Abstract
Objective
To quantify the association between accelerometer-assessed physical activity and coronavirus disease 2019 (COVID-19) outcomes.
Methods
Data from 82,253 UK Biobank participants with accelerometer data (measured 2013-2015), complete covariate data, and linked COVID-19 data from March 16, 2020, to March 16, 2021, were included. Two outcomes were investigated: severe COVID-19 (positive test result from in-hospital setting or COVID-19 as primary cause of death) and nonsevere COVID-19 (positive test result from community setting). Logistic regressions were used to assess associations with moderate to vigorous physical activity (MVPA), total activity, and intensity gradient. A higher intensity gradient indicates a higher proportion of vigorous activity.
Results
Average MVPA was 48.1 (32.7) min/d. Physical activity was associated with lower odds of severe COVID-19 (adjusted odds ratio per standard deviation increase: MVPA, 0.75 [95% CI, 0.67 to 0.85]; total, 0.83 [0.74 to 0.92]; intensity, 0.77 [0.70 to 0.86]), with stronger associations in women (MVPA, 0.63 [0.52 to 0.77]; total, 0.76 [0.64 to 0.90]; intensity, 0.63 [0.53 to 0.74]) than in men (MVPA, 0.84 [0.73 to 0.97]; total, 0.88 [0.77 to 1.01]; intensity, 0.88 [0.77 to 1.00]). In contrast, when mutually adjusted, total activity was associated with higher odds of a nonsevere infection (1.10 [1.04 to 1.16]), whereas the intensity gradient was associated with lower odds (0.91 [0.86 to 0.97]).
Conclusion
Odds of severe COVID-19 were approximately 25% lower per standard deviation (∼30 min/d) MVPA. A greater proportion of vigorous activity was associated with lower odds of severe and nonsevere infections. The association between total activity and higher odds of a nonsevere infection may be through greater community engagement and thus more exposure to the virus. Results support calls for public health messaging highlighting the potential of MVPA for reducing the odds of severe COVID-19.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019; MVPA, moderate to vigorous physical activity; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Poor outcomes from coronavirus disease 2019 (COVID-19) are more likely in people who are older,1 are more deprived,2 have comorbidities,3 or are from ethnic minority populations.4 As with chronic disease, research suggests that risk factors related to health behaviors, such as obesity5 and slow walking pace,6 also have a negative impact on COVID-19 outcomes.
Physical activity is a modifiable health behavior that may mitigate the risks of COVID-19.7 This could occur through reductions in chronic inflammation8,9 or cardiometabolic risk factors,10 which are associated with an increased risk of COVID-19,11 or through enhanced immunity.7 In the early months of the pandemic (up to July 2020), we reported initial observations from Biobank data,12 which was suggestive evidence for lower odds (up to 20%) of severe COVID-19 per 30 minutes of daily moderate to vigorous physical activity (MVPA; P=.06). Consistent with this finding, Sallis et al13 reported that being physically inactive is the strongest modifiable risk factor for severe COVID-19 and stressed the importance of this message for public health. They found that the risk of hospitalization or death with COVID-19 up to October 2020 in a sample of US health plan members was more than twice as high in people who self-reported consistently being inactive in the 2 years before the pandemic compared with those who self-reported consistently meeting the guidelines of 150 minutes of MVPA per week.13
Whereas most of the evidence on associations between physical activity and a wide range of health outcomes has similarly been gleaned from self-report methods,14 self-report has well-documented limitations, not least of which is the reliance on recall and consequently a focus on purposeful activity.14 Accelerometers directly measure movement, reducing measurement error and facilitating a more nuanced consideration of physical activity (eg, the relative importance of the total amount or intensity of physical activity).15,16 UK Biobank is the largest data set with accelerometer-assessed physical activity, having assessed about 100,000 participants.17
In this study, we use UK Biobank data from the first and second waves of the COVID-19 pandemic to determine the association between accelerometer-assessed physical activity (measured between 2013 and 2015) and severe and nonsevere COVID-19 outcomes. We hypothesized that physical activity would be associated with reduced odds of severe COVID-19. However, for nonsevere COVID-19, which reflects community transmission, we hypothesized that associations would be attenuated as higher physical activity levels may also reflect greater exposure to the virus.
Methods
Study Design
This is a retrospective observational study. Physical activity was assessed between June 2013 and December 2015, 4 to 7 years preceding the COVID-19 pandemic.
Setting and Cohort
This study uses data from UK Biobank (Application 36371), a prospective cohort of more than 500,000 adults aged 40 to 69 years with baseline assessments conducted between March 2006 and July 2010.18 Some participants took part in further touchscreen interviews between 2009 (n=8503) and 2018 (n=15,140). Methods were carried out in accordance with relevant guidelines and regulations, and all participants gave written informed consent before data collection. UK Biobank has full ethical approval from the NHS National Research Ethics Service (16/NW/0274). Data are linked to national severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) laboratory test data through Public Health England’s Second Generation Surveillance System and include specimen origin (hospital inpatient vs other). COVID-19 testing data are available for England from March 16, 2020; thus, participants from non-English centers and individuals who had died before March 16, 2020, were excluded. Analyses were based on March 16, 2021, refresh data. A flow chart detailing all participant exclusions is provided in Supplemental Figure 1 (available online at http://mcpiqojournal.org).
COVID-19 Outcomes
Two outcomes were reported: severe infection with SARS-CoV-2 and nonsevere infection with SARS-CoV-2. A positive test result for SARS-CoV-2 with hospitalization or death related to the disease (ie, any death with an International Statistical Classification of Diseases, Tenth Revision code of U07.1 or U07.2 as the primary cause of death on the death certificate) was considered evidence of a severe infection.19 A positive test result for SARS-CoV-2 from a community setting without a hospital diagnosis or death was considered evidence of probable mild disease or a nonsevere infection. Classifying a positive test result for SARS-CoV-2 in those admitted to the hospital as a marker of disease severity within UK Biobank is in line with guidance for this data set.19 However, actual disease severity cannot be confirmed from the linkage data available. Therefore, our reference to the composite of a test result for SARS-CoV-2 in those admitted to the hospital or death from COVID-19 as indicating “severe” disease is for descriptive purposes only.
Physical Activity
A subsample of approximately 100,000 adults were asked to wear the Axivity AX3 wrist-worn accelerometer 24 hours a day for 7 days between June 2013 and December 2015.17 For each participant, we extracted the accelerometer data (5-second epoch time series) from UK Biobank17 and converted it to R-format for processing and analysis with GGIR (version 1.11-0; http://cran.r-project.org).20 Participants were excluded if they failed calibration, they had fewer than 3 days of valid wear (defined as >16 h/d), or wear data were not present for each 15-minute period of the 24-hour cycle.12,15,21 Accelerometer outcomes, selected to describe total physical activity and its intensity, were as follows:
-
•
average acceleration during the 24-hour day (proxy for total physical activity, mg);
-
•
intensity gradient during 24 hours (intensity distribution of activity during the day; higher values indicate that a greater proportion of total activity is spent at high intensity)21; and
-
•
time spent in 1-minute bouts of MVPA (acceleration cut point 100 mg22).
Statistical Analyses
Logistic regression was used to analyze associations of physical activity with the COVID-19 outcomes:
Model 1: severe COVID-19 infection (N=434) with no test or negative test result (N=79,856) as comparator
Model 2: severe COVID-19 infection (N=434) with nonsevere infection (N=1963) as comparator
Model 3: nonsevere COVID-19 infection (N=1963) with no test or negative test result (N=79,856) as comparator
Models 1 and 3 (no test or negative test result as comparator) can be interpreted as the increased or decreased odds of being admitted to the hospital or dying of COVID-19 (severe disease; model 1) or of having a positive test result in the community (nonsevere disease; model 3) during the linkage period within UK Biobank. Model 2 can be interpreted as the risk of any positive test result being from a setting (hospital) or outcome (death) that indicates severe disease during the linkage period within UK Biobank. Assessing the odds of infection relative to the cohort (models 1 and 3) is commonly reported within COVID-19 risk factor research and enables comparison to the literature in terms of how the risk factors assessed compare with other commonly reported risk factors (eg, obesity5).
Three physical activity exposures were considered: total physical activity, intensity gradient, and MVPA. A mutually adjusted model was also run for total physical activity and the intensity gradient to test whether associations were independent of the alternative activity metric consistent with previous research assessing the relative contributions of total activity and intensity of activity for health.15,23 The variables were standardized before entry into the models and the odds ratios per cohort standard deviation reported for ease of comparison across exposures.12 Covariates were selected on the basis of current clinical knowledge and included age (at censoring), follow-up time (difference between age at accelerometer measure and age at censoring), season of accelerometer wear, sex, ethnicity (White, South Asian, Black and African or Caribbean), Townsend score (area-level measure of deprivation), employment status, and cardiovascular disease or cancer diagnosis before accelerometer baseline (self-reported history of heart attack, angina, stroke, or cancer variables or hospital episode with International Statistical Classification of Diseases, Tenth Revision code I20-25, I60-69, or C00-99). Additional health-related covariates potentially on the causal pathway from physical activity to COVID-19 risk were included in sensitivity analyses. These were body mass index, blood pressure or cholesterol medication, and prescribed insulin medication. Covariates from the assessment closest to the accelerometer time point were used.
Analyses were reported for the full population and stratified by sex. Effect modification by sex was tested using an interaction term (sex∗physical activity) in the model.
Statistical significance was set at a P value of less than .05; results are reported with a 95% CI. Interactions were considered significant at a P value of less than .1. All analyses were performed in Stata version 16.1 (StataCorp LLC).
Sensitivity Analyses
All models were further adjusted for covariates potentially on the causal pathway from physical activity to COVID-19 risk.
As testing in the United Kingdom has not been universal, particularly in the first wave of the pandemic, there is a risk of selection bias in models 1 and 3, where those with COVID-19 were compared with those with a negative test result or no test. Participants with COVID-19 may not have been tested and thus wrongly allocated to the comparator group. To address this, we carried out sensitivity analyses for models 1 and 3, restricting the comparator group to those who tested negative for COVID-19.
Results
Data from 82,253 UK Biobank participants with accelerometer data (2013-2015) and complete covariate and linked COVID-19 data to March 16, 2021, were included. Of these participants, 12,713 (15.5%) had been tested for COVID-19, 2388 (2.9%) had a positive test result, 1963 (2.4%) were classified as having a nonsevere infection, and 425 (0.5%) were classified as having a severe infection; another 9 (0.01%) were classified as severe without a positive test result. Participants accumulated a mean of 48.1 (SD 32.7) minutes of MVPA per day. Participants’ characteristics are reported in the Table.
Table.
| Characteristic | No COVID-19 |
Severe COVID-19 |
Nonsevere COVID-19 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Men | Women | All | Men | Women | All | Men | Women | |
| No. [%] | 79,856 [100] | 34,614 [43.4] | 45,242 [56.7] | 434 [100] | 250 [57.6] | 184 [42.4] | 1963 [100] | 812 [41.4] | 1151 [58.6] |
| Age at census (y) | 68.0 (7.77) | 68.7 (7.83) | 67.6 (7.70) | 68.5 (8.73) | 69.5 (8.85) | 67.2 (8.4) | 63.5 (7.72) | 64.2 (8.18) | 63.0 (7.35) |
| Follow-up time (y) | 5.6 (0.70) | 5.6 (0.70) | 5.6 (0.70) | 5.5 (0.76) | 5.5 (0.80) | 5.6 (0.69) | 5.6 (0.68) | 5.6 (0.68) | 5.6 (0.68) |
| Body mass index (kg/m2) | 26.6 (4.54) | 27.2 (4.03) | 26.2 (4.85) | 28.7 (5.07) | 28.7 (4.40) | 28.6 (6.01) | 27.1 (4.66) | 27.7 (4.12) | 26.6 (4.95) |
| Townsend scorec | −1.7 (2.79) | −1.8 (2.79) | −1.7 (2.79) | −1.4 (3.00) | −1.1 (3.14) | −1.7 (2.79) | −1.3 (2.94) | −1.4 (2.85) | −1.2 (2.99) |
| Ethnicity | |||||||||
| White | 78,470 [98.3] | 33,969 [98.1] | 44,501 [98.4] | 416 [95.9] | 239 [95.6] | 177 [96.2] | 1901 [96.8] | 788 [97.0] | 1113 [96.7] |
| South Asian | 657 [0.8] | 363 [1.1] | 294 [0.7] | 8 [1.8] | 6 [2.4] | 2 [1.1] | 30 [1.5] | 16 [2.0] | 14 [1.2] |
| Black | 729 [0.9] | 282 [0.8] | 447 [1.0] | 10 [2.3] | 5 [2.0] | 5 [2.7] | 32 [1.6] | 8 [1.0] | 24 [2.1] |
| In paid employment or self-employed | 43,206 [54.1] | 19,025 [55.0] | 24,181 [53.5] | 234 [53.9] | 123 [49.2] | 111 [60.3] | 1422 [72.4] | 592 [72.9] | 830 [72.1] |
| Prevalent CVD or cancer at baselined | 14,105 [17.7] | 6806 [19.7] | 7299 [16.1] | 110 [25.4] | 74 [29.6] | 36 [19.6] | 290 [14.8] | 139 [17.1] | 151 [13.1] |
| Blood pressure or cholesterol medication | 20,978 [26.3] | 11,714 [33.9] | 9264 [20.5] | 181 [41.7] | 124 [49.6] | 57 [31.0] | 386 [19.7] | 215 [26.5] | 171 [14.9] |
| Insulin medication | 3154 [4.0] | 1949 [5.6] | 1205 [2.7] | 43 [9.9] | 31 [12.4] | 12 [6.5] | 79 [4.0] | 40 [4.9] | 39 [3.4] |
| Physical activity | |||||||||
| Total physical activity (mg) | 28.3 (8.33) | 27.8 (8.66) | 28.7 (8.04) | 26.5 (8.86) | 26.2 (9.27) | 27.0 (8.29) | 29.9 (8.51) | 29.6 (9.09) | 30.1 (8.08) |
| Intensity gradient | −2.55 (0.193) | −2.51 (0.201) | −2.57 (0.182) | −2.59 (0.213) | −2.54 (0.209) | −2.64 (0.206) | −2.52 (0.194) | −2.48 (0.207) | −2.55 (0.179) |
| MVPA (minutes) | 48.1 (32.74) | 48.9 (32.85) | 47.6 (32.65) | 40.0 (33.61) | 42.3 (35.46) | 37.0 (30.75) | 51.7 (34.53) | 52.7 (34.96) | 51.0 (34.23) |
CVD, cardiovascular disease; MVPA, moderate to vigorous physical activity.
Values are reported are mean (standard deviation) or number [percentage].
Composite measure of deprivation based on unemployment, non–car ownership, non–home ownership, and household overcrowding; negative values represent less deprivation.
Self-reported history of heart attack, angina, stroke, or cancer variables or hospital episode with International Statistical Classification of Diseases, Tenth Revision code I20-25, I60-69, or C00-99.
Severe COVID-19 Relative to No COVID-19 (Model 1) and Relative to Nonsevere COVID-19 (Model 2)
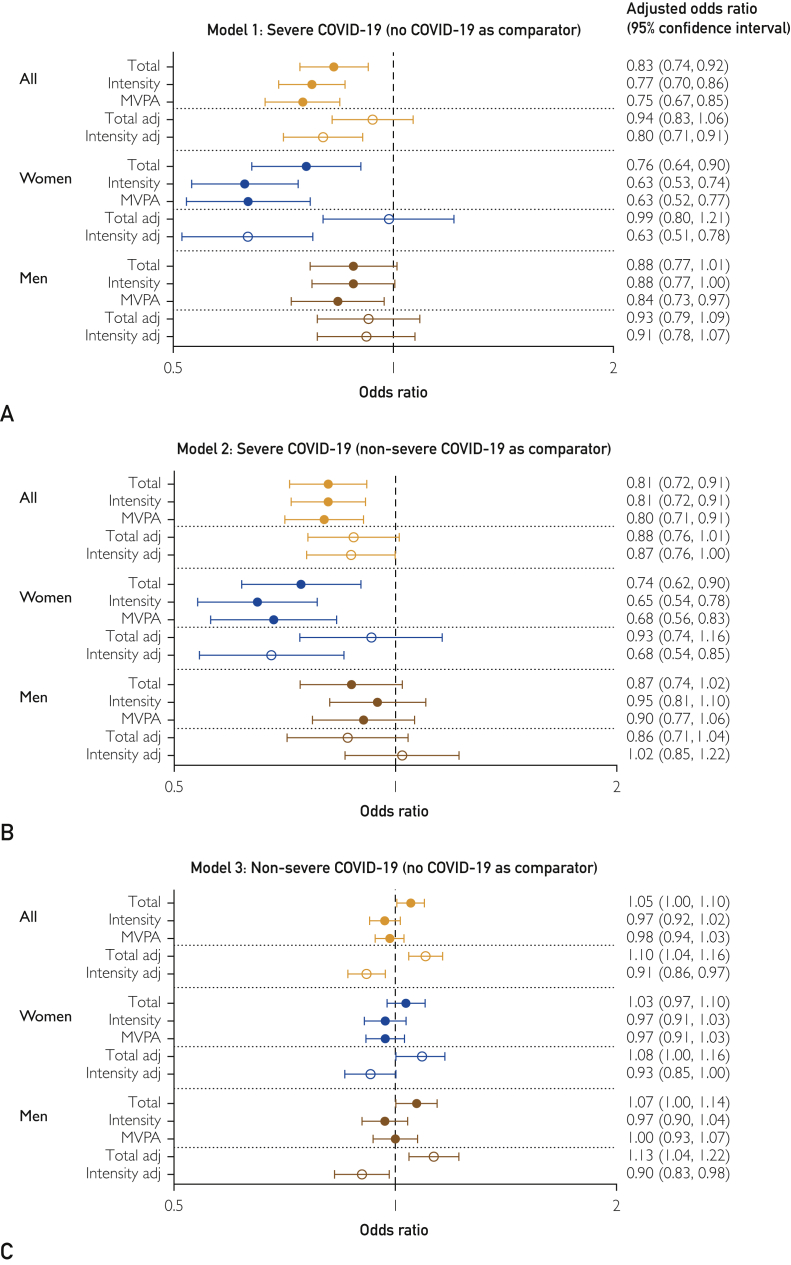
Total physical activity (adjusted odds ratio per standard deviation increase: 0.83 [95% CI, 0.74 to 0.92]), intensity gradient (0.77 [0.70 to 0.86]), and time in MVPA (0.75 [0.67 to 0.85]) were associated with lower odds of a severe infection relative to the cohort (Figure 1A). When mutually adjusted, the intensity gradient remained significant (0.80 [0.71 to 0.91]), but the association with total physical activity was attenuated.
Figure 1.
Association of total physical activity, intensity gradient, and moderate to vigorous physical activity (MVPA). A, Model 1, severe COVID-19 (no COVID-19 as comparator). B, Model 2, severe COVID-19 (nonsevere COVID-19 as comparator). C, Model 3, nonsevere COVID-19 (no COVID-19 as comparator). Odds ratios are expressed per standard deviation of each variable. Where adj follows the variable name, it indicates the 2 variables were mutually adjusted.
Interactions between sex and physical activity were significant for the intensity gradient (P=.03) and MVPA (P=.10). Associations were stronger in women for all exposures (total physical activity, 0.76 [0.64 to 0.90]; intensity gradient, 0.63 [0.53 to 0.74]; MVPA, 0.63 [0.52 to 0.77]) than in men (total, 0.88 [0.77 to 1.01]; intensity gradient, 0.88 [0.77 to 1.00]; MVPA, 0.84 [0.73 to 0.97]; Figure 1A).
Results for a severe infection relative to those with a nonsevere infection (model 2) were consistent with those for model 1 (Figure 1B).
Nonsevere COVID-19 Relative to No COVID-19 (Model 3)
There was an association between total physical activity and higher odds of a nonsevere infection (1.05 [1.00 to 1.10]; P=.03; Figure 1C). When mutually adjusted, the intensity gradient was associated with lower odds of a nonsevere infection (0.91 [0.86 to 0.97]), whereas total physical activity was associated with higher odds (1.10 [1.04 to 1.16]). No associations with MVPA were evident. The pattern of results was consistent for men and women.
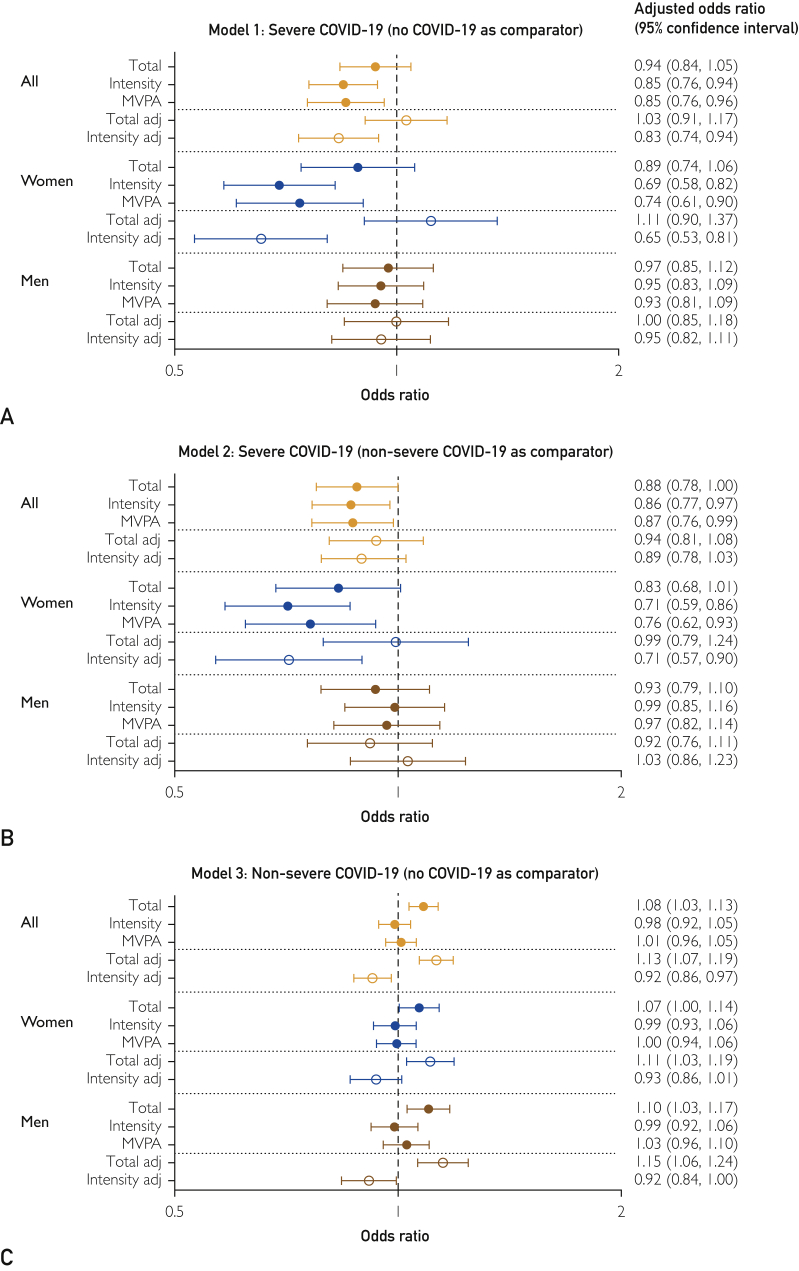
Sensitivity analyses with further adjustment for health-related covariates potentially on the causal pathway from physical activity to COVID-19 risk (Figure 2) slightly attenuated the effect estimates for models 1 and 2 but were broadly consistent with the main analyses. Models 1 and 3, with the comparator group restricted to people who tested negative for COVID-19 (Supplemental Figure 2, available online at http://mcpiqojournal.org; Supplemental Figure 3, further adjusted for covariates potentially on the causal pathway, available online at http://mcpiqojournal.org), and results of unadjusted models (Supplemental Figure 4, available online at http://mcpiqojournal.org) were broadly consistent with results of the main analyses. Adjusted odds ratios for the risk factors included in the analyses are shown in Supplemental Figures 5 and 6 (available online at http://mcpiqojournal.org).
Figure 2.
Sensitivity analysis with models further adjusted for health-related covariates potentially on the causal pathway. Association of total physical activity, intensity gradient, and moderate to vigorous physical activity (MVPA). A, Model 1, severe COVID-19 (no COVID-19 as comparator). B, Model 2, severe COVID-19 (nonsevere COVID-19 as comparator). C, Model 3, nonsevere COVID-19 (no COVID-19 as comparator). Odds ratios are expressed per standard deviation of each variable. Where adj follows the variable name, it indicates the 2 variables were mutually adjusted.
Discussion
Main Findings
Higher physical activity was associated with reduced odds of severe COVID-19; intensity of physical activity was the driving factor, with 20% to 25% lower odds per 30 minutes of daily MVPA (eg, walking). Associations were stronger in women, with 32% to 37% lower odds per 30 minutes of daily MVPA relative to 10% to 16% lower odds in men. Total physical activity appeared to increase the odds of nonsevere COVID-19. As the incidence of nonsevere infections reflects community transmission, this finding probably reflects greater exposure to the virus. In contrast, when adjusted for total activity, a greater proportion of high-intensity activity was associated with 7% to 10% lower odds of infection.
Increased odds of severe COVID-19 with lower total activity and MVPA is consistent with the recent findings from self-reported physical activity in the United States13 and from UK Biobank early in the pandemic.12 It is not clear why associations with severe COVID-19 tended to be weaker in men than in women for metrics reflecting the intensity of physical activity. Men are known to be at higher risk of severe COVID-19 than women, and although most studies included sex in their analyses as a potential confounder, relatively few studies have reported whether associations with risk factors differ by sex (ie, whether sex is an effect modifier). However, Gao et al24 recently reported no difference in associations between body mass index and COVID-19 severity by sex. Conversely, higher odds of severe COVID-19 have been reported for women who work shifts outside of health care (2.77 [2.14 to 3.59]) than for men who work shifts outside of health care (1.59 [1.23 to 2.05]).25
Total Amount and Intensity of Activity
The availability of accelerometer-assessed physical activity enabled us to explore whether the total amount of physical activity and the intensity of that activity were associated with COVID-19 outcomes independent of each other. Independent associations for the intensity gradient for the whole cohort and women for severe COVID-19, alongside reduced odds for MVPA, were observed. This suggests that the proportion of activity taken at a moderate to vigorous intensity is key (eg, walking and brisk walking), consistent with self-report of meeting physical activity guidelines13 or having a brisk walking pace.6 As time spent in MVPA is associated with cardiorespiratory fitness, this is consistent with evidence that cardiorespiratory fitness26 is associated with reduced risk of hospitalization due to COVID-19.27,28
Low levels of physical activity contribute to chronic disease10 and chronic inflammation,9 which could be a factor in the observed association with severe COVID-19.11 Given that COVID-19 is an acute inflammatory disease, inactivity may also exacerbate existing chronic inflammation and, alongside other risk factors (eg, genetic predisposition, psychological factors), be associated with a “cytokine storm” contributing to this increased risk of severe COVID-19.9
For nonsevere infections, when mutually adjusted, the intensity gradient was again independently associated with reduced odds of infection, but total physical activity was associated with elevated odds. MVPA, which combines moderate- and vigorous-intensity activity, was not associated with reduced odds. This suggests that intensities greater than moderate (eg, vigorous activity such as brisk walking and running) may be optimal to reduce the odds of a nonsevere infection. It is possible that the observed association between total physical activity and increased odds of a nonsevere infection is due to a bias in the people who were more likely to be tested for COVID-19.29 However, results were consistent in restricting the comparator group to people who tested negative for COVID-19. Accumulating evidence indicates that occupation type is associated with COVID-19 outcomes, with elevated risk in essential workers.30 Notably, approximately three-quarters of the people with nonsevere COVID-19 were employed, relative to approximately half of the cohort. It is possible that high levels of total physical activity accumulated at relatively low intensities reflect working in a job that requires high levels of routine movement/walking and more community engagement and thus increased exposure to the virus. Whereas we controlled for employment status and deprivation, it was not possible to determine the level of exposure to infection. Conversely, vigorous-intensity activity may reflect exercise or training-type activity that strengthens immunity.7
Strengths and Limitations
The main strength of this study is the availability of accelerometer-assessed physical activity, facilitating precise characterization of the physical activity profile of participants in a large population with linked COVID-19 data. In addition, UK Biobank is an extensively phenotyped population, differentiating it from many other data sets currently being analyzed to better understand COVID-19. However, there are also some limitations. First and foremost, the characteristics of participants were measured—and accelerometer data collected—some years before the pandemic. However, data from a longitudinal study of older adults in England31 found steady physical activity levels over time, albeit with a slight decline in vigorous activity. Second, the definition of severe COVID-19 was a positive test result from a hospital inpatient. Whereas this is consistent with the definition proposed by the researchers who developed the linkage method19 and with previous research using the UK Biobank data set to explore risk factors for COVID-19,2,5,6,12,30 actual disease severity cannot be confirmed from the linkage data available. Testing in the UK has not been universal, particularly in the first wave of the pandemic, making the analyses vulnerable to bias.29 Furthermore, this is an observational study; thus, we cannot exclude the risk of residual confounders due to unmeasured confounders or measurement error. Finally, UK Biobank participants are not representative of the wider population; however, participants may not need to be representative in estimating relative risk factor associations.32 Taking these limitations into consideration, our results point to the potential importance of physical activity as predictive of later risk of severe and nonsevere COVID-19 infection. Notably, however, the results are consistent with proposed mechanisms and the reduced risk of severe COVID-19 in participants who self-reported consistently meeting physical activity guidelines in the 2 years before the pandemic.13
Conclusion
Physical activity appeared to be associated with lower odds of severe COVID-19, with stronger associations for intensity of movement in women than in men. Odds of severe COVID-19 were lower by 37% in women and 16% in men per 30 minutes of daily MVPA. Higher total physical activity appeared to increase the odds of nonsevere infection, but a greater proportion of high-intensity activity was associated with 8% to 10% lower odds. Nonsevere infections reflect community transmission; thus, the greater odds associated with higher total physical activity levels, accumulated at lower intensity, are likely to reflect greater exposure to the virus (eg, through occupational activity).
Results from this study are consistent with self-reported data and provide further evidence for the role of physical activity in reducing the odds of a severe infection and the potential for vigorous activity to play a role in reducing the odds of nonsevere infection, possibly through more robust immunity. This is important as a reduction in nonsevere infections has potential for reducing community transmission. This study provides further support to calls for public health messaging to highlight the potential of physical activity, particularly of moderate to vigorous intensity, in reducing the risk of severe COVID-19.
Footnotes
Grant Support: This research was supported by the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre, the NIHR Applied Research Collaborations–East Midlands, and grants from the UK Research and Innovation–Department of Health and Social Care COVID-19 Rapid Response Rolling Call (MR/V020536/1) and Health Data Research UK (HDRUK2020.138). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Potential Competing Interests: K.K. is a member of the UK Scientific Advisory Group for Emergencies (SAGE), Chair of the SAGE subgroup on ethnicity and COVID-19, and member of Independent SAGE. All other authors declare that they have no competing interests.
Data Sharing: This research has been conducted using the UK Biobank Resource under Application 36371. The database supporting the conclusions of this article is available from the UK Biobank project site, subject to registration and application process. Further details can be found at https://www.ukbiobank.ac.uk.
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
References
- 1.Joint Committee on Vaccination and Immunisation Independent report. Priority groups for COVID-19 vaccination: advice from the JCVI, 2 December 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-2-december-2020/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-2-december-2020
- 2.Razieh C., Zaccardi F., Islam N. Ethnic minorities and COVID-19: examining whether excess risk is mediated through deprivation. Eur J Public Health. 2021;31(3):630–634. doi: 10.1093/eurpub/ckab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson R.M., Heesterbeek H., Klinkenberg D., Hollingsworth T.D. How will country-based mitigation . How will country-based mitigation measures influence the course of the COVID-19 epidemic? Lancet. 2020;395(10228):931–934. doi: 10.1016/S0140-6736(20)30567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathur R., Rentsch C.T., Morton C.E. Ethnic differences in SARS-CoV-2 infection and COVID-19–related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform [erratum appears in Lancet. 2021;397(10291):2252] Lancet. 2021;397(10286):1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yates T., Razieh C., Zaccardi F., Davies M.J., Khunti K. Obesity and risk of COVID-19: analysis of UK Biobank. Prim Care Diabetes. 2020;14(5):566–567. doi: 10.1016/j.pcd.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yates T., Razieh C., Zaccardi F. Obesity, walking pace and risk of severe COVID-19: analysis of UK Biobank. Int J Obes (Lond) 2021;45(5):115–1159. doi: 10.1038/s41366-021-00771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chastin S., Abaraogu U., Bourgois J.G. Effects of regular physical activity on the immune system, vaccination and risk of community-acquired infectious disease in the general population: systematic review and meta-analysis. Sports Med. 2021;51(8):1673–1686. doi: 10.1007/s40279-021-01466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallis J.F., Adlakha D., Oyeyemi A. An international physical activity and public health research agenda to inform coronavirus disease-2019 policies and practices. J Sport Health Sci. 2020;9(4):328–334. doi: 10.1016/j.jshs.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vepa A., Bae J.P., Ahmed F., Pareek M., Khunti K. COVID-19 and ethnicity: a novel pathophysiological role for inflammation. Diabetes Metab Syndr. 2020;14(5):1043–1051. doi: 10.1016/j.dsx.2020.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warburton D.E., Nicol C.W., Bredin S.S. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rowlands A.V., Kloecker D.E., Chudasama Y. Association of balance and timing of physical activity and rest with risk of COVID-19 in UK Biobank. Mayo Clin Proc. 2020;96(1):156–164. doi: 10.1016/j.mayocp.2020.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallis R., Young D.R., Tartof S.Y. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021 Apr 13 doi: 10.1136/bjsports-2021-104080. ;bjsports-2021-104080. [DOI] [PubMed] [Google Scholar]
- 14.Arvidsson D., Fridolfsson J., Börjesson M. Measurement of physical activity in clinical practice using accelerometers. J Intern Med. 2019;286(2):137–153. doi: 10.1111/joim.12908. [DOI] [PubMed] [Google Scholar]
- 15.Rowlands A.V., Yates T., Edwardson C.L. Activity intensity, volume and norms: utility and interpretation of accelerometer metrics. Med Sci Sports Exerc. 2019;51(11):2410–2422. doi: 10.1249/MSS.0000000000002047. [DOI] [PubMed] [Google Scholar]
- 16.Strain T., Wijendaele K., Dempsey P.C. Wearable-device-measured physical activity and future health risk. Nat Med. 2020;26(9):1385–1391. doi: 10.1038/s41591-020-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty A., Jackson D., Hammerla N. Large scale population assessment of physical activity using wrist worn accelerometers: the UK Biobank Study. PLoS One. 2017;12(2):e0169649. doi: 10.1371/journal.pone.0169649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudlow C., Gallacher J., Allen N. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong J., Rudkin J.K., Allen N. Dynamic linkage of COVID-19 test results between Public Health England’s Second Generation Surveillance System and UK Biobank. Microb Genom. 2020;6(7):mgen000397. doi: 10.1099/mgen.0.000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migueles J.H., Rowlands A.V., Huber F., Sabia S., van Hees V.T. GGIR: a research community-driven open-source R-package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Measure Phys Behav. 2019;2(3):188–196. [Google Scholar]
- 21.Rowlands A.V., Edwardson C.L., Davies M.J., Khunti K., Harrington D.M., Yates T. Beyond cut points: accelerometer metrics that capture the physical activity profile. Med Sci Sports Exerc. 2018;50(6):1323–1332. doi: 10.1249/MSS.0000000000001561. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrand M., van Hees V.T., Hansen B.H., Ekelund U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med Sci Sports Exerc. 2014;46(9):1816–1824. doi: 10.1249/MSS.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 23.Rowlands A.V., Edwardson C.L., Dawkins N., Maylor B., Metcalf K.M., Janz K.F. Physical activity for bone health: how much and/or how hard? Med Sci Sports Exerc. 2020;52(11):2331–2341. doi: 10.1249/MSS.0000000000002380. [DOI] [PubMed] [Google Scholar]
- 24.Gao M., Piernas C., Astbury N.M. Associations between body-mass index and COVID-19 severity in 6.9 million people in England: a prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 2021;9(6):350–359. doi: 10.1016/S2213-8587(21)00089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowlands A.V., Gillies C., Chudasama Y. Association of working shifts, inside and outside of healthcare, with severe COVID-19: an observational study. BMC Public Health. 2021;21(1):773. doi: 10.1186/s12889-021-10839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bull F.C., Al-Ansari S., Biddle S. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brawner C.A., Ehman J.K., Bole S. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc. 2021;96(1):32–39. doi: 10.1016/j.mayocp.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lavie C.J., Sanchis-Gomar F., Arena R. Fit is it in COVID-19, future pandemics, and overall healthy living. Mayo Clin Proc. 2021;96(1):7–9. doi: 10.1016/j.mayocp.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Griffith G.J., Morrus T.J., Tudball M.J. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Comm. 2020;11(1):5749. doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutambudzi M., Niedwiedz C., Macdonald E.B. Occupation and risk of severe COVID-19: prospective cohort study of 120,075 UK Biobank participants. Occup Environ Med. 2020 Dec 9 doi: 10.1136/oemed-2020-106731. oemed-2020-106731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith L., Gardner B., Fisher A., Hamer M. Patterns and correlates of physical activity behaviour over 10 years in older adults: prospective analyses from the English Longitudinal Study of Ageing. BMJ Open. 2015;5(4):e007423. doi: 10.1136/bmjopen-2014-007423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batty G.D., Gale C.R., Kivimäki M., Deary I.J., Bell S. Comparison of risk factor associations in UK Biobank against representative, general population-based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131. doi: 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.