To the editor:
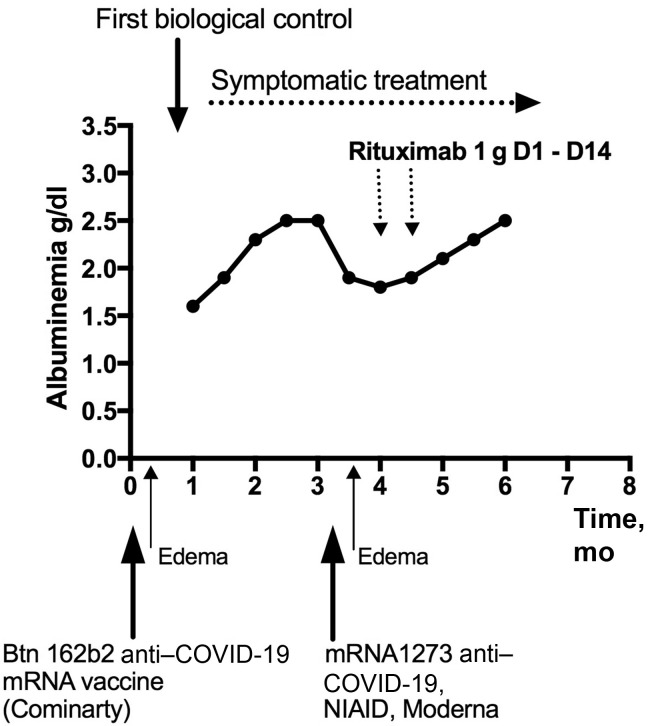
A 76-year-old man with a history of hypertension and UV-treated cutaneous mycosis fungoid was vaccinated in January 2021 for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with Bnt162b2 and developed an antibody response. He had not had prior coronavirus disease 2019 (COVID-19) infection. He developed edema 4 days after vaccination with a random spot urine protein-to-creatinine ratio of 6.5 g/g, hypoalbuminemia (1.6 g/dl), hematuria, and normal serum creatinine (0.86 mg/dl). His anti–phospholipase A2 receptor autoantibody titer was found to be 1:800 (maximal dilution for this assay), supporting a diagnosis of membranous nephropathy (MN).1 As there were no other clinical data to suggest an alternative diagnosis, a kidney biopsy was not performed. He was initially treated symptomatically, with dietary modification and renin-angiotensin system blockade, resulting in partial control of the nephrotic syndrome (body weight stabilized, serum albumin increased to 2.6 g/dl, urine protein-to-creatinine ratio decreased to 3 g/g, creatinine increased to 1.14 mg/dl, and the titer of anti–phospholipase A2 receptor did not change).
He was given the SARS-CoV-2 mRNA-1273 vaccine for his second dose to maintain mRNA vaccination but avoid a second dose of Bnt162b2. Two days later, his edema worsened, serum albumin decreased to 2.2 g/dl, urine protein-to-creatinine ratio increased to 3.8 g/g, and serum creatinine was stable at 1.15 mg/dl. At this time, rituximab treatment was initiated and resulted in a partial remission at 2 months (Figure 1 ).
Figure 1.
Timeline. Treatment and clinical and biological evolution of the nephrotic syndrome from the first anti–coronavirus disease 2019 (COVID-19) vaccine injection. D1, day 1 Rituximab perfusion 1 g; D14, day 14 Rituximab perfusion 1 g; NIAID, National Institute of Allergy and Infectious Diseases.
To our knowledge, this is the first case of MN occurring after anti–COVID-19 mRNA vaccination. A recurrence of previously diagnosed MN has been reported after administration of inactivated SARS-CoV-2 vaccination,2 and a case of minimal change disease has been reported after mRNA-1273 vaccination.3 Exacerbation of nephrotic syndrome following a second injection of an mRNA vaccine seems to suggest a role of these vaccines in triggering MN. Further studies are needed to elucidate the early postvaccination immune response mechanism.
References
- 1.Debiec H., Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 2.Aydin M.F., Yildiz A., Oruc A. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int. 2021;100:464–465. doi: 10.1016/j.kint.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lebedev L., Sapojnikov M., Wechsler A. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]