Abstract
Introduction
Stroke, a dreaded complication of SARS-CoV2, has been reported in 0.9 to 5% of SARS-CoV2 patients. There are concerns that SARS-CoV2 infection has a significant independent association with acute ischemic stroke, even in the absence of conventional cerebrovascular risk factors. Whether elevated levels of inflammatory biomarkers have predictive value in the occurrence of stroke in SARS-CoV2 is poorly understood.
Aim
To profile the characteristics of SARS-CoV2 positive patients with ischemic stroke (COVID-Stroke) and to identify the significance of elevated IBMs in the prediction of ischemic COVID-stroke.
Materials and methods
Clinical characteristics, stroke risk factors, laboratory parameters- including levels of inflammatory biomarkers, and outcome of SARS-CoV2 patients with stroke (n=60) were collected. SARS-CoV2 RT- PCR positive age, gender, and pulmonary severity matched non-stroke patients were taken as controls (n = 60). Binary multivariate logistic regression analysis was used to find the predictors of ischemic COVID-stroke.
Results
D-dimer > 441.8 ng/mL, LDH> 395U/L, ESR >19 mm/h and CRP> 0.2 mg/dL were independently found to be very strong predictors of occurrence of ischemic COVID-stroke (p < 0.001 for each). On multivariate analysis, D-dimer > 441.8 ng/mL, ESR > 19 mm/h, and RDW > 16.1% were found to be the most strong predictors of the occurrence of ischemic COVID-stroke. Conventional CVD risk factors- higher age (> 60years), presence of diabetes mellitus, and hypertension were not found to be significant predictors in multivariate analysis.
Conclusion
In SARS-CoV2 patients, D-dimer elevated beyond 441.8 ng/mL, ESR greater than 19 mm/h, and RDW widened more than 16.1% were the strongest predictors of the occurrence of ischemic stroke. This is the first study that attempts to find cut-off levels of IBMs in the prediction of ischemic COVID-stroke.
Key Words: Covid, COVID-19, Stroke, Covid stroke, Ischemic stroke
Introduction
Severe acute respiratory syndrome Coronavirus 2 (SARS-CoV2) has caused a global health crisis. Although lungs bear the main brunt of the infection, no organ system appears to be spared. The nervous system is no exception and is reported to get affected in up to 57.4% of the patients with SARS-CoV2 infection.1 Stroke, a dreaded complication of SARS-CoV2, has a profound effect on the ultimate outcome of the patients with SARS-CoV2. The reported rate of occurrence of the stroke in SARS-CoV2 varies between 0.9 to 5%,2 , 3 and the reported mortality rate in them varies from 21 to 83%.2, 3, 4, 5
Whether the relationship between SARS-CoV2 and stroke is incidental, triggered or causative is still evolving. A recent study from South India has highlighted the absence of conventional stroke- risk factors in 26% of COVID-stroke patients.4 In a retrospective case-control study, Belani et al have described that after adjustment for age, gender, and cerebrovascular disease (CVD) risk factors, SARS-CoV2 infection has a significant independent association with acute ischemic stroke.6
It is known that severity, complications, and mortality of SARS-CoV2 infection are associated with elevated levels of inflammatory biomarkers (IBMs) like Erythrocyte Sedimentation Rate (ESR), C-reactive protein (CRP), serum D-Dimer, serum Ferritin, and serum Lactate Dehydrogenase (LDH).7, 8, 9, 10 Higher levels of IBMs have been reported previously in COVID stroke.2 , 3 , 11, 12, 13, 14 However, the predictive value of these IBMs for the occurrence of stroke in SARS-CoV2, whether stand-alone or in combination, is poorly understood.
Therefore, we planned this prospective study with an aim to profile the characteristics of SARS-CoV2 positive patients with ischemic stroke (COVID-Stroke) and to identify the predictors of ischemic COVID-stroke.
Materials and methods
This prospective observational study was conducted at Sri Aurobindo Medical College and Post Graduate Institute from 1st July 2020 to 30th November 2020. The hospital is the largest designated COVID-19 care center in central India.
Study population
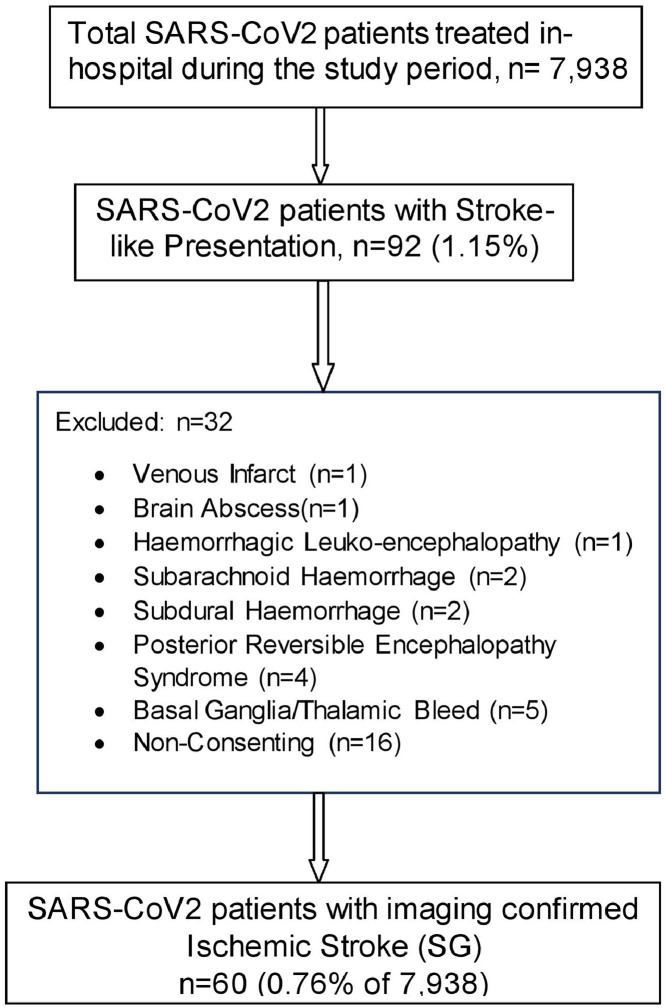
Patients with suspected stroke were recruited consecutively in the five months of the study period. During this period, a total of 7,938 nasopharyngeal SARS-CoV2 RT-PCR positive patients were admitted to our hospital. Of them, 92 (1.15%) developed stroke-like symptoms while hospitalized.
A detailed history of stroke symptoms and pre-existing CVD risk factors was obtained from the patients/ attendants/ previous medical records. The risk factors enquired for included diabetes mellitus,15 hypertension,16 prior cerebrovascular events, prior or current cardiovascular events or peripheral arterial disease (PAD), habits like smoking and alcohol consumption. All these patients were evaluated physically by one of the investigators [NG] and were subjected to brain imaging to identify the stroke type. The patients who were not found to have ischemic strokes (n = 16) were excluded from further analysis (Fig. 1 ).
Fig. 1.
Study population.
Simultaneously, nasopharyngeal SARS-CoV2 RT-PCR positive patients without neurological symptoms and signs, matched for age, gender, and SARS-CoV2 severity (as judged by chest involvement on High-Resolution Computed Tomography {HRCT} chest) were also recruited in 1:1 ratio as the control group.
Methodology
The study was approved by the institutional ethical committee. The data of the included patients was filled in research-approved proforma with informed consent.
Fifty-seven (95%) of the COVID-stroke group (n = 60) underwent Magnetic Resonance Imaging (MRI) brain (1.5 T, 18 channel system Magnetom Symphony, Siemens Medical Solutions, Germany). MR Angiography was performed using a 3D time of flight (TOF) technique. Three patients (5%) were judged unfit for MRI brain and underwent Non-Contrast Computed Tomography (NCCT) brain. The images were reviewed independently and discretely by a radiologist [HR] and one of the neurologists [AKS].
The acute infarct/s were evaluated for the laterality (unilateral or bilateral), arterial territory distribution (carotid/ vertebro-basilar), and the number of arterial territories involved (single or multiple intracranial arterial territory affection). The strokes were grouped as per the TOAST classification.17 The distribution pattern of the acute infarcts was further identified as “embolic pattern” and “non-embolic pattern”, using previously laid down MRI criteria.18
A set of laboratory tests were done in all the patients including complete hemogram (CBC), ESR, CRP, serum D-Dimer, serum Ferritin, and LDH. D-dimer levels were measured using a commercially available quantitative latex enhanced immunoassay (TECHNOLEIA® D-dimer latex kit). D-dimer values are reported in ng/mL D-dimer units (DDU). The stroke patients were also tested for lipid profile, glycosylated hemoglobin (HbA1c), serum homocysteine levels, Prothrombin Time (PT), Activated Partial Thromboplastin Time (APTT), Troponin-T (Trop-T), and Interleukin-6 (IL-6) levels. Dyslipidemia and hyper-homocysteinemia were defined as before.19 , 20 The values for all laboratory variables were measured within 24 h of the occurrence of stroke.
Additionally, all the subjects in the stroke group underwent a 12-lead Electrocardiogram (ECG) and conventional trans-thoracic 2-D Echocardiography (2D-Echo). The abnormalities noted on 2D-Echo included regional wall motion abnormality (RWMA), localized or global hypokinesia, left atrial (LA) dilatation, mitral valve (MV) or aortic valve (AV) vegetations, MV/AV stenosis or regurgitation, left ventricular ejection fraction (LVEF) < 55% and/or left ventricular diastolic dysfunction (LVDD) > Grade II. Also, all patients were monitored in the Intensive Care Unit (ICU) during hospitalization for the development of any cardiac arrhythmias.
Due to ethical reasons and institutional policies, brain imaging, certain laboratory tests, and 2D-Echo were not performed in the control group.
All patients were given similar treatment for SARS-CoV2, as per the governmental advisories and institutional guidelines.
The outcome of all patients in both groups was noted in the form of death or discharge from the hospital.
Data analysis
The responses obtained were sorted out in the form of an excel sheet, analyzed, and evaluated for fulfilling the objectives. Statistical software, SPSS version 17.0 Trial was used for analysis. Both, descriptive and inferential statistics were used. Descriptive statistics were used to depict the main features and characteristics of the collected data. Results on categorical measurements were presented in numbers/percentages. For continuous data, a statistical test for normality – Kolmogorov-Smirnov was applied using SPSS. Results of normally distributed continuous measurements are presented as mean ± standard deviation and that of non-normal data as a median, inter-quartile range (IQR). As and when required, a comparison of proportions (chi-square test) and a comparison of means (independent sample t-test) were performed for data following a normal distribution. Further, the effect size was calculated using Glass's delta to determine the strength of association. For non-normal data, Mann Whitney U test was performed, and the effect size was calculated using eta squared (η2). The level of significance was set at p<0.05.
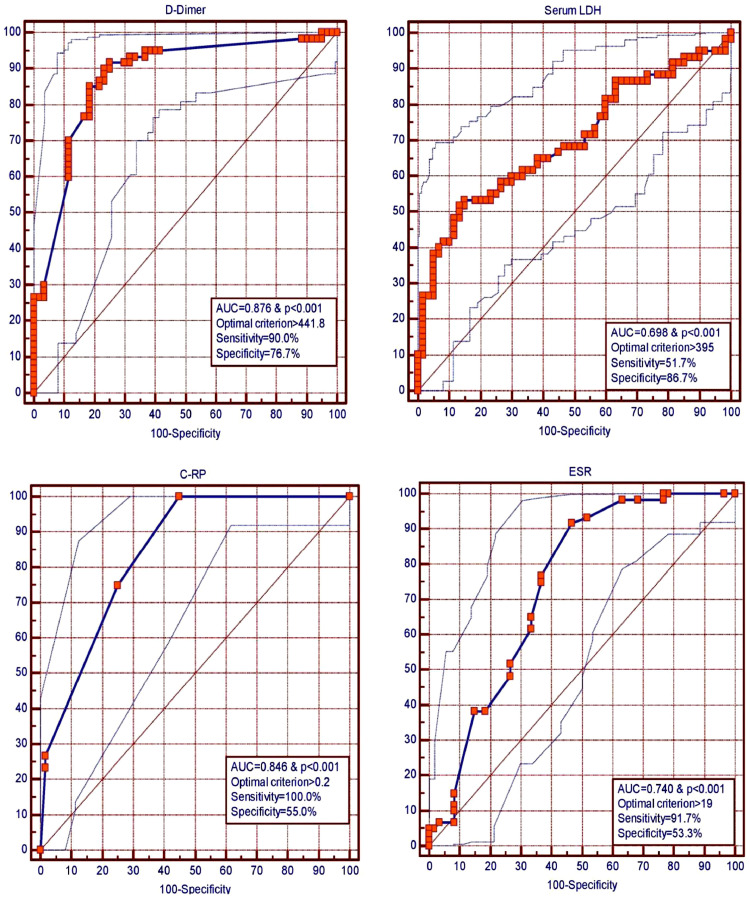
Cut-off values were determined for the occurrence of ischemic stroke for laboratory parameters- Haemoglobin, Red cell distribution width (RDW), ESR, CRP, D-Dimer, serum Ferritin, and serum LDH. For each of these parameters, the receiver operating characteristic (ROC) curve was drawn to find a possible cut-off to predict the occurrence of ischemic stroke by using values of cases and controls. Cut-offs with optimal sensitivity and specificity were chosen as per the optimal criterion, Youden's J statistic, and area under the curve (AUC).
Limits of normative laboratory values were not used as cut-offs in the binary multivariate logistic regression analysis (LRA), as the absolute values of all patients included in both groups (stroke and control) were significantly deranged in the same direction. This may be explained by the fact that both groups were selected from a cohort of SARS-CoV2 positive hospitalized patients and are known to have abnormalities in the above-mentioned laboratory parameters. Hence, there was a need to determine a cut-off in the deranged range, for the possible occurrence of ischemic stroke.
These cut-offs [for RDW (> 16.1%, AUC=0.656), ESR (> 19 mm/h, AUC = 0.740), CRP (> 0.2 mg/dL, AUC = 0.846), D-Dimer (> 441.8 ng/mL, AUC = 0.876), serum Ferritin (> 280.6 ng/mL, AUC = 0.608), and serum LDH (> 395 U/L, AUC = 0.698)] and pre-existing CVD risk factors (presence of diabetes, hypertension, age>60years) were used in a binary multivariate LRA for predicting the occurrence of ischemic stroke. Hemoglobin (Hb) was eliminated as a covariate in the LRA as the AUC was 0.590 with an insignificant p-value (0.085). These ROCs are depicted in Fig. 2 and Table 2.
Fig. 2.
Receiver operating characteristic curves for prediction cut-offs for occurrence of ischemic stroke in SARS-CoV2.
Table 2.
Possible cut-offs for occurrenceof ischemic stroke for various laboratory parameters (ROC Values)
| Normal Laboratory Value | Optimal Criterion | Sensitiv-ity (%) | Specific-ity (%) | Area under Curve | p-value | Youden’ J Statistic | |
|---|---|---|---|---|---|---|---|
| D-dimer | < 250 ng/mL | > 441.8 ng/mL | 90.0 | 76.7 | 0.876 | < 0.001 | 0.67 |
| Serum Ferritin | 10-291 ng/mL | > 280.6 ng/mL | 83.3 | 45.0 | 0.608 | 0.037 | 0.28 |
| Serum LDH | 313-618 U/L | > 395 U/L | 51.7 | 86.7 | 0.698 | < 0.001 | 0.38 |
| ESR | 0-9 mm/h | > 19 mm/h | 91.7 | 53.3 | 0.740 | < 0.001 | 0.45 |
| CRP | < 0.6 mg/dL | > 0.2 mg/dL | 100.0 | 55.0 | 0.846 | < 0.001 | 0.55 |
| Hemo-globin | 13-17 gm/dL | ≤ 11.5 gm/dL | 40.0 | 76.7 | 0.590 | 0.085 | 0.17 |
| RDW | 11.6–14.0% | > 16.1% | 45.0 | 86.7 | 0.656 | 0.002 | 0.32 |
Abbreviations: LDH: Lactate Dehydrogenase, ESR: Erythrocyte Sedimentation Rate, CRP: C-Reactive Protein, RDW= Red Cell Distribution Width
Results
A stroke-like presentation was found in 92 (1.15%) patients out of total COVID-19 patients (n = 7,938). The incidence of radiological-confirmed ischemic stroke was 0.76% (n = 60).
Clinical profile of study population
The profile of the study population (COVID-stroke and controls) is provided in Table 1 . The mean age, gender, and severity of lung involvement between the stroke patients and controls did not differ significantly. The number of diabetic and hypertensive patients was significantly more in the COVID-stroke group vis-à-vis the control group (32 vs 21, p = 0.044; and 34 vs 21, p = 0.177, respectively).
Table 1.
Profile of the study population (STROKE GROUP AND CONTROL GROUP).
| Demographic Characteristics | ||||||
|---|---|---|---|---|---|---|
| Stroke Group (n = 60) | Control Group (n = 60) | p-value | ||||
| Gender | Males | 46 (76.67%) | 44 (73.33%) | Matched (p = 0.674#, insignificant) | ||
| Females | 14 (23.33%) | 16 (23.67%) | ||||
| Age [Mean ± SD] (in years) |
61.53 ± 13.72 | 62.03 ± 13.66 | Matched (p = 0.842¥, insignificant) | |||
| Age > 60years (number of patients) | 37 (61.66%) | 39 (65%) | 0.7054# | |||
| Diabetes Mellitus (number of patients) | 32 (53.33%) | 21 (35%) | 0.0441# | |||
| Hypertension (number of patients) | 34 (56.67%) | 21 (35%) | 0.0177# | |||
| Outcome | Death | 34 (56.67%) | 8 (13.33%) | < 0.001# | ||
| Discharge | 26 (43.33%) | 52 (86.67%) | < 0.001# | |||
| Laboratory | ||||||
|
Stroke Group (n= 60) Mean ± SD; Median, IQR |
Control Group (n = 60) Mean ± SD; Median, IQR |
p-value | Effect Size | |||
| Hemogram | ||||||
| Hb (g/dL) | 12.01 ± 2.04; 12.25, 2.85 | 12.74 ± 1.95; 12.60, 2.43 | 0.045¥ | 0.374! | ||
| RDW (%) | 16.35 ± 3.20; 15.20, 3.40 | 14.80 ± 1.76; 14.40, 1.80 | 0.003@ | 0.073^ | ||
| Inflammatory Markers | ||||||
| ESR (mm/h) | 25.51 ± 6.88; 25.00, 6.25 | 20.00 ± 6.52; 19.00, 10.00 | < 0.00001@ | 0.172^ | ||
| CRP (mg/dL) | 2.67 ± 1.29; 2.40, 0.70 |
0.99 ± 1.04; 0.20, 1.30 |
< 0.00001@ | 0.358^ | ||
| Sr Ferritin (ng/ml) | 611.43 ± 454.76; 547.30, 421.60 |
530.52 ± 471.15; 333.10, 498.20 |
0.04136@ | 0.035^ | ||
| Sr LDH (U/L) | 496.65 ± 410.94; 401.50, 363.75 |
305.72 ± 110.80; 299.00, 121.75 |
0.0002@ | 0.117^ | ||
| D-Dimer (ng/mL) | 1511.61 ± 1305.70; 1000.00, 634.63 |
335.84 ± 327.56; 150.00, 260.45 |
< 0.0001@ | 0.423^ | ||
| HRCT Chest | ||||||
|
Stroke Group (n = 60) Mean ± SD |
Control Group (n = 60) Mean ± SD |
p-value | ||||
| Lung Involvement (%) | 42.91 ± 24.39 | 44.53 ± 24.58 | Matched (p = 0.719¥, insignificant) | |||
| CT SS | 12.51 ± 6.94 | 12.53 ± 6.84 | Matched (p = 0.987¥, insignificant) | |||
# chi-square test, ¥ independent sample t-test, @ Mann Whitney U test, ! Glass's delta, ^η2 (eta squared)
Abbreviations: Hb= Hemoglobin, RDW= Red Cell Distribution Width, ESR= Erythrocyte Sedimentation Rate, CRP= C-Reactive Protein, LDH= Lactate Dehydrogenase, CT SS= Computed Tomography Severity Score
Clinical profile of COVID-stroke group (n = 60, Table 3)
Table 3.
Profile of patients with radiological- confirmed ischemic stroke.
| Clinical Presentation of Stroke (n = 60) | |
|---|---|
| Presenting Symptom | Number of Patients (%) |
| Hemiplegia/paresis | 44 (73.33%) |
| Posterior Circulation Symptoms | 6 (10%) |
| Ataxic Hemiparesis | 6 (10%) |
| Facio-brachial plegia/paresis | 3 (5%) |
| Lower Limb Monoplegia/paresis | 1 (1.67%) |
| Cerebrovascular Disease Risk Factors | |
| Factor | Number of Patients (%) |
| Hypertension | 34 (56.67%) |
| Diabetes | 32 (53.33%) |
| Dyslipidemia | 15 (25%) |
| Peripheral Arterial Disease | 5 (8.33%) |
| Smoking | 9 (15%) |
| Hyper-homocysteinemia | 3 (5%) |
| Previous Stroke on Brain Imaging | 15 (25%) |
| Abnormal 2D Echocardiography | 3 (5%) |
| Laboratory Parameters | |
| Parameter (Normal Reference Value) | Number of patients (%) |
| Thrombocytopenia (< 1.5 lakh/cmm) | 17 (28.33%) |
| PT elevated (> 14 s) | 22 (36.67%) |
| APTT elevated (> 32 s) | 10 (16.67%) |
| APTT and PT- both elevated | 9 (15%) |
| IL-6 elevated (> 6.4 pg/ml) | 60 (100%) |
| Troponin-T elevated | 20 (33.33%) |
| TOAST Classification of Strokes (n = 60) | |
| Stroke Subtype | Number of Patients (%) |
| Large Artery Atherosclerosis | 17 (28.33%) |
| Cardio-embolism | 3 (5%) |
| Small- vessel Occlusion | 4 (6.67%) |
| Stroke of Other Determined Etiology | 3 (5%) |
| Stroke of Other Undetermined Etiology (Cryptogenic) | 33 (55%) |
| Brain Imaging | |
| Infarct Laterality | |
| Unilateral | 35 (58.33%) |
| Bilateral | 25 (41.66%) |
| Arterial Territory Distribution | |
| Carotid | 38 (63.33%) |
| Vertebro-basilar | 8 (13.33%) |
| Both | 14 (23.33%) |
| Number of Arterial Territories Involved | |
| Single | 27 (45%) |
| Multiple | 33 (55%) |
| Stroke Pattern (as per MRI criteria)18 | Number of Patients (%) |
| Embolic | 30 (50%) |
| Others | 30 (50%) |
| Angiography (n = 57)* | |
| Finding | Number of Patients (%) |
| Focal or Segmental Narrowing/Stenosis | 12 (21.05%) |
| Intra-arterial Thrombus | 12 (21.05%) |
| Normal | 33 (57.89%) |
*Angiography could not be done in 3 patients
Abbreviations: PT= Prothrombin Time, APTT= Activated Partial Thromboplastin Time, IL-6= Interleukin -6, TOAST = Trial of Org 10172 in Acute Stroke Treatment
Stroke was the reason for hospital admission or the presenting symptom of SARS-CoV2 illness in 23.33% (14/60) patients. In the rest of the patients (n = 46), index stroke was seen to occur within the first 3 weeks of the onset of SARS-CoV2 illness (mean= 7.37 ± 5.54days, minimum=1 day, maximum= 21days).
Hemiparesis/plegia (73.33%) was the most common presentation amongst the stroke patients (n = 44). The rest of them presented with visual disturbances, vomiting, ataxia, or monoparesis. The details of the same are provided in Table 3 .
One or more known CVD risk factors could be identified in most of the COVID-stroke patients (n = 52, 86.67%), hypertension (56.67%) and diabetes (53.33%) being the most common. None of the patients showed valvular dysfunctions or vegetation of transthoracic conventional echocardiography. The only abnormality detected was RWMA and/or reduced LVEF (n = 3).
Imaging characteristics (Table 3)
MRI could be performed in 95% COVID-stroke patients (57/60), and the remaining 3 underwent NCCT brain. According to TOAST classification,17 55% (n=33) had cryptogenic stroke, 28% (n = 17) had large artery atherosclerosis, 6% (n = 4) had small vessel occlusion, 5% (n = 3) had cardio-embolic stroke and 5% (n = 3) had stroke of other determined etiology. The number of single arterial territory infarcts was lesser (n = 27) than multiple territory lesions (n = 33). Strokes restricted to carotid circulation were more frequent (n=38, 63.33%) were more frequent, 13.33% (n = 8) had infarcts in the vertebrobasilar circulation, and 23.33% (n=14) had infarcts in both the territories. Bilateral hemispheric infarcts were observed in 41.67% (n = 25) patients. Evidence of prior stroke on imaging was present in 25% (n = 15) of the patients. MRA was normal in 57.89% (n = 33 out of 57). Either vascular narrowing or intra-arterial thrombus was detected in 42.11% (n = 24) patients. In 30 patients, brain imaging was suggestive of an embolic pattern of infarct;18 with the presence of multiple non-contiguous infarcts, bilateral infarcts, simultaneous infarcts in the anterior and posterior circulation, or isolated cortical infarcts.
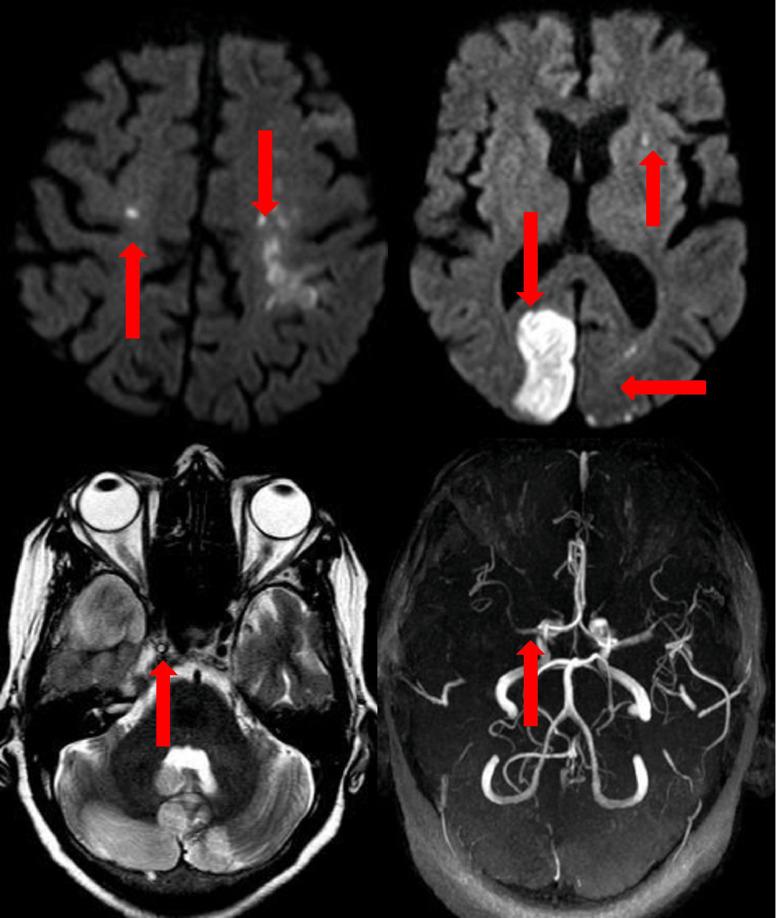
Representative images are given in Fig. 3 .
Fig. 3.
Representative brain images (Clockwise from left top corner: 3a bilateral anterior circulation infarcts, 3b) bilateral posterior circulation and left anterior circulation.
Laboratory parameters
The results of the various laboratory parameters are summarised in Tables 1 and 3. Compared to controls, the COVID-stroke group showed significant derangement in Hb and RDW values. The latter was significantly wider in the stroke group (16.35 ± 3.20 versus 14.80 ± 1.76, p = 0.001, 95% CI= -2.24 to – 0.62). The average values of inflammatory markers were significantly deranged in both groups. But the abnormalities were much more significant in the COVID-stroke group as compared with the control group. D-dimer was found to have the largest effect size (η2 = 0.423) followed by serum CRP (η2 = 0.358) and ESR (η2 = 0.172).
Coagulation parameters were tested in the COVID-stroke patients. They were deranged in 38.3% (n = 23) patients. Ubiquitous elevation of IL-6 levels was a notable finding in the COVID-stroke patients. The average IL-6 value was 206.74 ± 198.01 pg/mL (normal laboratory reference range was < 6.4 pg/mL).
Predictors of ischemic stroke in SARS-CoV2 patients
As per the ROCs, values of D-dimer > 441.8 ng/mL (AUC = 0.876), LDH> 395U/L (AUC = 0.698), ESR > 19 mm/h (AUC = 0.740) and CRP > 0.2 mg/dL (AUC = 0.846) were found to be strong predictors of occurrence of stroke (p < 0.001 for each).
A binary multivariate logistic regression model (summarised in Table 4 ) was used to determine the predictors for the occurrence of ischemic stroke in SARS-CoV2 patients. As per the model, the Log Odds Ratio for Prediction of Ischemic Stroke in a SARS-CoV2 patient was formed (Table 4).
Table 4.
Predictors of ischemic stroke in sars-CoV2-
a binary logistic regression analysis.
| Independent Predictor | β | Standard Error | p-value | Odds Ratio |
95% Confidence Interval |
|
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| Age > 60years | -.865 | .825 | .294 | .421 | .084 | 2.121 |
| Diabetes: Present | -.623 | .766 | .416 | .536 | .119 | 2.409 |
| Hypertension: Present | .252 | .691 | .716 | 1.286 | .332 | 4.988 |
| RDW > 16.1% | 2.050 | .924 | .027 | 7.764 | 1.270 | 47.469 |
| D-Dimer > 441.8 ng/mL | 2.229 | .761 | .003 | 9.293 | 2.092 | 41.285 |
| Serum Ferritin > 280.6 ng/mL | .377 | .931 | .685 | 1.458 | .235 | 9.041 |
| Serum LDH > 395 U/L | .251 | .762 | .742 | 1.286 | .289 | 5.719 |
| ESR > 19 mm/h | 2.569 | .870 | .003 | 13.054 | 2.374 | 71.777 |
| CRP > 0.2 mg/dL | 22.096 | 5741.242 | .997 | 3.947 | .000 | . |
| Constant | -24.725 | 5741.242 | .997 | .000 | ||
Abbreviations: RDW= Red Cell Distribution Width, LDH: Lactate Dehydrogenase, ESR: Erythrocyte Sedimentation Rate, CRP: C-Reactive Protein
As per the model, the Log Odds Ratio for Prediction of Ischemic Stroke in SARS-CoV2 patients = -24.725 + (-0.865) x Age {1, if >60years and 0, ≤60years} if + (-0.623) x Diabetes {1, if yes and 0, if no} + (0.252) x Hypertension {1, if yes and 0, if no} + 2.050 x RDW {1, if >16.1 and 0, if ≤16.1} + 2.229 x D-dimer {1, if >441.4 and 0, if ≤441.8} + 0.377 x Ferritin {1, if >280.6 and 0, if ≤280.6} + 0.251 x LDH {1, if >395 and 0, if ≤395} + 2.569 x ESR {1, if >19 and 0, if ≤19} + 22.096 x CRP {1, if >0.2 and 0, if ≤0.2}
The Log Odds Ratio for prediction of Ischemic Stroke in SARS-CoV2 patients = -24.725 + [-0.865 x Age {1, if > 60years and 0, ≤ 60years}] if + [-0.623 x Diabetes {1, if yes and 0, if no}] + [0.252 x Hypertension {1, if yes and 0, if no}] + [2.050 x RDW {1, if > 16.1 and 0, if ≤ 16.1}] + [2.229 x D-dimer {1, if > 441.4 and 0, if ≤ 441.8}] + [0.377 x Ferritin {1, if > 280.6 and 0, if ≤ 280.6}] + [0.251 x LDH {1, if > 395 and 0, if ≤ 395}] + [2.569 x ESR {1, if > 19 and 0, if ≤ 19}] + [22.096 x CRP {1, if > 0.2 and 0, if ≤ 0.2}]
This model has an excellent probability of prediction of COVID-stroke of 90.0%. The sensitivity and specificity of the prediction were found to be 90.0% each.
In multivariate analysis, RDW > 16.1% (Odds Ratio= 7.764, 95%CI = 1.27 to 47.47) was found to be the strongest predictor of the occurrence of stroke, along with D-dimer > 441.8 ng/mL (Odds Ratio= 9.293, 95%CI = 2.09 to 41.29) and ESR > 19 mm/h (Odds Ratio= 13.054, 95%CI= 2.37 to 71.78). The predictive values of conventional CVD risk factors, higher age (> 60years), presence of diabetes mellitus, and hypertension were found to be relatively low in this multivariate analysis.
In LRA, multicollinearity was checked using Variance Inflation Factor (VIF) values and they showed the absence of any correlation between the various independent predictors.
Outcomes
In the COVID-stroke group, the outcome was significantly poor, as compared to the controls (p < 0.001, shown in Table 1). In the stroke group, there were 56.67% (n = 34) deaths (modified Rankin scale 6 mRS6) and 33.33% (n = 20) patients remained severely neurologically affected (mRS5 = 1 patient, mRS4 = 19 patients). Only 10% (n = 6) patients in the COVID-stroke group had moderate to mild disability at discharge (mRS3 = 3 patients, mRS2 = 1 patient, mRS1 = 2 patients).
Discussion
The incidence of radiological-confirmed ischemic stroke in our study was 0.76% which is in tandem with previous studies.2 , 21, 22, 23 The mean age (61.53 ± 13.72years) and gender distribution (76.67% males) of our COVID-stroke population were comparable to previous research.7 , 12 , 21 Previously reported mortality in COVID-stroke patients varies from 21 to 83%.2, 3, 4, 5 The mortality in our COVID-stroke patients (56.67%) was significantly higher as compared to the controls.
In previous studies, stroke emerged as the first symptom in asymptomatic carriers of SARS-CoV2 infection.2 , 21 , 24 , 25 In our study, stroke was the presenting symptom of SARS-CoV2 illness in 23.33% (n = 14) patients. Further, in our cohort, hemiplegia was the most common stroke manifestation (66.7%), as was found in prior research on COVID-stroke.21 , 24, 25, 26
In previous studies on COVID-stroke patients, conventional CVD risk factors are not seen in up to 26% of the patients.4 We also did not find CVD risk factors in 13.33% of our COVID-stroke patients. Data regarding the association of conventional CVD risk factors with COVID-stroke is divergent. While some researchers have found a higher incidence of COVID-strokes in subjects with the conventional CVD risk factor, viz. hypertension and diabetes;3 , 7 , 12 , 21 , 27 others have reported SARS-CoV2 infection as an independent risk factor for COVID-stroke in a CVD matched cohort.6 , 28
In SARS-CoV2 infected patients, strokes have been found to co-occur with significantly higher levels of IBMs.2 , 3 , 11 We also found significantly higher levels of IBM in COVID-stroke patients, as compared with the SARS-CoV2 patients not developing stroke. In our research, we endured understanding the co-variate relationship of IBMs and conventional CVD risk factors in the prediction of COVID-stroke. Further, in our study population, the prevalence of hypertension and diabetes was significantly higher in COVID-stroke patients than that in controls. Despite this, on multivariate analysis, they did not emerge as significant predictors of ischemic stroke in COVID patients.
We found multiple vascular territory infarcts, bilateral infarcts, and simultaneous anterior and posterior circulation infarcts in 55%, 41.66%, and 23.33% patients respectively. According to TOAST classification, the majority of the patients (55%, n=33) had a cryptogenic stroke, as reported previously,2 , 5 , 29 and only 5% (n = 3) had a cardio-embolic stroke. However, on applying the MRI-based criterion18 for identifying the pattern of stroke, brain imaging was suggestive of an embolic pattern of infarction in 50% of the patients. This pattern of infarction might suggest the presence of an underlying vasculopathy, which in COVID-19 is attributed to a generalized systemic inflammatory response.30 This view is supported by postmortem studies of the brains of subjects with COVID-stroke that have revealed widespread micro-thrombi with patchy infarcts, intra-capillary cells resembling megakaryocytes, and vasculitis.31, 32, 33
Hypercoagulability has also been suggested to underlie COVID-stroke.3 , 34 , 35 Direct evidence of intra-arterial thrombosis was discernible in 21.05% (n = 12) patients. Derangement of the coagulation factors, particularly D-dimer, was seen in 93.33% of stroke patients in our study. In recent years, evidence has suggested that elevated RDW serves as a marker of increased inflammation, pro-thrombotic state, and results in an increased incidence of ischemic stroke.36 In SARS-CoV2, RDW is expected to be elevated as red blood cell production kinetics slows down in the setting of increased white blood cell and platelet kinetics.8 In our study, widened RDW was found to have a strong predictive value for the occurrence of COVID-stroke.
The few limitations of the study were single-center enrolment and the use of prophylactic anticoagulation in SARS-CoV2 patients (guided by institutional policies) which may have resulted in a lowered incidence of ischemic stroke. Additional shortcomings include non-performance of certain tests in the control population, viz IL-6, lipid profile, serum homocysteine, and 2D-Echo due to institutional policy constraints. Furthermore, the confidence intervals for our LRA model are wide, and the results of our study may be interpreted cautiously.
Conclusion
The present study is the first study to attempt to determine cut-offs of elevated levels of IBMs in the prediction of occurrence of ischemic stroke in SARS-CoV2. In SARS-CoV2 patients, D-dimer elevated beyond 441.8 ng/mL, ESR greater than 19 mm/h, and RDW widened more than 16.1% were the strongest predictors of the occurrence of ischemic stroke. Interestingly, conventional CVD risk factors- higher age (> 60years), presence of diabetes mellitus, and hypertension were not found to be significant predictors in a multivariate analysis. As these findings have important diagnostic, prognostic, and therapeutic implications in the management of ischemic strokes in SARS-CoV2, further research in a larger population is required to establish the same. Moreover, an association of elevated IL-6 (cytokine storm) with the occurrence of COVID-stroke needs to be investigated further.
Disclaimers
None.
Funding
The investigations done in this research were part of routine institutional treatment protocols and no additional cost was incurred by the patient/family. No additional funding was required.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Acknowledgments
I would like to thank Dr. Vinit Patel, MD, Psychiatrist and Statistician for his invaluable contribution to the preparation of the manuscript.
ETHICAL COMMITTEE APPROVAL for the study was taken from the institutional ethical committee at Sri Aurobindo Institute of Medical Sciences, Indore, Madhya Pradesh, India.
References
- 1.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaghi S., Ishida K., Torres J., Mac Grory B., Raz E., Humbert K., et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. 2020;(7):2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morassi M., Bagatto D., Cobelli M., D'Agostini S., Gigli G.L., Bnà C., Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathew T., John S.K., Sarma G., Nadig R., Kumar R.S., Murgod U., et al. COVID-19-related strokes are associated with increased mortality and morbidity: a multicenter comparative study from Bengaluru, South India. Int J Stroke. 2021;16(4):429–436. doi: 10.1177/1747493020968236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belani P., Schefflein J., Kihira S., Rigney B., Delman B.N., Mahmoudi K., et al. COVID-19 is an independent risk factor for acute ischemic stroke. AJNR Am J Neuroradiol. 2020;41(8):1361–1364. doi: 10.3174/ajnr.A6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao X., Liu S., Wang J., Zhao K., Long X., He X., et al. The clinical characteristics and prognosis of COVID-19 patients with cerebral stroke: a retrospective study of 113 cases from one single-centre. Eur J Neurosci. 2021;53(4):1350–1361. doi: 10.1111/ejn.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foy B.H., Carlson J.C.T., Reinertsen E., Padros I., Valls R., Pallares Lopez R., et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry B.M., Aggarwal G., Wong J., Benoit S., Vikse J., Plebani M., et al. Lactate dehydrogenase levels predict Coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722–1726. doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng F., Huang Y., Guo Y., Yin M., Chen X., Xiao L., Deng G. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z., Yang Y., Liang X., Gao B., Liu M., Li W., Chen Z., Wang Z. COVID-19 associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism, and management. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K.W., Yusof Khan A.H.K., Ching S.M., Chia P.K., Loh W.C., Abdul Rashid A.M., et al. Stroke and novel Coronavirus infection in humans: a systematic review and meta-analysis. Front Neurol. 2020;11 doi: 10.3389/fneur.2020.579070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alzoughool F., Alanagreh L., Abumweis S., Atoum M. Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients. Clin Hemorheol Microcirc. 2020 doi: 10.3233/CH-201002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Katz J.M., Libman R.B., Wang J.J., Filippi C.G., Sanelli P., Zlochower A., et al. COVID-19 severity and stroke: correlation of imaging and laboratory markers. AJNR Am J Neuroradiol. 2021;42(2):257–261. doi: 10.3174/ajnr.A6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010 Jan;33 Suppl 1(Suppl 1):S62-9. doi: 10.2337/dc10-S062. Erratum in: Diabetes Care. 2010 Apr;33(4):e57. PMID: 20042775; PMCID: PMC2797383. [DOI] [PMC free article] [PubMed]
- 16.Hernandez-Vila E. A review of the JNC 8 blood pressure guideline. Tex Heart Inst J. 2015;42(3):226–228. doi: 10.14503/THIJ-15-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams H.P., Jr, Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Ay H., Gungor L., Arsava E.M., Rosand J., Vangel M., Benner T., et al. A score to predict early risk of recurrence after ischemic stroke. Neurology. 2010;74(2):128–135. doi: 10.1212/WNL.0b013e3181ca9cff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fodor G. Primary prevention of CVD: treating dyslipidaemia. BMJ Clin Evid. 2008;2008:0215. [PMC free article] [PubMed] [Google Scholar]
- 20.Hankey G.J., Eikelboom J.W. Homocysteine and vascular disease. Lancet. 1999;354 doi: 10.1016/S0140-6736(98)11058-9. 407‑13. [DOI] [PubMed] [Google Scholar]
- 21.Siow I., Lee K.S., Zhang J.J.Y., Saffari S.E., Ng A., Young B. Stroke as a neurological complication of COVID-19: a systematic review and meta-analysis of incidence, outcomes and predictors. J Stroke Cerebrovasc Dis. 2021;30(3) doi: 10.1016/j.jstrokecerebrovasdis.2020.105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong W., Mu J., Guo J., Lu L., Liu D., Luo J., et al. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95(11):e1479–e1487. doi: 10.1212/WNL.0000000000010034. [DOI] [PubMed] [Google Scholar]
- 23.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., et al. Large-vessel stroke as a presenting feature of COVID-19 in the Young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamud A.Y., Griffith B., Rehman M., Miller D., Chebl A., Patel S.C., et al. Intraluminal carotid artery thrombus in COVID-19: another danger of cytokine storm? AJNR Am J Neuroradiol. 2020;41(9):1677–1682. doi: 10.3174/ajnr.A6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kremer S., Lersy F., de Sèze J., Ferré J.C., Maamar A., Carsin-Nicol B., et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020;297(2):E242–E251. doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.K. Wada, Y. Hashimoto, M. Nakajima, M. Ueda [COVID-19 and stroke]. Rinsho Shinkeigaku. 2020;60(12):822-839. [DOI] [PubMed]
- 28.JM Katz, RB Libman, JJ Wang, P Sanelli, CG. Filippi, M Gribko, SV Pacia, RI Kuzniecky, S Najjar, S Azhar Cerebrovascular complications of COVID-19. Stroke. 2020;51(9):e227-e231. [DOI] [PMC free article] [PubMed]
- 29.Grewal P., Pinna P., Hall J.P., Dafer R.M., Tavarez T., Pellack D.R., et al. Acute ischemic stroke and COVID-19: experience from a comprehensive stroke center in Midwest US. Front Neurol. 2020;11:910. doi: 10.3389/fneur.2020.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nauen D.W., Hooper J.E., Stewart C.M., Solomon IH. Assessing brain capillaries in Coronavirus disease 2019. JAMA Neurol. 2021;78(6):760–762. doi: 10.1001/jamaneurol.2021.0225. 2021; e210225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryce C., Grimes Z., Pujadas E., et al. Pathophysiology of SARS-CoV-2: The Mount Sinai COVID-19 autopsy experience. Mod Pathol. 2021;34:1456–1467. doi: 10.1038/s41379-021-00793-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen M.P., Le Quesne J., Officer-Jones L., Teodòsio A., Thaventhiran J., Ficken C., et al. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol. 2021;47(1):17–25. doi: 10.1111/nan.12662. [DOI] [PubMed] [Google Scholar]
- 34.Beyrouti R., Adams M.E., Benjamin L., Cohen H., Farmer S.F., Goh Y.Y., et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91(8):889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avula A., Nalleballe K., Narula N., Sapozhnikov S., Dandu V., Toom S., et al. COVID-19 presenting as stroke. Brain Behav. 2020;87:115. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song S.Y., Lin Hua C., Dornbors D., Kang R.J., Zhao X.X., Du X., He W., Ding Y.C., Meng R. Baseline red blood cell distribution width as a predictor of stroke occurrence and outcome: a comprehensive meta-analysis of 31 studies. Front Neurol. 2019;10:1237. doi: 10.3389/fneur.2019.01237. [DOI] [PMC free article] [PubMed] [Google Scholar]