al., 2009).
2.3. Data extraction and quality assessment
Two authors performed the data extraction process. An extraction form was developed to list the essential information on the authors, year of study, study design, number of participants, age, gender, number of patients with delirium symptoms, number of patients without delirium, and proportion of patients with each outcome of COVID-19.
The outcome of interest was poor outcomes from COVID-19 that consist of severe COVID-19 and mortality. Severe COVID-19 manifestation was the one having either of the mentioned features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (FiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure) or Intensive Care Unit (ICU) admission. Mortality outcome from COVID-19 was defined as the number of patients who were dead because of COVID-19.
Two investigators independently evaluated the quality of the included cohort and case-control studies using the Newcastle–Ottawa Scale (NOS) (Margulis et al., 2014). The selection, comparability, and exposure of each study were broadly assessed and studies were assigned a score from zero to nine. Studies with scores ≥7 were considered of good quality. Meanwhile, the quality of the included case-series studies was assessed by using the Joanna Briggs Institute (JBI) Critical Appraisal Tools For Case-Series (Moola et al., 2017).
2.4. Statistical analysis
Review Manager 5.4 (Cochrane Collaboration) software was used to perform the meta-analysis. Generic Inverse Variance formula with random-effects models was used to calculate each outcome's risk. The effect size was reported as odds ratio (OR) and its 95% confidence interval (CI). The heterogeneity was assessed by using the I2 statistic with a value of <25%, 26–50%, and >50% were considered as low, moderate, and high degrees of heterogeneity, respectively. P-value was two-tailed, and the statistical significance was set at ≤0.05. Subgroup analysis was performed for each component of poor outcomes from COVID-19. Random effects meta-regression was performed using a restricted-maximum likelihood for pre-specified variables including age, gender, dementia, and history of stroke. The qualitative risk of publication bias was assessed with Begg's funnel plot analysis.
3. Results
3.1. Study selection and characteristics
In electronic databases, 1580 studies were found. A total of 852 records remained following the elimination of duplicates. By screening the titles/abstracts and matching the inclusion and exclusion criteria, 818 studies were removed. Among the 34 full-text articles evaluated for their eligibility, 7 articles were excluded due to no control/comparison group in the studies, 5 articles because they do not have the outcome of interest, 2 articles because the articles were not in English. At last, the meta-analysis included 20 studies (Ticinesi et al., 2020; Garcez et al., 2020; Kennedy et al., 2020; Atkins et al., 2020; De Smet et al., 2020; Emmerton and Abdelhafiz, 2020; Hammes et al., 2020; Helms et al., 2020; Karlsson et al., 2020; Khan et al., 2020; Knopp et al., 2020; Marengoni et al., 2020; Mattace-Raso et al., 2020; Poloni et al., 2020; Rawle et al., 2020; Rebora et al., 2020; Romero-Sánchez et al., 2020; Steinmeyer et al., 2020; Vrillon et al., 2020; Zerah et al., 2020) with a total of 6659 COVID-19 patients. (Fig. 1 ). Amongst them, 15 were retrospective cohort studies, 4 studies were prospective cohort design, and the remaining 1 study was a case-series study. Table 1 presents the essential characteristic of the included studies.
Fig. 1.
PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis.
Table 1.
Characteristics of included studies.
| Study | Sample size | Design | Outcome | Age (years) | Male (%) | Patients with dementia (%) | Patients with history of stroke (%) | Patients with delirium n (%) | Delirium diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| Atkins JL et al. (Atkins et al., 2020) 2020 | 507 | Retrospective cohort | Mortality | 74.3 | 61.3 | 2.8 | 4.5 | 5 (1%) | Using the DSM-V criteria |
| De Smet R et al. (De Smet et al., 2020) 2020 | 81 | Retrospective cohort | Mortality | 85.3 (88 vs 84.5) | 41 (42 vs 40) | 44 (52.6 vs 43) | N/A | 34 (42%) | Not described |
| Emmerton D et al. (Emmerton and Abdelhafiz, 2020) 2020 | 71 | Case-series | Severity, Mortality | 73.5 (83 vs 68) | 57.7 (58.3 vs 57.6) | 14 (33 vs 10) | 33.8 (50 vs 31) | 12 (17%) | Using the 4AT tool |
| Garcez FB et al. (Garcez et al., 2020) 2020 | 707 | Retrospective cohort | Severity, Mortality | 66 (70 vs 64) | 57 (61 vs 55) | 4 (9 vs 2) | 7 (12 vs 5) | 234 (33%) | Using the Chart-Based Delirium Identification Instrument (CHART-DEL) |
| Hammes J et al. (Hammes et al., 2020) 2020 | 144 | Retrospective cohort | Mortality | N/A | N/A | N/A | N/A | 106 (73.6%) | Using the Confusion Assessment Method for the ICU (CAM-ICU) |
| Helms J et al. (Helms et al., 2020) 2020 | 140 | Prospective cohort | Mortality | 61.3 (62 vs 65) | 71.4 (75.4 vs 50) | 2.9 (2.7 vs 4.5) | 6.4 (8 vs 0) | 118 (81.9%) | Using the Confusion Assessment Method for the ICU (CAM-ICU) |
| Karlsson LK et al. (Karlsson et al., 2020) 2020 | 102 | Retrospective cohort | Mortality | 84.6 | 47 | N/A | N/A | 30 (29.4%) | Not described |
| Kennedy M et al. (Kennedy et al., 2020) 2020 | 817 | Prospective cohort | Mortality | 77.7 (83.2 vs 74.3) | 47 (45.5 vs 47.8) | 30 (42 vs 25.8) | 13 (20.3 vs 10.3) | 226 (28%) | Using the Confusion Assessment Method (CAM) |
| Khan SH et al. (Khan et al., 2020) 2020 | 268 | Retrospective cohort | Severity, Mortality | 58.4 (58.9 vs 55.7) | 59.7 (54.7 vs 65.8) | 3.5 (3.8 vs 2.6) | N/A | 215 (80.2%) | Using the Confusion Assessment Method for the ICU (CAM-ICU) |
| Knopp P et al. (Knopp et al., 2020) 2020 | 217 | Retrospective cohort | Mortality | 80 | 62 | 33 | N/A | 64 (29%) | Not described |
| Marengoni A et al. (Marengoni et al., 2020) 2020 | 91 | Retrospective cohort | Severity, Mortality | 79.5 (81.7 vs 78.6) | 60.4 (64 vs 59.1) | N/A | N/A | 25 (27.4%) | Screened by using 4AT tool and confirmed by the DSM-V criteria |
| Mattace-Raso F et al. (Mattace-Raso et al., 2020) 2020 | 123 | Retrospective cohort | Severity | 70.7 (71.3 vs 70.4) | 71.5 (78.7 vs 67) | N/A | N/A | 47 (38.2%) | Using the DSM-V criteria |
| Poloni TE et al. (Poloni et al., 2020) 2020 | 57 | Retrospective cohort | Mortality | 82.8 (85.4 vs 81.2) | 33.3 (42.9 vs 27.8) | 100 (100 vs 100) | N/A | 21 (36.8%) | Using the DSM-V criteria |
| Rawle MJ et al. (Rawle et al., 2020) 2020 | 134 | Retrospective cohort | Mortality | 86 | 54.5 | 26.1 | N/A | 55 (41%) | Using the 4AT tool |
| Rebora P et al. (Rebora et al., 2020) 2020 | 516 | Prospective cohort | Mortality | 78.3 (84 vs 77) | 62 (47 vs 64) | 16 (48 vs 11) | N/A | 73 (14.1%) | Screened by using 4AT tool and confirmed by the DSM-V criteria |
| Romero-Sanchez CM et al. (Romero-Sánchez et al., 2020) 2020 | 841 | Retrospective cohort | Severity | 66.4 (71.5 vs 63.1) | 56.2 (56.5 vs 56.1) | 8.4 (12.5 vs 5.9) | 6.3 (8.3 vs 5.1) | 69 (8.2%) | Not described |
| Steinmeyer Z et al. (Steinmeyer et al., 2020) 2020 | 94 | Retrospective cohort | Mortality | 85.5 (88.6 vs 84.8) | 44.7 (35.3 vs 46.8) | 45.7 (52.9 vs 44.2) | N/A | 8 (8.5%) | Using the Confusion Assessment Method (CAM) |
| Ticinesi A et al. (Ticinesi et al., 2020) 2020 | 852 | Retrospective cohort | Mortality | 73 (82 vs 75) | 52.9 (55 vs 53) | 18.3 (40 vs 16) | 6.3 (12 vs 6) | 758 (88.9%) | Using the Confusion Assessment Method (CAM) |
| Vrillon A et al. (Vrillon et al., 2020) 2020 | 76 | Prospective cohort | Mortality | 89.3 (89.5 vs 90) | 44.7 (68.2 vs 35.2) | 63.2 (45.5 vs 70.4) | 28.9 (22.7 vs 31.5) | 54 (71.1%) | Not described |
| Zerah L et al. (Zerah et al., 2020) 2020 | 821 | Retrospective cohort | Mortality | 86 (87 vs 86) | 42 (50 vs 39) | 54 (52 vs 55) | 22 (24 vs 21) | 205 (25%) | Using the Confusion Assessment Method (CAM) |
vs = Group A vs Group B.
3.2. Quality of study assessment
Studies with various study designs including cohort and case-control were included in this review and assessed accordingly with the appropriate scale or tool. The Newcastle Ottawa Scale (NOS) was used to assess the cohort and case-control studies (Table 2 ), while the Joanna Briggs Institute Critical Appraisal checklist was used for case series studies (Table 3 ). All included studies were rated ‘good’ based on the criteria used in the Newcastle Ottawa Scale (NOS) and the Joanna Briggs Institute Critical Appraisal checklist. In conclusion, all studies were deemed fit to be included in the meta-analysis.
Table 2.
Newcastle-Ottawa quality assessment of observational studies.
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Atkins JL et al. (Atkins et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| De Smet R et al. (De Smet et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Garcez FB et al. (Garcez et al., 2020) 2020 | Cohort | *** | ** | **** | 9 | Good |
| Hammes J et al. (Hammes et al., 2020) 2020 | Cohort | *** | ** | ** | 7 | Good |
| Helms J et al. (Helms et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Karlsson LK et al. (Karlsson et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Kennedy M et al. (Kennedy et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Khan SH et al. (Khan et al., 2020) 2020 | Cohort | *** | ** | **** | 9 | Good |
| Knopp P et al. (Knopp et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Marengoni A et al. (Marengoni et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Mattace-Raso F et al. (Mattace-Raso et al., 2020) 2020 | Cohort | ** | ** | *** | 7 | Good |
| Poloni TE et al. (Poloni et al., 2020) 2020 | Cohort | **** | ** | *** | 9 | Good |
| Rawle MJ et al. (Rawle et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Rebora P et al. (Rebora et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Romero-Sanchez CM et al. (Romero-Sánchez et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Steinmeyer Z et al. (Steinmeyer et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Ticinesi A et al. (Ticinesi et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Vrillon A et al. (Vrillon et al., 2020) 2020 | Cohort | *** | ** | *** | 8 | Good |
| Zerah L et al. (Zerah et al., 2020) 2020 | Cohort | *** | ** | **** | 9 | Good |
Table 3.
Joanna Briggs Institute Critical Appraisal tool for case series.
| Emmerton D et al. (Emmerton and Abdelhafiz, 2020) 2020 | |
|---|---|
| 1. Were the criteria for inclusion in the sample clearly defined? | No |
| 2. Were the study subjects and the setting described in detail? | Yes |
| 3. Was the exposure measured in a valid and reliable way? | Yes |
| 4. Were objective, standard criteria used for measurement of the condition? | Yes |
| 5. Were confounding factors identified? | Yes |
| 6. Were strategies to deal with confounding factors stated? | No |
| 7. Were the outcomes measured in a valid and reliable way? | Yes |
| 8. Was appropriate statistical analysis used? | Yes |
| Quality | Include study |
3.3. Delirium symptoms and outcomes
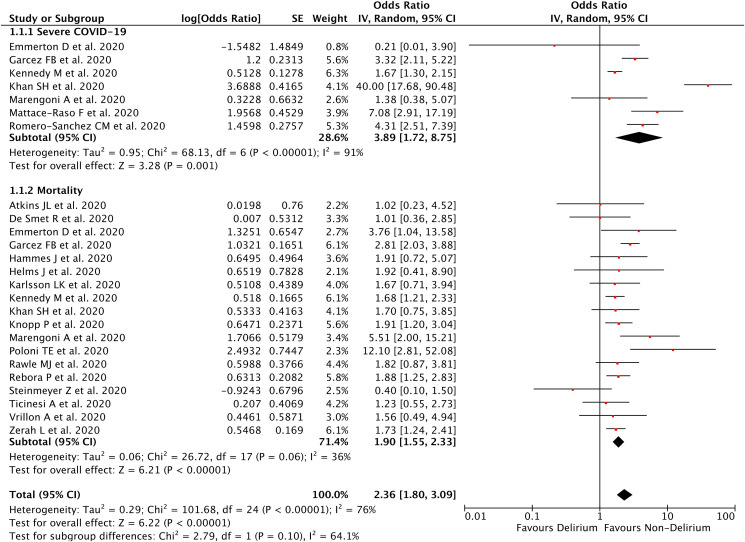
Our pooled analysis showed that delirium symptoms on admission was associated with poor outcomes from COVID-19, with high heterogeneity [OR 2.36 (95% CI 1.80–3.09), p < 0.00001, I 2 = 76%, random-effect modelling] (Fig. 2 ). Subgroup analysis showed that delirium symptoms on admission was associated with severe COVID-19 [OR 3.89 (95% CI 1.72–8.75), p = 0.001, I 2 = 91%, random-effect modelling], and mortality from COVID-19 [OR 1.90 (95% CI 1.55–2.33), p < 0.00001, I 2 = 36%, random-effect modelling].
Fig. 2.
Forest plot that demonstrates the association of delirium symptoms with poor outcomes and its subgroup which comprises of severe COVID-19 and mortality.
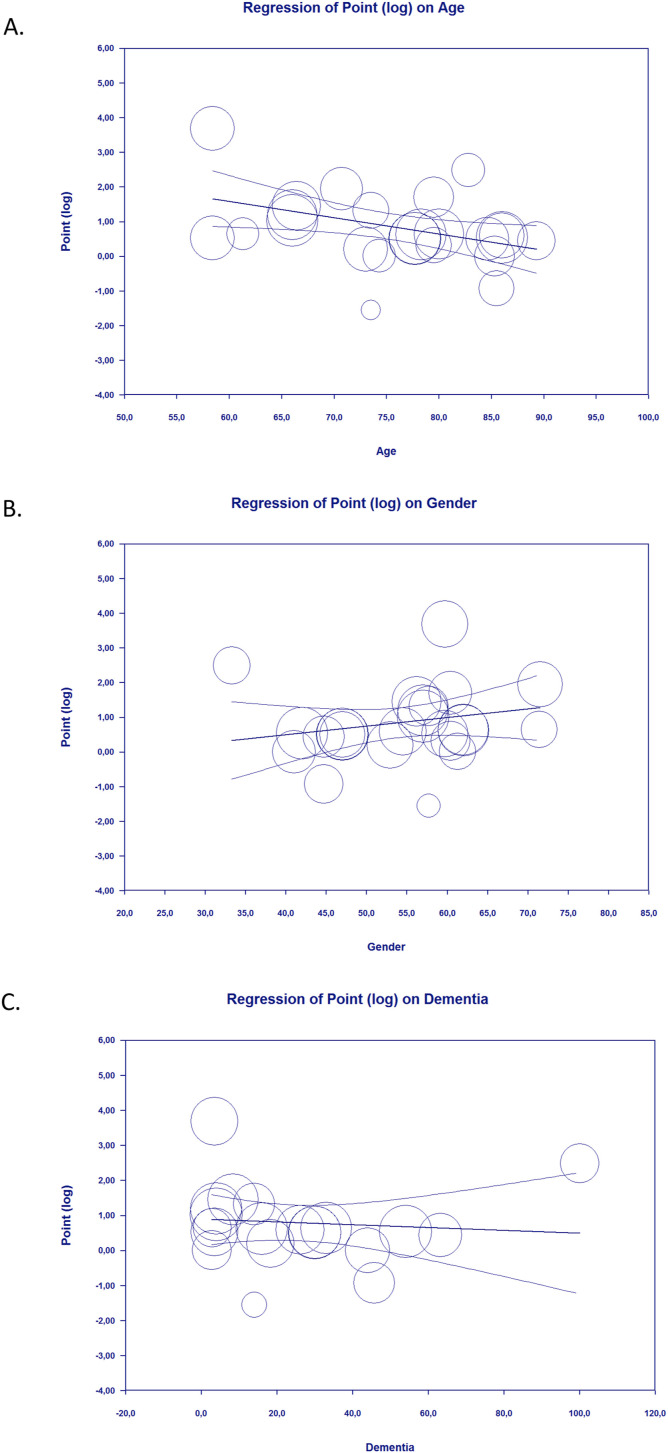
3.4. Meta-regression
Meta-regression showed that the association between delirium symptoms on admission and poor outcome from COVID-19 was affected by age (p = 0.005) (Fig. 3 A), meaning that the magnitude of poor COVID-19 outcomes in patients with delirium was increased according to age. However, the relationship between delirium symptoms on admission and poor COVID-19 outcome was not affected by gender (p = 0.215) (Fig. 3B), dementia (p = 0.656) (Fig. 3C), and history of stroke (p = 0.224).
Fig. 3.
Bubble-plot for Meta-regression. Meta-regression analysis showed that the association between delirium on admission and poor outcome was affected by age [A], but not by gender [B] and dementia [C].
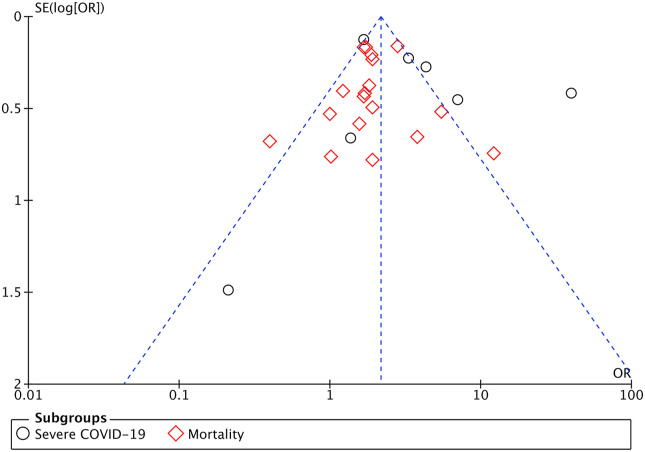
3.5. Publication bias
The funnel-plot analysis showed a qualitatively symmetrical inverted funnel-plot for the association between delirium symptoms and poor outcomes from COVID-19 (Fig. 4 ), showing no indication of publication bias.
Fig. 4.
Funnel plot analysis for the association of delirium symptoms with poor outcomes and its subgroup which comprises of severe COVID-19 and mortality.
4. Discussion
This is the first systematic review and meta-analysis which analyzes the potential ability of delirium symptoms on admission to predict poor outcomes from COVID-19. Based on our pooled analysis of available data, delirium is associated with greater morbidity and mortality among COVID-19 patients and this association was influenced by age. Therefore, delirium at presentation can serve as an important marker to identify COVID-19 patients at high risk of poor outcomes.
Several reasons can be proposed to explain these findings. First, delirium (either hypo- or hyperactive) is a common presenting symptom of COVID-19 infection in older adults (Nanda et al., 2020). The prevalence of delirium among older people admitted into the hospitals ranges from 11 to 25%, higher than the general population which is only 1–2% (Vasilevskis et al., 2012; Wass et al., 2008). Although the prevalence was quite high in older people, delirium often went unidentified during hospital admission or stay in two-third of the cases (Suffoletto et al., 2013b; Bellelli et al., 2015). In COVID-19 elderly patients, delirium may be the only presenting symptom in the absence of other typical COVID-19 symptoms. The prevalence of typical COVID-19 symptoms, such as fever (>38°) and cough were only 39–47% and 32–54%, respectively in elderly patients admitted into the hospital when compared with younger/middle-aged patients (77–80% and 58–75%, respectively) (Mori et al., 2021; Gómez-Belda et al., 2021). The presence of atypical symptoms such as delirium and the lack of typical COVID-19 symptoms may impede the early identification of COVID-19, and will increase the risk of developing poor outcomes, including prolonged ICU stay and death. Second, the old age itself in patients who present with delirium is an independent risk factor for poor COVID-19 outcomes. A previous meta-analysis study has shown an exponential relationship between age and infection fatality rate (IFR) for COVID-19 (Levin et al., 2020). Older people have disrupted the capability to fight against newly encountered antigens. These age-related changes in the immune system, called immunosenescence, lead to a progressive reduction in the ability to trigger antibody and cellular responses against infection. This phenomenon affects both acquired and innate immunity (Aiello et al., 2019). Older people usually also have other comorbidities inducing chronic inflammatory states such as hypertension, obesity, diabetes mellitus that contribute to the development of severe COVID-19 by activating RAS which then activates Angiotensin II type 1 receptor (AT1R) and produces proinflammatory cytokines, vasoconstriction, fibrosis, thrombosis, and Reactive Oxygen Species (ROS). Moreover, patients with old age have a reduced expression of ACE2 and therefore reduced capacity to produce vasodilator, anti-inflammation, anti-fibrosis, anti-thrombosis, and ROS Neutralizer (Czick et al., 2020). All of these conditions will contribute to the development of poor outcomes from COVID-19 in patients who present with delirium symptoms which usually have older age. Third, patients with central nervous system diseases, such as Alzheimer's Dementia (AD), are more prone to develop delirious states, and the diagnosis of delirium superimposed on dementia (DSD) is being studied recently (Maclullich et al., 2008; Morandi and Bellelli, 2020). In a study among dementia patients infected with COVID-19, delirium was the most common presenting symptom at admission (Bianchetti et al., 2020). On the other side, meta-analysis studies have reported that dementia was associated with poor outcomes of COVID-19 (Hariyanto et al., 2020b, 2020c). Fourth, delirium was associated with an increased level of blood urea nitrogen (BUN) (Chu et al., 2011). An increase in BUN levels is an indication of impaired perfusion and dehydration of peripheral organs, including the central nervous system in COVID-19 patients (Li et al., 2020). COVID-19 patients are indeed at higher risks of dehydration and related acute kidney failure due to negative fluid balance caused by fever, tachypnea, and oxygen supply. Elevated BUN was an independent risk factor for an unfavorable prognosis of COVID-19 (Cheng et al., 2020). Not only that, delirium was also associated with higher C-reactive protein (CRP) and pro-inflammatory cytokines levels such as IL-2, IL-6, and TNF-α, and these laboratory values were often used as biomarkers for delirium (Chu et al., 2011; Toft et al., 2019). Elevated levels of CRP and pro-inflammatory cytokines serve as an indication of hyperinflammatory response and cytokine storm which are associated with severe COVID-19 (Hariyanto et al., 2020a; Leisman et al., 2020). Therefore, the presence of delirium can be used as a marker of impaired peripheral perfusion and hyperinflammatory conditions in COVID-19 patients, which are associated with higher morbidity and mortality rates.
The limitation of this study is that the information regarding the other factors which can influence the relationship between delirium and COVID-19 outcomes such as patients’ nutritional status, daily medication, and duration of delirium symptoms are lacking in the included studies, therefore cannot be analyzed. Moreover, some of the included studies did not mention the criteria they used for delirium diagnosis, while the mentioned criteria for delirium diagnosis were varied among the included studies which may increase the heterogeneity in the analysis. However, with this study, we hope that delirium can further be considered as a marker for identifying patients that are at high risk of developing severe COVID-19 and mortality.
Our study suggests delirium as an important marker to identify patients at higher risk for developing severe COVID-19 and related death. Understanding the variations in COVID-19 presentation is essential to prompt early recognition and management of the disease. The physicians should add delirium as one of the common presenting symptoms of COVID-19 in older populations, to better identify COVID-19 cases that are at high risk of poor outcomes and related mortality.
Funding
None.
Author statement
Timotius Ivan Hariyanto: Conceptualization; Data curation; Methodology; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing. Cynthia Putri: Conceptualization; Data curation; Methodology; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing. Joshua Edward: Conceptualization; Data curation; Methodology; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing. Jessie Arisa: Conceptualization; Data curation; Methodology; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing. Rocksy Fransisca V Situmeang: Conceptualization; Validation; Resources; Writing – original draft; Writing – review & editing; Supervision. Andree Kurniawan: Conceptualization; Validation; Resources; Writing – original draft; Writing – review & editing; Supervision.
Data availability statement
The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/, reference number 14–16, and 21–37.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgment
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2021.08.031.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aiello A., Farzaneh F., Candore G., Caruso C., Davinelli S., Gambino C.M., et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019 Sep 25;10:2247. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J.L., Masoli J.A.H., Delgado J., Pilling L.C., Kuo C.L., Kuchel G.A., et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020 Oct 15;75(11):2224–2230. doi: 10.1093/gerona/glaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellelli G., Nobili A., Annoni G., Morandi A., Djade C.D., Meagher D.J., et al. Under-detection of delirium and impact of neurocognitive deficits on in-hospital mortality among acute geriatric and medical wards. Eur. J. Intern. Med. 2015 Nov;26(9):696–704. doi: 10.1016/j.ejim.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Bianchetti A., Rozzini R., Guerini F., Boffelli S., Ranieri P., Minelli G., et al. Clinical presentation of COVID19 in dementia patients. J. Nutr. Health Aging. 2020;24(6):560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Hu L., Wang Y., Huang L., Zhao L., Zhang C., et al. Diagnostic performance of initial blood urea nitrogen combined with D-dimer levels for predicting in-hospital mortality in COVID-19 patients. Int. J. Antimicrob. Agents. 2020 Sep;56(3):106110. doi: 10.1016/j.ijantimicag.2020.106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.L., Liang C.K., Lin Y.T., Chow P.C., Pan C.C., Chou M.Y., et al. Biomarkers of delirium: well evidenced or not? J. Clin. Gerontol. Geriatr. 2011;2:100–104. doi: 10.1016/j.jcgg.2011.11.005. [DOI] [Google Scholar]
- Czick M., Shapter C., Shapter R. COVID's razor: RAS imbalance, the common denominator across disparate, unexpected aspects of COVID-19. Diabet. Metab. Syndr. Obes. 2020 Sep 11;13:3169–3192. doi: 10.2147/DMSO.S265518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Adamo H., Yoshikawa T., Ouslander J.G. Coronavirus disease 2019 in geriatrics and long-term care: the ABCDs of COVID-19. J. Am. Geriatr. Soc. 2020 May;68(5):912–917. doi: 10.1111/jgs.16445. [DOI] [PubMed] [Google Scholar]
- De Smet R., Mellaerts B., Vandewinckele H., Lybeert P., Frans E., Ombelet S., et al. Frailty and mortality in hospitalized older adults with COVID-19: retrospective observational study. J. Am. Med. Dir. Assoc. 2020 Jul;21(7):928–932. doi: 10.1016/j.jamda.2020.06.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerton D., Abdelhafiz A. Delirium in older people with COVID-19: clinical scenario and literature review. SN Compr. Clin. Med. 2020 Aug 29:1–8. doi: 10.1007/s42399-020-00474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez F.B., Aliberti M.J.R., Poco P.C.E., Hiratsuka M., Takahashi S.F., Coelho V.A., et al. Delirium and adverse outcomes in hospitalized patients with COVID-19. J. Am. Geriatr. Soc. 2020 Nov;68(11):2440–2446. doi: 10.1111/jgs.16803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Belda A.B., Fernández-Garcés M., Mateo-Sanchis E., Madrazo M., Carmona M., Piles-Roger L., et al. COVID-19 in older adults: what are the differences with younger patients? Geriatr. Gerontol. Int. 2021 Jan;21(1):60–65. doi: 10.1111/ggi.14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes J., Khan S., Lindroth H., Khan B. Delirium incidence, duration and severity in critically ill patients with COVID-19. Proc. IMPRS. 2020 Dec;3(1) doi: 10.18060/24618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Kurniawan A. Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Sleep Med. 2021 Jun;82:47–53. doi: 10.1016/j.sleep.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Japar K.V., Kwenandar F., Damay V., Siregar J.I., Lugito N.P.H., et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID-19 infection: a systematic review and meta-analysis. Am. J. Emerg. Med. 2020 Dec 30;41:110–119. doi: 10.1016/j.ajem.2020.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Putri C., Arisa J., Situmeang R.F.V., Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020 Nov 19;93 doi: 10.1016/j.archger.2020.104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Putri C., Situmeang R.F.V., Kurniawan A. Dementia is a predictor for mortality outcome from coronavirus disease 2019 (COVID-19) infection. Eur. Arch. Psychiatr. Clin. Neurosci. 2020 Oct 26:1–3. doi: 10.1007/s00406-020-01205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Rosalind J., Christian K., Kurniawan A. Human immunodeficiency virus and mortality from coronavirus disease 2019: a systematic review and meta-analysis. South. Afr. J. HIV Med. 2021 Apr 15;22(1):1220. doi: 10.4102/sajhivmed.v22i1.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Halim D.A., Jodhinata C., Yanto T.A., Kurniawan A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2021 Jun;48(6):823–830. doi: 10.1111/1440-1681.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariyanto T.I., Halim D.A., Rosalind J., Gunawan C., Kurniawan A. Ivermectin and outcomes from Covid-19 pneumonia: a systematic review and meta-analysis of randomized clinical trial studies. Rev. Med. Virol. 2021 Jun 6 doi: 10.1002/rmv.2265. [DOI] [Google Scholar]
- Hariyanto T.I., Rizki N.A., Kurniawan A. Anosmia/hyposmia is a good predictor of coronavirus disease 2019 (COVID-19) infection: a meta-analysis. Int. Arch. Otorhinolaryngol. 2021 Jan;25(1):e170–e174. doi: 10.1055/s-0040-1719120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Schenck M., Severac F., Clere-Jehl R., et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit. Care. 2020 Aug 8;24(1):491. doi: 10.1186/s13054-020-03200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan Hariyanto T., Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J. Med. Virol. 2020 Nov 26 doi: 10.1002/jmv.26698. doi.org/10.1002/jmv.26698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson L.K., Jakobsen L.H., Hollensberg L., Ryg J., Midttun M., Frederiksen H., et al. Clinical presentation and mortality in hospitalized patients aged 80+ years with COVID-19-A retrospective cohort study. Arch. Gerontol. Geriatr. 2020 Dec 30;94 doi: 10.1016/j.archger.2020.104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M., Helfand B.K.I., Gou R.Y., Gartaganis S.L., Webb M., Moccia J.M., et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw. Open. 2020 Nov 2;3(11) doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.H., Lindroth H., Perkins A.J., Jamil Y., Wang S., Roberts S., et al. Delirium incidence, duration, and severity in critically ill patients with coronavirus disease 2019. Crit. Care Explor. 2020 Nov 25;2(12) doi: 10.1097/CCE.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp P., Miles A., Webb T.E., Mcloughlin B.C., Mannan I., Raja N., et al. Presenting features of COVID-19 in older people: relationships with frailty, inflammation and mortality. Eur. Geriatr. Med. 2020 Dec;11(6):1089–1094. doi: 10.1007/s41999-020-00373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwenandar F., Japar K.V., Damay V., Hariyanto T.I., Tanaka M., Lugito N.P.H., et al. Coronavirus disease 2019 and cardiovascular system: a narrative review. Int. J. Cardiol. Heart Vasc. 2020 Jun 3;29 doi: 10.1016/j.ijcha.2020.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leisman D.E., Ronner L., Pinotti R., Taylor M.D., Sinha P., Calfee C.S., et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020 Dec;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin A.T., Hanage W.P., Owusu-Boaitey N., Cochran K.B., Walsh S.P., Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 2020 Dec;35(12):1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020 May 9;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. 10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclullich A.M., Ferguson K.J., Miller T., de Rooij S.E., Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J. Psychosom. Res. 2008 Sep;65(3):229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020 Jun 1;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marengoni A., Zucchelli A., Grande G., Fratiglioni L., Rizzuto D. The impact of delirium on outcomes for older adults hospitalised with COVID-19. Age Ageing. 2020 Oct 23;49(6):923–926. doi: 10.1093/ageing/afaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis A.V., Pladevall M., Riera-Guardia N., Varas-Lorenzo C., Hazell L., Berkman N.D., et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin. Epidemiol. 2014;6:359–368. doi: 10.2147/CLEP.S66677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattace-Raso F., Polinder-Bos H., Oosterwijk B., van Bruchem-Visser R., Goudzwaard J., Oudshoorn C., et al. Delirium: a frequent manifestation in COVID-19 older patients. Clin. Interv. Aging. 2020 Dec 1;15:2245–2247. doi: 10.2147/CIA.S280189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. In: Joanna Briggs Institute Reviewer's Manual. Aromataris E., Munn Z., editors. The Joanna Briggs Institute; 2017. Chapter 7: systematic reviews of etiology and risk.https://reviewersmanual.joannabriggs.org/ Available from. [Google Scholar]
- Morandi A., Bellelli G. Delirium superimposed on dementia. Eur. Geriatr. Med. 2020 Feb;11(1):53–62. doi: 10.1007/s41999-019-00261-6. [DOI] [PubMed] [Google Scholar]
- Mori H., Obinata H., Murakami W., Tatsuya K., Sasaki H., Miyake Y., et al. Comparison of COVID-19 disease between young and elderly patients: hidden viral shedding of COVID-19. J. Infect. Chemother. 2021 Jan;27(1):70–75. doi: 10.1016/j.jiac.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Vura N.V.R.K., Gravenstein S. COVID-19 in older adults. Aging Clin. Exp. Res. 2020 Jul;32(7):1199–1202. doi: 10.1007/s40520-020-01581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloni T.E., Carlos A.F., Cairati M., Cutaia C., Medici V., Marelli E., et al. Prevalence and prognostic value of Delirium as the initial presentation of COVID-19 in the elderly with dementia: an Italian retrospective study. EClinicalMedicine. 2020 Sep;26:100490. doi: 10.1016/j.eclinm.2020.100490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putri C., Hariyanto T.I., Hananto J.E., Christian K., Situmeang R.F.V., Kurniawan A. Parkinson's disease may worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia in hospitalized patients: a systematic review, meta-analysis, and meta-regression. Park. Relat. Disord. 2021 Apr 24;(21):S1353–S8020. doi: 10.1016/j.parkreldis.2021.04.019. 00152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawle M.J., Bertfield D.L., Brill S.E. Atypical presentations of COVID-19 in care home residents presenting to secondary care: a UK single centre study. Aging Med. (Milton) 2020 Sep 17;3(4):237–244. doi: 10.1002/agm2.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebora P., Rozzini R., Bianchetti A., Blangiardo P., Marchegiani A., Piazzoli A., et al. Delirium in patients with SARS-CoV-2 infection: a multicenter study. J. Am. Geriatr. Soc. 2020 Nov 27 doi: 10.1111/jgs.16969. doi.org/10.1111/jgs.16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á, Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020 Aug 25;95(8) doi: 10.1212/WNL.0000000000009937. e1060-e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheleme T., Bekele F., Ayela T. Clinical presentation of patients infected with coronavirus disease 19: a systematic review. Infect Dis (Auckl). 2020 Sep 10;13 doi: 10.1177/1178633720952076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmeyer Z., Vienne-Noyes S., Bernard M., Steinmeyer A., Balardy L., Piau A., et al. Acute care of older patients with COVID-19: clinical characteristics and outcomes. Geriatrics. 2020 Sep 27;5(4):65. doi: 10.3390/geriatrics5040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suffoletto B., Miller T., Frisch A., Callaway C. Emergency physician recognition of delirium. Postgrad. Med. J. 2013;89(1057):621–625. doi: 10.1136/postgradmedj-2012-131608. [DOI] [PubMed] [Google Scholar]
- Suffoletto B., Miller T., Frisch A., Callaway C. Emergency physician recognition of delirium. Postgrad. Med. J. 2013;89(1057):621–625. doi: 10.1136/postgradmedj-2012-131608. [DOI] [PubMed] [Google Scholar]
- Ticinesi A., Cerundolo N., Parise A., Nouvenne A., Prati B., Guerra A., et al. Delirium in COVID-19: epidemiology and clinical correlations in a large group of patients admitted to an academic hospital. Aging Clin. Exp. Res. 2020 Oct;32(10):2159–2166. doi: 10.1007/s40520-020-01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft K., Tontsch J., Abdelhamid S., Steiner L., Siegemund M., Hollinger A. Serum biomarkers of delirium in the elderly: a narrative review. Ann. Intensive Care. 2019 Jul 1;9(1):76. doi: 10.1186/s13613-019-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilevskis E.E., Han J.H., Hughes C.G., Ely E.W. Epidemiology and risk factors for delirium across hospital settings. Best Pract. Res. Clin. Anaesthesiol. 2012 Sep;26(3):277–287. doi: 10.1016/j.bpa.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrillon A., Hourregue C., Azuar J., Grosset L., Boutelier A., Tan S., et al. COVID-19 in older adults: a series of 76 patients aged 85 Years and older with COVID-19. J. Am. Geriatr. Soc. 2020 Dec;68(12):2735–2743. doi: 10.1111/jgs.16894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass S., Webster P.J., Nair B.R. Delirium in the elderly: a review. Oman Med. J. 2008 Jul;23(3):150–157. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, Coronavirus disease (COVID-19): Weekly epidemiological update, https://www.who.int/publications/m/item/weekly-epidemiological-update---22-december-2020, Last updated December 22, 2020, Accessed on December 24, 2020.
- Zerah L., Baudouin É., Pépin M., Mary M., Krypciak S., Bianco C., et al. Clinical characteristics and outcomes of 821 older patients with SARS-cov-2 infection admitted to acute care geriatric wards. J. Gerontol. A Biol. Sci. Med. Sci. 2020 Aug 26 doi: 10.1093/gerona/glaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/, reference number 14–16, and 21–37.