Abstract
The periosteum covering the outer surface of bone contains skeletal stem/progenitor cells that can efficiently form cartilage and bone during bone repair. Several methods have been described to isolate periosteal cells based on bone scraping and/or enzymatic digestion. Here, we describe an explant culture method to isolate periosteum-derived stem/progenitor cells for subsequent in vitro and in vivo analyses. Periosteal cells (PCs) isolated using this protocol express mesenchymal markers, can be expanded in vitro, and exhibit high regenerative potential after in vivo transplantation at a fracture site, suggesting that this protocol can be employed for PC production to use in new cell-based therapies.
Keywords: Periosteum, Bone regeneration, Skeletal stem/progenitor cell, In vivo cell transplantation , In vitro differentiation
Background
Bone regeneration is a highly efficient process. After bone fracture, skeletal stem/progenitor cells are activated and differentiate into chondrocytes and osteoblasts that form cartilage and bone to consolidate the fracture. Skeletal stem/progenitor cells originate from the bone itself including bone-marrow and periosteum, as well as from adjacent soft tissue. The diverse origins of skeletal stem/progenitor cells during bone regeneration suggest that these cells can be obtained from various sources for stem cell therapies. Due to their accessibility, bone marrow stromal cells (BMSCs) are the most studied (Arthur and Gronthos, 2020); however, their variable osteogenic potential highlights the need for new sources of cells capable of contributing efficiently to the repair process. Recent studies have revealed the role of the periosteum as an essential source of stem/progenitor cells during bone regeneration ( Debnath et al., 2018 ; Duchamp de Lageneste et al., 2018 ; Ortinau et al., 2019 ). When transplanted to a bone fracture site, periosteal cells (PCs) display a higher regenerative potential than BMSCs and have the ability to correct a bone repair failure (Duchamp de Lageneste et al., 2018 ; Julien et al., 2020 ). Isolating mouse PCs is challenging since the periosteum is a very thin layer of tissue on the outer surface of bone. Several methods have been previously described to isolate PCs in mice, relying on enzymatic digestion and periosteum scraping or peeling ( Brownlow et al., 2000 ; Arnsdorf et al., 2009 ; Wang et al., 2010 ; Chang and Knothe Tate, 2012; van Gastel et al., 2012 ). Here, we describe a method to isolate PCs based on cell migration from bone explants without digestion or periosteum separation from the bone (Figure 1). We developed this method to analyze PC properties for direct comparison with BMSCs that are usually isolated by direct bone marrow flushing and plating. Long bones free of skeletal muscle, epiphyses, and bone-marrow are placed in culture to allow PC migration and proliferation. PCs isolated using this protocol express mesenchymal markers (Figure 2), display in vitro adipogenic, osteogenic, and chondrogenic differentiation capacities (Figure 3), and are able to form cartilage and bone upon in vivo transplantation at the site of a tibial fracture (Figure 4). PCs therefore maintain their osteochondrogenic capacities, offering new potential perspectives for the study of PCs and their use in cell-based therapies.
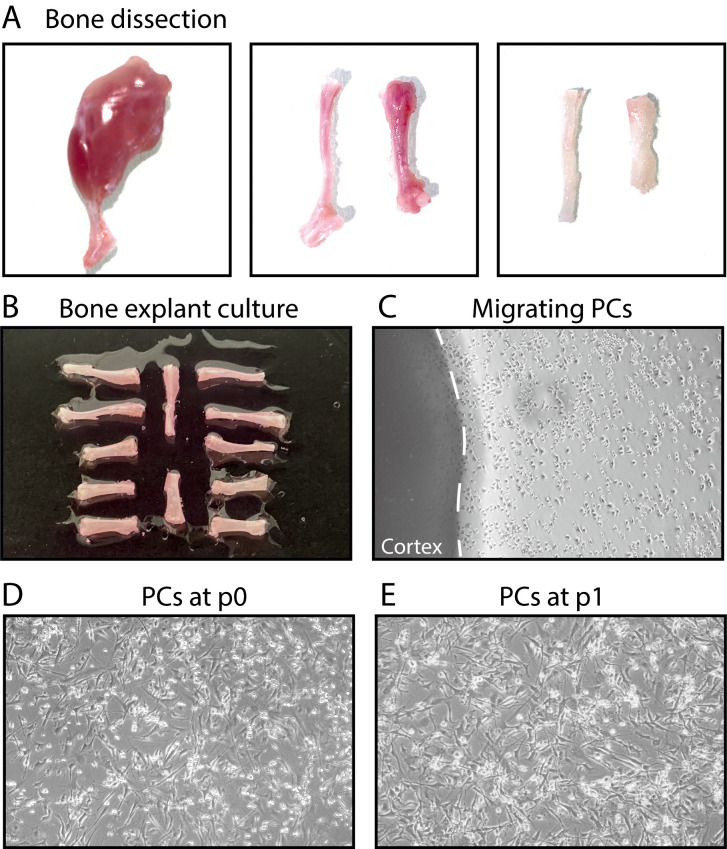
Figure 1. Steps of bone explant culture.
(A) Steps of bone dissection. Left, hindlimb free of skin. Middle, tibia, and femur free of muscle. Right, tibia and femur after cutting the epiphyses and flushing the bone marrow. (B) Tibias and femurs plated in a culture dish. (C) After a few days, periosteal cells (PCs) migrate out of the bone explants into the dish. (D-E) PCs at P0 and P1.
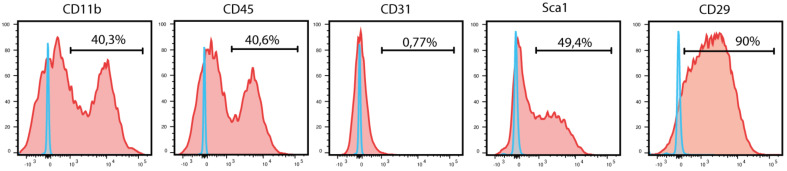
Figure 2. Flow cytometry analysis of periosteum-derived cells at P1.
Blue curves represent FMO controls, and red curves represent experimental samples.
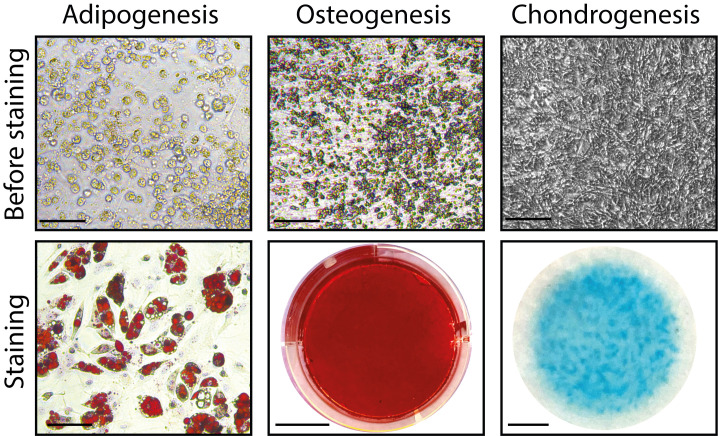
Figure 3. In vitro adipogenic, osteogenic, and chondrogenic differentiation of PCs before and after staining.
Scale bars: before staining: 200 μm; staining: 50 μm (adipogenesis), 1 cm (osteogenesis), 2.5 mm (chondrogenesis).
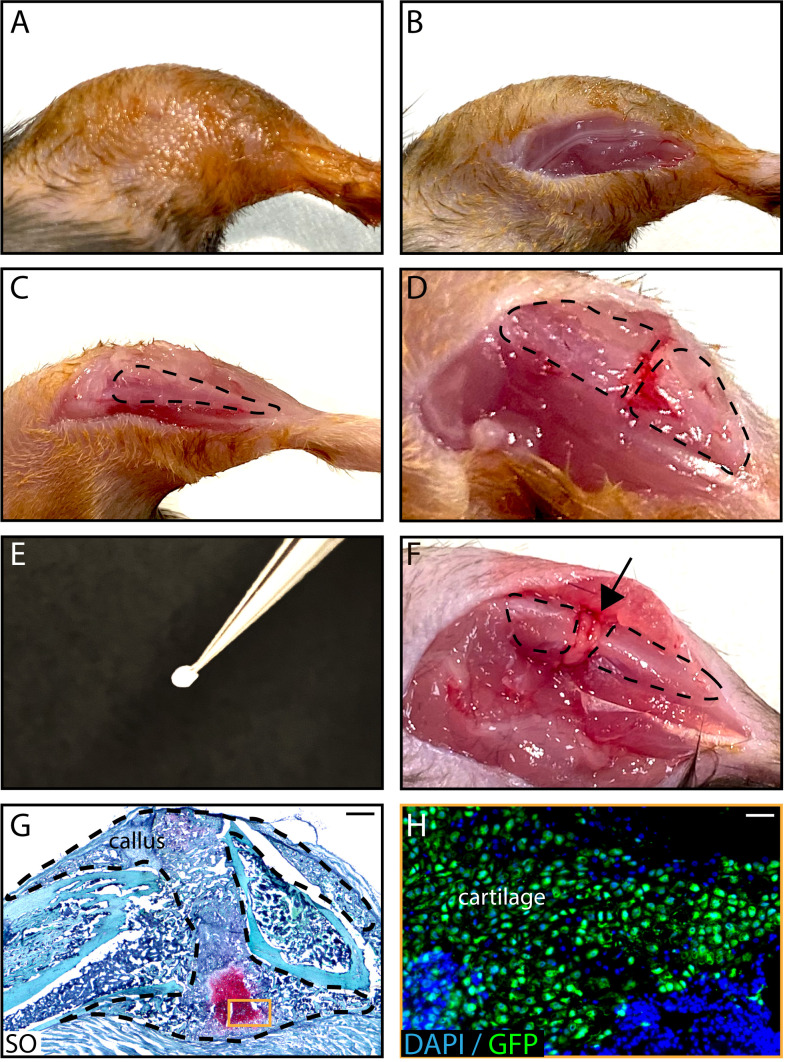
Figure 4. In vivo transplantation of PCs isolated from GFP-actin donors at the fracture site of wild-type hosts .
. After shaving and sanitizing the limb (A), an incision in the skin is performed (B) and the tibia is exposed (C, tibia is delineated by a dotted line) to induce a fracture (D). A Tisseel matrix pellet containing PCs (E) is transplanted at the fracture site (F, black arrow). (G) Representative image of a longitudinal callus section on day 14 post-fracture stained with SO. (H) GFP+ chondrocytes derived from PCs on an adjacent section of the callus. Scale bars: 1 mm (G) and 100 μm (H).
Materials and Reagents
Falcon® 5-ml round-bottomed polystyrene test tubes (Corning, catalog number: 352235)
40-μm cell strainer (Fisher Scientific, catalog number: 352340)
Conical tubes, 15-ml and 50-ml (Falcon, catalog numbers: 352097 [15 ml] and 352070 [50 ml] or equivalent)
25 G needles (Terumo, catalog number: AN*2516R1)
1-ml syringes (Terumo, catalog number: SS+01H1)
60-mm TPP culture dishes (TPP, catalog number: 93060)
100-mm TPP culture dishes (TPP, catalog number: 93100)
6-well plates (TPP, catalog number: 009206)
Greiner Bio-One Petri dishes (bacterial dish; Dutscher, catalog number: 633185)
10-ml and 25-ml pipets (Dutscher, catalog numbers: 357551 [10 ml] and 357535 [25 ml] or equivalent)
Cell scrapers (TPP, catalog number: 99010 or equivalent)
Kova® slides (Fisher Scientific, catalog number: 22-270141)
Falcon® 5-ml round-bottomed polystyrene test tubes (Corning, catalog number: 352235)
Glass slides, Superfrost Plus (Thermo Fisher, catalog number: J1800AMNZ)
Coverslips (Labellians, catalog number: LCO2460M)
For periosteum-derived cell culture: 4 to 8-week-old mice in the C57BL/6J background (see Note 1)
Hosts for in vivo cell transplantation: 10 to 14-week-old mice in the C57BL/6J background (see Note 2)
DMEM (Life Technologies, catalog number: 11966-025)
Penicillin-streptomycin (P/S; Life Technologies, catalog number:15140-122)
α-Modified Eagle’s Medium (α-MEM with GlutaMAX; Life Technologies, catalog number: 32561-029)
Lot-selected fetal bovine serum (FBS; Life Technologies, catalog number:10270-106)
Recombinant mouse basic fibroblast growth factor (bFGF; R&D, catalog number: 3139FB/CF)
Trypan Blue stain (Life Technologies, catalog number: 15250-061)
Trypsin-EDTA (0.25%), phenol red (Life Technologies, catalog number: 25200056)
Dexamethasone (Sigma-Aldrich, catalog number: D8893)
Human insulin solution (Sigma-Aldrich, catalog number: I9278)
Indomethacin (Sigma-Aldrich, catalog number: I7378)
3-Isobutyl-1-methylxantine (IBMX, Sigma-Aldrich, catalog number: I5879)
Oil Red O (Sigma-Aldrich, catalog number: O0625)
Isopropanol (Sigma-Aldrich, catalog number: I9516)
L-ascorbic acid (Sigma-Aldrich, catalog number: A8960)
Beta-glycerophosphate (Sigma-Aldrich, catalog number: G9422)
Alizarin Red S (Sigma-Aldrich, catalog number: A5533)
DMEM high glucose (Life Technologies, catalog number: 31966-021)
Sodium pyruvate (Sigma-Aldrich, catalog number: P5280)
L-proline (Sigma-Aldrich, catalog number: P5607)
Insulin-transferrin-sodium selenite (ITS; Sigma-Aldrich, catalog number: I1884)
TGF-β1 (Sigma-Aldrich, catalog number: T7039)
Alcian Blue (Sigma-Aldrich, catalog number: A5268)
Glutaraldehyde (Merck, catalog number:1-04239-0250)
Hydrochloric acid (Sigma-Aldrich, catalog number:H1758)
Phosphate-buffered saline (PBS; Life Technologies, catalog number: 14190-094)
Ethanol (Ethanol absolute ≥99.8%; VWR, catalog number: 20821.365)
Hematoxylin (Sigma-Aldrich, Hematoxylin Solution, Harris Modified, catalog number: HHS32-1L)
Sytox Blue dead cell stain (Life Technologies, catalog number: S34857)
Tisseel matrix (Thrombin and Fibrin solution, Baxter, catalog number: 3400894252443)
Sterile distilled water (Life Technologies, catalog number: 15230162)
Buprenorphine (Centravet, catalog number: BUP001)
Atipamezole (Centravet, catalog number: ANT201)
Ketamine (Centravet, catalog number: KET205)
Medetomidine (Centravet, catalog number: DOM003)
Vetedine Savon (Vetoquinol, catalog number: 2608436 7/1992)
Vetedine solution (Vetoquinol, catalog number: 4576889 5/1992)
Paraformaldehyde 4% (PFA; Clinisciences, catalog number: sc-281692)
Sucrose (VWR, catalog number: 443815S)
EDTA solution 0.5 M (Euromedex, catalog number: EU0084)
Tissue Freezing Medium (MMFrance, catalog number:F/TFM-C)
Neo-Clear (Sigma-Aldrich, catalog number: 109843)
Hematoxylin anhydrous (Sigma-Aldrich, catalog number: 109843)
Iron(III) chloride, 97% (Sigma-Aldrich, catalog number: 157740)
Fast Green (Sigma-Aldrich, catalog number: F7252)
Safranin O (Sigma-Aldrich, catalog number: S2255)
Neo-mount (Sigma-Aldrich, catalog number: 109016)
Fluoromount-G Mounting medium with DAPI (Thermo Fisher, catalog number: 00-4959-52)
PE-CyTM7 Rat Anti-Mouse CD31 Antibody (dilution 1/400; BD Bioscience, catalog number: 561410)
PE-CyTM7 Rat Anti-Mouse CD45 Antibody (dilution 1/400; BD Bioscience, catalog number: 552848)
PE-CyTM7 Rat Anti-Mouse CD11b Antibody (dilution 1/400; BD Bioscience, catalog number: 552850)
BV650 Rat Anti-Mouse Ly-6A/E Antibody (dilution 1/200; BD Bioscience, catalog number: 740450)
PE Hamster Anti-MouseCD29 Antibody (dilution 1/200; Miltenyi, catalog number: 130-102-994)
Washing medium (see Recipes)
Growth medium (see Recipes)
FACS medium (see Recipes)
Adipogenic medium (see Recipes)
Oil Red O stock solution (see Recipes)
Osteogenic medium (see Recipes)
Alizarin Red S staining solution (see Recipes)
Chondrogenic medium (see Recipes)
1% Alcian Blue staining solution (see Recipes)
Cryoprotection solution (see Recipes)
Weigert’s solution (see Recipes)
Fast Green solution (see Recipes)
Safranin O solution (see Recipes)
Equipment
Surgical forceps (×4) (Dumont AA Forceps; FST, catalog number: 11210-20, or equivalent)
Surgical scissors (×4) (Fine Scissors-ToughCut® 11 mm; FST, catalog number: 14058-11, or equivalent)
BD LSRFortessa machine (Becton Dickinson)
Sterile hood for cell culture
Centrifuge with temperature control
Water bath with temperature control
CO2 incubator set at 5% CO2 and 33°C or 37°C
Shaker (VWR, model: Mini nutating, 3D mixer)
Drill (Dremel, catalog number: 8050-15)
Drill bits (0.4 mm)
W/C3 NDL Silk BL Braid sutures (Havard Apparatus, catalog number: 72-3318)
Heating pad (Harvard Apparatus, catalog number: 55-7033)
Mouse mower (Kerbl, catalog number: GT416)
Sterile scalpels (Dutscher, catalog number: 132622)
Cryostat (Leica Biosystems)
MM35P blade (MMFrance, F/MM35P)
Zeiss Imager D1 AX10 light microscope (Carl Zeiss Microscopy)
Procedures
-
Bone explant culture
Prepare washing and growth media before mouse sacrifice.
Keep 10 ml washing medium on ice to keep the bones in until culture.
Place the growth medium in a 37°C water bath.
Sacrifice the mice by cervical dislocation (or any other appropriate method).
Rinse the animal thoroughly with 10 ml 70% ethanol.
-
Bone isolation (Time: 10 min/mouse, requires 2 scissors and 2 forceps)
Incise the skin of the inguinal region using scissors and remove it entirely from the hindlimbs. Disconnect the hindlimbs (femur with attached tibia) from the trunk by cutting at the femoral head with scissors. Place the hindlimbs in a sterile 100-mm Petri dish (Note 3, see Figure 1A).
Cut at the knee junction to separate the tibia from the femur. Remove the soft tissue using forceps and scissors. Avoid taking hair and fat (see Figure 1A).
Place the bones (femurs and tibias) in a 50-ml conical tube containing 15 ml washing medium on ice until all the bones have been isolated (see Notes 4 and 5).
-
Bone marrow cell removal (Time: 2 min/bone, requires 2 scissors and 2 forceps)
Under a cell culture hood, place the bones into a dish containing 10 ml washing medium.
Cut the epiphyses just below the end of the marrow cavity using sterile scissors.
Place the diaphysis into an empty Petri dish.
Insert a 25 G needle with a 1-ml syringe filled with 1 ml growth medium into the bone marrow cavity and flush out the bone marrow. Repeat this step at least 3 times, until the bone becomes white (Notes 6 and 7, see Figure 1A).
Place the flushed bones into a 50-ml tube containing 5 ml ice-cold growth medium for periosteal cell isolation.
-
Periosteal cell culture
Remove the growth medium and wash the bones by rinsing 3 times with fresh growth medium.
Place all 12 bones into a 60-mm TPP culture dish (in the center of the dish, each bone separated by 0.3 cm, see Figure 1B).
Cover the bones by adding drops of growth medium at 37°C (around 1 ml in total, see Note 8).
Place the bones in a humid CO2 incubator at 33°C or 37°C and 5% CO2 (see Note 9).
Change the medium daily. Carefully aspirate the medium and replace with fresh growth medium. Place the dish back at 33°C or 37°C (see Notes 10 and 11). Cell migration usually starts after 2 or 3 days.
When cell migration is observed (Figure 1C), add 2-3 ml growth medium. Change the medium daily.
When PCs have sufficiently migrated from the explant (usually after 2 weeks), gently remove the explants from the dish using sterile forceps, wash once with 1× PBS, and add 5 ml fresh growth medium to cover the cells (Figure 1C).
Allow the cells to grow for 4-5 more days. Change the medium daily.
When PCs reach 80% confluence around the explant site, remove the medium and add 3 ml trypsin. Place the plate back into the incubator for 3 min. Detach PCs using a cell scraper and transfer the cells to a 50-ml Falcon tube containing 10 ml growth medium.
Centrifuge at 300 × g for 10 min.
Discard the supernatant and resuspend the cells in 10 ml growth medium. Plate the cells into a 100-mm TPP plate.
Replace the medium every 2-3 days with fresh growth medium.
When PCs reach 80% confluence, cells can be trypsinized for further passage (Figure 1D). For experiments, PCs can be used from P0 (Notes 12, 13, and 14).
-
Flow cytometry analysis of PCs
When PCs reach 80% confluence, remove medium and add 3 ml trypsin. Place the plate back into the incubator for 3 min. Detach PCs using a cell scraper, and transfer the cells into a 50-ml Falcon tube containing 10 ml growth medium.
Centrifuge at 300 × g for 10 min.
Resuspend cells in 10 ml growth medium.
Filter through a 40-μm cell strainer.
Count the living cells using Trypan Blue and process the number of cells needed for the experiment: 1.5 × 105 cells per tube.
Centrifuge at 300 × g for 10 min.
Resuspend the cells in 200 μl FACS medium per 1.5 × 105 cells.
Split the cells into FACS tubes.
Add antibody mix according to each tube and mix well (Notes 15 and 16).
Incubate for 15 min on ice in the dark.
Add 1 ml FACS medium to each tube.
Centrifuge at 300 × g for 10 min.
Remove the supernatant and resuspend the cells in 200 μl FACS medium.
Add 0.5 μl Sytox Blue per tube immediately before analysis.
Perform flow cytometry analysis using a BD LSR Fortessa (Figure 2).
-
In vitro differentiation
-
In vitro adipogenesis
Plate 1.5 × 105 PCs in a 6-well plate in duplicate.
Allow the cells to reach 100% confluence in growth medium.
When confluency is reached, induce adipogenesis by covering the cells with adipogenic medium.
Incubate the cells at 37°C for up to 3 weeks; change adipogenesis medium twice a week.
-
After 3 weeks of differentiation, proceed to Oil Red O staining for lipid droplets:
Discard medium.
Rinse gently twice with PBS.
Fix cells with 3 ml 70% ethanol for 30 min at room temperature.
Rinse gently twice with H2O.
Cover the cells with 3 ml 60% isopropanol for 5 min.
Discard isopropanol and stain with 3 ml filtered Oil Red O staining solution for 50 min at room temperature.
Rinse twice with H2O.
Counterstain nuclei with 3 ml hematoxylin for 1 min.
Rinse twice with H2O.
Leave H2O and take an image within 2 h using an inverted microscope (Figure 3).
-
In vitro osteogenesis
Plate 1.5 × 105 PCs in a 6-well plate in duplicate.
Allow the cells to reach 100% confluence in growth medium.
When confluency is reached, induce osteogenesis by covering the cells with osteogenic medium.
Incubate the cells at 37°C for up to 3 weeks; change the osteogenic medium twice a week.
-
After 3 weeks of differentiation, proceed to Alizarin Red staining for hydroxyapatite crystals.
Discard medium.
Rinse twice with PBS.
Fix cells with 3 ml 70% ethanol for 30 min at room temperature.
Rinse twice with H2O.
Stain with 3 ml Alizarin Red S solution for 45 min at room temperature, under agitation and protected from light.
Rinse twice with H2O.
Allow the plate to dry before taking images (Figure 3).
-
In vitro chondrogenesis
Resuspend the cells at a concentration of 5 × 105 cells in 200 μl growth medium.
Using a 200-μl pipet, seed the PCs in drops of 200 μl containing 5 × 105 cells. Place 3 drops in a 60-mm TPP culture dish.
Carefully place the plates at 37°C (Note 17).
Allow the cells to attach to the plate for 6-8 h.
Check cell attachment before starting differentiation (leave longer if necessary).
Remove growth medium and induce chondrogenesis by covering the cell drops with chondrogenic medium.
Incubate the cells at 37°C for 3 days.
-
Proceed to Alcian Blue staining for sulfated GAG:
Discard medium.
Rinse twice with PBS.
Fix cells with 2% glutaraldehyde in H2O for 1 h at room temperature.
Rinse with 0.1 M HCl.
Stain with 1% Alcian Blue for 2 h at room temperature.
Rinse twice with 0.1 M HCl.
Allow the plate to dry before taking images (Figure 3).
-
-
In vivo PC transplantation at the fracture site (Note 18)
-
Tisseel matrix pellet formation
When PCs reach 80% confluence, remove the medium and add 3 ml trypsin. Place the plate back into the incubator for 3 min. Detach PCs using a cell scraper and transfer the cells to a 50-ml Falcon tube containing 10 ml growth medium.
Centrifuge at 300 × g for 10 min.
Count the cells and keep only the number of cells needed for the experiment: 105 cells per host animal.
Place 105 cells in a 1.5-ml sterile Eppendorf tube (one tube for each host animal). Centrifuge for 10 min at 300 × g and remove as much medium as possible.
Prepare appropriate volumes of fibrin (F) and thrombin (T) diluted 1:4 in distilled water: 105 cells are resuspended in 15 μl F (diluted 1:4) + 15 μl T (diluted 1:4) per host animal (see Note 19).
Add 15 μl diluted fibrin to the tubes containing cells. Resuspend by pipetting without forming bubbles.
Add 15 μl diluted thrombin to form a solid gel (see Note 20).
Allow the matrix to polymerize for 15 min on ice. If the pellet is well formed, it can be easily grabbed with the tip of forceps (Figure 4E).
Keep the tubes at 4°C on ice until transplantation.
-
Surgery for in vivo transplantation
Anesthetize mice with intraperitoneal injection of 50 mg/kg ketamine and 1 mg/kg medetomidine (Note 21).
Inject 0.1 mg/kg buprenorphine subcutaneously for analgesia.
After 15 min, check the quality of anesthesia by foot pinching.
Shave the right limb and sanitize using vetedine soap and solution or any other skin disinfectant solution (Figure 4A).
Perform a 2-cm incision on the skin above the tibia using a sterile scalpel (Figure 4B).
Expose the anterior tibial surface by carefully separating the muscle from the bone surface (Figure 4C).
Create 3 holes at the mid-diaphysis perpendicular to the longitudinal axis of the tibia using a drill and a 0.4-mm drill bit.
Induce an osteotomy by cutting the bone along the 3 holes with scissors (see Note 22) (Figure 4D).
Gently position the Tisseel matrix pellet containing PCs at the fracture site between the two bone cortices (Figure 4F).
Close the skin wound using 5-0 non-resorbable sutures.
Revive the mice with an intraperitoneal injection of 1 mg/kg atipamezole.
Place the mice on a 37°C heating pad until revived.
Perform two additional subcutaneous injections of buprenorphine 0.1 mg/kg at 12 h and 24 h post-surgery.
-
Histological analysis of the PC contribution to the callus
Sacrifice the mice by cervical dislocation (or any other appropriate method) and rinse the limb with 70% ethanol (Note 23).
Incise the skin and remove it entirely from the hindlimbs. Disconnect the femur and the tibia by cutting at the knee junction and at the feet. Remove the soft tissue around the callus using forceps and scissors (Note 24).
Place the sample in a 15-ml Falcon tube containing 8 ml 4% ice-cold PFA for 24 h with constant shaking at 4°C.
Remove the PFA solution, wash the sample with ice-cold PBS, and add 8 ml EDTA solution. Place at 4°C with constant shaking for 21 days; change the EDTA solution every other day (see Note 25).
Remove the EDTA solution, wash the sample with ice-cold PBS, and add 8 ml cryoprotection solution. Place at 4°C for 24 h without shaking.
Remove the cryoprotection solution and wash 3 times with ice-cold PBS.
On dry ice, fill a plastic mold with Tissue Freezing Medium, embed the bone, and place the sample at -80°C.
Cut the sample into 10 μm-thick sections using a cryostat.
-
Stain the sections with Safranin O to allow cartilage visualization (Figure 4G):
Allow the slides to dry for 30 min at room temperature.
Hydrate the slides in PBS for 5 min.
Place the slides in Weigert’s solution for 5 min.
Wash in tap water for 3 min.
Place the slides in Fast Green solution for 30 s.
Place the slides in 1% acetic acid for 30 s.
Place the slides in Safranin O solution for 30 min.
Wash in distilled water for 3 min.
Place the slides in 70% ethanol for 3 min.
Place the slides in 95% ethanol for 3 min.
Place the slides in 100% ethanol for 5 min, twice.
Place the slides in Neo-Clear for 3 min, twice.
Mount the slides with Neo-mount.
Take images using a brightfield microscope.
-
Mount the slides with DAPI to visualize the PC contribution to the callus (Figure 4H):
Allow the slides to dry for 30 min at room temperature.
Hydrate the slides in PBS for 5 min.
Mount the slides with Fluoromount-G with DAPI.
Take images using a fluorescence microscope.
-
Notes
We recommend using the hindlimbs (femur and tibia bones) of at least 3 mice per culture. Donor mice for PC culture should carry a reporter transgene in the C57BL/6J background in order to perform cell tracing following in vivo transplantation in C57BL/6J hosts.
All procedures must be approved by the local Ethics Committee.
Two sets of sterilized surgical tools (2 scissors and 2 forceps per set) are needed for bone explant culture: one for bone dissection and one for bone marrow removal under a culture hood. To avoid contamination, tools used to cut and remove the skin should not be used to touch bones and soft tissue. Bones should only be handled using forceps to avoid any contamination.
Remove soft tissue gently along the bone using scissors, instead of pulling out tissues, to avoid detaching the periosteum from the cortex.
Isolated bones can be kept on ice in washing medium for up to 2 h.
Be careful not to drop the bones into the Petri dish containing the bone marrow cells in order to avoid contamination of PC cultures with BMSCs. Any bone dropped in the plate containing BMSCs should be discarded.
BMSCs can be cultured from the flushed bone marrow. Centrifuge the medium containing the flushed bone marrow at 300 × g for 10 min, resuspend the pellet in growth medium, and plate in a 100-mm TPP culture dish. On subsequent days, wash the cells with PBS once a day to eliminate floating hematopoietic cells and obtain adherent bone marrow cells.
When covering the bones with medium, drops covering each bone can touch and merge. However, be careful that the bones are not floating or detaching, as it is critical for bones to be in contact with the plate to allow for cell migration.
The protocol was initially optimized using a 33°C incubator, but PC migration and growth are also observed when cultured in a 37°C incubator.
Do not wash the bones and proceed gently to avoid moving bones that have started to attach to the bottom of the dish.
Change the medium daily to avoid bone explant drying.
We recommend using PCs between passages 0 and 2 for optimal cell growth.
PCs can be frozen down for later use. For optimal cell viability after thawing, we recommend freezing cells in growth medium supplemented with 10% DMSO.
As observed in Figure 2, primary cultures of PCs also contain hematopoietic cells. An additional step of cell sorting or antibody-based column depletion can be used to discard hematopoietic cells and increase the purity of periosteal stem/progenitor populations.
Each antibody has to be titrated prior to the experiment.
For each experiment, FMO (Fluorescence Minus One) controls can be included to determine positive vs. negative signals, and isotype controls can be used to check the specificity of the antibodies.
After plating cell drops for chondrogenesis, handle the plates with caution, as the drop should remain intact to allow cell deposition at the right confluency to mimic a 3D environment.
For tracing of PC contribution to the fracture callus, donor mice should express a reporter gene. As an example, we use GFP-actin mice in this protocol.
Fibrin is a highly viscous solution; be careful to pipet the right volume of fibrin when diluting. If needed, cut the extremity of the tip for easier pipetting of the viscous solution.
Coagulation is very fast. After adding thrombin, remove the tip from the gel within 2 s.
Anesthetized mice should be kept on a 37°C heating pad to avoid temperature loss and improve well-being.
Tibia can be cut without drilling holes, but drilling holes prevents bone fragments.
The time of sacrifice should be decided according to the biological question. We recommend choosing days 7-14 post-fracture to observe the contribution to cartilage and days 14-21 to observe the contribution to bone.
Be careful to remove enough soft tissue without affecting the callus structure, especially when collecting on days 3, 5, and 7 post-fracture.
The pH of the EDTA solution must be between 7.4 and 7.6 to allow for proper decalcification.
Recipes
-
Washing medium
DMEM
1% penicillin/streptomycin
-
Growth medium
α-MEM with GlutaMAX
1% penicillin/streptomycin
20% lot-selected fetal bovine serum
10 ng/ml bFGF
-
FACS medium
PBS
1% penicillin/streptomycin
2% fetal bovine serum
-
Adipogenic medium (make fresh)
α-MEM with GlutaMAX
1% penicillin/streptomycin
10% fetal bovine serum
0.1 μM dexamethasone
100 μM indomethacin
0.5 mM IBMX
10 μg/ml insulin
-
Oil Red O stock solution
Stock solution: 300 mg Oil Red O powder in 100 ml 99% isopropanol
Ready-to-use solution (make fresh):
Mix 3 parts Oil Red O stock solution with 2 parts H2O
Allow to sit at room temperature for 10 min
Filter the staining solution through coffee filter paper
-
Osteogenic medium (make fresh)
α-MEM with GlutaMAX
1% penicillin/streptomycin
10% fetal bovine serum
0.1 μM dexamethasone
0.05 mM L-ascorbic acid
10 mM β-glycerophosphate
-
Alizarin Red S staining solution
Alizarin Red S 0.2% in distilled water
Adjust the pH to 4.1-4.3.
The pH is critical; make fresh or check the pH if the solution is more than one month old.
Store at 4°C.
-
Chondrogenic medium (make fresh)
DMEM high glucose
1% penicillin/streptomycin
10% fetal bovine serum
0.1 μM dexamethasone
100 μg/ml sodium pyruvate
40 μg/ml L-proline
50 μg/ml L-ascorbic acid
50 mg/ml ITS
10 ng/ml TGFβ1
-
1% Alcian Blue staining solution
Prepare 5% Alcian Blue solution in PBS
Dilute 5% Alcian Blue stock solution in 0.1 M HCl to obtain 1% Alcian Blue
-
Cryoprotection solution
30 g sucrose
100 ml H2O
-
Weigert’s solution (make fresh)
100 ml solution A and 100 ml solution B
Solution A:
5.0 g hematoxylin
500 ml ethanol 95%
Solution B:
20 ml 29% aqueous iron(III) chloride
5 ml HCl
475 ml distilled water
-
Fast Green solution
0.2 g Fast Green
1 L distilled water
-
Safranin O solution
1 g Safranin O
1 L distilled water
Acknowledgments
This work was supported by Fondation de l’Avenir, Osteosynthesis and Trauma Care Foundation, ANR-18-CE14-0033, NIAMS R01 AR072707 to C.C. A.J., S.P., and O.D.L were supported by Ph.D. fellowships from Paris University. This protocol was first described in Duchamp de Lageneste et al. (2018) and in Julien et al. (2020).
Competing interests
The authors declare no competing interests.
Ethics
All procedures involving animals were approved by the Creteil University (agreement #19295-2019052015468705) Ethical Committee.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Arnsdorf E. J., Luis M. J., Dennis R. C. and Christopher R. J.(2009). The periosteum as a cellular source for functional tissue engineering. Tissue Eng Part A 15(9): 2637-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur A. and Gronthos S.(2020). Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int J Mol Sci 21(24): 9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlow H. C., Reed A., Joyner C., Simpson A.H.(2000). Anatomical effects of periosteal elevation. J Orthop Res 18(3): 500-502. [DOI] [PubMed] [Google Scholar]
- 4.Chang H. and Knothe Tate M. L.(2012) Concise review: the periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med 1(6): 480-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debnath S., Yallowitz A.R., McCormick J., Lalani S., Zhang T., Xu R., Li N., Liu Y. F., Yang Y. S., Eiseman M., Shim J. H., Hameed M., Healey J. H., Bostrom M. P., Landau D. A., Greenblatt M. B.(2018). Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 562(7725): 133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Lageneste Duchamp, Julien O., Abou-Khalil A., Frangi R., Carvalho G., Cagnard C., Cordier N., Conway C., Colnot S. J., C. (2018). Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun 9(1): 773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Gastel N., Torrekens S., Roberts S. J., Moermans K., Schrooten J., Carmeliet P., Luttun A., Luyten F. P., Carmeliet G.(2012). Engineering vascularized bone: osteogenic and proangiogenic potential of murine periosteal cells. Stem Cells 30(11): 2460-2471. [DOI] [PubMed] [Google Scholar]
- 8.Julien A. Perrin, S. Duchamp de Lageneste, Carvalho O., Bensidhoum C., Legeai-Mallet M., Colnot L., C. (2020). FGFR3 in Periosteal Cells Drives Cartilage-to-Bone Transformation in Bone Repair. Stem Cell Reports 15(4): 955-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortinau L. C., Wang H., Lei K., Deveza L., Jeong Y., Hara Y., Grafe I., Rosenfeld S. B., Lee D., Lee D., Scadden D. T., Park D.(2019). Identification of Functionally Distinct Mx1+αSMA+ Periosteal Skeletal Stem Cells. Cell Stem Cell 25(6): 784–796..e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q., Huang C., Zeng F., Xue M., Zhang X.(2010). Activation of the Hh pathway in periosteum-derived mesenchymal stem cells induces bone formation in vivo: implication for postnatal bone repair . Am J Pathol 177(6): 3100-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]