Abstract
Respiratory symptoms are one of COVID-19 manifestations, and the metalloproteinases (MMPs) have essential roles in the lung physiology. We sought to characterize the plasmatic levels of matrix metalloproteinase-2 and 9 (MMP-2 and MMP-9) in patients with severe COVID-19 and to investigate an association between plasma MMP-2 and MMP-9 levels and clinical outcomes and mortality. MMP-2 and MMP-9 levels in plasma from patients with COVID-19 treated in the ICU (COVID-19 group) and Control patients were measured with the zymography. The study groups were matched for age, sex, hypertension, diabetes, BMI, and obesity profile. MMP-2 levels were lower and MMP-9 levels were higher in a COVID-19 group (p < 0.0001) compared to Controls. MMP-9 levels in COVID-19 patients were not affected by comorbidity such as hypertension or obesity. MMP-2 levels were affected by hypertension (p < 0.05), but unaffected by obesity status. Notably, hypertensive COVID-19 patients had higher MMP-2 levels compared to the non-hypertensive COVID-19 group, albeit still lower than Controls (p < 0.05). No association between MMP-2 and MMP-9 plasmatic levels and corticosteroid treatment or acute kidney injury was found in COVID-19 patients. The survival analysis showed that COVID-19 mortality was associated with increased MMP-2 and MMP-9 levels. Age, hypertension, BMI, and MMP-2 and MMP-9 were better predictors of mortality during hospitalization than SAPS3 and SOFA scores at hospital admission. In conclusion, a significant association between MMP-2 and MMP-9 levels and COVID-19 was found. Notably, MMP-2 and MMP-9 levels predicted the risk of in-hospital death suggesting possible pathophysiologic and prognostic roles.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ACE, angiotensin-converting enzyme; ACE2, angiotensin-converting enzyme 2; AKI, Acute Kidney Injury; Ang, angiotensin; ARB, angiotensin receptor blockers; COVID-19, Coronavirus Disease 2019; iRAAs, inhibitors of the renin-angiotensin system; MMPs, metalloproteinases; MMP-9, Matrix Metalloproteinase 9; MMP-2, Matrix Metalloproteinase 2; RAS, renin-angiotensin system; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; SAPS3, Simplified Acute Physiology Score III; SOFA, sequential organ failure assessment score
Keywords: SARS-COV2 infection, COVID-19 pathophysiology, Metalloproteinases, MMP-2, MMP-9
Graphical Abstract
1. Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is responsible for Coronavirus Disease 2019 (COVID-19). COVID-19 has already caused over 4340,000 deaths (August 2021) and infected over 200 million cases in 220 countries, according to the John Hopkins Coronavirus Resource Center [1]. SARS-CoV-2 infects cells using the angiotensin-converting enzyme 2 (ACE2), similarly to SARS-CoV [2], and activates the immune system leading to cytokine storm [3] characterized by markedly increased levels of cytokines (interleukins (IL)− 1α, IL-1β, IL-6 and TNF-α) [4], increased count of neutrophils and decreased count of lymphocytes [5]. COVID infection also stimulates ROS generation [6] and coagulation cascade increasing the risk for thrombosis in micro and macro vasculature [7]. COVID-19 might also trigger lung injury that progresses to acute respiratory distress syndrome, leading to respiratory failure, sepsis, septic shock [8], and death [9], [10]. In adult patients, the risk of developing severe COVID-19, which requires intensive care unit admission (ICU), is higher with age and comorbidities such as obesity, hypertension, and other cardiovascular diseases [11], [12].
Respiratory symptoms are one of COVID-19 manifestations [9] and the metalloproteinases (MMPs) have essential roles in lung disease [13], [14]. It has been shown recently that the expression of metalloproteinases-9 (MMP-9) gene is upregulated in COVID-19 patients [15] and that MMP-9 levels, as measured by immunoassay, are directly proportional to a risk of respiratory failure [16]. Duda et al.[17] found that MMP-9 plasmatic levels are greater at day 7 of hospitalization in non-survivors in non-COVID-19 ICU patients [17], suggesting prognostic relevance of this enzyme. Also, MMP-9 levels have been shown to be increased in severe COVID-19 and to be associated with mortality in those patients [18].
The severe form of COVID-19 has many similar features to sepsis [19], and both metalloproteinases-2 (MMP-2) and MMP-9 have been considered as potential biomarkers for septic patients [17], [20]. Aguirre et al.[20] have shown reduced MMP-2 levels in patients meeting criteria for sepsis [20]. Despite the recognized role of MMP-2 as an anti-inflammatory factor [21], [22], little is known about the role of MMP-2 in COVID-19.
Despite the aforementioned current understanding of COVID-19 disease as a new infection, there is still much to be understood, more insight into the pathophysiology of the disease is critically needed. This study sought to investigate the association between the plasmatic levels of MMP-2 and MMP-9 and COVID-19 outcomes.
2. Material and methods
2.1. Patients, Plasma samples and demographic
The COVID-19 group included 53 patients who were admitted to the Intensive Care Unit (ICU). COVID-19 infection was confirmed by a positive RT-PCR test. The Control group consisted of 28 apparently healthy subjects whose blood was collected before the COVID-19 pandemic. Blood samples were drawn from all patients in citrate blood collectors, and the COVID-19 group had blood collected within 48 H of the ICU admission.
2.2. Ethics
This study was approved by the Ethic Committee of the Hospital das Clínicas de Ribeirão Preto- University of São Paulo, Brazil (CAAE: 30816620.0.0000.5440). All participants or family members have provided informed consent.
2.3. Demographic and clinical data
Demographic, clinical, and laboratory data were obtained from the hospital's digital database. The parameters considered in the analysis were: age, sex, body mass index (BMI), use of inhibitors of the renin-angiotensin system (iRAAs) such as angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB), use of corticosteroids during hospitalization, days of symptoms, days after worsening of symptoms out of ICU, length of hospitalization, development of acute kidney injury (AKI), dialysis during hospitalization, and previous comorbidities such as diabetes, systemic hypertension and chronic cardiovascular diseases (CVD). The following laboratory parameters were collected and analyzed: neutrophil, lymphocyte and platelet count, bilirubin, creatinine, lactate, C-reactive protein, and PaO2 / FiO2 ratio.
The scores used to predict the diagnosis of sepsis and the probability of death were the sequential assessment score for organ failure (SOFA score) [23] and the SAPS-3 [24], respectively. The SOFA score is used to diagnose sepsis and a score ranges from 0 to 28 [23], where 0 is considered a patient without organ failure and a score greater than or equal to 2 is considered a diagnosis of sepsis. The SOFA score considers both clinical and laboratory data from cardiovascular, respiratory and neurological systems such as Mean Blood Pressure (MAP) less than 70 mmHg and/or use of vasoactive drugs, PaO2/FiO2 with or without the need for ventilatory support and the Glasgow Coma Scale (ECG). The assessment of hepatic, renal and coagulation systems are based on bilirubin, creatinine and platelet count. The highest SOFA score obtained within the first 24 h of hospitalization were used in the analysis. The calculator used to quantify the SOFA (severity score) is available at the website of the Brazilian Institute of Intensive Medicine (http://www.medicinaintensiva.com.br/sofa.html).
The SAPS-3 is used to predict the probability of death in patients admitted to the ICU. It uses logarithmic equations for calculations and therefore score can range from 0 to 217 [24] with higher numbers reflecting higher probability of death. The SAPS − 3 score includes, in addition to the parameters used to calculate the SOFA score, leukocyte count, blood pH, heart rate, temperature, systolic blood pressure, comorbidities (such as cancer treatment, liver, kidney or cardiovascular disease, immunosuppression), age, length of hospitalization and location (other ICU, emergency room, ward) before admission to the ICU and a reason for admission. The highest SAPS-3 score (the highest probability of death) obtained in the first 24 h of hospitalization was used in the analysis. SAPS-3 calculations were adjusted to the geographic location of the individuals involved in the study (South America). The SAPS-3 calculator used in this study is available at https://www.rccc.eu/ppc/indicators/saps3.html.
2.4. Quantification of MMPs levels by zymography
The MMP-2 and MMP-9 levels were measured in plasma samples by gelatin zymography [25] Gels were stained with Coomassie Blue G-250 for 30 mins and unstained with 10% acetic acid and 30% methanol. The molecular weight for: MMP-2 (64KDa), and for MMP-9 + pro-MMP-9 (86–92 kDa). The results were then scanned at 400dpi and analyzed with ImageJ. We used this approach in accordance with an established protocol from our lab based on references found in the literature [26], [27], [28], [29].
2.5. Statistical analysis
Clinical data are presented as mean ± SEM. The Shapiro Wilk test was used to define distribution of continuous variables. Continuous variables were compared by the Mann-Whitney test for non-parametrically distributed variables and by unpaired t-test for parametrically distributed variables. Dichotomous variables were analyzed using the chi-square test. The Kruskal Wallis test with Dunn's multiple comparisons post-test was used to compare three or more groups. MMPs levels were all normalized to the control group in each graph. Multiple regression was used to analyze models with more than one variable to predict the outcome. ROC curves were designed and the area under the curve (AUC) was calculated. All statistical analyses were performed in Graph Pad Prism 8.
3. Results
3.1. Demographic and clinical data
COVID-19 and Control groups were matched for age and BMI, and distribution of male sex, hypertension, diabetes, usage of iRAAs prior to COVID-19 related hospitalization ( Table 1). As expected, the leukocyte (10.8 ± 0.6 ×10 ³/uL), neutrophil (8.7 ± 0.6 ×10 ³/uL) and platelet (243.6 ± 13.2 ×10 ³/L) counts were higher in the COVID-19 group than in the Control group (p < 0.01), but the average of lymphocytes was lower (1.1 ± 0.1 ×10 ³/mm³) (p < 0.0001). COVID-19 group showed an average SAPS-3 score of 59.8 (± 1.5) and average SOFA score of 8.8 (± 0.5). On average, patients who tested positive for SARS-CoV-2 were admitted to the ICU 10 days after the onset of symptoms, and 2.9 days after worsening of symptoms. The average ICU length of stay for COVID-19 patients was 15 days and mortality rate was 43% mortality.
Table 1.
Demographic and Clinical Data of Control and COVID-19 patients. COVID-19 and Control group were matched for age and BMI, and distribution of male sex, hypertension, diabetes, usage of pre-hospitalization iRAAS prior to COVID-19 related hospitalization. Clinical data are expressed as mean ± standard error of mean. BMI - Body Max Index, iRAAS- inhibitor of the renin angiotensin system (either angiotensin converting enzyme inhibitors or angiotensin receptor blockers), AKI - acute kidney injury, CVD - known history of Cardiovascular disease, SAPS3 - Simplified Acute Physiology Score III, SOFA - Sequential Organ Failure Assessment score, n/a- not applicable.
| Control (n = 29) | COVID-19 (53) | p | |
|---|---|---|---|
| Age | 57.8 ± 2.4 | 59.54 ± 1.7 | 0.561 |
| Male sex | 17(59) | 36(68) | 0.4 |
| Diabetes | 3(10) | 10(19) | 0.312 |
| Hypertension | 14(48) | 29(58) | 0.32 |
| BMI (kg/m2) | 30.0 ± 1.1 | 31.0 ± 1.18 | 0.823 |
| Obese individuals (BMI>30) | 13(44.8) | 27(50.9) | 0.596 |
| iRAAS prehospitalization | 7(24.1) | 24(45.3) | 0.059 |
| Leukocytes (x10/ mm3) | 6.6 ± 0.4 | 10.8 ± 0.6 * | < 0.0001 |
| Neutrophiles (x10/ mm3) | 4.7 ± 0.3 | 8.7 ± 0.6 * | < 0.0001 |
| Lymphocytes (x10/ mm3) | 1.6 ± 0.16 | 1.1 ± 0.1 * | < 0.0001 |
| Platelets (x103/l) | 186.8 ± 8.8 | 243.6 ± 13.23* | 0.004 |
| Days of symptoms | 10 ± 1 | ||
| Days from symptoms worsening | 2.9 ± 0.5 | ||
| SAPS3 | 59.8 ± 1.5 | ||
| SOFA | 8.8 ± 0.5 | ||
| Hospitalization days | 15.8 ± 1.6 | ||
| In hospital mortality | 23(43) |
3.2. Association between severe COVID-19 status and MMPs levels
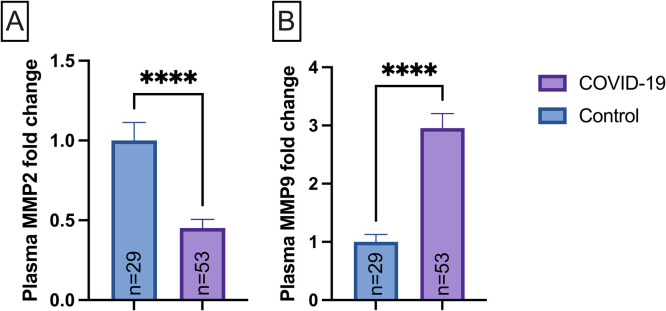
Firstly, we sought to determine whether the plasmatic MMP-2 and MMP-9 levels differed between COVID-19 and Control group ( Fig. 1). The plasma MMP-2 levels were 54.8% lower in the COVID-19 (p < 0.0001) as compared to the Control group (Fig. 1A). In contrast, plasma MMP-9 levels were 195.4% augmented in the COVID-19 group (p < 0.0001) compared to Control groups (Fig. 1B).
Fig. 1.
Plasmatic levels of metalloproteinase-2 (MMP-2, (A)) and metalloproteinase-9 (MMP-9, (B) in control (n = 29) and COVID-19 (n = 53) subjects’ group. Data are presented as mean ± SEM, normalized to control group, * ** *p < 0.0001 determined by Mann-Whitney test. AU = arbitrary unit.
3.3. Effect of hypertension and obesity on MMP-2 levels
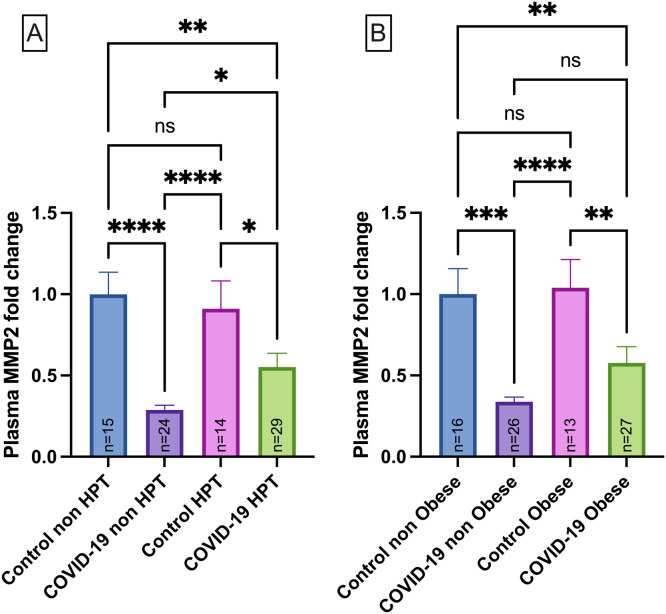
COVID-19 patients were categorized according to hypertension and obesity status. Interestingly, MMP-2 levels were upregulated in COVID-19 patients who were hypertensive (p < 0.05) but still downregulated in comparison to Controls ( Fig. 2A). No significant association between MMP-2 levels and obesity was found in COVID-19 patients (Fig. 2B).
Fig. 2.
MMP-2 plasma levels in normotensive Control group (Control non-HPT, n = 15) and Hypertensive Control group (HPT, n = 14), normotensive COVID-19 patients (COVID-19 non HPT, n = 24) and hypertensive COVID-19 patients (COVID-19 HPT, n = 29) (A); MMP-2 plasma levels in Control group with normal BMI (Control non-obese, n = 16) and Control group with obesity (Control obese, n = 13) and COVID-19 group with normal BMI (COVID-19 non obese, n = 26) and COVID-19 group with obesity (COVID-19 obese, n = 27) (B). Data are presented as mean ± SEM, normalized to control group *p< 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001,determined by Kruskal-Wallis test and Dunn’s post hoc test. AU = arbitrary unit. No differences were observed in the MMP-2 levels for intra group comparisons between non-HPT vs HPT or non-obese vs obese.
3.4. Effect of hypertension and obesity on MMP-9 levels
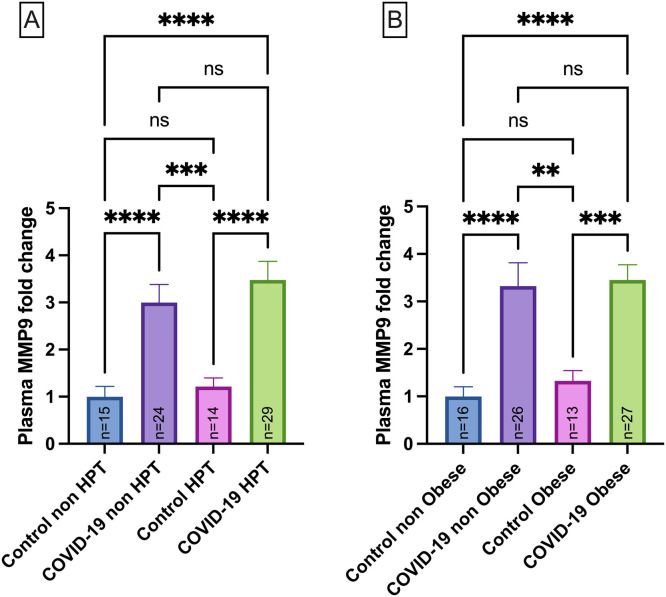
The MMP-9 levels were also evaluated according to the hypertension profile, and data demonstrated that MMP-9 upregulation in the COVID-19 occurs independently of hypertension (p < 0.05) ( Fig. 3A) and obesity (Fig. 3B) (p < 0.05).
Fig. 3.
MMP-9 plasma levels in normotensive Control group (Control non-HPT, n = 15) and Hypertensive Control group (Control HPT, n = 14), normotensive COVID-19 patients (COVID-19 non-HPT, n = 24) and hypertensive COVID-19 patients (COVID-19 HPT, n = 29) (A); MMP-2 plasma levels in Control group with normal BMI (control non-obese, n = 16) and Control group with obesity (Control obese, n = 13) and COVID-19 group with normal BMI (COVID-19 non obese,n = 26) and COVID-19 group with obesity (COVID-19 obese, n = 27) (B). Data are presented as mean ± SEM, normalized to control group ***p < 0.001, ****p < 0.0001 determined by Kruskal-Wallis test and Dunn’s post hoc test. AU = arbitrary unit. No differences were observed for the MMP-9 levels for intra group comparisons between non-HPT vs HPT or non-obese vs obese.
3.5. Effect of medications and AKI on MMP-2 and MMP-9 levels
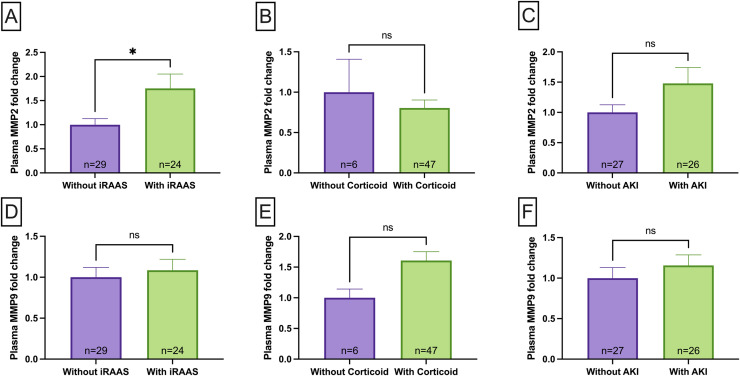
We investigated the association between MMP-2 and MMP-9 levels and usage of corticosteroids and iRAAs. Additionally, we analyzed whether AKI affects MMPs alterations. COVID-19 patients who received iRAAs treatment had significantly higher MMP-2 levels compared to patients without such treatment (p < 0,05, Fig. 4A). In contrast, no difference in MMP-2 levels in COVID-19 patients who were treated with corticosteroids in the hospital (Fig. 4B) were found. Neither corticosteroids nor iRAAs treatment were associated with the MMP-9 changes in the COVID-19 group (Fig. 4D and E). No significant association between MMP-2 or MMP-9 levels and AKI was found in COVID-19 patients (Fig. 4C and F).
Fig. 4.
MMP-2 plasma levels in and COVID-19 patients that received inhibitor of renin-angiotensin system (iRAAS) treatment before hospitalization (COVID-19 on-iRAAS, n = 24) and COVID-19 patients without iRAAS treatment before hospitalization (COVID-19 off-iRAAS, n = 29)(A); MMP-2 plasma levels in COVID-19 patients that received corticosteroid treatment (COVID-19 on-corticosteroid, n = 47) and COVID-19 patients that without corticosteroid treatment (COVID-19 off-corticosteroid, n = 6) in the first day of hospitalization (B); MMP-2 plasma levels in and COVID-19 patients that had Acute Kidney Injury (AKI) during hospitalisation (without AKI, n = 27) and COVID-19 patients with AKI during hospitalization (with AKI, n = 26) (C); MMP-9 plasma levels in COVID-19 patients that received iRAAS treatment before hospitalization (COVID-19 on-iRAAS, n = 24) and COVID-19 patients without iRAAS treatment before hospitalization (COVID-19 off-iRAAS, n = 29) (D); MMP-9 plasma levels in COVID-19 patients that received corticosteroid treatment (COVID-19 on-corticosteroid, n = 47) and COVID-19 patients that without corticosteroid treatment (COVID-19 off-corticosteroid, n = 6) in the first day of hospitalization (E); MMP-2 plasma levels in and COVID-19 patients that had Acute Kidney Injury (AKI) during hospitalisation (without AKI, n = 27) and COVID-19 patients with AKI during hospitalization (with AKI, n = 26) (F). Data are presented as mean ± SEM, normalized to group not on medication or who had no AKI, *p < 0.05 determined by Mann-Whitney test. AU = arbitrary unit, ns = non significant.
3.6. MMP-2, MMP-9 and prognosis of COVID-19
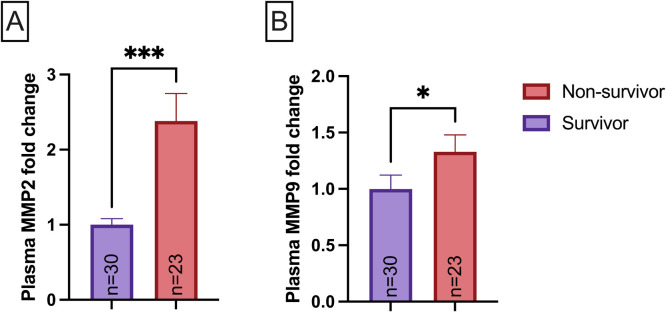
To investigate whether MMP-2 and MMP-9 levels were associated with mortality in COVID-19, patients were classified into subgroups referred as discharged from the ICU (survivor group, n = 30) and those that did not survive albeit the ICU care received (non-survivor group, n = 23). The non-survivor group showed increased MMP-2 (p < 0.05, Fig. 5A) and MMP-9 (p < 0.05, Fig. 5B) levels on the first day of ICU care.
Fig. 5.
Relationship between MMP-2 plasmatic levels (A), MMP-9 plasmatic levels (B), and death in survivor (n = 30) and non-survivor (n = 23) groups on the first day after admission in ICU care. Data are presented as mean ± SEM, normalized to survivor group, *p < 0.05, ***p < 0.001, determined by Mann-Whitney test.
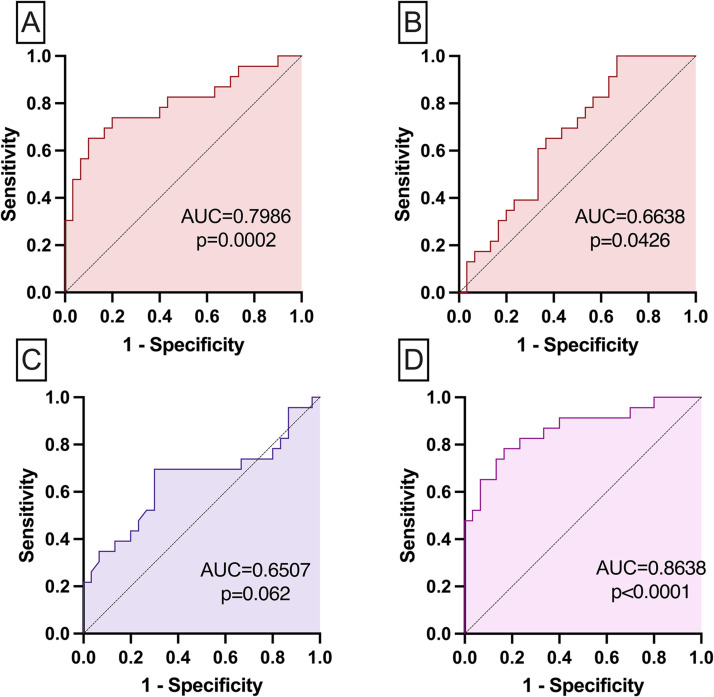
ROC curve data were used to define cutoff points for each MMP evaluated. The ROC curves were analyzed using the performance variables expressed as a percentage (sensitivity and specificity) as well as the area under the curve (AUC) as an indicator of global accuracy. Fig. 6 shows the analysis of the performance of models for prognostic markers in critically ill patients with COVID-19 according to an outcome.
Fig. 6.
Mortality prediction showed on the ROC curve including SAPS3 and SOFA scores ability to predict death on COVID-19 severe patients analyzed by multiple logistic regression AUC= 0.651, p = 0.062 (A); Mortality prediction showed on the ROC curve including MMP-2, MMP-9 associated with BMI, age, AKI and hypertension ability to predict death on COVID-19 severe patients analyzed by multiple logistic regression AUC= 0.864, p < 0.0001 (B).
The ROC curve analysis is shown in Fig. 6(A and B) and indicated that both MMP-2 (AUC=0.7986, p = 0.0002, cutoff point >0.46 AU, 74% sensitivity and 80% specificity, Fig. 6A) and MMP-9 (AUC=0.6634, p = 0.0426, cutoff point >7.12 AU, 65% sensitivity and 63% specificity, Fig. 6B) had good global accuracy on differentiating survivors and non-survivors. Fig. 6C and D show the analysis of the performance of two prognostic models in critically ill patients with COVID-19 according to an outcome. The model including SAPS3 and SOFA had low accuracy (AUC=0.6507, p = 0.062) to discriminate between survivors vs. non-survivors (Fig. 6C), while the model with age, hypertension, BMI and MMP2, MMP9 had high accuracy (AUC=0.8638, p < 0.0001) to discriminate between survivors and non-survivors.
Baseline clinical data of COVID-19 severe patients according to outcome after ICU care is presented in Table 2. The survivor group was younger than the non-survivor group (56.1 ± 2.2 vs 64.0 ± 2.4 years old, p < 0,05). The sex distribution was similar between both groups, male sex predominanting in both groups. The non-survivor group had significantly higher prevalence of hypertension (69.6% vs. 36.6% survivor group, p < 0,05), diabetes (34.8% vs. 6.7%, p < 0.05), cardiovascular diseases (52.2% vs. 22.3%, p < 0.05) and dialysis (56.5% vs. 13.3%, p < 0.05), had higher BMI (33.7 ± 2 vs.29.7 ± 1.2 kg/m2, p < 0.05), AKI (65.2% vs; 36.7%, p < 0.05). The other parameters such as days of symptoms, length of ICU stay, pre-hospitalization iRAAs use, in hospital corticosteroid use, SAPS3 and SOFA scores at hospital admission, PO2/FiO2 ratio, white cell blood counts, creatinine, bilirubin, and lactate were not statistically different between survivor groups and non-survivor groups.
Table 2.
Clinical data of COVID-19 severe patients according to outcome after ICU care. The survivor group was younger than the non-survivor group and the sex distribution was similar between both groups. In the non-survivor group there was a significantly higher prevalence of hypertension diabetes, cardiovascular diseases and dialysis, higher BMI and, AKI. The other parameters such as days of symptoms, length of ICU stay, pre-hospitalization iRAAS use, in hospital corticosteroid use, SAPS3 and SOFA scores at hospital admission, PO2/FiO2 ratio, white cell blood counts, RCP, creatinine, bilirubin, and lactate were not statistically different between survivor groups and non-survivor groups. Clinical data are expressed as mean ± standard error of mean. BMI=Body max index, RCP=Reactive C-Protein.
| Survivors (n = 30) | Non-survivors (n = 23) | p | |
|---|---|---|---|
| Age | 56.1 ± 2.2. | 64.0 ± 2.4 * | 0.0223 |
| Male sex | 20 (66.7%) | 16 (69.6%) | 0.823 |
| Diabetes | 2 (6.7%) | 8 (34.8%) * | 0.009 |
| Hypertension | 11 (36.6%) | 18 (78.3%) * | 0.006 |
| BMI, kg/m2 | 29.7 ± 1 | 33.7 ± 2 * | 0.0442 |
| CVD | 7 (23.3%) | 12 (52.2%) * | 0.03 |
| iRAAS prehospitalization | 13 (43.3%) | 11 (47.8%) | 0.744 |
| Days of symptoms | 9.7 ± 1 | 10.4 ± 2 | 0.581 |
| Days from symptoms worsening | 2.7 ± 1 | 3.2 ± 1 | 0.333 |
| SAPS3 | 58.3 ± 2.1 | 61.2 ± 2.2 | 0.432 |
| SOFA | 8.2 ± 0.5 | 9.6 ± 0.7 | 0.067 |
| Hospitalization days | 17.5 ± 3 | 13.6 ± 2 | 0.692 |
| In hospital corticoid use | 26 (86.7%) | 21 (91.30%) | 0.597 |
| PaO2/FiO2, ratio | 138.4 ± 20 | 138.3 ± 19 | 0.996 |
| Leukocytes | 10.6 ± 0.8 | 10.9 + 0.95 | 0.805 |
| Neutrophiles | 8.4 + 0.8 | 9.3 + 0.91 | 0.459 |
| Lymphocytes x10/ mm3 | 1.2 + 0.1 | 0.9 + 0.2 | 0.259 |
| Platelets x 103/l | 250 ± 150 | 234 ± 23 | 0.532 |
| Bilirubin (mg/dL) | 0.54 ± 0.1 | 1.14 ± 0.3 | 0.0547 |
| Creatinine (mg/dL) | 1.50 ± 0.2 | 1.65 ± 0.2 | 0.244 |
| Lactate (mg/dL) | 2.19 ± 0.1 | 2.37 ± 0.2 | 0.58 |
| CRP (mg/dL) | 12.55 ± 1.5 | 13.20 ± 1.7 | 0.775 |
| AKI (mg/dL) | 11(36.7%) | 15(65.2%) * | 0.039 |
| Dialysis | 4(13.3%) | 13(56.5%) * | 0.0008 |
4. Discussion
The main finding of this study is that plasma MMP-2 levels were downregulated while MMP-9 levels were highly augmented in patients with severe COVID-19. To our knowledge, this is the first study that shows that MMP-2 level independently correlates with mortality in COVID-19 infection on the first day of ICU admission.
Hypertension, obesity, and CVD are associated with an increased risk of severity and mortality in patients with COVID-19 [30]. The MMPs are involved in many physiological processes and pathophysiological conditions such as lung disease, vascular alterations, cardiovascular disease, and obesity [31], [32], [33]. Systemic arterial hypertension drives significant vascular changes such as vascular remodeling in which MMPs [34], especially MMP-2, play the pathophysiological role [35]. Therefore, to understand whether comorbidities such as hypertension affect MMP-9 and MMP-2 levels in COVID-19 subjects is of great importance. Several studies explored an association between MMP-2 and MMP-9 levels and hypertension [32], [36]. MMP-9 levels are not only highly increased in hypertension [32], [37], [38], but are also poor prognostic factors in stroke independently of hypertension history [39]. On the other hand, a meta-analysis showed no significant difference in MMP-2 levels between hypertensive and non-hypertensive patients [32]. Our study shows no significant difference in both MMP-2 and MMP-9 levels between the hypertensive control group and the normotensive control group.
In contrast, MMP-2 plasmatic levels were upregulated in hypertensive COVID-19 patients compared to normotensive COVID-19 patients, albeit still downregulated compared to normotensive and hypertensive controls. Generally downregulated MMP-2 levels in COVID-19 patients signify a state of severe inflammation similar to what is seen in septic patients [20]. On the other hand, increased MMP-2 levels specifically seen in our cohort of hypertensive COVID-19, although surprising, are consistent with the finding of elevated MMP-2 levels in bronchial cells infected with SARS-CoV-2 [40]. We hypothesize that upregulated MMP-2 levels in hypertensive COVID-19 patients might be reflective of the overactivated renin-angiotensin system (RAS) [41], [42]. Indeed, it has been demonstrated that elevated levels of angiotensin (Ang) II observed in COVID-19 disease are associated with endothelial injury [43] and that this peptide increases MMP-2 protein expression [44]. Therefore, we speculate that downregulated levels of MMP-2, which is generally associated with severe inflammation, are overridden by overactivated RAS leading to small, yet significant increases of MMP-2 levels in hypertensive COVID-19 patients.
Obesity is one of the most critical comorbidities for a worst prognostic in COVID-19 infection [45], [46], [47]. Interestingly, adipose tissue is characterized by high expression of the ACE2 [48], which SARS-CoV-2 exploits to enter in the host cell [2]. As a result, adipose tissue becomes a reservoir for viruses, increasing the integral viral charge [49]. Bouloumié et al. [50] showed that human adipose tissue produces and secretes MMP-2 and MMP-9 which are key-regulators in the adipocyte differentiation process [50]. Our data show that COVID-19 patients had increased MMP-9 and decreased MMP-2 levels independently of obesity, which might suggest that this comorbidity is not associated with MMPs level in our study.
Corticosteroids are used in a treatment of COVID-19 patients [51]. It has been shown that the use of corticosteroids can alter MMP-2 and MMP-9 expression [52], [53]. Dexamethasone treatment, a corticosteroid drug, inhibits neointimal hyperplasia by suppressing MMP-2 levels and secretion in a dose-dependent manner in vitro [54]. Lohi et al. [55] suggested that TIMP-MMPs are regulated by corticosteroids, cytokines, and growth factors [55], which explains the reduction in active MMP-2 levels in response to cortisol in sheep lung fetal [56]. The present study shows insignificant change in MMP-2 or MMP-9 levels in response to corticosteroids, which might suggest that SARS-CoV-2 alone promotes a significant reduction in MMP-2 and increasing in MMP-9. The effect of corticosteroids on MMP-2 and MMP-9 levels seems to be insignificant in COVID-19.
ACEi and ARBs may decrease the risk of developing acute respiratory distress syndrome in patients with COVID-19, which is suggested to be related to diminished less Ang II formation [57]. ACEi prevent Ang I into Ang II cleavage, reducing blood pressure and heart remodelling [58]. In experimental studies, ACE2 protected against lung injuries [59] and chronic treatment with ARBs (losartan and olmesartan) promoted ACE2 overload [60]. Since SARS-CoV-2 binds to the ACE2 for intracellular invasion with a mechanism of acute lung injury mediated by RAS [2], and ACE2 is an important molecule in maintaining the balance of RAS, its binding to the virus causes an immediate imbalance and increased Ang II and, consequently, increased vasoconstriction and inflammation [61].
It is well known that both MMP-2 and MMP-9 are related to inflammatory processes [22] and induced by Ang II [44], [62], and therefore we investigated the relation between the RAS inhibitor treatment and plasmatic metalloproteinases levels. The treatment with iRAAs was not associated with MMP-9 levels in COVID-19 subjects, suggesting that increased MMP-9 levels might be related to COVID-19 infection. However, patients receiving iRAAs had higher MMP-2 levels compared to patients without iRAAs treatment. This is contradictory with the result of a study that showed a reduction in MMP-2 due to the use of iRAAS [34]. Our hypothesis is that a reduction in MMP-2 levels might be a consequence of systemic changes promoted by SARS-CoV-2 and related to cytokine storm and the ACE2 imbalance. It is known that MMP-2 deficiency predisposes to inflammation, with low levels being as harmful as high levels for the cardiovascular system [63]. Thus, the raised hypothesis is that the iRAAs act to restore the balance of proteinases such as iRAAS treatment reduces MMP-2 levels in inflammation while the treatment increases its expression as seen in COVID-19 patients.
Clinical and laboratory data are an important tool for clarifying pathophysiology and prognosis for patients with COVID-19 admitted to the ICU. In our study, the comparison between COVID-19 and Control subjects showed relevant differences in leukocytes, neutrophils, lymphocytes, and platelets counts as expected due to the highly inflammatory state in COVID-19 [4]. No differences were seen regarding age, sex, and comorbidities (diabetes, hypertension, and BMI - including obese individuals with BMI> 30 kg/m2) and use of pre-iRAAS hospitalization. Our data are consistent with the meta-analysis concerning the increased neutrophil/lymphocyte ratio in severe COVID-19 patients [5].
The COVID-19 patients who did not survive were more likely to develop AKI during hospitalization and to undergo dialysis. In contrast, patients who survived showed less severe renal dysfunction in the ICU, and were 23% less likely to require dialysis. No significant association was found between MMP-2 or MMP-9 levels and AKI in COVID-19 patients. When comparing patients who did not survive with survivors, we showed that 63.2% had a history of cardiovascular diseases in the first group, while in the second, only 36.8%.
In our study, we show that MMP-2 levels were generally downregulated in COVID-19 patients compared to Controls. However, in a subanalysis, we found that COVID-19 non-survivors had higher levels of MMP-2 compared with COVID-19 survivors. We speculate that elevated levels of MMP-2 signals a higher activation of the RAS, which is critical both in the pathophysiology of hypertension [64] and COVID-19 [41]. Another hypothesis is that vascular lesions were more severe in the patients who already had the predisposing factor of hypertension and thus higher levels of AngII, which induces MMP-2 via AT-1receptor [44]. However, more studies are necessary to clarify the mechanism involved in MMP-2 levels and COVID-19.
Consistently with our data, Petito et al. [65] showed recently (December 2020) that COVID-19 patients (n = 32) had greater MMP-9 levels in comparison to healthy controls by ELISA test [65]. Another study using genomic analyses showed that MMP-9 and MMP-2 genes were altered in COVID-19 [40]. The biomarker screening study with 175 Italian patients showed an association between increased MMP-9 in the first sample and mortality [18]. Interestingly Abers et al. [18] did not find augmented MMP-9 to be consistently associated with mortality through hospitalization, which is consistent with our result that MMP-9 level was not an independent predictor of mortality in a model adjusted for BMI, age, and hypertension. Another study found that respiratory failure as defined by a pO2/FiO2 was associated with increased plasma MMP-9 and that this clinical indicator (P/F) correlated directly with MMP-9 values [16]. We could not replicate this finding either because of the different MMP-9 measurement method used in our study or because almost all our patients had P/F much below acute respiratory distress syndrome criteria or because the cited study had a smaller number of patients included (39 patients).
It has been widely discussed whether severe COVID-19 should be considered viral sepsis [19]. The controversy is held in that sepsis has unifying characteristics that apply very well to severe COVID-19 patients, a clinical situation in which the infection causes a disruptive inflammatory response in the host and, if not treated, will likely lead to multiorgan failure and death [9], [10]. One could argue that our study was a study of sepsis since all of the patients had the diagnostic criteria for sepsis as defined in the third consensus [23], SOFA score greater or equal to 2. In septic patients, it has already been suggested that MMP-2 is diminished [20]. Nevertheless, sepsis prognosis and pathophysiology depend on pathogenic factors [23], and it is not guaranteed that the known hallmarks and treatments applied in septic patients will provide the best care for severe COVID-19 patients. Hence, it seems clear that studies similar to ours that investigate severe COVID-19 alterations already seen in sepsis, namely MMP-2, are warranted.
5. Conclusion
In conclusion, our data show that MMP-2 was downregulated and MMP-9 was upregulated in severe COVID-19 patients. The MMP-2 was independently correlated with mortality in COVID-19 diseases and might be a potential prognostic predictor in COVID-19.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil supported: Christiane Becari (2017/21539-7; 2018/23718-8), Mauricio S. Ribeiro (2019/11485-1), Maria Auxiliadora Martins (2019/11485-1), Carolina D`Avila-Mesquita (2019/24369-0), Ariel E. S. Couto (2020/03308-0), Ligia C.B. Campos (2020/09999-4), Bruno C.P. Moraes (2021/01195-6), Jessyca M. Barbosa (2019/21721-4). CNPq, Brazil supported Maria Julia Garbellini Diab (137525/2020-6).
Disclosures
The authors declare that there are no conflict of interest.
CRediT authorship contribution statement
Carolina D`Avila-Mesquita: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Ariel E.S. Couto: Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Ligia C.B. Campos: Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Tauana F. Vasconcelos: Methodology, Investigation, Writing – original draft, Writing – review & editing. Jessyca M. Barbosa: Methodology, Investigation, Writing – review & editing. Carlos A.C. Corsi: Methodology, Investigation, Writing – review & editing. Fabiola Mestriner Methodology, Investigation, Writing – review & editing. Bruno C.P. Moraes: Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Maria J.G. Diab: Methodology, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Daniel M.S. Couto: Methodology, Formal analysis, Investigation, Writing – review & editin. Maria C. Jordani: Methodology, Writing – review & editing. Denise Ferro: Methodology, Investigation, Writing – review & editing. Lourenço Sbragia-Neto: Writing – review & editing. Edwaldo E. Joviliano: Writing – review & editing. Paulo R. Evora: Writing – original draft, Writing – review & editing. Rodrigo C. Santana: Methodology, Writing – review & editing. Olindo Assis Martins-Filho: Writing – review & editing. Katarzyna Polonis: Writing – review & editing. Mayra G. Menegueti: Conceptualization, Formal analysis, Writing – review & editing. Mauricio S. Ribeiro: Conceptualization, Writing – review & editing. M. Auxiliadora-Martins: Conceptualization, Formal analysis, Writing – review & editing. Christiane Becari: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Acknowledgments
none.
Data Availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html (Accessed 1 August 2021).
- 2.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ace2 and tmprss2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang D., Comish P., Kang R. The hallmarks of COVID-19 disease. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong M., Zhang H., Cao X., Mao X., Lu Z. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miripour Z.S., Sarrami-Forooshani R., Sanati H., Makarem J., Taheri M.S., Shojaeian F., Eskafi A.H., Abbasvandi F., Namdar N., Ghafari H., Aghaee P., Zandi A., Faramarzpour M., Hoseinyazdi M., Tayebi M., Abdolahad M. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patell R., Bogue T., Koshy A., Bindal P., Merrill M., Aird W.C., Bauer K.A., Zwicker J.I. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond. Engl. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 11.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., the Northwell COVID-19 Research Consortium, Barnaby D.P., Becker L.B., Chelico J.D., Cohen S.L., Cookingham J., Coppa K., Diefenbach M.A., Dominello A.J., Duer-Hefele J., Falzon L., Gitlin J., Hajizadeh N., Harvin T.G., Hirschwerk D.A., Kim E.J., Kozel Z.M., Marrast L.M., Mogavero J.N., Osorio G.A., Qiu M., Zanos T.P. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat. Rev. Cardiol. 2020:1–16. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davey A., McAuley D.F., O’Kane C.M. Matrix metalloproteinases in acute lung injury: mediators of injury and drivers of repair. Eur. Respir. J. 2011;38:959–970. doi: 10.1183/09031936.00032111. [DOI] [PubMed] [Google Scholar]
- 14.Fligiel S.E.G., Standiford T., Fligiel H.M., Tashkin D., Strieter R.M., Warner R.L., Johnson K.J., Varani J. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum. Pathol. 2006;37:422–430. doi: 10.1016/j.humpath.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Hazra S., Chaudhuri A.G., Tiwary B.K., Chakrabarti N. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: a network-based meta-analysis. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ueland T., Holter J., Holten A., Müller K., Lind A., Bekken G., Dudman S., Aukrust P., Dyrhol-Riise A., Heggelund L. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. J. Infect. 2020;81:e41–e43. doi: 10.1016/j.jinf.2020.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duda I., Krzych Ł., Jędrzejowska-Szypułka H., Lewin-Kowalik J. Plasma matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 as prognostic biomarkers in critically ill patients. Open Med. 2020;15:50–56. doi: 10.1515/med-2020-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abers M.S., Delmonte O.M., Ricotta E.E., Fintzi J., Fink D., de Jesus A.A., Zarember K.A., Alehashemi S., Oikonomou V., Desai J.V., Canna S.W., Shakoory B., Dobbs K., Imberti L., Sottini A., Quiros-Roldan E., Castelli F., Rossi C., Brugnoni D., Biondi A., Bettini L.R., D’Angio’ M., Bonfanti P., Castagnoli R., Montagna D., Licari A., Marseglia G.L., Gliniewicz E., Shaw E.R., Kahle D., Rastegar A.T., Stack M.A., Myint-Hpu K., Levinson S.L., DiNubile M.J., Chertow D.W., Burbelo P., Cohen J.I., Calvo K.R., Tsang J.S., Su H.C., Gallin J.I., Kuhns D.B., Goldbach-Mansky R., Lionakis M.S., Notarangelo L.D. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2020 doi: 10.1172/jci.insight.144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beltrán-García J., Osca-Verdegal R., Pallardó F.V., Ferreres J., Rodríguez M., Mulet S., Ferrando-Sánchez C., Carbonell N., García-Giménez J.L. Sepsis and coronavirus disease 2019: common features and anti-inflammatory therapeutic approaches. Crit. Care Med. 2020;48:1841–1844. doi: 10.1097/CCM.0000000000004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguirre A., Blázquez-Prieto J., Amado-Rodriguez L., López-Alonso I., Batalla-Solís E., González-López A., Sánchez-Pérez M., Mayoral-Garcia C., Gutiérrez-Fernández A., Albaiceta G.M. Matrix metalloproteinase-14 triggers an anti-inflammatory proteolytic cascade in endotoxemia. J. Mol. Med. 2017;95:487–497. doi: 10.1007/s00109-017-1510-z. [DOI] [PubMed] [Google Scholar]
- 21.McQuibban G.A., Gong J.-H., Wong J.P., Wallace J.L., Clark-Lewis I., Overall C.M. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- 22.Fingleton B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:2036–2042. doi: 10.1016/j.bbamcr.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Seymour C.W., Liu V.X., Iwashyna T.J., Brunkhorst F.M., Rea T.D., Scherag A., Rubenfeld G., Kahn J.M., Shankar-Hari M., Singer M., Deutschman C.S., Escobar G.J., Angus D.C. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno R.P., Metnitz P.G.H., Almeida E., Jordan B., Bauer P., Campos R.A., Iapichino G., Edbrooke D., Capuzzo M., Le Gall J.-R. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–1355. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toth M., Fridman R. Assessment of gelatinases (MMP-2 and MMP-9 by gelatin zymography. Methods Mol. Med. 2001;57:163–174. doi: 10.1385/1-59259-136-1:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bencsik P., Bartekova M., Görbe A., Kiss K., Pálóczi J., Radosinska J., Szűcs G., Ferdinandy P. MMP activity detection in zymograms. Methods Mol. Biol. Clifton NJ. 2017;1626:53–70. doi: 10.1007/978-1-4939-7111-4_6. [DOI] [PubMed] [Google Scholar]
- 27.J V., N G., E M., den V., Pe S., G O. Zymography methods for visualizing hydrolytic enzymes. Nat. Methods. 2013;10 doi: 10.1038/nmeth.2371. [DOI] [PubMed] [Google Scholar]
- 28.Tajhya R.B., Patel R.S., Beeton C. Detection of matrix metalloproteinases by zymography. Methods Mol. Biol. Clifton Nj. 2017;1579:231–244. doi: 10.1007/978-1-4939-6863-3_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S., Meng F., Chen Z., Tomlinson B.N., Wesley J.M., Sun G.Y., Whaley-Connell A.T., Sowers J.R., Cui J., Gu Z. Two-dimensional zymography differentiates gelatinase isoforms in stimulated microglial cells and in brain tissues of acute brain injuries. PLOS ONE. 2015;10 doi: 10.1371/journal.pone.0123852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., Ye C., Zhang P., Xing Y., Guo H., Tang W. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Unal R., Yao-Borengasser A., Varma V., Rasouli N., Labbate C., Kern P.A., Ranganathan G. Matrix metalloproteinase-9 is increased in obese subjects and decreases in response to pioglitazone. J. Clin. Endocrinol. Metab. 2010;95:2993–3001. doi: 10.1210/jc.2009-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchesi C., Dentali F., Nicolini E., Maresca A.M., Tayebjee M.H., Franz M., Guasti L., Venco A., Schiffrin E.L., Lip G.Y.H., Grandi A.M. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: a systematic review and meta-analysis. J. Hypertens. 2012;30:3–16. doi: 10.1097/HJH.0b013e32834d249a. [DOI] [PubMed] [Google Scholar]
- 33.Kapoor C., Vaidya S., Wadhwan V., Hitesh, Kaur G., Pathak A. Seesaw of matrix metalloproteinases (MMPs) J. Cancer Res. Ther. 2016;12:28–35. doi: 10.4103/0973-1482.157337. [DOI] [PubMed] [Google Scholar]
- 34.Hopps E., Lo Presti R., Caimi G. Matrix metalloproteases in arterial hypertension and their trend after antihypertensive treatment. Kidney Blood Press. Res. 2017;42:347–357. doi: 10.1159/000477785. [DOI] [PubMed] [Google Scholar]
- 35.Belo V.A., Guimarães D.A., Castro M.M. Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J. Vasc. Res. 2015;52:221–231. doi: 10.1159/000441621. [DOI] [PubMed] [Google Scholar]
- 36.Fontana V., Silva P.S., Gerlach R.F., Tanus-Santos J.E. Circulating matrix metalloproteinases and their inhibitors in hypertension. Clin. Chim. Acta Int. J. Clin. Chem. 2012;413:656–662. doi: 10.1016/j.cca.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Valente F.M., de Andrade D.O., Cosenso-Martin L.N., Cesarino C.B., Guimarães S.M., Guimarães V.B., Lacchini R., Tanus-Santos J.E., Yugar-Toledo J.C., Vilela-Martin J.F. Plasma levels of matrix metalloproteinase-9 are elevated in individuals with hypertensive crisis. BMC Cardiovasc. Disord. 2020;20:132. doi: 10.1186/s12872-020-01412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tayebjee M.H., Nadar S., Blann A.D., Beevers D.G., MacFadyen R.J., Lip G.Y.H. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in hypertension and their relationship to cardiovascular risk and treatmentA substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Am. J. Hypertens. 2004;17:764–769. doi: 10.1016/j.amjhyper.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Zhong C., Yang J., Xu T., Xu T., Peng Y., Wang A., Wang J., Peng H., Li Q., Ju Z., Geng D., Zhang Y., He J. CATIS Investigators, Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke. Neurology. 2017;89:805–812. doi: 10.1212/WNL.0000000000004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karakurt H.U., Pİr P. Integration of transcriptomic profile of SARS-CoV-2 infected normal human bronchial epithelial cells with metabolic and protein-protein interaction networks. Turk. J. Biol. Turk. Biyol. Derg. 2020;44:168–177. doi: 10.3906/biy-2005-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miesbach W. Pathological role of angiotensin ii in severe COVID-19. TH Open Companion J. Thromb. Haemost. 2020;4:e138–e144. doi: 10.1055/s-0040-1713678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osman I.O., Melenotte C., Brouqui P., Million M., Lagier J.-C., Parola P., Stein A., La Scola B., Meddeb L., Mege J.-L., Raoult D., Devaux C.A. Expression of ACE2, soluble ACE2, angiotensin i, angiotensin ii and angiotensin-(1-7) is modulated in COVID-19 patients. Front. Immunol. 2021;0 doi: 10.3389/fimmu.2021.625732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oz M., Lorke D.E. Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury. Biomed. Pharmacother. 2021;136 doi: 10.1016/j.biopha.2020.111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C., Qian X., Sun X., Chang Q. Angiotensin II increases matrix metalloproteinase 2 expression in human aortic smooth muscle cells via AT1R and ERK1/2. Exp. Biol. Med. 2015;240:1564–1571. doi: 10.1177/1535370215576312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekiz T., Pazarlı A.C. Relationship between COVID-19 and obesity. Diabetes Metab. Syndr. 2020;14:761–763. doi: 10.1016/j.dsx.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefan N., Birkenfeld A.L., Schulze M.B., Ludwig D.S. Obesity and impaired metabolic health in patients with COVID-19. Nat. Rev. Endocrinol. 2020;16:341–342. doi: 10.1038/s41574-020-0364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popkin B.M., Du S., Green W.D., Beck M.A., Algaith T., Herbst C.H., Alsukait R.F., Alluhidan M., Alazemi N., Shekar M. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes. Rev. 2020;21 doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Benna S. Association of high level gene expression of ACE2 in adipose tissue with mortality of COVID-19 infection in obese patients. Obes. Med. 2020;19 doi: 10.1016/j.obmed.2020.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kruglikov I.L., Shah M., Scherer P.E. Obesity and diabetes as comorbidities for COVID-19: Underlying mechanisms and the role of viral-bacterial interactions. ELife. 2020;9 doi: 10.7554/eLife.61330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bouloumié A., Sengenès C., Portolan G., Galitzky J., Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001;50:2080–2086. doi: 10.2337/diabetes.50.9.2080. [DOI] [PubMed] [Google Scholar]
- 51.Yang Z., Liu J., Zhou Y., Zhao X., Zhao Q., Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J. Infect. 2020;81:e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eberhardt W., Schulze M., Engels C., Klasmeier E., Pfeilschifter J. Glucocorticoid-mediated suppression of cytokine-induced matrix metalloproteinase-9 expression in rat mesangial cells: involvement of nuclear factor-kappaB and Ets transcription factors. Mol. Endocrinol. Baltim. Md. 2002;16:1752–1766. doi: 10.1210/me.2001-0278. [DOI] [PubMed] [Google Scholar]
- 53.Beppu L., Yang T., Luk M., Newbury R.O., Palmquist J., Dohil R., Kurten R.C., Broide D.H., Aceves S.S. MMPs-2 and -14 are elevated in eosinophilic esophagitis and reduced following topical corticosteroid therapy. J. Pediatr. Gastroenterol. Nutr. 2015;61:194–199. doi: 10.1097/MPG.0000000000000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pross C., Farooq M.M., Angle N., Lane J.S., Cerveira J.J., Xavier A.E., Freischlag J.A., Law R.E., Gelabert H.A. Dexamethasone inhibits vascular smooth muscle cell migration via modulation of matrix metalloproteinase activity. J. Surg. Res. 2002;102:57–62. doi: 10.1006/jsre.2001.6220. [DOI] [PubMed] [Google Scholar]
- 55.Lohi J., Lehti K., Westermarck J., Kähäri V.M., Keski-Oja J. Regulation of membrane-type matrix metalloproteinase-1 expression by growth factors and phorbol 12-myristate 13-acetate. Eur. J. Biochem. 1996;239:239–247. doi: 10.1111/j.1432-1033.1996.0239u.x. [DOI] [PubMed] [Google Scholar]
- 56.Boland R., Joyce B.J., Wallace M.J., Stanton H., Fosang A.J., Pierce R.A., Harding R., Hooper S.B. Cortisol enhances structural maturation of the hypoplastic fetal lung in sheep. J. Physiol. 2004;554:505–517. doi: 10.1113/jphysiol.2003.055111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schiffrin E.L., Flack J.M., Ito S., Muntner P., Webb R.C. Hypertension and COVID-19. Am. J. Hypertens. 2020;33:373–374. doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.-C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Furuhashi M., Moniwa N., Mita T., Fuseya T., Ishimura S., Ohno K., Shibata S., Tanaka M., Watanabe Y., Akasaka H., Ohnishi H., Yoshida H., Takizawa H., Saitoh S., Ura N., Shimamoto K., Miura T. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am. J. Hypertens. 2015;28:15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- 61.Murray E., Tomaszewski M., Guzik T.J. Binding of SARS-CoV-2 and angiotensin-converting enzyme 2: clinical implications. Cardiovasc. Res. 2020;116:e87–e89. doi: 10.1093/cvr/cvaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo R., Yang L., Wang H., Liu B., Wang L. Angiotensin II induces matrix metalloproteinase-9 expression via a nuclear factor-kappaB-dependent pathway in vascular smooth muscle cells. Regul. Pept. 2008;147:37–44. doi: 10.1016/j.regpep.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Hardy E., Hardy-Sosa A., Fernandez-Patron C. MMP-2: is too low as bad as too high in the cardiovascular system? Am. J. Physiol. Heart Circ. Physiol. 2018;315:H1332–H1340. doi: 10.1152/ajpheart.00198.2018. [DOI] [PubMed] [Google Scholar]
- 64.Forrester S.J., Booz G.W., Sigmund C.D., Coffman T.M., Kawai T., Rizzo V., Scalia R., Eguchi S. Angiotensin ii signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018;98:1627–1738. doi: 10.1152/physrev.00038.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petito E., Falcinelli E., Paliani U., Cesari E., Vaudo G., Sebastiano M., Cerotto V., Guglielmini G., Gori F., Malvestiti M., Becattini C., Paciullo F., De Robertis E., Bury L., Lazzarini T., Gresele P. COVIR study investigators, Neutrophil more than platelet activation associates with thrombotic complications in COVID-19 patients. J. Infect. Dis. 2020 doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.