Abstract
Platelets and coagulation have long been known to be essential for metastasis in experimental models. In order to study the interactions between tumor cells, platelets and endothelium, we have adapted methods used in coagulation research for the isolation of platelets and their reintroduction into mice. Anti-coagulated murine blood served as the source for platelets. Platelets were separated from other elements of the whole blood by centrifugation. Here the critical elements are first inhibition of coagulation and second isolation and maintenance of the platelets in the presence of inhibitors of platelet activation. We then used the vital dye PKH26 to fluorescently label the platelets. Infusion of these labelled platelets allows microscopic observation of the introduced platelets. After reintroduction, these platelets appear to function normally and comprise approximately 50% of the total platelets. Because they are fluorescently labelled, they can easily be identified. Finally it would be possible to use these methods for the determination of specific effects of altered gene expression in platelets by using platelets from genetically engineered mice. These methods have facilitated study of the interactions between platelets and tumor cells in tissue culture and in murine models. They would also be applicable to video microscopy. Here we provide details of the methods we have used for platelet isolation from mice and their staining for further microscopy and re-introduction into mice.
Keywords: Platelets, Coagulation, Fluorescent labeling, Vital dye, Clot
Background
Platelets are known to be essential for metastasis, but also to play roles during tumor growth not to mention clot formation. In order to readily identify and track platelets we developed the means for fluorescently labeling and reinfusing platelets. This allows them to be readily identified in tissues without immunostaining. Using these methods, we showed that interactions between tumor cells and platelets play key roles in survival of the tumour cells early during metastasis ( Im et al., 2004 ; Gil- Bernabe et al., 2012 ). Platelets formed clots with tumor cells in the blood stream and this coagulation promoted spreading and subsequent retention of the tumor cells during lung metastasis ( Im et al., 2004 ). Tissue factor expressed by tumor cells is capable of mediating clot formation with platelets and recruitment of macrophages (Gil- Bernabe et al., 2012 ). The interaction of platelets, thrombin and fibrin also have been reported to promote metastasis by generating epithelial-mesenchymal transition of the cancer cells and evasion from the immune system by protection from NK cells as well as secretion of pro-metastatic chemokines and cytokines ( Labelle et al., 2011 ; Nieswandt et al., 1999 ; Palumbo et al., 2005 and 2007). Precise tracking of platelets will provide opportunities to uncover how tumor cells utilize the host for their survival.
Materials and Reagents
Scalpel (Swann Morton, catalog number: 0208)
Syringe, 1 ml (BD, catalog number: 300013)
15 ml conical bottom polypropylene tube (SARSTEDT, catalog number: 62.554.502)
5 ml pipette
Needle (27 G)
Mice (4-6 weeks old, weight over 20 g, Charles Liver, UK)
Isoflurane (Abbott, catalog number: 0044-5260-05)
EGTA (Sigma-Aldrich, catalog number: E3889)
PKH26 Kit (Sigma-Aldrich, catalog number: PKH26GL-1KT)
Sodium chloride, NaCl (Thermo Fisher Scientific, Fisher Scientific, catalog number: 10378573)
Potassium chloride, KCl (Thermo Fisher Scientific, Fisher Scientific, catalog number: 10427460)
Sodium phosphate monobasic monohydrate, NaH2PO4.H2O (Sigma-Aldrich, catalog number: S9638)
HEPES (Sigma-Aldrich, catalog number: H3375)
Glucose (Thermo Fisher Scientific, GibcoTM, catalog number: 15023021)
Magnesium chloride, MgCl2 (Sigma-Aldrich, catalog number: M8266)
Trisodium citrate (Thermo Fisher Scientific, Fisher Scientific, catalog number: 10362234)
Citric acid (Sigma-Aldrich, catalog number: 251275)
Dextrose (Thermo Fisher Scientific, Fisher Scientific, catalog number: D16-1)
Prostaglandin E1 (Sigma-Aldrich, catalog number: P5515)
100% ethanol
Sodium bicarbonate, NaHCO3 (Thermo Fisher Scientific, Fisher Scientific, catalog number: S637-212)
Distilled water
Bovine serum albumin, BSA (Sigma-Aldrich, catalog number: A2058)
Sodium bicarbonate, Na2HPO4 (Thermo Fisher Scientific, Fisher Scientific, catalog number: S374)
Sodium citrate (Sigma-Aldrich, catalog number: 71498)
Modified Tyrode’s calcium-free buffer (see Recipes)
ACD buffer (see Recipes)
Undiluted prostaglandin E1 solution
Resuspension buffer (see Recipes)
Washing buffer (see Recipes)
Citrate-albumin buffer (see Recipes)
Equipment
Coulter counter (CDC Technology, model: HEMAVET® 1500)
Centrifuge (Thermo Fisher Scientific, model: Jouan CR4i)
Procedure
-
Platelet isolation
The mouse is placed under terminal anaesthesia by inhalation of isoflurane (2.5% with oxygen at medical grade).
With a scalpel the abdomen is opened and the bowel pushed aside to expose the major abdominal vessels.
A syringe with a needle (27 G) containing ACD buffer (150 µl/ml blood) is inserted into the vena cava above the renal veins to collect blood (Figure 1). Blood volume collected in this way is usually from 800-1,000 µl for a mouse of 20.0 g weight. After the collection of blood, the mouse was completely exsanguinated by transection of the vena cava.
The blood should be anti-coagulated by rapid mixing with the ACD buffer during collection. The blood is then transferred to a 15 ml conical bottom polypropylene tube and mixed with washing buffer (3 ml/ml, 1 ml of 100 mmol/L EGTA [pH 6.8] and 2 ml of resuspension buffer).
The tube is centrifuged at 180 × g at 22 °C for 10 min. This results in pelleting of the larger cellular elements such as red blood cells and leukocytes.
Platelet-rich plasma is collected and transferred to a 15 ml conical bottom polypropylene tube. The pellet discarded. The washing buffer detailed in step A4 now with the addition of prostaglandin E1 (0.25 µmol/L) is added to yield a total volume of 10 ml. The mixture was gently pipetted 3-5 times with a 5 ml pipette. The prostaglandin is essential at this point to prevent platelet activation.
The tube is centrifuged at 1,250 × g and 22 °C for 10 min.
The pellet now consists mainly of platelets. Remove the supernatant and wash two more times using the buffer in step A6 with prostaglandin and centrifuge as in step A7.
After suspension at this point in 10 ml of washing buffer including 0.25 µmol/L prostaglandin E1 the platelets can be counted.
To count the platelets, mix the platelet suspension (15 µl) and undiluted prostaglandin E1 (5 µl, 500 µM). Counting can be done in a Coulter counter. Note that the counter must be adjusted for the size of mouse platelets. The diameter of the mouse platelet is approximately 0.5 µm, which is smaller than human platelets (1-2 µm).
At this point the platelets can be used for experimentation remembering that until an experiment is initiated, it is important to block activation with 0.25 µmol/L prostaglandin E1. This will then need to be removed- often by dilution- to assess platelet action.
-
Platelet staining
We have used the vital dye PKH26 to fluorescently tag the collected platelets following the manufacturer’s directions with some modifications. At this point the platelets from step A9 can be centrifuged as in A7. They should be resuspended in 1 ml of Diluent C (PKH26 Kit) to be 8 x 105/µl with added 0.25 µmol/L prostaglandin E1.
Prepare a stock solution of PKH26 (1 mM) by dilution of PKH26 dye solution in 100% ethanol.
Add 2 µl of both PKH26 and prostaglandin E1 (2.5 µg/ml) into 1 ml of platelet suspension.
Immediately mix the sample by gentle pipetting.
Incubate the sample at 22 °C for 10 min.
The staining reaction is stopped by addition of 10 ml of a mixture of citrate-albumin buffer, 0.35% bovine serum albumin (pH 6.5) and 0.25 µmol/L prostaglandin E1.
Incubate the mixture at 22 °C for 1 min.
Pellet the platelets by centrifugation at 1,250 × g at 22 °C for 20 min.
Remove the supernatant and resuspend the platelets at 6 x 106/µl in the resuspension buffer for use (as described in Recipe 4).
For in vivo experiments, 100 µl of platelet suspended solution can be injected with a 1 ml syringe a needle (27 G) into the mouse tail vein.
We find that the number of platelets harvested in this way from 5 mice usually supplies enough for in vivo injections into 3 mice. The resultant reconstitution usually results in approximately 50% of the platelets being newly introduced and fluorescent. They remain functional for at least 24 h. We have not looked in detail after that time, but in principle a longer time span should be possible.
Figure 1. Blood collection from the vena cava in a mouse.
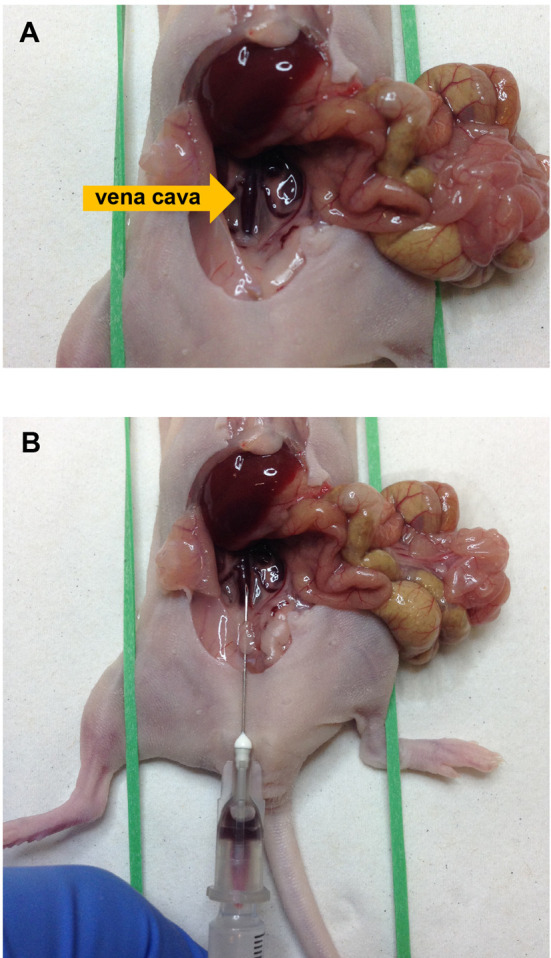
A shows position of the vena cava and B shows blood collection with a syringe containing ACD buffer.
Data analysis
The details of data analysis are presented at the article by Im et al. (2004) and Gil-Bernabe et al. (2012) .
Notes
PGE1 must be added freshly for each use of the buffer and cannot be stockpiled. The most variable part of the procedure is the reinjection of platelets intravenously.
Recipes
-
Modified Tyrode’s calcium-free buffer (pH 7.2)
134 mmol/L NaCl
3 mmol/L KCl
0.3 mmol/L NaH2PO4.H2O
5 mmol/L HEPES
5 mmol/L glucose
2 mmol/L MgCl2
Adjust pH to 7.2 with HCl (36.5%-38%)
-
ACD buffer (150 µl/ml blood, pH 4.5)
85 mmol/L trisodium citrate
71 mmol/L citric acid
111 mmol/L dextrose
Note: The solution has a pH of about 4.5 and thus adjustment does not needed.
-
Undiluted prostaglandin E1 solution
50 µmol prostaglandin E1/100 ml ethanol
-
Resuspension buffer
100 ml of 7.5% NaHCO3
133 ml of distilled water
300 mg of bovine serum albumin
15 ml modified Tyrode’s calcium-free buffer
-
Washing buffer
1 ml of 100 mmol/L EGTA (pH 6.8)
2 ml of resuspension buffer
-
Citrate-albumin buffer (pH 6.5)
11 mmol/L glucose
128 mmol/L NaCl
4.3 mmol/L NaH2PO4.H2O
7.5 mmol/L Na2HPO4
4.8 mmol/L sodium citrate
2.4 mmol/L citric acid
0.35 % (w/v) bovine serum albumin
Adjust pH to 6.5 with HCl (36.5%-38%)
Acknowledgments
The authors were supported by NIH grants R01 CA89188, R01 CA46830 and currently are funded by Cancer Research UK. This protocol was adapted from the article by Im et al. (2004).
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Gil-Bernabe A. M., Ferjancic S., Tlalka M., Zhao L., Allen P. D., Im J. H., Watson K., Hill S. A., Amirkhosravi A., Francis J. L., Pollard J. W., Ruf W. and Muschel R. J.(2012). Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood 119(13): 3164-3175. [DOI] [PubMed] [Google Scholar]
- 2.Im J. H., Fu W., Wang H., Bhatia S. K., Hammer D. A., Kowalska M. A. and Muschel R. J.(2004). Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res 64(23): 8613-8619. [DOI] [PubMed] [Google Scholar]
- 3.Labelle M., Begum S. and Hynes R. O.(2011). Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 20(5): 576-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieswandt B., Hafner M., Echtenacher B. and Mannel D. N.(1999). Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59(6): 1295-1300. [PubMed] [Google Scholar]
- 5.Palumbo J. S., Talmage K. E., Massari J. V., La Jeunesse C. M., Flick M. J., Kombrinck K. W., Jirouskova M. and Degen J. L.(2005). Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105(1): 178-185. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo J. S., Talmage K. E., Massari J. V., La Jeunesse C. M., Flick M. J., Kombrinck K. W., Hu Z., Barney K. A. and Degen J. L.(2007). Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood 110(1): 133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]


