Abstract
Peripheral blood mononuclear cell (PBMC) isolation is commonly done via density gradient centrifugation over Ficoll-Hypaque, a labor-intensive procedure that requires skilled technicians and can contribute to sample variability. Cellular Preparation Tubes (CPTs) are Vacutainer blood draw tubes that contain Ficoll-Hypaque and a gel plug that separates the Ficoll solution from the blood to be drawn. Once blood is drawn into CPTs, they can be centrifuged to separate the PBMC, then shipped (if desired) to a processing lab. The processing lab removes the PBMC from the upper compartment of the tube (above the gel plug), washes the PBMC, and can cryopreserve them using DMSO-containing media, as detailed in this protocol.
Keywords: PBMC, Cell preparation, CPT, Ficoll, Blood, Stabilization
Background
Isolation and cryopreservation of peripheral blood mononuclear cells (PBMC) is common practice in clinical studies that employ cellular immune assays. Cryopreservation allows for batching of samples, which is convenient and improves the comparability of data. Cryopreservation also allows cells to be stored for unknown future purposes. Because erythrocytes and granulocytes are much more fragile to freezing and thawing, PBMC isolation is a common prerequisite to cryopreservation of blood cells; and the most common method for PBMC isolation is density gradient centrifugation using Ficoll-Hypaque, a high molecular weight carbohydrate solution.
Cellular Preparation Tubes (CPTs), which contain Ficoll-Hypaque, simplify the standard procedure for density gradient centrifugation in two ways: (1) blood is collected into the same tube that is then used to isolate the PBMC; and (2) the tube is pre-loaded with Ficoll-Hypaque, which is separated by a gel plug so that it is not disturbed by the entry of blood into the tube. After blood draw, the tubes are centrifuged, and the PBMCs and plasma become separated from the erythrocytes and granulocytes by the gel plug (see Figure 1). This allows the spun tubes to be shipped, maintaining the PBMC in an isolated environment from the erythrocytes and granulocytes, which may improve their viability and function.
Figure 1. Empty CPT (left), after blood draw (middle), and after centrifugation (right).
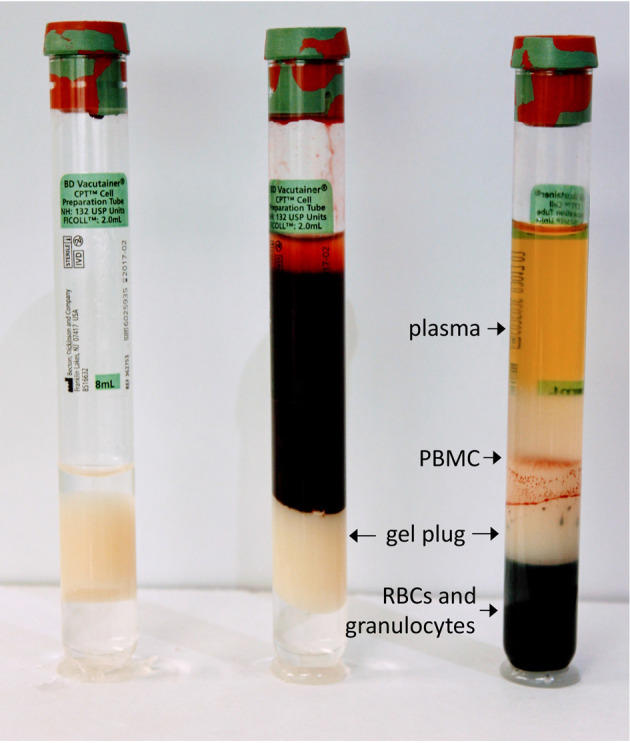
Location of gel plug and sample layers after centrifugation are shown.
CPTs are ideal for use in studies which collect whole blood across multiple sites and ship to a central processing laboratory ( Ruitenberg et al., 2006 ); this reduces variability in PBMC isolation between technicians. Studies have found no significant difference in PBMCs isolated using the CPT system or by traditional layover methods via density gradient separation ( Corkum et al., 2015 ; Ruitenberg et al., 2006 ). While material cost can be high, CPTs reduce the length of processing time in addition to decreasing inconsistency between operators; both of these are key to reducing cost and increasing sample quality and consistency. Furthermore, when used with studies or sites which ship blood samples overnight, PBMCs from CPTs have a higher purity and less infiltrate from other (contaminating) cell types, such as red blood cells, in contrast to samples shipped in standard blood collection tubes over a 24-48 h period ( Schlenke et al., 1998 ).
Materials and Reagents
1.8 ml Nunc (or similar) cryovials
BD Vactuainer® Mononuclear Cell Preparation Tubes (CPTTM), 8 ml, with sodium heparin (BD, catalog number: 362753)
P10 and P200 pipette tips (any vendor)
P10 and P200 mechanical pipettors (any vendor)
50 ml Falcon (or similar) conical polypropylene tubes
Serological pipettes (assorted volumes)
BioCision CoolCell® (BioCision, catalog number: BCS172) – alcohol free controlled-rate freezing container
Foam packing material and shipping containers
Phosphate buffer saline (PBS),Ca2+ and Mg2+ free (e.g., Thermo Fisher Scientific, GibcoTM, catalog number: 10010-023)
Trypan blue (e.g., GE Healthcare, HycloneTM, catalog number: SV30084.01 or Sigma-Aldrich, catalog number: 93595)
Human AB Serum (e.g., Valley Biomedical, catalog number: HP1022)
Dimethyl sulfoxide (e.g., Sigma-Aldrich, catalog number: D8418)
Equipment
A centrifuge capable of reaching speeds of 1,800 × g (e.g., Beckman Coulter, model: Allegra X-14 Series)
50 ml conical adapters and buckets for centrifuge
Pipette gun (any vendor)
-
Automated cell counter or microscope and hemacytometer (any vendor)
Note: Cell counting methods vary in their throughput, cost, and flexibility for different cell types. For consistency, the same counting method should be used throughout a study.
Biosafety cabinet level A2 (BSC) (any vendor)
-80 °C freezer (for initial cryopreservation) (any vendor)
-
Liquid nitrogen (LN2) freezer (for long-term cryopreservation) (any vendor)
Note: LN2 systems range from small and simple to large and highly automated. Auto-filling and self-monitoring systems are recommended, especially for larger studies.
Procedure
-
Aliquot tube preparation
Print out labels containing the study name, study number, de-identified patient number and visit number, and paste on cryovials.
-
Sample preparation
Mix the blood by gentle inversion several times to ensure a homogeneous suspension.
Centrifuge the tubes at 1,800 × g for 20 min (brake can be on) at room temperature. Be sure that the tubes can clear the rotor in swinging-bucket configurations; some tube locations can cause collision with the rotor and result in broken tubes.
-
If shipping to another location, wrap in protective foam and ship via same-day or overnight courier to the processing lab at room temperature.
Note: Shipping sample at 4 °C (or on wet ice), can result in platelet activation and other unwanted physiological and phenotypic changes.
-
PBMC isolation
In BSC, gently invert the CPT tubes several times, then pipette the plasma and PBMCs into a sterile 50 ml conical tube.
Fill tube containing the plasma and PBMCs with PBS for a final volume of 50 ml in conical. Centrifuge at 250 × g for 10 min at room temperature with the brake on.
-
Aspirate the supernatant carefully to not disturb cell pellet.
A Pasteur pipette and vacuum can be used for aspirating as well as manually aspirating with serological pipettes and a pipette gun.
Gently re-suspend the cells in 10 ml of PBS and remove an aliquot for cell counting. Use a 10 μl sample for a microscope using trypan blue exclusion, or amount needed for automated cell counter.
After removing the aliquot for counting, fill the tube to final volume of 50 ml with PBS and centrifuge for 10 min at 250 × g with the brake on.
-
Determining the number of aliquots
Depending on your cell count methods (manual or automated counter), determine the total amount of cells. This will be used to calculate how many aliquots of PBMCs are frozen down.
Cells should be frozen in approximately 107 cells per ml of freezing media; when the total cell count is divided by 107, the result is the total number of aliquots.
Place the appropriate number of cryovials on ice or in a cooling rack in the 4 °C to begin chilling the vials in preparation for freezing.
-
Determining the volume of freezing media
-
Freezing media is broken into two parts: ‘A’ and ‘B’. Part ‘A’ is made up of 100% Human AB Serum and Part ‘B’ is made up of 80% Human AB Serum and 20% DMSO.
Note: Human AB Serum must be heat inactivated prior to use.
Your samples will be frozen in a 50:50 mixture of Part ‘A’ and Part ‘B’, totaling a final concentration of 10% DMSO.
Each aliquot will have a total of 1 ml of freezing media. Divide your total number of aliquots in half to determine what volume of each media you will need.
Keep both parts of the freezing media on ice (or at 4 °C) until use.
-
-
Freezing PBMC ALiquots (for viable cryopreservation)
Aspirate the supernatant from the cells and re-suspend them with the volume of ‘A’ calculated in step 5c.
At an approximate rate of 1 drop per second, and while swirling the sample, add the necessary volume of ‘B’.
Do not re-freeze unused ‘B’ media.
-
After all the freezing media has been added, gently pipette the sample up and down a couple times to ensure re-suspension. Over pipetting can result in cell rupture.
Note: It is important to be efficient through this process, as warming DMSO is toxic to the PBMCs.
Add 1 ml of the cell suspension to each pre-chilled cryovial and place the vials in a CoolCell or Mr. Frosty.
Place the cells in a -80 °C freezer for 24 h.
-
After 24 h, remove cells to a long-term LN2 freezer.
Note: PBMC can be kept for days or even weeks at -80 °C before transfer to LN2; however, there is progressive loss of viability and function with longer storage at -80 °C. Also, repeated cycling between -80 °C (or dry ice) and LN2 will negatively affect viability and function. Thus, if cells are to be shipped on dry ice, it’s preferable to store them short-term at -80 °C, ship, then move to LN2.
Data analysis
Typical assessment of a PBMC isolation protocol includes monitoring yield and purity. To some degree, the latter can be determined visually, as erythrocyte contamination will redden the pellet of PBMC acquired after centrifugation. This is sometimes seen with CPT, to a greater extent than traditional Ficoll protocols. However, these contaminating erythrocytes are lost after downstream procedures such as cryopreservation, and have not been observed to influence function ( Ruitenberg et al., 2006 ).
Yield is easily determined by either manual or automated cell counting, and has also been reported to be roughly equivalent between CPT and manual Ficoll methods ( Ruitenberg et al., 2006 ). An example is shown below in Figure 2 from one of our own lab’s studies.
Representative data
Figure 2. Comparison of cell recovery from CPTs versus conventional Ficoll procedure (using SepMate tubes, Stem Cell Technologies).
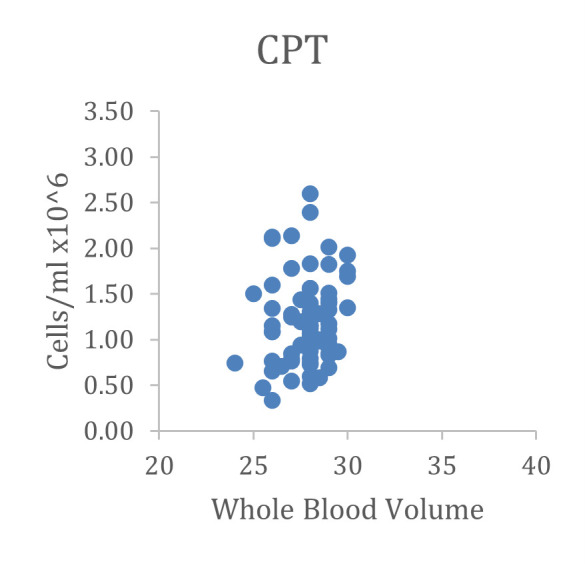
Data were derived from two different studies, one drawing 4 x 8-cc CPT, the other 4 x 10-cc sodium heparin tubes. Because of the different maximum draw volumes, there is a wider range of blood volumes for the SepMate study; but recovered cells/ml were very similar with CPT and SepMate tubes. Only samples in good condition (no visible clumping or erythrocyte contamination) with > 20-cc blood volume were included for comparison.
Notes
Shipping Temperature. When using CPT in the context of a multisite study, we have seen that gel plugs can loosen and the cell separation can fail when tubes become too cold during shipping. For this reason, an insulated shipping container is highly recommended, especially in cold winter climates.
Consistency of centrifugation step. Similarly, when multiple sites are involved, it is critical to ensure success that the correct centrifugation G force is calculated and implemented across sites with potentially different instrumentation. Both spin time and G force are important to success with the procedure.
Acknowledgments
We thank BD Biosciences for development of the CPT method, on which this protocol is based.
References
- 1.Corkum C., Ings D., Burgess C., Karwowska S., Kroll W. and Michalak T.(2015). Immune cell subsets and their gene expression profiles from human PBMC isolated by Vacutainer Cell Preparation Tube(CPTTM) and standard density gradient . BMC Immunology 16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruitenberg J. J., Mulder C. B., Maino V. C., Landay A. L. and Ghanekar S. A.(2006). VACUTAINER CPT and Ficoll density gradient separation perform equivalently in maintaining the quality and function of PBMC from HIV seropositive blood samples. BMC Immunol 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlenke P., Klüter H., Müller-Steinhardt M., Hammers H. J., Borchert K. and Bein G.(1998). Evaluation of a novel mononuclear cell isolation procedure for serological HLA typing. Clin Diagn Lab Immun 5(6): 808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]


