Highlights
-
•
Specific immunotherapy is the ‘holy grail’ for treatment of autoimmunity.
-
•
Antigens are delivered by either direct or indirect presentation mechanisms.
-
•
Liver APC and steady state DC mediate distinct forms of immune regulation.
-
•
Tr1 cell induction involves epigenetic modification of tolerance associated genes.
-
•
Trials reveal that antigen-specific immunotherapy can control autoimmune diseases.
Abstract
Current treatments for autoimmune diseases do not address the immune pathology underlying their initiation and progression and too often rely on non-specific immunosuppressive drugs for control of symptoms. Antigen-specific immunotherapy aims to induce tolerance selectively among the cells causing the disease while leaving the rest of the adaptive immune system capable of protecting against infectious diseases and cancers. Here we describe how novel approaches for antigen-specific immunotherapy are designed to manipulate antigen presentation and promote tolerance to specific self-antigens. This analysis points to liver antigen presenting cells, targeted by carrier particles, and steady-state dendritic cells, to which antigen-processing independent T-cell epitopes (apitopes) bind directly, as the principal targets for antigen-specific immunotherapy. Delivery of antigens to these cells holds great promise for effective control of this rapidly expanding group of diseases.
Current Opinion in Immunology 2021, 70:75–81
This review comes from a themed issue on Antigen processing
Edited by Scheherazade Sadegh-Nasseri and Sebastian Springer
For a complete overview see the Issue and the Editorial
Available online 18th April 2021
https://doi.org/10.1016/j.coi.2021.03.019
0952-7915/© 2021 University of Birmingham, U.K. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Autoimmune diseases arise from immune responses to self-antigens and reflect a breakdown in immunological tolerance. Polymorphisms linked to autoimmune diseases map to genes regulating the adaptive immune system and their dysregulation leads to ineffective control of autoreactive T-cells and associated production of autoantibodies [1]. Most classical autoimmune diseases have associations with genes in the major histocompatibility complex (MHC) class II region and are often more common in women than men [2]. Recent years have seen a dramatic increase in the incidence and prevalence of autoimmune diseases [3] with an alarming increase in type I diabetes (T1D) amongst children of 0–4-years [4].
Current treatments for autoimmune diseases depend on replacement therapies or the continued use of non-specific immunosuppressive drugs. Immunosuppressive drugs fail to resolve the immune pathology underlying autoimmune diseases and reduce the ability of the treated individual to combat infections and cancers. An alternative strategy is antigen-specific immunotherapy (AIT) designed to reinstate immunological tolerance to self-antigen/s while leaving the immune system to function effectively.
AIT has been practiced by allergists for over a century. Noon and Freeman described how inoculation with ‘pollen toxin’ led to ‘a distinct amelioration of symptoms’ in affected individuals [5]. The ‘pollen toxin’ was an aqueous extract of pollen and suppression of disease by repeated injection laid the foundations for the allergic desensitization practiced today. Incremental improvements in desensitization protocols have included allergen purification; however, desensitization with whole allergen retains the risk of immune activation through cross-linking of mast cell bound IgE. This hazard is avoided by targeting allergen-specific CD4+ T-cells with T-cell epitopes since CD4+ cells orchestrate the allergic response [6]. Various attempts have been made to use intact proteins for treatment of autoimmune diseases. Oral tolerance induction proved successful in experimental models of autoimmune disease but did not translate to the clinic [7], presumably because an effective dose could not be reached [8]. Further attempts to treat autoimmune conditions with intact antigen even led to exacerbation of disease in models of MS [9], T1D [10] and Graves’ disease [11]. These examples strengthen the case for using T-cell epitopes for AIT of autoimmune diseases [12].
In developing AIT, several studies have designed altered peptide ligands (APL) based on the T-cell epitope in order to modulate interactions with either MHC or the T-cell receptor (TCR). Our laboratory has designed analogues of Ac1-9, the N-terminal epitope of myelin basic protein (MBP) that induces experimental autoimmune encephalomyelitis (EAE) in H-2u mice. A heteroclitic MHC-binding analogue was shown to suppress disease by activation induced cell death in Ac1-9 specific T-cells [13]. APL were designed as TCR antagonists by altering two TCR interaction residues in Ac1-9 [14]. These were shown to antagonise activation of Ac1-9 specific T-cell clones in vitro. However, it was noted that an APL that served as an antagonist in vitro would induce EAE in vivo demonstrating that one peptide could be an antagonist for clone A but an agonist for clone B. Subsequent clinical trials of immunotherapy with an APL from MBP:83-99 led to disease exacerbation in an individual with MS [15]. In parallel trials of the APL, patients developed urticaria proving that this peptide was indeed immunogenic when injected subcutaneously at high dose [16]. These studies warn against the use of APL for AIT and raise important questions concerning the dose and solubility of the peptide that caused urticaria: subsequent studies have shown that soluble, native epitopes are well tolerated and effective for AIT of allergic [6,17•] or autoimmune diseases [18••,19••].
Rationale for AIT in autoimmune diseases
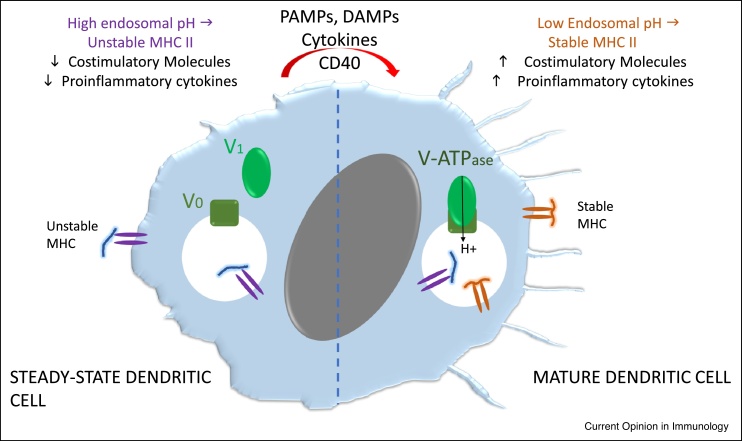
The immune response to self-antigens is controlled by both central (thymic) and peripheral tolerance. The properties of dendritic cells (DC) (Figure 1) and their role in peripheral tolerance are instructive. First, elimination of ‘steady-state’ DC (ssDC) in mice provokes uncontrolled lymphoproliferation and autoimmunity indicating that CD11c+ ssDC maintain peripheral tolerance [20,21]. Second, targeting antigen to ssDC using antibodies against surface receptors such as DEC-205 induced tolerance characterised by transient T-cell activation followed by cell death, anergy and the generation of regulatory T (Treg) cells [22,23]. The key point here is that this approach kept the targeted DC in the steady-state since no DC-activating inflammatory signals were provided.
Figure 1.
Properties of steady-state dendritic cells: ssDC in situ are relatively immature. They have low levels of both class II MHC and costimulatory molecules and secrete cytokines at low levels. ssDC undergo maturation under the influence of pathogen-associated molecular patterns (PAMPS), damage-associated molecular patterns (DAMPS), inflammatory cytokines such as TNF-α or CD40 ligation. The endosomal compartment in ssDC has a relatively high pH because of a low level of V-ATPase formed from its two subunits V0 and V1. The high pH in this compartment results in surface expression of unstable/peptide-receptive MHC II molecules. Endosomal acidification following formation of the V-ATPase in mature DC results in optimal function of the antigen presenting machinery stable MHC which with increased expression of costimulatory molecules results in T cell activation and differentiation into effector cells.
Novel approaches for AIT in autoimmune diseases
Current approaches being developed for AIT in autoimmune diseases include those targeting ssDC or directing antigens to liver APC (Table 1).
Table 1.
Novel approaches in development for AIT of autoimmune diseases
| Company | Delivery approach | Proposed mechanism of action | Impact on T cell response | Efficacy in experimental models | Clinical trial progress |
|---|---|---|---|---|---|
| Anokion | Antigens modified with polymeric forms of either N-acetylgalactosamine or N-acetylglucosamine | Target hepatic antigen-presenting cells | Induce CD4+ and CD8+ T-cell deletion and anergy | EAEb, T1D | Enrolling patients for KAN-101 trial in coeliac disease |
| Apitope International NV | Synthetic peptides designed as antigen processing independent CD4+ T cell epitopes (apitopes) injected in saline i.d. or s.c. | Bind selectively to steady-state DC in lymphoid organs | Induction of anergy and generation of regulatory T cells (Tr1 and Foxp3) | EAE and Graves’ disease models | Completed trials in MS and Graves’ disease |
| Phase Ia in SPMS | |||||
| Phase Ib in RRMS | |||||
| Phase II in RRMS | |||||
| Phase I in Graves’ disease | |||||
| Cellerys | Red blood cells (RBC) coupled with peptides from myelin in MS | Cell target macrophages and Kupffer cells in spleen and liver. | Increase in Tr1 cell response to antigen with reduced IFN-γ | Phase 1 in RRMSc | |
| Cour/Takeda | Antigen encapsulated in PLG (poly(lactide-co-glycolide)) nanoparticles | Ag-PLG internalized by splenic marginal zone macrophages and liver phagocytic cells via scavenger receptors (MARCO) | Increase in Foxp3 Treg cells, dependent on CTLA-4, PD-1 and IL-10 | EAE, T1D and Coeliac disease models | Phase I in patients with coeliac diseasee |
| Dendright/Janssen Biotech Inc | Antigen with calcitriol in liposomes | Liposomes (105−135 nm) target steady-state DC in draining lymph nodes | Increase in Foxp3 Treg cells | Autoimmune arthritis and experimental Goodpasture’s vasculitis | Phase I in ACPA + rheumatoid arthritisa |
| Imcyse | T cell epitopes modified by addition of a thioredox motif (CXXC), injected in alum adjuvant | Promotes cytotoxic activity in T cells through increasing expression of granzyme B and FasL | Cytotoxic cells delete B cells in cognate recognition | T1D | Phase I in T1D recruiting for phase IIf |
| Novo Nordisk | Plasmid DNA encoding proinsulin and co-expressing IL-10 and TGF-β | Promotes Treg cells | Promotes Treg cell differentiation | T1D with vector expressing GAD antigen | |
| Parvus | Nanoparticles coated with MHC II proteins and antigenic peptides | Bind directly to CD4+ effector cells | Drives differentiation of Tr1 cells from Th1 precursors in mice | EAE, CIA, T1D and autoimmune liver diseases | Phase I in coeliac disease planned for 2023 |
| Selecta | PLG nanoparticles containing rapamycin co-administered with antigen | Nanoparticles found in dendritic cells in spleen and LSEC and Kupffer cells in the liver where they mediate down-regulation of CD80, 86, class II MHC and upregulation of PDL-1 | Promotes Treg cell differentiation | EAE and anti-drug antibodies | Phase II in gout designed to block the anti-drug antibody response to pegadricased |
| Tolerion | DNA encoding self-antigen | CpG islands in DNA replaced with GpG to reduce immunogenicity of antigen delivery | Promote immune regulatory response to self-antigen | BHT-3021 prevents T1D in mouse model | Phase I trial in T1D completed and phase II enrolling |
| Topaz | Ferromagnetic nanoparticles coupled to T cell epitopes | Nanoparticle-based autoantigen delivery to liver sinusoidal endothelial cells | Induction of Foxp3+ Treg cells in the liver | EAE | Phase I trial of TPM203 in pemphigus vulgaris |
MULTIPLE SCLEROSIS JOURNAL Volume: 25 Special Issue: SI Supplement: 2 Pages: 894−894 Published: SEP 2019.
Volume: 158 Issue: 6 Supplement: 1 Pages: S135-S135 Published: MAY 2020.
https://clinicaltrials.gov/ct2/show/NCT03272269 (no results posted at time of review).
As reviewed elsewhere, the liver is especially important for tolerance induction [24]. The liver recycles cells and material from the rest of the body and, therefore, needs fail-safe mechanisms to maintain tolerance to self-antigens. A prime example is ageing or damaged red blood cells [25]: this recycling pathway is now being targeted for tolerance induction via liver APC [26]. Liver sinusoidal endothelial cells (LSECs) are highly effective in promoting TGF-β-dependent, Foxp3+ Treg when compared with other liver cell types [27]. Myelin peptides targeted to LSECs with iron oxide nanoparticles induced antigen-specific Treg that controlled EAE in mice [28••].
Nanoparticles and liposomes allow encapsulation of antigen or delivery with immunosuppressants and are believed to reduce the risk of immunogenicity of poorly soluble peptides and proteins. The fate of antigenic particles, from peptides to nanoparticles, depends on their size. Berkland et al. have shown that particles >200 nm are retained in the liver while those <4 nm are excreted [29]. Small particles, including soluble peptides, drain rapidly from sites of injection with particles of 4–10 nm penetrating the lymph node cortex to encounter ssDCs. Particles >100 nm, on the other hand, are retained in the subcapsular space where they are processed by macrophages. Nanoparticle approaches in clinical development range from medium sized particles enclosing rapamycin [30••] to larger particles taken up by monocytes that then traffic to lymphoid tissues and liver where they release antigen [31••]. Subcutaneous administration of liposomes encapsulating peptide antigen and calcitriol targets PD-L1hi DCs, reduces their MHC class II expression, suppresses expansion of T effector cells and induces both antigen-specific Foxp3+ and IL-10+ Tregs [32••].
Recently, Krienke et al. described the use of RNA vaccines as a means of delivering autoantigenic peptides for AIT [33••]. Systemic delivery of 1-methylpseudouridine-modified mRNA coding for disease-associated T-cell epitopes resulted in their expression and antigen processing by CD11c+ ssDC, a reduction of effector T-cells and differentiation of Tregs capable of bystander suppression. It would be a major advantage if this approach could safely deliver whole antigens for AIT.
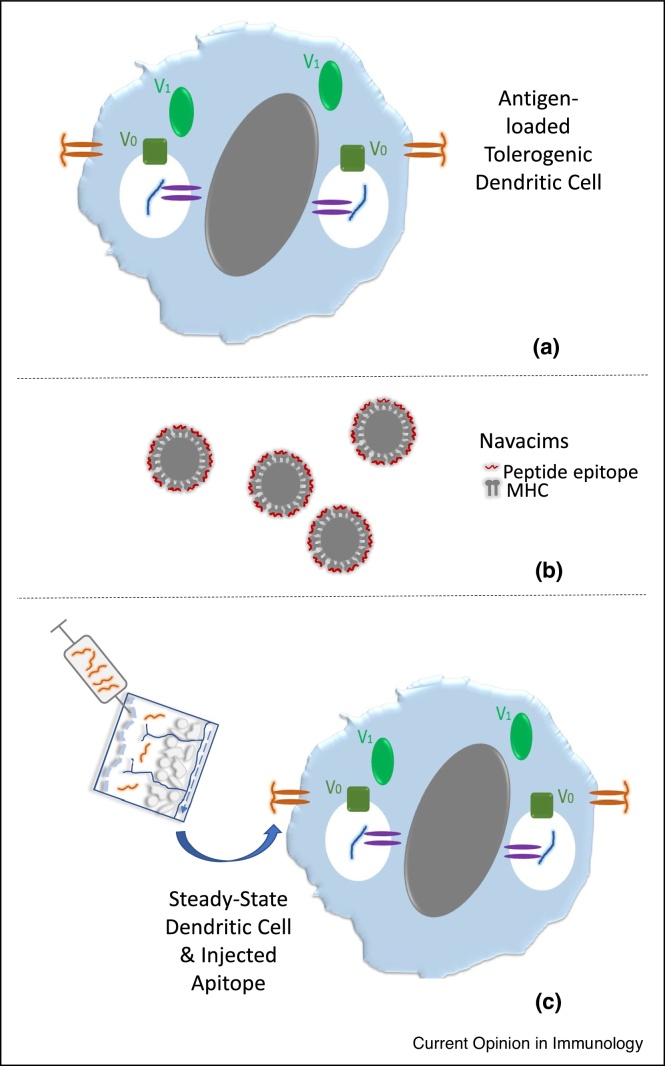
‘Direct’ antigen presentation for AIT has been achieved in three ways (Figure 2). First, human DC are generated in vitro, rendered tolerogenic by culture with vitamin D3, NFκB inhibitors or IL-10, combined with peptide epitopes and injected by intravenous, intradermal or intranodal routes. Phase I clinical trials have shown that this approach is well tolerated with preliminary evidence of efficacy [34•,35•,36].
Figure 2.
‘Direct’ delivery of antigenic epitopes for tolerance induction: (a) immature DCs generated in vitro and treated with drugs to block maturation are incubated with peptide antigens in vitro, the peptides bind to MHC and stabilise the complex. The resulting tolerogenic DC are injected by intravenous, intradermal or intranodal routes for direct presentation of these epitopes to T cells. (b) Navacims are nanoparticles coated with MHC-peptide complexes. In mice, repeated injection of Navacims (∼10x) induces antigen-specific Tr1 cells. (c) Apitopes, soluble T cell epitopes, are injected intradermally or subcutaneously. Peptides migrate rapidly to lymphoid organs via blood or lymph where they bind directly to and stabilise MHC II proteins on or in ssDC. Repeated injection of apitopes induces antigen-specific Treg and Tr1 cells to control autoimmune disease.
Secondly, ‘direct’ exposure of T-cells to peptide epitopes may be achieved by loading them onto MHC-coupled nanoparticles (Navacims). Navacims have been shown to suppress a range of autoimmune disease models in mice [37•,38••]. They modify the function of previously activated Th1 cells rather than naïve T-cells converting them into cells with a Tr1 phenotype. The nanoparticles depend on accurate spacing of MHC molecules for optimal efficacy [39] and this could complicate large-scale manufacture.
Finally, peptides defined as apitopes (antigen processing independent T-cell epitopes) also bind directly to MHC II. Apitopes mimic naturally processed epitopes from self-antigens, suppress inflammatory cytokine secretion and promote differentiation of Tr1 cells, reviewed in Ref. [40]. Apitope mediated Tr1 cell differentiation is directly related to affinity for MHC with the resulting cells mediating negative feedback regulation by suppressing DC maturation. Repeated administration of apitopes drives a sequential change in gene expression [41•] with expression of tolerance genes similar to that seen in tumour infiltrating lymphocytes and other forms of induced tolerance [42••]. Analysis of cell signalling following tolerance induction showed that the resulting anergic, Tr1 cells have a membrane proximal block in signalling substantially limiting levels of downstream transcription factors [43••].
Recent work has shown that tolerance-associated genes induced in Tr1 cells, including IL-10, inhibitory receptors (CTLA-4, TIGIT, Tim-3 etc.) and IL-10-regulating transcription factors, are all modified by epigenetic priming [43••]. This opens chromatin close to tolerance-associated genes making them sensitive to low levels of transcription factors. This novel mechanism explains how potentially pathogenic T-cells transform into regulatory Tr1 cells mediating the negative feedback associated with apitope-induced AIT. This novel mechanism combining reduced TCR-mediated signalling with selective epigenetic changes may also account for the Th1-Tr1 conversion by navacims [37•] or the negative feedback regulation seen in chronic infections [44] and tumour infiltrating cells [42••].
Apitopes selectively bind steady state DC
Apitopes administered by subcutaneous or intranasal delivery migrate rapidly to lymphoid organs and can be detected on splenic APC within minutes [45]. Recent work has revealed that apitopes bind rapidly and selectively to ssDC rather than B cells and monocytes following subcutaneous injection [46•]. Santambrogio et al. have shown that splenic ssDC have unstable MHC II at the cell surface that is stabilised by exogenous peptide whereas B cells and monocytes do not [47]. DC maturation is controlled by TLRs, cytokine receptors and costimulation [48] and correlates with both the loss of peptide-receptive MHC II at the cell surface [47] and upregulation of costimulatory molecules (Figure 1). Soluble apitopes, therefore, selectively target tolerogenic ssDC because these cells express unstable MHC II; but, why do ssDC have peptide-receptive MHC at the cell surface? There are various properties that distinguish tolerogenic ssDC from mature DC; however, the critical, distinguishing changes affecting antigen processing and presentation are governed by endosomal pH. Trombetta et al. have shown that the endosomal pH in immature DCs is high compared to mature DC [49] and this is governed by the 2 subunit V-ATPase. DC maturation induces the recruitment of cytosolic V-ATPase subunits to the endosomal/lysosomal membrane to create the ion pump required for acidification. Acidification is essential for the optimal function of pH-dependent proteases and chaperone proteins such as DM [50] in antigen processing and presentation. This explains why immature ssDC fail to process and present antigens effectively and why peptide-receptive MHC II appears at the cell surface. The low level of costimulatory molecules expressed by ssDC [48] renders them tolerogenic. This makes ssDC the ideal target for AIT since they remain tolerogenic if the peptide epitope is delivered without PAMPs or DAMPs (Figure 1).
Clinical development of AIT with apitopes
The ultimate test of any therapeutic approach is its safe and effective application in the clinic. As yet, few of the approaches described above, other than tolerogenic DCs [34•,35•] or apitopes [18••,19••], have published outcomes of clinical trials (Table 1). In total, 78 patients have been treated with apitopes with no unexpected side effects. Furthermore, a phase I trial of apitopes in Graves’ disease, with epitopes from the TSHR [51•], noted a reduction in both thyroid hormone secretion and anti-TSHR antibodies in 7/10 patients [19••]. A phase 1b trial of intradermal apitopes from MBP in relapsing-MS [52•], reported a significant (p = 0.03) reduction in gadolinium-enhancing lesions while a phase 2 study revealed a marked improvement in cognition that correlated with suppression of CNS inflammation [18••]. The results of these trials show that the use of ssDC-targeting apitopes holds promise as a specific immunotherapy for a range of autoimmune conditions.
Conclusions
In 2000, Harrison and Hafler stated that AIT for autoimmune diseases was ‘closer to the Holy Grail’ [53]. The intervening years have seen evolution of novel approaches to target self-antigens to APCs including ssDC and liver APC. In our view, there are three questions that must be addressed by each approach. What is the mechanism of action in affected individuals and does this allow their safe administration to individuals? Does the approach induce bystander suppression? Most autoimmune diseases involve more than one antigen: a bystander-suppressive mechanism will be needed to control epitope spreading [54]. Does the approach allow repeated administration? Our experience of AIT with apitopes shows that repeated exposure will be required for maintenance of tolerance. Continuing progress in this field convinces us that AIT for autoimmune diseases is now ‘reaching the Holy Grail’.
Conflict of interest statement
HS and DW declare stock ownership in Apitope International NV. DW serves as a consultant to Apitope International NV and has sat on scientific advisory boards for Actelion Pharma and Zealand Pharma; is a senior editor for Immunotherapy; holds patents for peptides, tolerisation-inducing composition, FVIII peptides and their use in tolerising haemophiliacs, composition, disease markers, tolerogenic peptides from myelin basic protein, peptide selection method, and improvements relating to influenza vaccine; has consulted for Peptide Therapeutics Ltd., Teva, GSK Bio, Hoffmann-La Roche, Novartis, DTI, and the Food Standards Agency; received research support within the past 3 years from UCB Celltech and was an expert witness for Geron.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
The authors acknowledge support from the University of Birmingham, Medical Research Council (MR/K007645/1, MR/K015990/1), Wellcome Trust (204565/Z/16/Z), EU-IMI (RTCure consortium), Diabetes UK, Helmsley Trust & Children’s Liver Disease Foundation.
References
- 1.Barrett J.C., Clayton D.G., Concannon P., Akolkar B., Cooper J.D., Erlich H.A., Julier C., Morahan G., Nerup J., Nierras C. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libert C., Dejager L., Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 3.Lerner A., Jeremias P., Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int J Celiac Dis. 2015;3:151–155. [Google Scholar]
- 4.Patterson C.C., Dahlquist G.G., Gyurus E., Green A., Soltesz G., Group E.S. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 5.Noon L. Prophylactic innoculation against hay fever. Lancet. 1911:1572–1573. [Google Scholar]
- 6.Patel D., Couroux P., Hickey P., Salapatek A.M., Laidler P., Larche M., Hafner R.P. Fel d 1-derived peptide antigen desensitization shows a persistent treatment effect 1 year after the start of dosing: a randomized, placebo-controlled study. J Allergy Clin Immunol. 2013;131:103–109. doi: 10.1016/j.jaci.2012.07.028. e101-e107. [DOI] [PubMed] [Google Scholar]
- 7.Faria A.M., Weiner H.L. Oral tolerance: therapeutic implications for autoimmune diseases. Clin Dev Immunol. 2006;13:143–157. doi: 10.1080/17402520600876804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benson J.M., Stuckman S.S., Cox K.L., Wardrop R.M., Gienapp I.E., Cross A.H., Trotter J.L., Whitacre C.C. Oral administration of myelin basic protein is superior to myelin in suppressing established relapsing experimental autoimmune encephalomyelitis. J Immunol. 1999;162:6247–6254. [PubMed] [Google Scholar]
- 9.Genain C.P., Abel K., Belmar N., Villinger F., Rosenberg D.P., Linington C., Raine C.S., Hauser S.L. Late complications of immune deviation therapy in nonhuman primate. Science. 1996;274:2054–2057. doi: 10.1126/science.274.5295.2054. [DOI] [PubMed] [Google Scholar]
- 10.Hanninen A., Braakhuis A., Heath W.R., Harrison L.C. Mucosal antigen primes diabetogenic cytotoxic T-lymphocytes regardless of dose or delivery route. Diabetes. 2001;50:771–775. doi: 10.2337/diabetes.50.4.771. [DOI] [PubMed] [Google Scholar]
- 11.Rapoport B., Banuelos B., Aliesky H.A., Hartwig Trier N., McLachlan S.M. Critical differences between induced and spontaneous mouse models of Graves’ disease with implications for antigen-specific immunotherapy in humans. J Immunol. 2016;197:4560–4568. doi: 10.4049/jimmunol.1601393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith E.L., Peakman M. Peptide immunotherapy for type 1 diabetes-clinical advances. Front Immunol. 2018;9:392. doi: 10.3389/fimmu.2018.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderton S.M., Radu C.G., Lowrey P.A., Ward E.S., Wraith D.C. Negative selection during the peripheral immune response to antigen. J Exp Med. 2001;193:1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderton S.M., Kissler S., Lamont A.G., Wraith D.C. Therapeutic potential of TCR antagonists is determined by their ability to modulate a diverse repertoire of autoreactive T cells. Eur J Immunol. 1999;29:1850–1857. doi: 10.1002/(SICI)1521-4141(199906)29:06<1850::AID-IMMU1850>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 15.Bielekova B., Goodwin B., Richert N., Cortese I., Kondo T., Afshar G., Gran B., Eaton J., Antel J., Frank J.A. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83-99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–1175. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 16.Kappos L., Comi G., Panitch H., Oger J., Antel J., Conlon P., Steinman L. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. Nat Med. 2000;6:1176–1182. doi: 10.1038/80525. [DOI] [PubMed] [Google Scholar]
- 17•.Ellis A.K., Frankish C.W., O’Hehir R.E., Armstrong K., Steacy L., Larche M., Hafner R.P. Treatment with grass allergen peptides improves symptoms of grass pollen-induced allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2017;140:486–496. doi: 10.1016/j.jaci.2016.11.043. [DOI] [PubMed] [Google Scholar]
- 18••.Chataway J., Martin K., Barrell K., Sharrack B., Stolt P., Wraith D.C., Group A-MS Effects of ATX-MS-1467 immunotherapy over 16 weeks in relapsing multiple sclerosis. Neurology. 2018;90:e955–e962. doi: 10.1212/WNL.0000000000005118. [DOI] [PubMed] [Google Scholar]; Evidence that apitopes from MBP (see Ref. [52•]) are well tolerated with preliminary evidence of efficacy as shown by suppression of CNS inflammation and improvement in cognition.
- 19••.Pearce S.H.S., Dayan C., Wraith D.C., Barrell K., Olive N., Jansson L., Walker-Smith T., Carnegie C., Martin K.F., Boelaert K. Antigen-specific immunotherapy with thyrotropin receptor peptides in Graves’ hyperthyroidism: a phase I study. Thyroid. 2019;29:1003–1011. doi: 10.1089/thy.2019.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that apitopes from TSHR (see Ref. [51•]) are well tolerated with preliminary evidence of efficacy as shown by suppression of hyperthyroidism in 7/10 individuals treated.
- 20.Birnberg T., Bar-On L., Sapoznikov A., Caton M.L., Cervantes-Barragan L., Makia D., Krauthgamer R., Brenner O., Ludewig B., Brockschnieder D. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Ohnmacht C., Pullner A., King S.B., Drexler I., Meier S., Brocker T., Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawiger D., Inaba K., Dorsett Y., Guo M., Mahnke K., Rivera M., Ravetch J.V., Steinman R.M., Nussenzweig M.C. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 24.Richardson N., Ng S.T.H., Wraith D.C. Antigen-specific immunotherapy for treatment of autoimmune liver diseases. Front Immunol. 2020;11:1586. doi: 10.3389/fimmu.2020.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theurl I., Hilgendorf I., Nairz M., Tymoszuk P., Haschka D., Asshoff M., He S., Gerhardt L.M., Holderried T.A., Seifert M. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22:945–951. doi: 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kontos S., Kourtis I.C., Dane K.Y., Hubbell J.A. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci U S A. 2013;110:E60–E68. doi: 10.1073/pnas.1216353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carambia A., Freund B., Schwinge D., Heine M., Laschtowitz A., Huber S., Wraith D.C., Korn T., Schramm C., Lohse A.W. TGF-beta-dependent induction of CD4(+)CD25(+)Foxp3(+) Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61:594–599. doi: 10.1016/j.jhep.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 28••.Carambia A., Freund B., Schwinge D., Bruns O.T., Salmen S.C., Ittrich H., Reimer R., Heine M., Huber S., Waurisch C. Nanoparticle-based autoantigen delivery to Treg-inducing liver sinusoidal endothelial cells enables control of autoimmunity in mice. J Hepatol. 2015;62:1349–1356. doi: 10.1016/j.jhep.2015.01.006. [DOI] [PubMed] [Google Scholar]; Antigen carrying nanoparticles target liver sinusoidal endothelial cells and induce Treg cells that control inflammatory autoimmune disease in mice.
- 29.Hartwell B.L., Antunez L., Sullivan B.P., Thati S., Sestak J.O., Berkland C. Multivalent nanomaterials: learning from vaccines and progressing to antigen-specific immunotherapies. J Pharm Sci. 2015;104:346–361. doi: 10.1002/jps.24273. [DOI] [PubMed] [Google Scholar]
- 30••.Meliani A., Boisgerault F., Hardet R., Marmier S., Collaud F., Ronzitti G., Leborgne C., Costa Verdera H., Simon Sola M., Charles S. Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat Commun. 2018;9 doi: 10.1038/s41467-018-06621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that focusing rapamycin on APC can suppress the immune response to adeno associated virus with important implications for gene therapy.
- 31••.Freitag T.L., Podojil J.R., Pearson R.M., Fokta F.J., Sahl C., Messing M., Andersson L.C., Leskinen K., Saavalainen P., Hoover L.I. Gliadin nanoparticles induce immune tolerance to gliadin in mouse models of celiac disease. Gastroenterology. 2020;158:1667–1681. doi: 10.1053/j.gastro.2020.01.045. e1612. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that delivery of whole proteins associated with nanoparticles can induce tolerance to gliadin in mice. Results of clinical trials in coeliac disease await publication.
- 32••.Galea R., Nel H.J., Talekar M., Liu X., Ooi J.D., Huynh M., Hadjigol S., Robson K.J., Ting Y.T., Cole S. PD-L1- and calcitriol-dependent liposomal antigen-specific regulation of systemic inflammatory autoimmune disease. JCI Insight. 2019;4 doi: 10.1172/jci.insight.126025. [DOI] [PMC free article] [PubMed] [Google Scholar]; Evidence that liposomes carrying peptide and calcitriol can suppress the immune response to antigen in mice.
- 33••.Krienke C., Kolb L., Diken E., Streuber M., Kirchhoff S., Bukur T., Akilli-Ozturk O., Kranz L.M., Berger H., Petschenka J. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–153. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]; A novel approach using hypoimmunogenic RNA to express antigenic peptide. Peptide presented by steady-state DC induces tolerance and induced regulatory cells that mediate bystander suppression in mice.
- 34•.Bell G.M., Anderson A.E., Diboll J., Reece R., Eltherington O., Harry R.A., Fouweather T., MacDonald C., Chadwick T., McColl E. Autologous tolerogenic dendritic cells for rheumatoid and inflammatory arthritis. Ann Rheum Dis. 2017;76:227–234. doi: 10.1136/annrheumdis-2015-208456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Benham H., Nel H.J., Law S.C., Mehdi A.M., Street S., Ramnoruth N., Pahau H., Lee B.T., Ng J., Brunck M.E. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa9301. [DOI] [PubMed] [Google Scholar]
- 36.Willekens B., Presas-Rodriguez S., Mansilla M.J., Derdelinckx J., Lee W.P., Nijs G., De Laere M., Wens I., Cras P., Parizel P. Tolerogenic dendritic cell-based treatment for multiple sclerosis (MS): a harmonised study protocol for two phase I clinical trials comparing intradermal and intranodal cell administration. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Clemente-Casares X., Blanco J., Ambalavanan P., Yamanouchi J., Singha S., Fandos C., Tsai S., Wang J., Garabatos N., Izquierdo C. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530:434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 38••.Umeshappa C.S., Singha S., Blanco J., Shao K., Nanjundappa R.H., Yamanouchi J., Pares A., Serra P., Yang Y., Santamaria P. Suppression of a broad spectrum of liver autoimmune pathologies by single peptide-MHC-based nanomedicines. Nat Commun. 2019;10 doi: 10.1038/s41467-019-09893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; With Ref. [37•] this provides evidence that nanoparticles displaying MHC II and peptide antigen promote Tr1 cells capable of bystander suppression that control a range of tissue specific autoimmune conditions.
- 39.Singha S., Shao K., Yang Y., Clemente-Casares X., Sole P., Clemente A., Blanco J., Dai Q., Song F., Liu S.W. Peptide-MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nat Nanotechnol. 2017;12:701–710. doi: 10.1038/nnano.2017.56. [DOI] [PubMed] [Google Scholar]
- 40.Wraith D.C. Designing antigens for the prevention and treatment of autoimmune diseases. Curr Opin Chem Eng. 2018;19:35–42. [Google Scholar]
- 41•.Burton B.R., Britton G.J., Fang H., Verhagen J., Smithers B., Sabatos-Peyton C.A., Carney L.J., Gough J., Strobel S., Wraith D.C. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nat Commun. 2014;5 doi: 10.1038/ncomms5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Chihara N., Madi A., Kondo T., Zhang H., Acharya N., Singer M., Nyman J., Marjanovic N.D., Kowalczyk M.S., Wang C. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature. 2018;558:454–459. doi: 10.1038/s41586-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reveals a transcriptional signature shared between regulatory T cells and tumour infiltrating lymphocytes including genes for inhibitory receptors, IL-10 and transcription factors regulating IL-10 secretion.
- 43••.Bevington S.L., Ng S.T.H., Britton G.J., Keane P., Wraith D.C., Cockerill P.N. Chromatin priming renders T cell tolerance-associated genes sensitive to activation below the signaling threshold for immune response genes. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107748. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes a novel mechanism regulating gene expression that controls antigen-specific tolerance induction (Ref. [41•]) whereby signalling blockade is coupled with epigenetic priming at tolerance associated genes.
- 44.Trinchieri G. Interleukin-10 production by effector T cells: Th1 cells show self control. J Exp Med. 2007;204:239–243. doi: 10.1084/jem.20070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Metzler B., Anderton S.M., Manickasingham S.P., Wraith D.C. Kinetics of peptide uptake and tissue distribution following a single intranasal dose of peptide. Immunol Invest. 2000;29:61–70. doi: 10.3109/08820130009105145. [DOI] [PubMed] [Google Scholar]
- 46•.Shepard E.R., Wegner A., Hill E.V., Burton B.R., Aerts S., Schurgers E., Hoedemaekers B., Ng T.H.S., Streeter H.B., Jansson L. The mechanism of action of antigen processing independent T cells epitopes for immunotherapy of autoimmune diseases. Front Immunol. 2021;12:1–14. doi: 10.3389/fimmu.2021.654201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santambrogio L., Sato A.K., Fischer F.R., Dorf M.E., Stern L.J. Abundant empty class II MHC molecules on the surface of immature dendritic cells. Proc Natl Acad Sci U S A. 1999;96:15050–15055. doi: 10.1073/pnas.96.26.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammer G.E., Ma A. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Annu Rev Immunol. 2013;31:743–791. doi: 10.1146/annurev-immunol-020711-074929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trombetta E.S., Ebersold M., Garrett W., Pypaert M., Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400–1403. doi: 10.1126/science.1080106. [DOI] [PubMed] [Google Scholar]
- 50.Busch R., Reich Z., Zaller D.M., Sloan V., Mellins E.D. Secondary structure composition and pH-dependent conformational changes of soluble recombinant HLA-DM. J Biol Chem. 1998;273:27557–27564. doi: 10.1074/jbc.273.42.27557. [DOI] [PubMed] [Google Scholar]
- 51•.Jansson L., Vrolix K., Jahraus A., Martin K.F., Wraith D.C. Immunotherapy with apitopes blocks the immune response to thyroid stimulating hormone receptor in HLA-DR transgenic mice. Endocrinology. 2018;159:3446–3457. doi: 10.1210/en.2018-00306. en.2018-00306. [DOI] [PubMed] [Google Scholar]
- 52•.Streeter H.B., Rigden R., Martin K.F., Scolding N.J., Wraith D.C. Preclinical development and first-in-human study of ATX-MS-1467 for immunotherapy of MS. Neurol Neuroimmunol Neuroinflamm. 2015;2 doi: 10.1212/NXI.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harrison L.C., Hafler D.A. Antigen-specific therapy for autoimmune disease. Curr Opin Immunol. 2000;12:704–711. doi: 10.1016/s0952-7915(00)00166-7. [DOI] [PubMed] [Google Scholar]
- 54.Vanderlugt C.L., Miller S.D. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]