Abstract
Lignin is the second most abundant biopolymer on Earth, providing plants with mechanical support in secondary cell walls and defense against abiotic and biotic stresses. However, lignin also acts as a barrier to biomass saccharification for biofuel generation (Carroll and Somerville, 2009; Zhao and Dixon, 2011; Wang et al., 2013 ). For these reasons, studying the properties of lignin is of great interest to researchers in agriculture and bioenergy fields. This protocol describes the acetyl bromide method of total lignin extraction and quantification, which is favored among other methods for its high recovery, consistency, and insensitivity to different tissue types ( Johnson et al., 1961 ; Chang et al., 2008 ; Moreira- Vilar et al., 2014 ; Kapp et al., 2015 ). In brief, acetyl bromide digestion causes the formation of acetyl derivatives on free hydroxyl groups and bromide substitution of α-carbon hydroxyl groups on the lignin backbone to cause a complete solubilization of lignin, which can be quantified using known extinction coefficients and absorbance at 280 nm (Moreira- Vilar et al., 2014 ).
Keywords: Lignin, Biochemical measurement, Plant biomass, Acetyl bromide, Plant cell walls
Background
The acetyl bromide method for quantification of lignin from plant biomass has been used to accurately measure total lignin content for decades ( Johnson et al., 1961 ). Recently, this method has gained support as an optimal procedure for lignin quantification, as opposed to the alternative thioglycolic acid and Klason lignin methods (Moreira- Vilar et al., 2014 ). Comparison of these three methods has empirically shown that the acetyl bromide method consistently results in the highest recovery of lignin, and is insensitive to tissue type, extent of lignification, and lignin composition (Moreira- Vilar et al., 2014 ). In our previous work ( Kapp et al., 2015 ), we adapted the scale of the acetyl bromide assay to facilitate a rapid, small-scale determination of lignin that uses a small amount of alcohol insoluble residue (AIR) derived from Brachypodium distachyon, based on a protocol described in the ‘Microscale Method for Cuvettes’ method detailed by Chang et al. (2008) . The protocol described below can be performed with standard laboratory equipment and requires 5-9 days total after harvesting plant material, which can be derived from a variety of tissues or developmental stages.
Materials and Reagents
-
Personal protective equipment (PPE; these should be worn at all times when dealing with concentrated acids and alkali. see Note 1)
Safety glasses
Lab coat
Gloves
Pipette tips
Pipette set
Ice bucket
2 ml Sarstedt tubes (SARSTEDT, catalog number: 72.694.007)
Plant material or cell wall preparation of choice
Liquid nitrogen
Acetone, ≥ 99.5% (EMD Millipore, catalog number: AX0120)
Lugol’s Iodine staining solution (Sigma-Aldrich, catalog number: 32922)
1.5 N sodium hydroxide (NaOH, strong base), ≥ 97% (Fisher Scientific, catalog number: BP359-500)
0.5 M hydroxylamine hydrochloride (strong reducing agent; store in Drierite container, make fresh in water), 98% (Sigma-Aldrich, catalog number: 255580)
Chloroform, ≥ 99.5% (Sigma-Aldrich, catalog number: C2432)
Methanol, ≥ 99.8% (Fisher Scientific, catalog number: A412P)
Deionized water
Ethanol, 100%, 200 proof (Decon Labs, catalog number: V1001)
Acetyl bromide (strong acid, violently reacts with water; dilute in acetic acid), 99% (Sigma-Aldrich, catalog number: 135968)
Glacial acetic acid, ≥ 99.7% (EMD Millipore, catalog number: AX0073)
DMSO (Dimethyl sulfoxide), ≥ 99.9% (Sigma-Aldrich, catalog number: 276855)
Chloroform-methanol mixture (see Recipes)
70% ethanol (see Recipes)
25% acetyl bromide (see Recipes)
90% DMSO (see Recipes)
Equipment
-80 °C freezer
Wiley Mini-Mill (Thomas Scientific, catalog number: 3383L10)
Cryogenic-compatible containers
CryoMill ball mill along with all sizes of steel balls, grinding jars, safety valves, and Autofill (Retsch, catalog numbers: 20.749.0001; 02.480.0002)
Microcentrifuge capable of spinning at 10,000 × g (e.g., Eppendorf, catalog number: 5424)
Chemical hood
Platform rocker (VWR, catalog number: 12620-906)
10 ml glass graduated cylinder (Kimble Chase Life Science and Research Products, catalog number: 20024D-10)
NanoDrop 2000C spectrophotometer, or other UV-Vis (Thermo Fisher Scientific, Thermo ScientificTM, model: ND-2000C-PC)
Quartz cuvette, 0.7 ml volume (Sigma-Aldrich, catalog number: Z600199)
Pipette
Water bath or incubator capable of reaching 70 °C (e.g., VWR, catalog number: 89032-216)
Analytical balance (Mettler Toledo, Excellence Series)
Vortex mixer (VWR, catalog number: 58816-123)
Tissue lyophilizer (4.5 Liter Freeze Dry Freeze Dryer) (Labconco, catalog number: 7750020)
Solid cap with PTFE Liner 15 mm, for 7 ml glass screw-cap vial (Sigma-Aldrich, catalog number: 27152)
Optional: Glass beads (Kimble Chase Life Science and Research Products, catalog number: 13500-4)
Optional: Reacti-Therm Heating and Stirring Module (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: TS-18823)
Procedure
-
Plant material preparation (requires 2-4 d after harvesting of tissue)
After harvesting approximately 5 g (fresh weight) of desired tissue for lignin content determination, flash freeze in liquid nitrogen and store at -80 °C for at least 1 h until use.
Lyophilize tissue until completely dry; duration of lyophilization often depends on tissue density, but commonly takes at least 24 h and up to 72 h for complete drying.
Grind freeze-dried tissue to pass through a 60-mesh screen using a Wiley Mini-Mill. Wiley Mini-Mills are equipped to grind dried samples ranging from long, thin Arabidopsis stems (several mm thick) to thicker woody tissues (~1 cm).
Transfer coarsely-ground samples to cryogenic-compatible containers with steel balls (leave 1/3 of the container empty for air space) and mill using a Retsch CryoMill ball mill with a pre-cooling cryo setting at 5 Hz for ~3 min, followed by grinding at 5-10 min at 30 Hz. If the sample is not a homogenously fine powder after this initial grinding, another round of cryogenic milling should be performed. It is essential to obtain a fine powder with high surface area to facilitate the complete digestion of lignin present in the AIR (Figure 1). This tissue will be used to prepare alcohol insoluble residue (AIR).
-
Alcohol insoluble residue (AIR) preparation (requires 2-3 d)
Weigh roughly 70 mg of finely ground tissue into a 2 ml Sarstedt tube (see Note 2).
Add 1.5 ml of 70% ethanol, vortex thoroughly, centrifuge at ≥ 10,000 × g for 10 min to pellet residue, remove as much supernatant as possible without disturbing the pellet.
Wash with 1.5 ml 1:1 chloroform:methanol, vortex to resuspend pellet, then centrifuge and remove supernatant as described in the previous step.
Add 1.5 ml acetone, vortex, and then centrifuge and remove supernatant as previously described. Allow material suspended in residual acetone to air dry in a chemical hood overnight with the tube cap removed, or dry under a stream of air at 35 °C on a Reacti-Therm apparatus until completely dry (see Note 3). The remaining material is AIR.
To destarch AIR, add 1.5 ml 90% DMSO to the pellet, vortex thoroughly, and allow to shake overnight on a platform rocker at a speed of at least 50 rpm speed at the highest angle to facilitate mixing. The following day, centrifuge and remove supernatant as previously described (see Note 4).
Wash once in 1.5 ml 90% DMSO, vortex, centrifuge, remove supernatant as described above.
Wash six times in 1.5 ml 70% ethanol; vortex, then centrifuge and remove supernatant each time as previously described.
Add 1.5 ml acetone, vortex, and then centrifuge and remove supernatant as previously described. Allow material suspended in residual acetone to air dry in a chemical hood overnight, or dry under a stream of air at 35 °C on a Reacti-Therm apparatus (see Note 3). The remaining material is de-starched AIR.
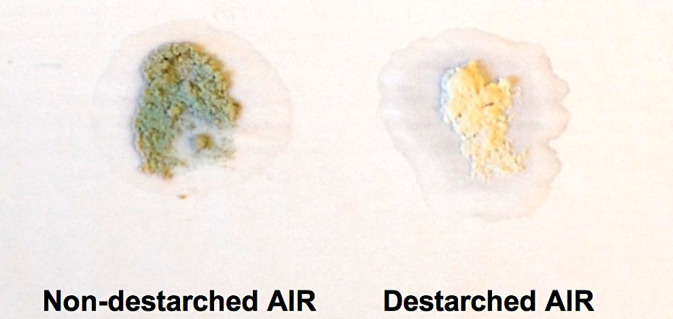
Verify the absence of starch by staining a small portion of de-starched AIR with Lugol’s Iodine solution and washing with water once. For staining, use at least 2 mg AIR for ease of visualizing stained starch. Add 500 µl Lugol’s Iodine solution to AIR and vortex, incubate at room temperature for 5 min, centrifuge and remove supernatant as previously described, wash with 1 ml water, centrifuge and remove supernatant as previously described, and observe color of AIR. If starch is present, the AIR will appear dark in color and the destarching protocol should be repeated (see Note 5; Figure 2).
-
Acetyl bromide soluble lignin determination (requires 1-2 d)
Weigh out approximately 5 mg of destarched AIR and put into a 7 ml glass screw-cap vial; record the exact mass. Small aggregates of particulate AIR below ~0.5 mm in diameter will not affect lignin solubilization as small aggregates should be permeable to the 25% acetyl bromide due to cryo-milling and will likely disintegrate during digestion. For each biological sample, perform technical replicates in triplicate.
In a chemical hood, prepare 25% acetyl bromide by diluting in glacial acetic acid (see Note 6). Be sure to wear appropriate PPE and use a glass graduated cylinder for measuring and only glass containers for all steps involving acetyl bromide.
To each glass tube containing destarched AIR, gently add 1 ml of 25% acetyl bromide, swirl gently to mix AIR into 25% acetyl bromide. Also add 1 ml of 25% acetyl bromide to an empty glass tube, which will serve as a blank.
-
Put all samples at 70 °C for 1 h with gentle swirling every 10 min (see Note 7).
Note: If using a water bath to fit the large 7 ml tubes, make sure to use a glass secondary container, such as a tall glass beaker, to prevent the tubes from tipping over.
Immediately put samples on ice to cool. While samples are on ice, add 5 ml of glacial acetic acid and vortex thoroughly in the chemical hood.
Allow any residual AIR to settle to the bottom of the glass vials for at least 1 h to overnight at room temperature. The reaction is complete and stable after the addition of glacial acetic acid and the absorbance corresponding to ABSL values will not be significantly affected; waiting for any residue to settle is important because suspended particles may interfere with the accuracy of the spectrophotometer (see Note 8).
Once settled, gently transfer 300 µl from the top of each acetyl bromide solution to a quartz cuvette while avoiding resuspending any residual AIR. Add 400 µl 1.5 N NaOH, then 300 µl 0.5 M freshly made hydroxylamine hydrochloride. Pipette solutions gently into the cuvette until fully mixed together after addition of the hydroxylamine hydrochloride (pipette up and down ~15-20 times for thorough mixing, look for complete miscibility of the mixed solutions), and measure absorption at 280 nm against a blank on a spectrophotometer immediately after mixing is complete. The blank consists of the blank acetyl bromide digestion sample mixed with 1.5 N NaOH and hydroxylamine hydrochloride in the same ratio as previously described. Due to the utilization of a single quartz cuvette, each sample must be prepared in the cuvette immediately before reading A280. If the A280 measurement exceeds 1.000, it is recommended to dilute the mixture with acetic acid to ensure accurate readings and to best observe the resulting curve for any aberrant characteristics (see Figure 3 for expected spectrum characteristics). Be sure to account for this dilution factor in addition to the other dilutions from the 1 ml digestion during calculation of percentage of acetyl bromide soluble lignin (see Note 9).
Between samples, wash the inside of the cuvette with glacial acetic acid and wipe the outside of the cuvette clean with 70% ethanol.
Use Beer’s Law to calculate the percentage of acetyl bromide soluble lignin with the proper extinction coefficient (see Table 1; see Note 10).
Figure 1. Representation of insufficiently and sufficiently ground dry plant material.
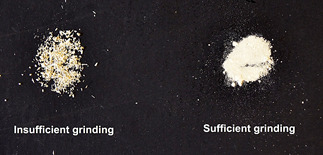
Figure 2. Image of non-destarched and destarched AIR following staining with Lugol’s Iodine solution.
Figure 3. Representative absorbance spectrum of ABSL reading measured against a blank.
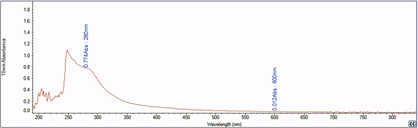
A280 absorbance is due to the presence of ABSL, and A600 is depicted to show that there is minimal residual AIR particulate matter present in the sample during absorbance reading.
Table 1. List of previously determined extinction coefficients for the acetyl bromide soluble lignin method of lignin quantification.
| Specimen | Extinction Coefficient (g-1 L cm-1) | Reference |
|---|---|---|
|
Arabidopsis thaliana, various ecotypes, stem tissues |
23.35 | Chang et al. (2008) |
| Poplar (Populus sp.) | 18.21 | Foster et al. (2010) |
|
Arabidopsis thaliana, alternative |
15.69 | Foster et al. (2010) |
| Grasses | 17.75 | Foster et al. (2010) |
| Brachypodium distachyon | 18.126 | Fukushima and Hatfield (2004) |
| Zea mays | 17.747 | Fukushima and Hatfield (2004) |
|
Medicago sativa, whole plant mean |
14.23 | Fukushima and Hatfield (2004) |
| Trifolium pretense L., Red clover | 14.49 | Fukushima and Hatfield (2004) |
| Bromus inermis Leyss., Bromegrass boot stage; anthesis; postseed stems | 17.11; 16.73; 17.40, respectively | Fukushima and Hatfield (2004) |
|
Avena sativa L., oat straw leaf; stem |
20.10; 18.91, respectively | Fukushima and Hatfield (2004) |
| Triticum aestivum L., wheat straw leaf; stem | 19.81; 17.54, respectively | Fukushima and Hatfield (2004) |
Data analysis
-
The following formula can be used to determine the mass percentage of AIR that is ABSL, and can be converted to µg mg-1 AIR:
Where,
A280 = Absorbance at 280 nm (Blank corrected),
= extinction coefficient (g-1 L cm-1),
L = spectrophotometer path length (cm),
D = dilution factor from digested AIR,
m = mass of de-starched AIR (mg).
The extinction coefficient has been determined for numerous organisms and tissues in several studies to account for a lack of lignin standards within the cell wall field (Table 1). Additional values for softwoods and hardwoods can be found in Johnson et al. (1961) , but are in the range of 23.3-23.6 g-1 L cm-1 (see Note 11).
After calculation of percent ABSL, apply a two-tailed t-test for comparing two samples or One-Way ANOVA for comparing multiple samples.
Notes
Always wear PPE when dealing with concentrated acids and bases such as acetyl bromide, glacial acetic acid, and sodium hydroxide.
A 3 mm glass bead can be added to each tube before preparing AIR to facilitate pellet resuspension and mixing after centrifugation. This will speed up the process considerably, extract lipids more efficiently, and maintain high yields of AIR, especially when dealing with a large number of samples.
All steps of AIR preparation are performed at room temperature, with the option of drying at 35 °C under a stream of air at steps B4 and B8. Use of a Reacti-Therm module is not required, but will dry samples faster and more evenly due to the supply of gentle airflow and heat. Drying in the Reacti-Therm module usually takes < 1 h, and the pellet will appear cracked when it is completely dry. This method, as opposed to allowing samples to dry in the chemical hood overnight, accounts for the differences in estimated time given for each sub-heading of the Procedure section.
Other destarching protocols are available, such as the enzymatic method used by Hatfield et al. (2009) . However, in our experience ( Kapp et al., 2015 ), using 90% DMSO appeared to be the more effective technique.
Lugol’s Iodine staining is recommended to verify the presence or absence of starch due to the facile nature of staining and noticeable darker coloration of AIR containing starch after staining. Lugol’s Iodine staining is commonly used to stain starch granules in intact plant tissues, with sensitivity of detection largely limited to visual restraints ( Ovecka et al., 2012 ). Removal of starch is necessary for accurate determination of lignin content because the ABSL quantification method quantifies lignin content with respect to the mass of cell wall material (AIR). If AIR contains starch, starch will contribute to the overall weight of the AIR in the acetyl bromide digestion and result in a lower and variable weight of cell wall material in each sample, thus diminishing the accuracy of lignin determination.
When diluting acetyl bromide to 25% in acetic acid in step 2 of Procedure C (Acetyl bromide soluble lignin determination), be sure to measure fuming acetyl bromide into glass containers such as a glass graduated cylinder or using a glass Pasteur pipette. Using a micropipette will result in damage to the micropipette and the need for repair.
It has been reported that excessive duration of the acetyl bromide digestion or increased temperature of the reaction causes xylan degradation ( Hatfield et al., 1999 ). Xylans are present in various amounts in all lignified plant tissues; xylan degradation produces furfural derivatives, which absorb in the range of 270-280 nm and can influence absorbance at 280 nm during ABSL measurements. Hatfield et al. (1999) recommend lowering the digestion temperature to 50 °C and extending the incubation time two-fold if xylan degradation is suspected of producing variable results. Occasionally, researchers have included perchloric acid in their acetyl bromide digestion, but Hatfield et al. (1999) recommend excluding perchloric acid as this also increases the propensity for xylan degradation.
We recommend allowing remaining AIR to settle to the bottom of the tube for 12-24 h after addition of 5 ml glacial acetic acid to sample to prevent residual AIR colloids from interfering with A280 measurements. When comparing measurements of A280 after 1 h of settling to after 24 h of settling of the same sample, A280 values at 24 h vary from 0.4-4.4% of the A280 measurement recorded at 1 h, indicating that the A280 readings are consistent following digestion and mixing of glacial acetic acid.
Dilution factor should be calculated from with respect to the volume of the 25% acetyl bromide digestion of 1 ml. For a sample yielding A280 readings in the range of 0-1.000 without the need for dilution, the following dilutions should be accounted for when using the protocol as read above: a 6x dilution upon addition of 5 ml of glacial acetic acid following digestion, and a (10/3)-fold dilution during mixing of 300 µl digested AIR material, with 400 µl 1.5 N NaOH, and 300 µl hydroxylamine hydrochloride, to produce a total dilution factor of 20.
The method described above produced highly consistent results between different biological samples of Arabidopsis basal stem tissue ( Xiao et al., 2017 ) and Brachypodium distachyon ( Kapp et al., 2015 ). The higher variation in the study performed by Kapp et al. (2015) is likely derived from the heterogeneity of tissues samples, as percentage ABSL was determined for the entirety of aerial tissue from 2-12 week-old Brachypodium distachyon plants, with variation in leaf:stem:tiller biomass ratios. Determination of ABSL content in the basal three internodes of Arabidopsis stems yielded a standard deviation ranging from 3.7-8% ABSL of the mean across three biological replicates, with standard deviation between technical replicates within a single biological replicate ranging from 0.07-1.12% ABSL, indicating highly precise results ( Xiao et al., 2017 ).
For determination of ABSL content in Arabidopsis stem tissue using this method in Xiao et al. (2017), we chose to use the extinction coefficient determined by Chang et al. (2008) because this extinction coefficient was determined using a method most similar to the protocol above on Arabidopsis thaliana (Col-0) stem tissues, and was experimentally determined across various Arabidopsis thaliana ecotypes, allowing our values to be readily compared to other studies using this method in the future.
Recipes
-
Chloroform-methanol mixture
Chloroform:methanol (1:1, v/v)
-
70% ethanol
100% ethanol (200 proof):double distilled water (7:3, v/v)
-
25% acetyl bromide
Acetyl bromide:glacial acetic acid (1:3, v/v)
-
90% DMSO
Double distilled Water:DMSO (1:9, v/v)
Note: The final volume of each mixture required by the procedure is dependent on the number of samples.
Acknowledgments
This work was supported as part of The Center for Lignocellulose Structure and Formation, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under Award # DE-SC0001090. Thanks to the authors of Foster et al. (2010) for guidelines and tips on AIR preparation, and the authors of Chang et al. (2008) for developing the ABSL method across different scales and providing excellent insight and expertise on the subject matter. The authors have no conflicts of interest in submitting this work.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Carroll A. and Somerville C.(2009). Cellulosic biofuels. Annu Rev Plant Biol 60: 165-182. [DOI] [PubMed] [Google Scholar]
- 2.Chang X. F., Chandra R., Berleth T. and Beatson R. P.(2008). Rapid, microscale, acetyl bromide-based method for high-throughput determination of lignin content in Arabidopsis thaliana . J Agric Food Chem 56(16): 6825-6834. [DOI] [PubMed] [Google Scholar]
- 3.Foster C. E., Martin T. M. and Pauly M.(2010). Comprehensive compositional analysis of plant cell walls(Lignocellulosic biomass) part I: lignin. J Vis Exp (37). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukushima R. S. and Hatfield R. D.(2004). Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. J Agric Food Chem 52(12): 3713-3720. [DOI] [PubMed] [Google Scholar]
- 5.Hatfield R. D., Grabber J., Ralph J., and Brei K.(1999). Using the acetyl bromide assay to determine lignin concentrations in herbaceous plants: some cautionary notes. J Agric Food Chem, 47, 628-632. [DOI] [PubMed] [Google Scholar]
- 6.Hatfield R. D., Marita J. M., Frost K., Grabber J., Ralph J., Lu F. and Kim H.(2009). Grass lignin acylation: p-coumaroyl transferase activity and cell wall characteristics of C3 and C4 grasses. Planta 229(6): 1253-1267. [DOI] [PubMed] [Google Scholar]
- 7.Johnson D. B., Moore W. E., and Zank L. C.(1961). The spectrophotometric determination of lignin in small wood samples. Tappi, 44, 793-798. [Google Scholar]
- 8.Kapp N., Barnes W. J., Richard T. L. and Anderson C. T.(2015). Imaging with the fluorogenic dye Basic Fuchsin reveals subcellular patterning and ecotype variation of lignification in Brachypodium distachyon . J Exp Bot 66(14): 4295-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira-Vilar F. C., Siqueira-Soares Rde. C., Finger-Teixeira A., de Oliveira D. M., Ferro A. P., da Rocha G. J., Ferrarese Mde. L., dos Santos W. D., and Ferrarese-Filho O.(2014). The acetyl bromide method is faster, simpler and presents best recovery of lignin in different herbaceous tissues than klason and thioglycolic acid methods. PloS One, 9(10), e110000. doi: 10.1371/journal.pone.0110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovecka M., Bahaji A., Munoz F. J., Almagro G., Ezquer I., Baroja-Fernandez E., Li J. and Pozueta-Romero J.(2012). A sensitive method for confocal fluorescence microscopic visualization of starch granules in iodine stained samples. Plant Signal Behav 7(9): 1146-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Chantreau M., Sibout R. and Hawkins S.(2013). Plant cell wall lignification and monolignol metabolism. Front Plant Sci 4: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao C., Barnes W. J., Zamil M. S., Yi H., Puri V. M. and Anderson C. T.(2016). Activation tagging of Arabidopsis POLYGALACTURONASE INVOLVED IN EXPANSION2 promotes hypocotyl elongation, leaf expansion, stem lignification, mechanical stiffening, and lodging . Plant J. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Q. and Dixon R. A.(2011). Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci 16(4): 227-233. [DOI] [PubMed] [Google Scholar]