Abstract
Determination of the relative distribution of Ca2+ and Mn2+ is an important tool for analyzing mutants showing altered levels of calcium and/or manganese transporters in the chloroplast envelope or thylakoid membrane. The method described in this protocol allows quantitative analyses of the relative distribution of calcium and manganese ions between chloroplast stroma and thylakoids using the isotopes [45Ca] and [54Mn] as radioactive tracers. To avoid contaminations with non chloroplastidic membrane systems, the method is designed for isolating pure and intact chloroplasts of Arabidopsis thaliana. Intact chloroplasts are isolated via Percoll gradient centrifugation. Chloroplasts are then allowed to take up [45Ca] or [54Mn] during a light incubation step. After incubation, chloroplasts are either kept intact or osmotically/mechanically treated to release thylakoids. The amount of incorporated [45Ca] or [54Mn] can be determined by liquid scintillation counting and the relative distribution calculated.
Keywords: Arabidopsis, Chloroplast, Ion transport, Thylakoid membrane, Envelope membrane, Photosynthesis
Background
Calcium and manganese are structural components of photosystem II and form the inorganic Mn4CaO5 cluster, where water oxidation takes place with the outcome of electrons, protons and molecular oxygen. Ca2+ and Mn2+ fluxes across the chloroplast envelope membrane and the thylakoid membrane are fundamental processes enabling the plant cell to meet the high demand of PSII for these cations. In a previous study, a Ca2+/H+ antiport activity was analyzed using isolated thylakoid membranes from pea plants ( Ettinger et al., 1999 ). In the model plant Arabidopsis thaliana hardly any Ca2+/H+ antiport activity is detectable in isolated thylakoid membranes ( Schneider et al., 2016 ), thus a protocol which allows the thylakoid membrane system to reside in its naturally physiological environment, namely the chloroplast is presented. Furthermore, this protocol permits the relative distribution of Ca2+ and Mn2+ in chloroplasts to be determined. The protocol given here has been tested with Arabidopsis as well as with pea plants.
Materials and Reagents
-
Corex® centrifuge tubes (30 ml) (Corning, USA)
Note: This product has been discontinued. Replaceable item: 30 ml glass tubes from Krackeler Scientific, catalog number: 6-45500-30.
Miracloth (pore size: 22-25 µm) (EMD Millipore, catalog number: 475855)
Pipette tips (10 µl, 200 µl, 1 ml) and ‘cut-off’ pipette tips (1 ml) with the top (~2 mm) cut with a scissors
Falcon tubes (50 ml) (Greiner Bio One, catalog number: 227261)
2 ml tubes
Aluminum foil
Scintillation vials (SARSTEDT, catalog number: 58.536) and push caps (SARSTEDT, catalog number: 65.816)
Arabidopsis plants, approximately 4 weeks old (see Notes)
Percoll (GE Healthcare, catalog number: 17-0891-01)
H2Obidest
80% (v/v) acetone (Carl Roth, catalog number: 9372.2)
45Ca stock solution (1 mCi [37 MBq], CaCl2 > 10 Ci/g) (PerkinElmer, catalog number NEZ013001MC)
54Mn stock solution (200 µCi [7.4 MBq], MnCl2 in 0.5 N HCl > 20 Ci/g) (PerkinElmer, catalog number: NEZ040200UC)
Calciumchlorid (CaCl2) (Carl Roth, catalog number: A119.1)
Mangan(II)-sulfat monohydrate (MnSO4.H2O) (Carl Roth, catalog number: 4487.1)
0.1% (w/v) SDS (Carl Roth, catalog number: CN30.4)
Rotiszint® Eco Plus scintillation cocktail (Carl Roth, catalog number: 0016.3)
Sorbitol (Carl Roth, catalog number: 6213.2)
Tricine (Carl Roth, catalog number: 6977.5)
Natriumhydroxid (NaOH) (Carl Roth, catalog number: P031.2)
EDTA (Carl Roth, catalog number: 8040.2)
Magnesiumchlorid (MgCl2) (Carl Roth, catalog number: KK36.1)
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S5761)
BSA (Carl Roth, catalog number: 8076.3)
Manganese(II) chloride dehydrate (MnCl2) (EMD Millipore, catalog number: 105934)
EGTA (Carl Roth, catalog number: 3054.2)
HEPES
45Ca working solution (see Recipes)
54Mn working solution (see Recipes)
5x resuspension buffer, pH 8.4 (see Recipes)
Homogenization buffer, pH 8.4 (see Recipes)
Import buffer, pH 8.0 (see Recipes)
Washing buffer, pH 8.0 (see Recipes)
Lysis buffer, pH 7.6 (see Recipes)
Equipment
Cold room equipped with green light (500-530 nm)
Automatic pipette controller (BrandTech Scientific, model: Accu-Jet® Pro) and a 10 ml glass pipette
Waring blender (Waring Laboratory Science, model: 7011S) with a steel container and four razor blades
-
Beckman-AvantiTM J-25 centrifuge with rotors JA-14 and JS-13.1 (Beckman Coulter, model: AvantiTM J-25)
Note: This product has been discontinued. Replaceable item: Beckman Avanti J-E series centrifuge (Beckman Coulter, model: Avanti J-E).
Centrifuge Type 4K15C for Falcon tubes (Sigma Laborzentrifugen, model: 4K15C) or any other centrifuge, which is adequate for Falcon tubes
UV/Vis spectrophotometer (Biochrom, model: UltrospecTM 2100) with quartz cuvettes
Light source Faser-Illuminator (Heinz Walz, model: FL-460) set at 90 µmol photons m-2 sec-1
Vortex shaker (Scientific Industries, model: Genie2)
Centrifuge 5418 R with rotor FA 45-18-11 for 2 ml tubes (Eppendorf, model: 5418 R)
Liquid scintillation counter (PerkinElmer, model: Tri-Carb® 2910TR)
Isotope lab with permission for 54Mn handling, lead coat and lead shields (approximately 5 cm)
Procedure
-
Preparation of a Percoll gradient
Place all materials, reagents and buffers in the cold room for temperature equilibration at 4 °C.
Prepare 10 ml Percoll solution (40%) by mixing 4 ml Percoll, 2 ml 5x resuspension buffer, pH 8.4 and 4 ml H2Obidest.
Prepare 10 ml Percoll solution (80%) by mixing 8 ml Percoll and 2 ml 5x resuspension buffer, pH 8.4.
Fill into a Corex tube 9 ml of 40% Percoll solution and carefully underlay 9 ml of 80% Percoll solution using a 10 ml glass pipette and an automatic pipette controller, keep the gradient on ice until further use (Figure 1).
-
Isolation of intact chloroplasts
Harvest 12 g fresh leaf material of overnight dark adapted Arabidopsis plants (see Notes). Perform all steps in a cold room equipped with green light (500-530 nm).
Add 180 ml of homogenization buffer, pH 8.4 to the leaf material and homogenize in a Warring blender for 4 x 3 sec at 18,000 rpm (corresponding to button ‘Hi’).
Filter the homogenate through two layers of Miracloth and centrifuge at 1,500 × g for 5 min at 4 °C in a JA-14 rotor.
Remove supernatant and resuspend the pellet in 1 ml of 1x resuspension buffer pH 8.4 by carefully inverting the tube.
Transfer the resuspended pellet (= chloroplast suspension) by using a cut-off pipette tip carefully on top of the Percoll gradient (Figure 1).
The density gradient centrifugation is carried out in a JS-13.1 rotor for 15 min at 4 °C and 7,000 × g, use a slow acceleration and turn deceleration off. After centrifugation broken chloroplasts reside in the top fraction, intact chloroplasts reside in the interface of the two Percoll layers (Figure 1).
Remove broken chloroplasts and the upper solution and carefully transfer intact chloroplasts using a cut-off pipette tip into a Falcon tube.
Wash intact chloroplasts with 25 ml of 1x resuspension buffer pH 8.4. This step removes residual Percoll. Centrifuge at 1,500 × g for 5 min at 4 °C.
Resuspend pellet (= Intact chloroplasts) in 500 µl import buffer, pH 8.0 ( Ettinger et al., 1999 ), transfer to a 2 ml tube, wrap in aluminum foil and keep on ice.
-
Determination of chlorophyll concentration
Dilute 10 µl of intact chloroplast solution with 990 µl 80% acetone and incubate for 10 min on ice and in the dark.
Centrifuge at 16,000 × g (centrifuge 5418 R) for 2 min at room temperature and immediately transfer supernatant to a quartz cuvette for measurement at 750 nm (for baseline correction), 663 nm and 646 nm in a UV/Vis spectrophotometer. Use 80% acetone as a blank sample.
-
Calculate the chlorophyll content with following formulae (Porra, 2002):
Chla = 12.25 x A663 - 2.55 x A646
Chlb = 20.31 x A646 - 4.91 x A663
(Chla + Chlb) x 100 = xxx µg Chl/ml
Adjust chlorophyll concentration to 0.3 mg/ml with import buffer, pH 8.0 and divide sample into 45 µl aliquots (= Intact chloroplasts), wrap in aluminum foil and keep on ice. The whole procedure requires 15 aliquots per isotope experiment.
-
Preparation of 45Ca or 54Mn working solution
Perform all steps in the isotope lab. For handling 54Mn it is necessary to wear a lead coat and to shield radioactive samples with a lead wall (5 cm). Perform 45Ca assays and 54Mn assays separately, e.g., on two following days, but use freshly prepared chloroplasts.
Take 4 µl of 45Ca stock solution and dilute it (1:20) with 76 µl of CaCl2 (15 µM). Mix well, this mixture corresponds to the 45Ca working solution and it is suitable for 15 (+1) reactions.
Take 4 µl of 54Mn stock solution and dilute it (1:20) with 76 µl of MnSO4 (1.5 µM). Mix well, this mixture corresponds to the 54Mn working solution and it is suitable for 15 (+1) reactions.
-
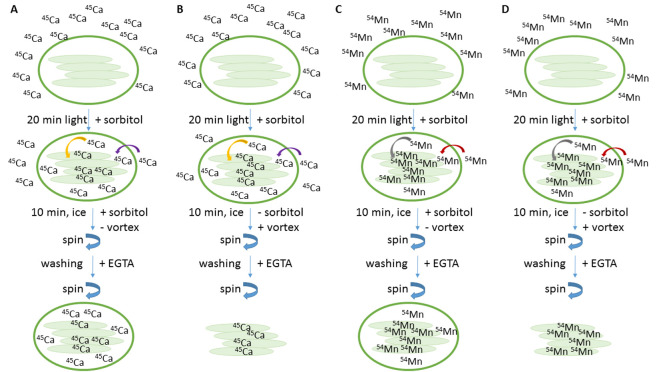
Uptake of 45Ca or 54Mn into chloroplasts (Figure 2)
-
To start the import reaction, mix a 45 µl aliquot (= Intact chloroplasts, step C3) with 5 µl of 45Ca working solution (step D2) or with 54Mn working solution (step D3), remove aluminum foil and immediately expose samples for 20 min to 90 µmol photons m-2 sec-1.
Set up the reaction with three aliquots corresponding to three replicates.
Add 1 ml import buffer, pH 8.0 to each aliquot and incubate on ice, in the dark for 10 min (the total incubation time is 30 min). The import buffer contains sorbitol therefore the chloroplasts are kept intact during this incubation step.
Centrifuge at 16,000 × g (centrifuge 5418 R) for 1 min at 4 °C and remove supernatant. Resuspend pellet in 1 ml washing buffer, pH 8.0 and vortex for 2 sec. This step removes 45Ca or 54Mn bound at the surface of the membrane, because the washing buffer contains EGTA as chelating agent.
Centrifuge at 16,000 × g (centrifuge 5418 R) for 1 min at 4 °C and remove supernatant. Resuspend pellet in 50 µl of 0.1% SDS and immediately transfer to 4 ml scintillation cocktail. Mix well and keep samples at room temperature until counting.
-
-
Uptake of 45Ca or 54Mn into chloroplasts and subsequent release of thylakoids (Figure 2)
-
To start the import reaction, mix a 45 µl aliquot (= Intact chloroplasts, step C3) with 5 µl of 45Ca working solution (step D2) or with a 54Mn working solution (step D3), remove aluminum foil and immediately expose samples for 20 min to 90 µmol photons m-2 sec-1.
Set up the reaction with three aliquots corresponding to three replicates.
Add 1 ml lysis buffer, pH 7.6 to each aliquot and incubate for 10 min on ice, in the dark and vortex every minute for 2 sec (the total incubation time is 30 min). The lysis buffer does not contain sorbitol. Therefore chloroplasts are osmotically shocked and, by vortexing mechanically broken to release thylakoids.
Centrifuge at 16,000 × g (centrifuge 5418 R) for 1 min at 4 °C and remove supernatant. Resuspend pellet in 1 ml washing buffer, pH 8.0 and vortex for 2 sec. This step removes 45Ca or 54Mn bound at the surface of the membrane, because the washing buffer contains EGTA as chelating agent.
Centrifuge at 16,000 × g (centrifuge 5418 R) for 1 min at 4 °C and remove supernatant. Resuspend pellet in 50 µl of 0.1% SDS and immediately transfer to 4 ml scintillation cocktail. Mix well and keep samples at room temperature until counting.
-
-
Additional reaction for estimating uptake of 45Ca or 54Mn during 10 min incubation step on ice
To start the import reaction, mix a 45 µl aliquot (= Intact chloroplasts, step C3) with 5 µl of 45Ca working solution (step D2) or with 54Mn working solution (step D3), remove aluminum foil and immediately perform steps E2 to E4 and F2 to F4. The total incubation time is therefore 10 min on ice in the dark. Set up the reactions with three aliquots each corresponding to three replicates.
-
Additional reaction for estimating background radiation
To estimate background radiation, mix a 45 µl aliquot (= Intact chloroplasts, step C3) with 5 µl of 45Ca working solution (step D2) or with 54Mn working solution (step D3), remove aluminum foil and immediately perform steps E3 to E4. Set up the reaction with three aliquots each corresponding to three replicates.
-
Determination of incorporated radioactivity
Collect scintillation vials form steps E4, F4, G and H and determine counts per minutes (cpm) in a liquid scintillation counter Tri-Carb® 2910 TR. Determine 45Ca values using program #5 and determine 54Mn values using program #8 with 5 min count time per individual scintillation vial.
Figure 1. Preparation of Percoll gradient and isolation of intact chloroplasts (Procedures A and B).
Figure 2. Schema of experimental set-up.
Incubation of intact chloroplasts with 45Ca (A and B) or 54Mn (C and D) is done for 20 min in 90 µmol photons m-2 sec-1. After light incubation samples are incubated in sorbitol, without vortexing to keep chloroplasts intact (A and C) or samples are incubated without sorbitol, but with vortexing to release thylakoids (B and D). EGTA is used as chelating agent to complex divalent cations, attached to the outside of the membranes.
Data analysis
Collect cpm values from step I and insert values into an Excel sheet.
Subtract cpm values for mean background radiation (step H) from all other cpm values (steps E4, F4 and G). Although the value derived after 10 min ice-incubation (step G) is not used for subsequent calculations, it should be clearly lower than the value obtained after 30 min incubation. If this is not the case, results can not be interpreted as ‘light dependent uptake’.
Convert cpm values into relative units, e.g., take the mean cpm value after 30 min incubation time from step E4 and set it as a relative unit to ‘one’. Experiments performed with independent grown plants and isotopes can thus easily be compared.
The thylakoid to stroma distribution in chloroplasts can be estimated from the amount of radioactivity separately detected in thylakoid fractions (step F4) and in intact chloroplast fractions (step E4). Set the cpm values obtained from intact chloroplast fraction (step E4) to 100% and calculate the percentage of cpm values from thylakoid fractions (step F4).
The whole procedure (steps A to I) is described for three technical replicates (see step F1). It should be performed two to three times with independently grown plants (= independent experiments).
Notes
Arabidopsis growth can be performed as described in Steinberger et al., 2015 . This protocol is also suitable for two-week old pea plants. For isolation of intact chloroplasts, it is important to avoid any starch granules, which accumulate during the light period. Starch granules inside chloroplasts might prevent accumulation of intact chloroplasts at the interface of the two Percoll layers. Therefore, dark adapt plants for at least 16 h; during the night starch degradation takes place.
Recipes
-
45Ca working solution (use a fresh preparation)
4 µl of 45Ca stock solution
76 µl of CaCl2 (15 µM)
-
54Mn working solution (use a fresh preparation)
4 µl of 54Mn stock solution
76 µl of MnSO4 (1.5 µM)
-
5x resuspension buffer, pH 8.4 (can be stored at 4 °C for a few days)
0.4 M sorbitol
20 mM Tricine/NaOH, pH 8.4
2.5 mM EDTA
5 mM MgCl2
-
Homogenization buffer, pH 8.4 (can be stored at 4 °C for a few days)
0.4 M sorbitol
20 mM Tricine/NaOH, pH 8.4
10 mM EDTA
5 mM NaHCO3
0.1% (w/v) BSA
1 mM MgCl2
1 mM MnCl2
-
Import buffer, pH 8.0 (can be stored at 4 °C for a few days)
0.33 M sorbitol
50 mM Tricine/NaOH, pH 8.0
5 mM MgCl2
-
Washing buffer, pH 8.0 (can be stored at 4 °C for a few days)
0.33 M sorbitol
50 mM Tricine/NaOH, pH 8.0
50 mM EGTA
-
Lysis buffer, pH 7.6 (can be stored at 4 °C for a few weeks)
10 mM HEPES/NaOH, pH 7.6
5 mM MgCl2
Acknowledgments
We thank Gabi Burkhard for excellent technical assistance. This work was carried out in the laboratory of Prof. Dario Leister (Biozentrum der LMU München, Department Biologie I, Munich, Germany) and supported by funds from the Deutsche Forschungsgesellschaft. This protocol is adapted from Schneider et al., 2016 .
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Ettinger W. F., Clear A. M., Fanning K. J., and Peck M. L.(1999). Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane . Plant Physiol 119: 1379-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porra R. J.(2002). The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b . Photosynth Res 73(1-3): 149-156. [DOI] [PubMed] [Google Scholar]
- 3.Schneider A., Steinberger I., Herdean A., Gandini C., Eisenhut M., Kurz S., Morper A., Hoecker N., Ruhle T., Labs M., Flugge U. I., Geimer S., Schmidt S. B., Husted S., Weber A. P., Spetea C. and Leister D.(2016). The evolutionarily conserved protein photosynthesis affected mutant71 is required for efficient manganese uptake at the thylakoid membrane in Arabidopsis . Plant Cell 28(4): 892-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberger I., Egidi F., and Schneider A.(2015). Chlorophyll fluorescence measurements in Arabidopsis wild-type and photosystem II mutant leaves . Bio-Protocol 5: e1532. [Google Scholar]