Abstract
Objective
To compare the effect of temporary fixed restorations to final fixed restorations on gingival health of abutment tooth by evaluating the level of IL-1β in gingival crevicular fluid (GCF) as an inflammatory biomarker.
Subjects and methods
It was a nonrandomized prospective observational study. Samples of GCF were collected from 24 participants with provisional and permanent definitive full-coverage restorations with subgingival margin placements at three intervals for each participant: Interval 1, after preparation and immediately before cementation of temporary fixed restoration. Interval 2, after two weeks of temporization and before receiving the final fixed restoration. Interval 3, after two weeks of final fixed restorations. GCF were collected from gingival crevice of the abutment tooth by pre-prepared filter paper with dimensions of 2mmx13mm. They were prepared for IL-1β concentration assay by enzyme-linked immuno sorbent assay – analysis (ELIZA).
Results
Were analyzed by SAS® software, Descriptive means and medians were used and the repeated ANVOA test was applied (1st interval) showed the highest level of IL-β (13.587 ± 5.735). In 2nd interval the level of IL-β was significantly reduced (9.602 ± 3.279). While, in 3rd interval the level of IL-β was the lowest (6.293 ± 3.279).
Conclusion
The material and technique of fabrication of both temporary and final fixed restorations are critical for gingival health. PMMA CAD-CAM based temporary restorations showed to be compatible to gingival health by decreasing the level of IL-β level, but the zirconia as a final fixed restoration showed to have optimum compatible effect on gingival health.
Keywords: Crevicular fluid, Gingival crevicular fluid, Interleukin-1β, Final fixed restorations, CAD-CAM temporary fixed restorations, Provisional restorations
1. Introduction
The main goal of restorative dental treatment is to restore the function of lost teeth in terms of esthetic, mastication, and periodontal health. While the restoration has an impact on periodontal health, periodontal health is basic for successful restoration (Gracis et al., 2001).
Temporary restoration provides a template for final restorations and helps in the recovery of any traumatized soft tissues. They may remain from 2 to 6 weeks up to months, so they should be well fabricated and fulfill many criteria, including occlusal stability, pulp protection, wear resistance, reasonable esthetics, margin accuracy and easy cleansing to enhance the periodontal health that may influence the outcome of final fixed restoration if becomes inflamed (Regish et al., 2011, Savadi et al., 2011).
The temporary restorations material and technique of fabrication may influence the gingival health due to polymerization heat generation and toxicity to the resident cells, surface roughness enhancing plaque accumulation and margin integrity (Rakhshan, 2015).
There are different materials for temporary restorations as Polymethyl methacrylate blocks (PMMA), polymethyl methacrylate (PEMA), bis-acryl composite, and urethane dimethacrylate (UDMA). They may be ready or custom made fabricated. The custom made technique is applied directly to tooth using a template, indirectly by taking an impression, or both directly and indirectly. The indirect technique has the main advantage of avoiding the direct contact of free monomer to tooth and soft tissue, polymerization heat and superior marginal adaptation (Tom et al., 2016, Regish et al., 2011).
There are different materials for the fabrication of fixed restorations such as metal alloys, metals and nonmetals, and all ceramics like Zirconia with superior esthetic appearance and biocompatibility (Poggio et al., 2017).
Gingival crevicular fluid (GCF) is a physiologic transudate present in a healthy gingival crevice in a small amount that increases during periodontal inflammation and becomes an inflammatory exudate. It is a promising diagnostic and prognostic fluid with many advantages such as site-specific, noninvasiveness and contains a wide variety of microbial and host mediators like pro-inflammatory cytokines such as IL-1 β, IL-6, IL-8 (Kapoor et al., 2014, Gupta et al., 2018).
Periodontal diseases are chronic inflammation mediated by host response related to dysbiotic biofilm, where IL-1 β plays a key role in pathogenesis as it increases the blood flow recruiting neutrophils, stimulates other collagenolytic enzymes that destruct tissues and bones, and intensifies other inflammatory mediators such as prostaglandin PGE2 and IL-6. Its concentration in GCF is greater in disease sites than in healthy ones and it is directly related to disease severity in terms of depth of pockets, bleeding on probing, and bone loss (Nazar Majeed et al., 2016, Cheng et al., 2020).
Different studies have investigated the effect of temporary and final fixed restorations on periodontal health; they were in vitro and in vivo studies to evaluate the clinical periodontal health indices (Bluma et al., 2016, Jubhari, et al., 2019). Recently, a study has investigated the effect of different final fixed restoration materials on gingival inflammation by measuring the level of IL-1β (Saravanakumar et al., 2017). To our knowledge, no previous study has compared the effect of temporary and final fixed restorations on gingival health. The purpose of this study was to compare the effect of temporary fixed restoration to that of final fixed restoration on gingival health of abutment tooth by evaluating the level of IL-1β in GCF as an inflammatory biomarker. The null hypothesis is that there is no difference between the level of IL-1β in GCF of abutment teeth in case of temporary and final fixed restorations. However, the alternative hypothesis is that the level of IL-1β in the case of temporary restoration is significantly higher than that in the case of final fixed restoration.
2. Subjects and methods
2.1. Ethical statement
The study protocol was approved by the Faculty of Dentistry's Ethical Research Committee (ERC), Beni-Suef University (FDBSU_REC-#FDBSUREC/09072020/ME). Written consents for the study design were provided by all participants.
2.2. Sample size
The study was conducted by 24 participants in a private dental clinic in Giza, Egypt. The sample size was calculated using G* Power 3.1.9.2 software (Franz Faul, Unvirsität Kiel, Germany) – with input data, effect size of 0.27, α 0.5, power of 0.80, and the chosen test ANOVA: repeated measures between-within factors.
2.3. Inclusion and exclusion criteria
It was a nonrandomized prospective observational study. The periodontal condition of abutment teeth was evaluated in each visit by using plaque index (PI) (Silness and Loe, 1964) for oral hygiene status, gingival index (GI) (Loe and Silness, 1963) for gingival health, probing depth (PD), and clinical attachment loss (CAL). Only participants with good oral hygiene (PI ≤ 1), having no gingival inflammation (GI ≤ 1) and no CAL of endodontically treated teeth for full coverage were included. The participants were also instructed to follow the routine oral hygiene measures. The periodontally affected sites, cases of local or systemic conditions such as diabetes were excluded.
2.4. Clinical preparation and prosthesis fabrication
All prosthetic steps were performed by the clinic’s operator and the fixed prosthodontist, while the periodontal evaluation and sample collection were performed by the periodontist.
During the 1st visit, upper and lower alginate impressions were taken with an anatomical stainless-steel tray to obtain the study cast (Hydrogum 5 – Zhermack Alginate dental material). Then the teeth were prepared with a subgingival shoulder finish line giving enough crown length for maximum retention (MAX300 min-1 DIA-BURS Dental High-Speed, and Air Turbine Handpiece ISO199\018FG Diamond Burs TR-12SC LOT D20A1600900). After tooth preparation, a secondary impression was taken. An addition silicone impression material (zetaplus - silicone impression putty (1 × 900ml.) - Zhermack S.P.A. via Bovazecchino, 100 45021Badia Polsine, Rovigo, Italy) and Zhermack Elite HD Light Body (Fast Set, 2 × 50ml Cartridges) were used to duplicate the prepared teeth and type IV stone powder was poured to obtain the master cast for construction of custom-made temporary crown according to laboratory instruction (CAD ivory Temp (PMMA), Poly methyl-methacrylate Crowns and Bridges casting disc, on dent Karacaoglan Mah. Erena Plaza 6166 Sk. No:32/1J 35,070 Bornova / İZMİR Turkey).
During the 2nd visit, within 1–3 days, the 1st sample was collected immediately before cementation of the temporary crown. The custom-made CAD ivory PMMA temporary crown was cemented using an Urbical self-curing calcium hydroxide paste/paste system with a flowable consistency (Promedica Dental Material, GmbH Domagkstrasse 31 24537, Neumuenster, Germany).
2 weeks later and during the 3rd visit, the 2nd sample was collected immediately before the removal of the temporary crown. The temporary crown and all the remaining cement were then cleaned, and the tooth was polished, irrigated, and dried. The constructed permanent zirconium crowns were cemented according to the manufactural instruction (ITENA Dento Bond Porcelain Fix), 10 ml, bonding process for ceramic 5 ml Etching + 5 ml Silane where the crown was etched by a specially buffered viscous hydrofluoric acid (8%) to make a micro-porous surface that provides a strong mechanical interlock with composite resin materials. Porcelain Silane (97% ethyl alcohol) was then applied to the etched surfaces (31 Avenue Georges Clemenceau, 93420 Villepinte, France). Finally, transparent Multilink Speed – (Ivoclar Vivadent) self-adhesive-resin-cement was applied and excess cement was removed. Two weeks later, the final sample was collected.
2.5. Samples collection
According to the previous protocol, the samples were collected at three intervals for each participant:
The 1st Interval (Group 1): Immediately before cementation of temporary fixed restoration.
The 2nd Interval (Group 2): 2 weeks after temporization and before receipt of final fixed restoration.
The 3rd Interval (Group 3): 2 weeks after receipt of final fixed restoration.
The GCF samples were collected from the gingival crevice of the abutment tooth by pre-prepared filter paper with dimensions of 2mmx13mm (Filter paper circles, MN 614, Qualitative, Medium fast (20 s), Embossed, MACHEREY-NAGEL GmbH& Co.KG, Germany). After gentle scaling to remove any plaque or deposits, isolation by cotton roll and dryness, the filter paper was inserted into the gingival crevice at the mesial or distal buccal line angle of the abutment tooth till slight resistance was found (Fig. 1) for 30 s then removed and checked to confirm that they were free from blood. They were kept in labeled Eppendorf tubes containing 100 μl of Hanks balanced salt solution (HBSS, Gibco /Invitrogen, Grand Island, New York, USA). They were kept frozen at −70 °C for assay (Saravanakumar et al.,2017).
Fig. 1.
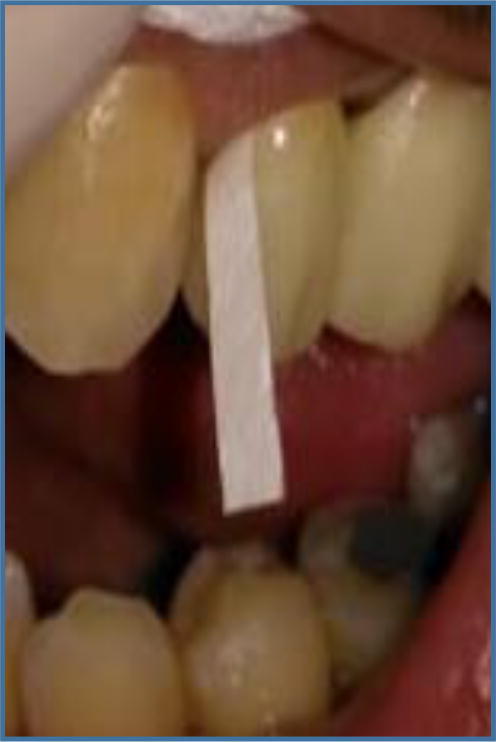
Filter paper inserted in mesial line angle of abutment tooth with zirconia crown.
2.6. IL-1β concentration assay by ELIZA
It was conducted in the Department of Medical Biochemistry, Faculty of Medicine, Cairo University. After the collection of all samples, they were prepared for IL-1β concentration assay by carrying out enzyme-linked immunosorbent assay - analysis (ELISA, Human IL-1 Beta ELISA Kit PicoKine™, Boster Biological Technology, and Pleasanton CA, USA, Catalog # MBS175901). The samples were kept at room temperature for at least 15 min before performing the assay and protease inhibitor and 0.1 mm phenylmethylsulfonyl fluoride were added. GCF was eluted from the paper by a rapid vortex mixer (HercuvanLab Systems Sdn Bhd, Malaysia). The papers were removed and the elutes were centrifuged for several minutes with the sample standardized volume of 100 μl. The samples and control were arranged in a microtitre plate. The sample diluent was gently mixed with samples and control. The plate was covered at room temperature for 120 min and then the excess was discarded. Biotinylated Anti-Human IL1B antibody was added, and the plate was covered for 90 min at room temperature. The plate was washed 3 times with wash buffer. Avidin-Biotin-Peroxidase Complex was added, and the plate was covered for 90 min at room temperature, and then it was washed 5 times with wash buffer. Color Developing Reagent was added, and the plate was covered and incubated in the dark for 30 min at room temperature. Stop Solution was added and within 30 min the reaction was stopped and the color absorbance was read at OD range 450 to 630 nm using an ELISA plate reader (Stat Fax 2200, Awareness Technologies, Florida, USA). The readings for each standard, sample, and control were duplicated. We plotted the mean OD absorbance for each standard against the concentration. The standard curve was created to generate a four parameter logistic (4-PL) curve-fit by using free software (www.myassays.com/four-parameter-logisticcurve.assay), then the measured concentration in the sample was interpolated by using linear regression of each average relative OD against the 4-PL curve.
2.7. Statistical analysis
The results were analyzed using SAS® software (SAS Institute Inc 2013. SAS/ACCESS® 9.4 Interface to ADABAS: Reference. Cary, NC: SAS Institute Inc). The data were tested by both Shapiro-Wilk and Kolmogorov-Smirnov normality tests, and then the ANOVA test was applied.
3. Results
3.1. Clinical parameters descriptive results:
The mean and standard deviation (SD) of clinical parameters of abutment teeth are shown in Table 1.
Table 1.
Mean and SD of the clinical periodontal parameters of abutment teeth during the 3 intervals.
| Plaque index | Gingival index | Pocket depth |
|---|---|---|
| 1.1666 ± 0.3806 | 1.2083 ± 0.4148 | 1.5 ± 0.3611 mm |
3.2. The IL-1β concentration results
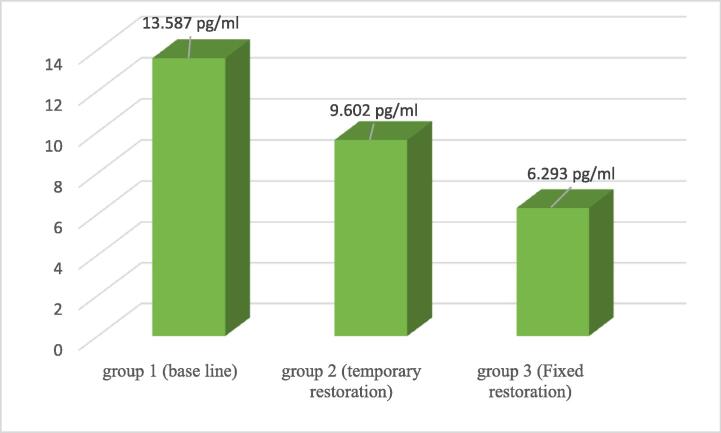
The results of IL-1β concentration are shown in Table 2, Fig. 2, in the form of the mean and SD, and on the application of ANOVA test for repeated measures. There was a statistically significant difference between all groups (p. value 0.0001).
Table 2.
Mean and SD of all groups and comparison with ANOVA test.
| GROUP | Mean | SD |
|---|---|---|
| GR1 | 13.587a pg/ml | 5.735 |
| GR2 | 9.602b pg/ml | 3.975 |
| GR3 | 6.293c pg/ml | 3.279 |
| P VALUE | 0.0001 | |
A-C = Means with the same letter in the column are not significantly different at p ≤ 0.05.
Fig. 2.
Bar Chart shows the comparison between inflammatory response (IL-β) level in pg/ml related to different groups.
The highest level of IL-1β was related to Group 1, before temporization, (13.587 ± 5.735 pg/ml), and then it was significantly decreased after temporization, Group 2, (9.602 ± 3.279 pg/ml). However, the lowest level was related to after final restoration, Group 3 (6.293 ± 3.279 pg/ml).
4. Discussion
The current study compared the effect of temporary fixed restorations and finally fixed restorations on gingival health of abutment tooth by evaluating the level of IL-1β in GCF as an inflammatory biomarker.
In the current study, the subgingival placement of both temporary and fixed restoration margins did not interfere with improvement in gingival health condition, where the IL-β levels were significantly lower than that level after preparation (p-value = 0.0001). This is consistent with (Kancyper and Koka, 2001) study results that the intracrevicular placement of crown restoration did not enhance the colonization of dental plaque by periodontal pathogens P gingivalis, P intermedia, and A actinomycetemcomitans in case of proper construction and proper oral hygiene,
About the current study, the most severe gingival inflammation was related to the interval after preparation and before temporization, where the IL-β level in GCF was (13.587 ± 5.735 pg/ml). These results can be explained through the possible traumatic effect of tooth structure preparation, retraction cord placement, and impression taking (Harish et al., 2015).
PMMA CAD/CAM indirectly based temporary restorations provide many advantages as they have a high strength to withstand the milling process, less polymerization shrinkage, natural appearance, better esthetic, and accurate marginal adaptation producing long term clinical success of final restoration (Yao et al., 2014). As for the current study, the CAD-CAM fabricated temporary restoration showed improvement of gingival health indicated by a significant decrease of the IL-β level in GCF after two weeks (9.602 ± 3.279 pg/ml, p-value 0.0001). This effect can be explained through high margin fitness, less surface roughness with subsequent less bacterial colonization as well as biocompatibility of PMMA CAD-CAM (Dureja et al., 2018). According to (Herráez-Galindo et al., 2019), the PMMA CAD-CAM material showed biologic compatibility with gingival health as they facilitate the gingival fibroblasts adhesion and proliferation with subsequent formation of gingival barrier decreasing the bacterial colonization. These results are consistent with (Ramkumar et al., 2012, Naqash et al., 2019, Jubhari, et al., 2019) that showed a better effect of the indirect fabrication technique of temporary restorations than that of direct techniques on gingival health.
Zirconium is considered one of the most selected materials for fixed final restorations with superior mechanical properties including flexural strength and toughness, good optical and natural esthetic appearance, and high biocompatible proprietaries that make the zirconia crowns a perfect choice for preserving the periodontal health (Zarone et al., 2019). About the current study, the zirconia final fixed restorations had a better effect on gingival health than temporary restorations presented by a significant decrease of the IL-β level in GCF after two weeks (6.293 ± 3.279 pg/ml, p-value 0.0001). These results can be explained through the biocompatibility of zirconia related to their low surface roughness inhibiting the bacterial colonization (Scarano et al., 2004). It is not cytotoxic for gingival fibroblasts as it does not affect their viability (Shin et al., 2016), highly polished surface facilitating oral hygiene as well as excellent marginal adaption provided by the CAD/CAM technique (Tang et al.,2019). These results are consistent with (Saravanakumar et al., 2017) that showed the zirconia crowns had minimal gingival inflammation indicated by the least IL-β level in GCF compared to other materials including metal and ceramic crowns.
Further studies are required to compare the effect of different materials of temporization and final fixed restorations over different intervals of temporization on periodontal health to decrease the incidence of periodontal inflammation.
5. Conclusion
It is recommended to shorten the temporization period as much as possible. The technique and material of both temporary and final fixed restorations are critical to the gingival health as the indirect technique of temporization as well as the CAD-CAM technique of both temporary and final fixed restorations fabrication improved the gingival health. The PMMA as a temporary restoration improved the gingival health after preparation, while zirconia as a final restoration material had a better effect indicated by a significant reduction in IL-1β level in GCF of abutment teeth.
CRediT authorship contribution statement
Amal Abdallah A. Abo Elmagd: Conceptualization, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Dina sabry: Data curation, Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Ebtehal Mohammed: Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to acknowledge Dr. Noha Mohammed Abu Baker's role for her great help in conducting this study in her private clinic and also would like to thank her patients who volunteered to help accomplish this work. Moreover, the authors appreciate the role played by Dr. Nazem Shalaby Zedan, Professor of Agriculture at Mansoura University, in performing the statistical analysis.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Amal Abdallah A. Abo-Elmagd, Email: amal.abdallah4660@gmail.com, dr.amal.abdallah@qudent.org, amal.abdallah@must.edu.eg.
Dina Sabry, Email: dinasabry@kasralainy.edu.eg, dinnasabry69@yahoo.com.
Ebtehal Mohammed, Email: ebtehalmaawad@dent.bsu.edu.eg, dr.ebtehalmoawad@gmail.com.
References
- Bluma E., Vidzis A., Zigurs G. The influence of fixed prostheses on periodontal health. Stomatologija. 2016;18(4):112–121. PMID: 28980541. [PubMed] [Google Scholar]
- Cheng R., Wu Z., Li M., Shao M., Hu T. Interleukin-1β is a potential therapeutic target for periodontitis: a narrative review. Int. J. Oral Sci. 2020;12(1):2. doi: 10.1038/s41368-019-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dureja I., Yadav B., Malhotra P., Dabas N., Bhargava A., Pahwa R. A comparative evaluation of vertical marginal fit of provisional crowns fabricated by computer-aided design/computer-aided manufacturing technique and direct (intraoral technique) and flexural strength of the materials: An in vitro study. J. Indian Prosthodont. Soc. 2018;18:314–320. doi: 10.4103/jips.jips_306_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracis S., Fradeani M., Celletti R., Brachhetti G. Biological integration of aesthetic restorations: factors influencing appearance and long-term success. Periodontol. 2001;2000(27):29–44. doi: 10.1034/j.1600-0757.2001.027001029.x. [DOI] [PubMed] [Google Scholar]
- Gupta S., Chhina S., Arora S.A. A systematic review of biomarkers of gingival crevicular fluid: Their predictive role in diagnosis of periodontal disease status. J. Oral. Biol. Craniofac. Res. 2018;8(2):98–104. doi: 10.1016/j.jobcr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harish, P-V., Joseph, S-A., Sirajuddin, S., Gundapaneni, V., Chungkham, S-A., 2015. Iatrogenic Damage to the Periodontium Caused by Fixed Prosthodontic Treatment Procedures. Open Dentistry J. Jun 26; 9:190–6. doi: 10.2174/1874210601509010190. PMCID: PMC4541334.
- Herráez-Galindo, C., Rizo-Gorrita, M., Luna-Oliva, I., Serrera-Figallo, MÁ., Castillo-Oyagüe, R., Torres-Lagares, D., 2019. In vitro Comparative Study of Fibroblastic Behaviour on Polymethacrylate (PMMA) and Lithium Disilicate Polymer Surfaces. Polymers (Basel). Apr 25;11(4):744. doi: 10.3390/polym11040744. PMID: 31027245; PMCID: PMC6523339. [DOI] [PMC free article] [PubMed]
- Jubhari, E.H., Machmud, E., Hasmina, et al., 2019. The effect of fabrication techniques of temporary crowns on the gingiva health. Journal of Dento maxillofacial Science (J Dentomaxillofac Sci) December 4(3): 133-136 | doi: 10.15562/jdmfs. v4i3.853.
- Kapoor P., Kharbanda O.P., Monga N., Miglani R., Kapila S. Effect of orthodontic forces on cytokine and receptor levels in gingival crevicular fluid: a systematic review. Prog. Orthod. 2014;15(1):65. doi: 10.1186/s40510-014-0065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kancyper, S.G., Koka, S., 2001. The influence of intracrevicular crown margins on gingival health: preliminary findings. J. Prosthet. Dent. May;85(5):461-5. doi: 10.1067/mpr.2001.115386. PMID: 11357072. [DOI] [PubMed]
- Loe H., Silness I. Periodontal disease in pregnancy. Acta Odont. Scand. 1963;21(6):533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Naqash Talib Amin. Marginal accuracy of provisional crowns using three material systems and two techniques: A scanning electron microscope study. Pakistan J. Med. Sci. 2019;35(1):55–60. doi: 10.12669/pjms.35.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar Majeed Z., Philip K., Alabsi A.M., Pushparajan S., Swaminathan D. Identification of Gingival Crevicular Fluid Sampling, Analytical Methods, and Oral Biomarkers for the Diagnosis and Monitoring of Periodontal Diseases: A Systematic Review. Dis. Markers. 2016;2016:1804727. doi: 10.1155/2016/1804727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio C.E., Ercoli C., Rispoli L., Maiorana C., Esposito M. Metal-free materials for fixed prosthodontic restorations. Cochrane Database Syst. Rev. 2017;12(12):CD009606. doi: 10.1002/14651858.CD009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhshan V. Marginal integrity of provisional resin restoration materials: A review of the literature. Saudi J. Dental Res. 2015;6:33–40. [Google Scholar]
- Ramkumar V., Sangeetha A., Kumar V. Effect of water temperature on the fit of provisional crown margins during polymerization: An in vitro study. J. Pharm. Bioallied. Sci. 2012;4:376–383. doi: 10.4103/0975-7406.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regish K.M., Sharma Deeksha, Prithviraj D.R. Techniques of Fabrication of Provisional Restoration: An Overview Review Article. Int. J. Dent. 2011 doi: 10.1155/2011/134659. Open Access. |Article ID 134659 |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanakumar P., Thallam Veeravalli P., Kumar V.A. Effect of Different Crown Materials on the InterLeukin-One Beta Content of Gingival Crevicular Fluid in Endodontically Treated Molars: An Original Research. Cureus. 2017;9(6) doi: 10.7759/cureus.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savadi A., Rangarajan V., Savadi R.C., Satheesh P. Biologic perspectives in restorative treatment. J. Indian Prosthodont. Soc. 2011;11(3):143–148. doi: 10.1007/s13191-011-0101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarano, A., Piattel, li M., Caputi, S., Favero, GA., Piattelli, A., 2004. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. Feb;75(2):292-6. doi: 10.1902/jop.2004.75.2.292. PMID: 15068118. [DOI] [PubMed]
- Shin H., Ko H., Kim M. Cytotoxicity and biocompatibility of Zirconia (Y-TZP) posts with various dental cements. Restor, Dent, Endod. 2016;41(3):167–175. doi: 10.5395/rde.2016.41.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silness J., Loe H. Periodontal Disease in Pregnancy. II. Correlation between Oral Hygiene and Periodontal Condition. Acta Odontologica Scandinavica. 1964;(22):121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Tang Z., Zhao X., Wang H., Liu B. Clinical evaluation of monolithic zirconia crowns for posterior teeth restorations. Medicine. 2019;98(40) doi: 10.1097/MD.0000000000017385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom N.T., Uthappa M.A., Sunny K., Begum F., Nautiyal M., Tamore S. Provisional restorations: An overview of materials used. J. Adv. Clin. Res. Insights. 2016;3:212–214. [Google Scholar]
- Yao, J., Li, J., Wang, Y., Huang, H., 2014. Comparison of the flexural strength and marginal accuracy of traditional and CAD/CAM interim materials before and after thermal cycling. J. Prosthet. Dent. Sep; 112(3):649-57. [DOI] [PubMed]
- Zarone F., Di Mauro M.I., Ausiello P. Current status on lithium disilicate and zirconia: a narrative review. BMC Oral Health. 2019;19:134. doi: 10.1186/s12903-019-0838-x. [DOI] [PMC free article] [PubMed] [Google Scholar]