Abstract
Making predictions about the world and responding appropriately to unexpected events are essential functions of the healthy brain. In neurodegenerative disorders, such as frontotemporal dementia and Alzheimer’s disease, impaired processing of ‘surprise’ may underpin a diverse array of symptoms, particularly abnormalities of social and emotional behaviour, but is challenging to characterize. Here, we addressed this issue using a novel paradigm: music. We studied 62 patients (24 female; aged 53–88) representing major syndromes of frontotemporal dementia (behavioural variant, semantic variant primary progressive aphasia, non-fluent-agrammatic variant primary progressive aphasia) and typical amnestic Alzheimer’s disease, in relation to 33 healthy controls (18 female; aged 54–78). Participants heard famous melodies containing no deviants or one of three types of deviant note—acoustic (white-noise burst), syntactic (key-violating pitch change) or semantic (key-preserving pitch change). Using a regression model that took elementary perceptual, executive and musical competence into account, we assessed accuracy detecting melodic deviants and simultaneously recorded pupillary responses and related these to deviant surprise value (information-content) and carrier melody predictability (entropy), calculated using an unsupervised machine learning model of music. Neuroanatomical associations of deviant detection accuracy and coupling of detection to deviant surprise value were assessed using voxel-based morphometry of patients’ brain MRI. Whereas Alzheimer’s disease was associated with normal deviant detection accuracy, behavioural and semantic variant frontotemporal dementia syndromes were associated with strikingly similar profiles of impaired syntactic and semantic deviant detection accuracy and impaired behavioural and autonomic sensitivity to deviant information-content (all P < 0.05). On the other hand, non-fluent-agrammatic primary progressive aphasia was associated with generalized impairment of deviant discriminability (P < 0.05) due to excessive false-alarms, despite retained behavioural and autonomic sensitivity to deviant information-content and melody predictability. Across the patient cohort, grey matter correlates of acoustic deviant detection accuracy were identified in precuneus, mid and mesial temporal regions; correlates of syntactic deviant detection accuracy and information-content processing, in inferior frontal and anterior temporal cortices, putamen and nucleus accumbens; and a common correlate of musical salience coding in supplementary motor area (all P < 0.05, corrected for multiple comparisons in pre-specified regions of interest). Our findings suggest that major dementias have distinct profiles of sensory ‘surprise’ processing, as instantiated in music. Music may be a useful and informative paradigm for probing the predictive decoding of complex sensory environments in neurodegenerative proteinopathies, with implications for understanding and measuring the core pathophysiology of these diseases.
Keywords: music, surprise, frontotemporal dementia, pupillometry, voxel-based morphometry
Benhamou et al. report that major dementias have distinct behavioural, autonomic and neuroanatomical profiles of musical ‘surprise’ processing. While Alzheimer’s disease patients showed normal reactivity to deviants in familiar melodies, frontotemporal dementia patients had specific impairments, suggesting that musical ‘tools’ could measure complex behavioural changes in these diseases.
Graphical Abstract
Graphical Abstract.
Introduction
Predicting the future based on past experience and responding appropriately to unexpected, ‘surprising’ events are fundamental functions of the healthy brain. These functions are targeted prominently and early in a number of neurodegenerative disorders. Impaired understanding of social norms, ‘rules’ and boundaries define behavioural variant frontotemporal dementia (bvFTD),1–3 signifying deficient predictive ‘modelling’ of the socio-emotional milieu4 and implicating more fundamental processes, such as error detection and monitoring, ambiguity and conflict resolution, probabilistic learning, risk evaluation and decision making.1,5–11 These processes are also affected in other FTD syndromes—semantic variant primary progressive aphasia (svPPA) and non-fluent-agrammatic (nfv) PPA—and Alzheimer’s disease.6,7,11–20
A plausible unifying mechanism for this diverse phenotypic spectrum is impaired integration of expectation (established by environmental context) with surprise (unexpected events). This mechanism has a neurophysiological signal in altered mismatch negativity21–27 and a neuroanatomical signature in dysfunctional frontotemporal neural circuitry.21,22,28–32 Impaired deviance detection may develop early in the course of neurodegeneration and indexes a core mechanism of the culprit proteinopathy in animal models.33–35 Taken together, this evidence suggests that neural processes establishing expectations and decoding ‘surprise’ may underpin diverse pathophysiological and clinical effects of dementias, such as FTD and Alzheimer’s disease. However, these processes remain poorly characterized and difficult to quantify, particularly in the setting of neurodegenerative disease.
As a paradigm for addressing these issues, music is particularly promising. Music is ubiquitous in daily life and constitutes a model ‘environment’ that is bound by a finite set of implicit ‘rules’. Even musically untrained listeners internalize these automatically through lifelong exposure to the dominant musical culture and accordingly acquire strong and reliable psychological expectations about musical patterns and events.36 Moreover, ‘surprise’ of various kinds is easily engendered in music by violating these rules. The inherently rule-based nature of music and the role of expectation and surprise in achieving many of its psychological and physiological effects37,38 together suggest that the neural processing of music might follow the principles of ‘predictive coding’. Predictive coding has gained wide currency as a paradigm of brain operation and in particular, the role of active neural inference in making and iteratively refining predictions about the environment, thereby minimizing error and optimizing neural representations of the world at large.39,40 The probabilistic structure of musical sequences (melodies) can be analysed using information-theoretic approaches that quantify both the listener’s degree of uncertainty about the prevailing musical environment (its entropy or unpredictability) and the information-content (IC; degree of unexpectedness or ‘surprise’) associated with a particular musical event (such as a deviant note).41
Our emerging picture of the musical brain, derived from cognitive and functional neuroimaging studies in both health and disease,42 provides candidate neural substrates for predictive coding of musical information. In the healthy brain, a parametric correlate of musical note unexpectedness (high IC) has been identified in anterior cingulate and insula43 and neural correlates of processing surprise and prior uncertainty in amygdala, hippocampus and nucleus accumbens.44 Canonical syndromes of FTD and Alzheimer’s disease have distinctive profiles of musical perceptual, semantic and affective impairment42,45–49 that reflect the targeting of these distributed networks by neurodegenerative proteinopathies. Dementias are therefore anticipated to produce separable but overlapping cognitive and physiological profiles of musical ‘surprise’ processing. Further, processing of higher order spectrotemporal statistics of speech signals is affected in nfvPPA and svPPA.50,51 A predictive coding account of the impaired understanding of degraded speech has been developed for nfvPPA52 while changes in the macro- and meso-scale functional organization of neural circuits that support predictive coding have been described in bvFTD.22,29,32 Moreover, the processing of music and social and emotional signals share key neural resources that are targeted in FTD and Alzheimer’s disease.42,53,54 However, music remains unexplored as a paradigm of sensory ‘surprise’ processing (and by extension, complex behavioural alterations) in neurodegenerative disease.
Here, we addressed this issue in a cohort of patients representing major FTD syndromes—bvFTD, svPPA and nfvPPA—and typical Alzheimer’s disease, referenced to healthy older individuals. We manipulated musical surprise—in both its traditional psychological and information-theoretic senses—by inserting notes representing three kinds of deviant ‘event’ condition into an ‘environment’ of familiar melodies with variable predictability levels. These deviant conditions targeted three levels of musical organization: basic acoustic structure, the general harmonic rules governing musical sequences or musical ‘syntax’ and the regularities or ‘semantics’ specifying melodies as individual musical objects.55 We simultaneously assessed performance at deviant detection and pupillary responses to deviant notes. Pupil dilatation, as a marker of physiological arousal potentially dissociates from cognitive processing and robustly signals violation of expectations and statistical regularities of sensory input in the healthy brain.56–61 We estimated the information-theoretic properties of our musical stimuli using a computational model of musical informational dynamics,62 in order to assess how environmental predictability (entropy) and the sensory informational structure of deviant ‘surprising’ events related to behavioural and physiological responses in different dementia syndromes. Structural neuroanatomical associations of behavioural reactivity to melodic deviants were assessed using voxel-based morphometry (VBM) of patients’ brain MRI.
Based on previous work addressing more general musical and auditory cognitive and perceptual functions in dementia,45–49,63–65 we hypothesized that FTD syndromes would be associated with more severe impairments of musical deviant detection and autonomic reactivity than would Alzheimer’s disease; and further, that FTD syndromes would be stratified by their relative degree of impairment in processing particular deviant conditions: impaired detection of semantic and syntactic deviants in bvFTD and svPPA45,49 and a generalized impairment of auditory deviant detection in nfvPPA.50,66 We additionally hypothesized that sensitivity to information-theoretic parameters of melodies (deviant surprise, melody entropy) would be relatively more severely reduced in bvFTD and svPPA than in other participant groups.8,22,32,50,51 Finally, we hypothesized that the cognitive coding of musical surprise in the patient cohort would have separable neuroanatomical correlates within the hierarchical distributed brain networks previously implicated in processing different kinds of musical information.42,43,49,67–72
Methods and materials
Participants
Nineteen patients with typical Alzheimer’s disease, 21 with bvFTD, 12 with svPPA and 12 with nfvPPA were recruited. All patients fulfilled consensus clinical criteria for their syndromic diagnosis,73–75 of mild to moderate severity. Thirty-three healthy older individuals with no history of neurological or psychiatric disorders also participated. None of the participants had a history of clinically relevant hearing loss, congenital amusia or pupillary disease. All participants had a comprehensive general neuropsychological assessment.
To index participants’ musical experience, patients’ caregivers and healthy control participants completed a questionnaire detailing years spent learning or playing an instrument (prior musical expertise, scored on a 4-point scale ranging from 0 (never played an instrument or sang in a choir) to 3 (learned an instrument for >10 years) and hours per week on average currently spent listening to music.76 All participants had audiometric screening of peripheral hearing function (indexed as a mean hearing threshold via the better ear over 0.5, 1, 2, 4 and 8 kHz) and assessment of their ability to discriminate the direction of pitch changes relevant to melodies. Details of these procedures are in Supplementary material. Demographic details (including musical background), clinical and general neuropsychological characteristics of the study cohort are summarized in Table 1.
Table 1.
Demographic, clinical and general neuropsychological characteristics of participant groups
| Characteristics | Controls | AD | bvFTD | svPPA | nfvPPA |
|---|---|---|---|---|---|
| Demographic and clinical | |||||
| No. (male:female) | 15:18 | 9:10 | 16:5 | 7:5 | 8:4 |
| Age (years) | 64.8 (5.1) | 71.4 (8.6)a | 65 (5.7) | 65.5 (6.0) | 70.6 (7.3) |
| Handedness (R:L) | 31:2 | 17:2 | 19:2 | 12:0 | 12:0 |
| Education (years) | 15.9 (2.3) | 15.1 (2.9) | 14.6 (2.9) | 15 (2.6) | 13.2 (2.6) |
| Symptom duration (years) | N/A | 6.1 (4.0) | 8.5 (4.5) | 5.9 (2.2) | 3.7 (1.8)a |
| MMSE (/30) | 29.4 (0.9)* | 21.1 (5.5) | 25.9 (3.4) | 23 (7.4) | 20 (8.4)a |
| Hearing threshold (dB)b | 27.6 (9.4) | 31.6 (6.6) | 28.5 (5.6) | 25.7 (8.1) | 32.8 (7.5) |
| Genetic mutations | N/A | 0 |
|
0 | 2 GRN |
| Acetylcholinesterase inhibitor use (n) | N/A | 11 | 0 | 0 | 0 |
| Neuropsychological functions | |||||
| Episodic memory | |||||
| RMT words (/50) | 40.9 (6.3)** | 31.7 (6.9) | 34.3 (7.6) | 30.7 (3.4)c | 35.4 (5.6) |
| RMT faces (/50) | 48 (2.3)** | 30.9 (7.5) | 38.6 (7.7) | 34.8 (6.5)c | 37.9 (9.5) |
| Camden PAL (/24) | 20.2 (2.0)** | 6.8 (5.4)c | 11.1 (7.2) | 9.4 (8.1) | 14.4 (5.0) |
| Executive skills | |||||
| WASI block design (/71) | 51 (12.7)*** | 15.1 (8.5)b,d | 31.2 (13.4) | 41.1 (17.9) | 19.3 (20.4)b,d |
| WASI matrices (/32) | 25.5 (5.2)*** | 13.2 (4.7)d | 17.3 (7.2)d | 26.4 (5.0) | 16.6 (9.7)d |
| WMS-R digit span forward (max) | 7.1 (1.2)*** | 6.2 (1.2) | 6.2 (1.2) | 6.5 (1.1) | 4.6 (1.3)b,d,e |
| WMS-R digit span reverse (max) | 6 (1.1)*** | 3.8 (1.5)d | 4.6 (1.5) | 5.4 (1.6) | 3.4 (0.8)d |
| D-KEFS stroop colour naming (s) | 28.4 (4.8)** | 52.5 (14.2)b | 44.1 (15.9) | 43.2 (14.9) | 77.7 (14.9)b,d,e |
| D-KEFS stroop word reading (s) | 21.7 (4.5)** | 36.4 (16.9)b,d | 26.2 (6.0) | 27.6 (10.2) | 68.7 (20.5)b,d,e |
| D-KEFS stroop interference (s) | 56 (15.8)** | 126.3 (38.8)b,d | 78.9 (24.9) | 83.3 (26.9) | 139.6 (24.3)b,d |
| Trails A (s) | 28.5 (10.6)** | 85.6 (40.2)b,c,d | 48 (21.2) | 45.4 (18.1) | 60.1 (43.2) |
| Trails B (s) | 65.2 (23.7)** | 219.5 (83.1)d | 157.3 (90.4) | 134.4 (97) | 147.2 (64.7) |
| Letter fluency (F, 1 min) | 17.9 (5.5)** | 9.7 (4.3) | 10.6 (5.9) | 8.1 (4.6) | 6.6 (6.3) |
| Category fluency (animals, 1 min) | 25 (4.7)** | 10.8 (4.7) | 13.5 (6.6) | 6.1 (5.6)b | 10.1 (5.3) |
| Language skills | |||||
| WASI vocabulary (/80) | 71.6 (5.2)** | 56.8 (13.1) | 47.9 (19.9) | 27 (21.4)b,e | 24 (21.4)b,e |
| Graded naming test (/30) | 24.9 (3.0)** | 14.6 (8.3) | 14.8 (9.9) | 1.9 (5.3)b,c,e | 14.9 (7.7) |
| BPVS (/150) | 147.9 (1.2)*** | 133.4 (24.2) | 115.7 (44.9) | 64.6 (46.1)b,e | 105.3 (48.8) |
| PALPA 55 (/24) | 23.7 (0.6) | N/A | N/A | 21 (2.9) | 16.6 (4.4)d |
| Other skills | |||||
| Graded difficulty arithmetic (/24) | 17 (4.2)** | 5.4 (5.8)d | 8.7 (6.7) | 13.4 (5.8) | 6.4 (6.7) |
| VOSP object decision (/20) | 18.5 (1.7)** | 10.6 (5.1)b | 15.1 (5.2) | 11.5 (6.2) | 14.3 (6.6) |
| Musical skills and background | |||||
| Pitch direction discrimination (/10)a | 9.1 (1.1) | 8.6 (1.3) | 7.9 (1.5)c | 9.6 (0.5) | 8.3 (2.2) |
| Recognition of melodies: % correct | 0.94 (0.08) | 0.93 (0.08) | 0.85 (0.16) | 0.89 (0.04) | 0.84 (0.17) |
| d-Prime | 3.25 (0.54) | 2.56 (0.60) | 2.16 (1.07) | 1.95 (1.61) | 2.61 (1.10) |
| Prior musical expertise (/3)f | 0.8 (0.9) | 0.8 (0.6) | 1 (0.8) | 0.9 (1.0) | 0.5 (0.7) |
| Current music listening (h/week) | 7.1 (7.6) | 5.8 (5.7) | 7.6 (6.6) | 7.7 (10.5) | 5.2 (5.5) |
Mean (standard deviation) scores are shown unless otherwise indicated; maximum scores are shown after tests (in parentheses). Significant differences (P < 0.05) from healthy control values are indicated in bold; *data from 19 healthy controls; **data from 23 healthy controls; ***data from 25 healthy controls.
AD, patient group with typical Alzheimer’s disease; BPVS, British Picture Vocabulary Scale; bvFTD, patient group with behavioural variant frontotemporal dementia; C9orf72, pathogenic mutation in open reading frame of chromosome 9; Category fluency totals for animal category and letter fluency for the letter F in 1 min; Controls, healthy control group; D-KEFS, Delis Kaplan Executive System; Graded Difficulty Arithmetic test; Graded Naming Test; GRN, pathogenic mutation in progranulin gene; MAPT, pathogenic mutation in microtubule-associated protein tau gene; MMSE, Mini-Mental State Examination score; N/A, not assessed; nfvPPA, patient group with non-fluent-agrammatic variant primary progressive aphasia; PAL, Paired Associate Learning test; PALPA 55, Psycholinguistic Assessments of Language Processing in Aphasia subtest for Auditory Sentence Comprehension; RMT, Recognition Memory Test; svPPA, patient group with semantic variant primary progressive aphasia; Trails-making scores based on maximum time achievable of 2.5 min on task A and 5 min on task B; VOSP, Visual Object and Spatial Perception Battery—Object Decision test; WASI, Wechsler Abbreviated Scale of Intelligence; WMS, Wechsler Memory Scale.
See text and Supplementary material for details.
Significantly lower than bvFTD group.
Significantly lower than nvfPPA group.
Significantly lower than svPPA group.
Significantly lower than AD group.
Musical training assessed on a four-point scale: (0: never played an instrument or sang in a choir, to 3: learned an instrument for >10 years; all participants were lifelong British residents sharing a similar socio-economic and musical milieu).
All participants gave informed consent for their involvement in the study. Ethical approval was granted by the University College London and National Hospital for Neurology and Neurosurgery Joint Research Ethics Committees in accordance with Declaration of Helsinki guidelines.
Experimental design and stimuli
Based on a survey of 15 older British individuals who did not participate in the main study, 48 melodies rated as highly familiar on a 5-point scale [ranging from 1 (completely unfamiliar) to 5 (very familiar)] were selected and all transcribed for solo piano (details of all stimuli in Supplementary Table 1; stimulus examples in Supplementary material). From these famous melodies, we created four experimental conditions, comprising melodies containing either: no deviant note; a single note that deviated from the canonical melody while preserving its key (‘semantic’ deviants); a single note that deviated from the canonical melody by violating its key or tonality (‘syntactic’ deviants); or a single note that deviated in timbre (‘acoustic’ deviants, formed from white-noise bursts). The final stimulus set comprised 48 trials (12 exemplars of each condition).
Experimental procedure
All participants were first familiarized with the experiment to ensure they understood and could comply with the procedure. The participant was seated fixating a desktop computer monitor in a dimly and uniformly illuminated, quiet room. Pupil area was measured continuously using an infrared camera (details in Supplementary material). Stimuli were presented in randomized order from a notebook computer running Experiment Builder® (www.sr-research.com/experiment-builder) binaurally via headphones at a constant, comfortable listening level. The task on completion of each trial was to decide whether the melody contained a ‘wrong note’.
Following the pupillometry session, we conducted a test to assess each participant’s recognition of stimulus melodies, based on a two-alternative forced-choice decision between famous tunes (the pupillometry stimuli) and newly composed melodies (details in Supplementary material).
All participant responses were stored for offline analysis; no feedback about performance was given and no time limits on responses were imposed.
Analysis of behavioural data
All behavioural data were analysed using Stata14.1®. Demographic characteristics and neuropsychological data were compared between participant groups using chi-squared tests for categorical variables, and for continuous variables, either one-way ANOVA followed by two-sample t-tests or non-parametric Kruskal–Wallis tests followed by Wilcoxon rank-sum tests when t-test assumptions were violated. d-Prime values were calculated as a measure of each participant’s sensitivity for detection of deviants in each condition. Restricted maximum likelihood mixed effects’ models, with participant identity as a random effect, were used to analyse d-prime (discriminability) and hit rate (deviant detection accuracy). Joint Wald tests of the relevant coefficients were used to examine the main effects of diagnostic group and experimental condition (deviant type) and their interaction. Post-hoc pairwise group comparisons were performed where main effects were found. To take account of potentially confounding factors, age, gender, pitch direction discrimination performance (indexing pitch contour perception, musical working memory and task decision-making), prior musical expertise score and mean hours of listening to music per week were included as covariates in the regression model. In addition, we used Pearson’s correlation tests to separately assess any relationship between d-primes with age, gender, prior musical expertise and hours of listening, ability to recognize familiar melodies, peripheral hearing function, pitch contour processing (pitch direction discrimination score) and general disease factors [Mini-Mental State Examination (MMSE) score, symptom duration], across the patient cohort.
In order to examine the sensitivity and specificity of deviant detection profiles for predicting disease, we conducted a receiver-operating-characteristic (ROC) analysis and calculated area under the curve (AUC) coefficients assessing how well melodic deviant detection rate (d-primes) in each experimental condition discriminated syndromic groups from the healthy control group (further details are in Supplementary material).
A threshold P < 0.05 was accepted as the criterion of statistical significance for all tests.
Analysis of pupillometric data
Pupil diameter data were pre-processed using previously described procedures77 (details in Supplementary material), normalized to baseline and time-domain-averaged across trials for each condition. To identify time windows with significant discrepancies between deviant and ‘standard’ time series, a non-parametric bootstrap-based statistical analysis was used78 (10 000 iterations; with replacement) with family-wise error (FWE)-corrected cluster-size threshold P < 0.05.
To assess effects of participant group and experimental conditions on pupillary response magnitude, we extracted the maximum of the normalized pupil dilatation response (averaged across trials for each participant) during the 2 s following deviant onset, an interval chosen based on both the permutation analysis and previous studies.79 We used the same linear mixed model employed for the behavioural data (with the same covariates) to compare pupil response amplitudes between participant groups and experimental conditions.
Information-theoretic modelling
To quantify the extent to which behavioural and autonomic responses were related to the level of predictability and surprise in the musical environment, an unsupervised machine-learning model of auditory expectancy (Information Dynamics of Music model80) was used to quantify the predictability of each note within our experimental melody stimuli (further details in Supplementary material and Supplementary Fig. 1). For each melody containing a pitch deviant (or, for melodies without deviants, equivalently positioned ‘standard’ notes), we calculated two key information-theoretic parameters: the IC of the deviant note and the mean entropy (ENT) of the melody stem (from the first note to the note immediately preceding the deviant note; see details and Supplementary Table 1 in Supplementary material). The IC parameter allowed us to determine whether deviant detection accuracy and pupillary reactivity were related to deviant unexpectedness. The ENT parameter allowed us to determine the effect of melody (musical environmental) predictability, for melodies categorized as more predictable (low ENT) or less predictable (high ENT) relative to the median ENT value for the entire stimulus set.
For each participant group, we assessed correlations of stimulus IC score with trial-by-trial deviant detection accuracy (hit rate) and maximum pupillary dilatation response (during the 2 s following deviant onset) over the combined melody set and separately for each melody ENT category, using non-parametric Spearman’s correlation tests. We compared correlation strengths between participant groups using one-tailed z-tests, after transforming correlations to Fischer z-scores.81 A threshold P < 0.05 was accepted as the criterion of statistical significance for all tests.
Brain image acquisition and analysis
For 58 patients (15 Alzheimer’s disease, 20 bvFTD, 12 svPPA, 11 nfvPPA), T1-weighted volumetric brain MRI were acquired on a Prisma 3 T MRI scanner using a 32-channel phased-array head-coil and pre-processed using standard procedures in SPM12 (www.fil.ion.ucl.ac.uk/spm82; details in Supplementary material).
In a VBM analysis of the combined patient cohort, full factorial linear regression models with diagnostic group as the main factor were used to assess associations between regional grey matter volume (indexed as voxel intensity) separately with detection accuracy for each deviant condition and with Spearman’s rho values for the correlation between detection accuracy and deviant IC. Age, total intracranial volume and pitch direction score were incorporated as covariates of no interest. Statistical parametric maps of regional grey matter associations were generated using an initial peak voxel and cluster-forming uncorrected threshold P < 0.001 and evaluated at peak voxel statistical significance level P < 0.05 after FWE correction for multiple voxel-wise comparisons within pre-specified anatomical regions of interest (see Supplementary Fig. 2). These regions were informed by previous studies of music and musical surprise processing in both the healthy brain and neurodegenerative disease and comprised: a posterior temporo-parietal network involved in sensory pattern analysis (posterior superior temporal gyrus, angular and supramarginal gyri and precuneus65,83,84) an anteroventral network involved in semantic appraisal (anterior superior temporal gyrus, middle temporal gyrus, temporal pole and inferior frontal gyrus48,49,65,71,85,86) a striato-limbic network involved in emotion and reward evaluation (putamen, caudate, nucleus accumbens, hippocampus and amygdala44,45,70,87–89) and a cingulo-insular network involved in salience processing and motor output (anterior insula, anterior cingulate cortex and supplementary motor area90–92).
Data availability
We are precluded by institutional ethics agreements from publishing the full dataset in the public domain. However, data and stimuli will be made available on reasonable request to Prof Warren, under appropriate data transfer agreements.
Results
General participant characteristics
Patient groups did not differ significantly from healthy controls in gender distribution, handedness, years in formal education or composite audiometry score (all P > 0.05; Table 1). Patients with Alzheimer’s disease were on average significantly older than healthy controls (P < 0.05). The patient groups did not differ in mean symptom duration but did differ in overall severity of cognitive impairment (MMSE score; Kruskal–Wallis H = 102.9, P < 0.001). Participant groups did not differ significantly in musical expertise or average time spent listening to music each week (all P > 0.05; Table 1). However, the bvFTD group performed significantly worse than both the healthy control and svPPA groups on the pitch direction discrimination test (Kruskal–Wallis H = 10.9, P < 0.05 and post-hoc two-sample Wilcoxon tests, P < 0.05).
Behavioural data: detection of deviants in melodies
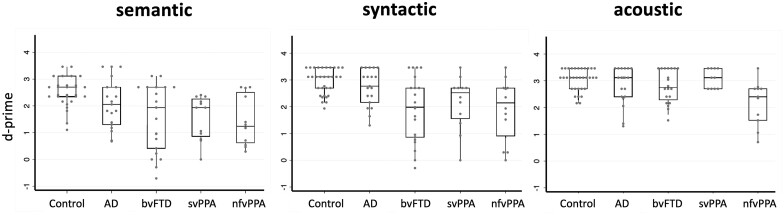
Group data for detection of deviants in melodies (hit rate and d-primes) are summarized in Table 2 and Supplementary Table 2. Individual d-primes data are plotted in Fig. 1. Results of detection accuracy (hit rate) are reported in Table 2.
Table 2.
Summary of melodic deviant detection accuracy and pupillary response profiles for participant groups and conditions
| Group | Condition | Behavioural response |
Pupil peak response |
|||
|---|---|---|---|---|---|---|
| Detection accuracya | d-Prime | IC correlate | Amplitudeb | IC correlate | ||
| Controls | No deviant | 0.90 (0.10) | NA | rho = 0.41 (0.14)c | 0.35 (0.22) | rho = 0.56 (0.14)c |
| Semantic | 0.89 (0.10) | 2.58 (0.55) | 0.53 (0.26) | |||
| Syntactic | 0.98 (0.05) | 2.95 (0.47) | 0.61 (0.25) | |||
| Acoustic | 1 (0) | 3.04 (0.41) | 0.78 (0.26) | |||
| AD | No deviant | 0.87 (0.16) | NA | rho = 0.10 (0.16) | 0.47 (0.25) | rho = 0.53 (0.15)c |
| Semantic | 0.75 (0.26) | 2.08 (0.91) | 0.53 (0.27) | |||
| Syntactic | 0.94 (0.14) | 2.72 (0.68) | 0.71 (0.44) | |||
| Acoustic | 0.97 (0.07) | 2.82 (0.68) | 0.81 (0.46) | |||
| bvFTD | No deviant | 0.85 (0.16) | NA | rho = −0.16 (0.19)d | 0.27 (0.31) | rho = 0.20 (0.15) |
| Semantic | 0.62 (0.35) | 1.52 (1.24) | 0.39 (0.30) | |||
| Syntactic | 0.73 (0.35) | 1.91 (1.21)e | 0.42 (0.28) | |||
| Acoustic | 0.99 (0.05) | 2.80 (0.62) | 0.68 (0.38) | |||
| svPPA | No deviant | 0.89 (0.10) | NA | rho = −0.43 (0.14)c,d | 0.29 (0.32) | rho = 0.21 (0.16) |
| Semantic | 0.61 (0.31) | 1.56 (0.81) | 0.32 (0.26) | |||
| Syntactic | 0.77 (0.32) | 2.16 (0.99)e | 0.36 (0.29) | |||
| Acoustic | 1 (0) | 3.09 (0.36) | 0.71 (0.33) | |||
| nfvPPA | No deviant | 0.74 (0.26) | NA | rho = 0.39 (0.13)c | 0.40 (0.31) | rho = 0.54 (0.11)1 |
| Semantic | 0.71 (0.24) | 1.45 (0.92) e | 0.53 (0.48) | |||
| Syntactic | 0.84 (0.23) | 1.90 (1.16)e | 0.76 (0.44) | |||
| Acoustic | 0.93 (0.15) | 2.19 (0.83) | 0.90 (0.47) | |||
Mean (standard deviation) values are shown; 12 trials were presented in each condition. Significant differences (P < 0.05) from healthy control values are indicated in bold; details of pair-wise comparisons between participant groups and deviant conditions are presented in Supplementary Tables 2 (d-prime data), 3 and 4 (pupil data), and Supplementary Tables 5 and 6 (IC correlation data) in Supplementary material.
AD, patient group with typical Alzheimer’s disease; bvFTD, patient group with behavioural variant frontotemporal dementia; Controls, healthy control group; IC, deviant information-content (see text); NA, not applicable; nfvPPA, patient group with non-fluent-agrammatic variant primary progressive aphasia; svPPA, patient group with semantic variant primary progressive aphasia.
For no-deviant condition, values refer to correctly rejected trials (1—false-alarm rate), thus a reduced deviant detection accuracy (hit rate) in this condition signifies a raised false-alarm rate.
In arbitrary units, for reference only.
Significantly different from null hypothesis (no correlation).
Significantly lower than nvfPPA group.
Significantly lower than AD group.
Figure 1.
Performance of detection of different types of deviants in melodies, for all participant groups. For each panel (deviant condition), d-prime value is plotted for individuals within each participant group (see also Table 2). Each dot corresponds to an individual data point; boxes code the interquartile range, whiskers represent the ranges for the bottom 25% and the top 25% of the data values (excluding outliers) and the horizontal line in each box represents the median d-prime value. AD, patient group with Alzheimer’s disease; bvFTD, patient group with behavioural variant frontotemporal dementia; Control, healthy control group; nfvPPA, patient group with non-fluent-agrammatic variant primary progressive aphasia; svPPA, patient group with semantic variant primary progressive aphasia.
There were significant main effects on performance (d-prime) of participant group [χ2(4) = 20.4, P < 0.001] and deviant condition [χ2(2) = 127.36, P < 0.001] and a significant interaction between group and condition [χ2(8) = 33.3, P < 0.001].
Comparing each patient group with the healthy control group, the bvFTD, svPPA and nfvPPA groups were significantly less sensitive in detecting semantic deviants (all P < 0.001) and syntactic deviants (all P < 0.005) and the nfvPPA group was additionally less sensitive in detecting acoustic deviants (P = 0.031), reflecting excessive false-alarms (Table 2). Comparing between patient groups, the bvFTD and svPPA groups were significantly less sensitive than the Alzheimer’s disease group in detecting syntactic deviants (all P < 0.042); while the nfvPPA group was significantly less sensitive than the Alzheimer’s disease group in detecting both syntactic deviants (P = 0.013) and semantic deviants (P = 0.045).
Comparing experimental conditions within participant groups, all groups were significantly more sensitive in detecting syntactic and acoustic deviants than semantic deviants (all P < 0.05). The bvFTD and svPPA groups were additionally significantly more sensitive in detecting acoustic than syntactic deviants (all P < 0.001).
Overall auditory deviant discriminability (d-prime) across the patient cohort correlated with musical background (r = 0.31, P = 0.01), pitch direction discrimination score (r = 0.39 = 0, P = 0.003), musical expertise score (rho = 0.31, P = 0.012) and musical familiarity d-prime (rho = 0.53, P < 0.001). There was no correlation of d-primes with age, gender, symptom duration, MMSE or peripheral hearing score.
Results of the ROC analysis with corresponding AUC coefficients are presented in Supplementary Fig. 3. Detection of both syntactic and semantic deviants discriminated well between each FTD syndromic group and healthy controls (AUC 0.81–0.87). No significant AUC differences were found between deviant conditions.
Pupillometric data: autonomic responses to deviants in melodies
Participant groups did not differ significantly in resting baseline pupil size [χ2(4) = 6.6, P = 0.16] or overall pupil dynamics (mean pupil response across all trials) [χ2(4) = 8.2, P = 0.09].
Group data for pupillary responses to melody deviants are summarized in Table 2 and Supplementary Tables 3 and 4; mean time courses of pupillary dilation responses are shown in Fig. 2 and Supplementary Fig. 4. There were significant main effects on mean pupillary response magnitude of participant group [χ2(4) = 10.3; P = 0.03] and deviant condition [χ2(3) = 123.18; P < 0.001] but no interaction [χ2(12) = 13.8; P = 0.31].
Figure 2.
Time course of pupil dilatation responses to melodic deviants in each of the experimental conditions, for all participant groups. Onset of the deviant note is at time 0 (indicated by vertical grey line on each panel). To generate these pupil time series, trial-by-trial pupil time series from individual participants were first filtered, smoothed, converted to z-scores based on the signal mean and standard deviation for that participant’s dataset and baseline-corrected by subtracting the pre-deviant baseline (details in Supplementary material); the plots show the mean normalized pupil time series flanked by error envelopes representing the standard deviation of the group pupillary response, for each experimental condition (coded at lower left). AD, patient group with Alzheimer’s disease; bvFTD, patient group with behavioural variant frontotemporal dementia; Controls, healthy control group; nfvPPA, patient group with non-fluent-agrammatic variant primary progressive aphasia; svPPA, patient group with semantic variant primary progressive aphasia.
Post-hoc tests revealed that the nfvPPA and Alzheimer’s disease groups had significantly larger pupillary responses than the svPPA and bvFTD groups (all P < 0.05). There were no significant differences between any patient group and healthy controls (see Supplementary Table 3). When comparing conditions for all groups combined, there were significant differences between all types of deviants with pairwise comparisons preserving the following order: acoustic deviant > syntactic deviant > semantic deviant > no-deviant (all P < 0.001; see Supplementary Table 4).
Information-theoretic modelling: processing statistical structure of melodies and deviants
Across the combined stimulus set, IC was positively correlated with deviant detection accuracy in the healthy control group (rho = 0.41, P = 0.01) and nfvPPA group (rho = 0.40, P = 0.02), whereas IC was negatively correlated with deviant detection accuracy in the svPPA group (rho = −0.43, P = 0.008). Correlations were significantly stronger in healthy controls and nfvPPA groups than both the bvFTD and svPPA groups (all P < 0.009; see Table 2, Supplementary Fig. 5 and Table 5). We found that for low ENT (more predictable) melodies, IC was significantly positively correlated with deviant detection accuracy in the healthy control group (rho = 0.52, P = 0.03), whereas IC was significantly negatively correlated with deviant detection accuracy in the svPPA group (rho = −0.57, P = 0.01), driven by a very low false-alarm rate in the no-deviant condition (see also Table 2). For high ENT (less predictable) melodies, there were no significant correlations between IC and deviant detection accuracy in any participant group.
Across the combined stimulus set, IC was positively correlated with pupillary dilatation magnitude in the healthy control group (rho = 0.56, P < 0.001), Alzheimer’s disease group (rho = 0.53, P < 0.001) and nfvPPA group (rho = 0.54, P < 0.001). The correlation was significantly stronger in healthy controls than the svPPA group (P = 0.04) and the bvFTD group (P = 0.04; see Table 2, Supplementary Fig. 5 and Table 6). For low ENT melodies, IC was significantly positively correlated with pupillary dilatation magnitude in the healthy control group (rho = 0.58, 0.01) and Alzheimer’s disease group (rho = 0.71, P < 0.001). For high ENT melodies, IC was significantly positively correlated with pupillary dilatation magnitude in the healthy control group (rho = 0.58, P = 0.01) and nfvPPA group (rho = 0.63, P = 0.01).
Neuroanatomical associations
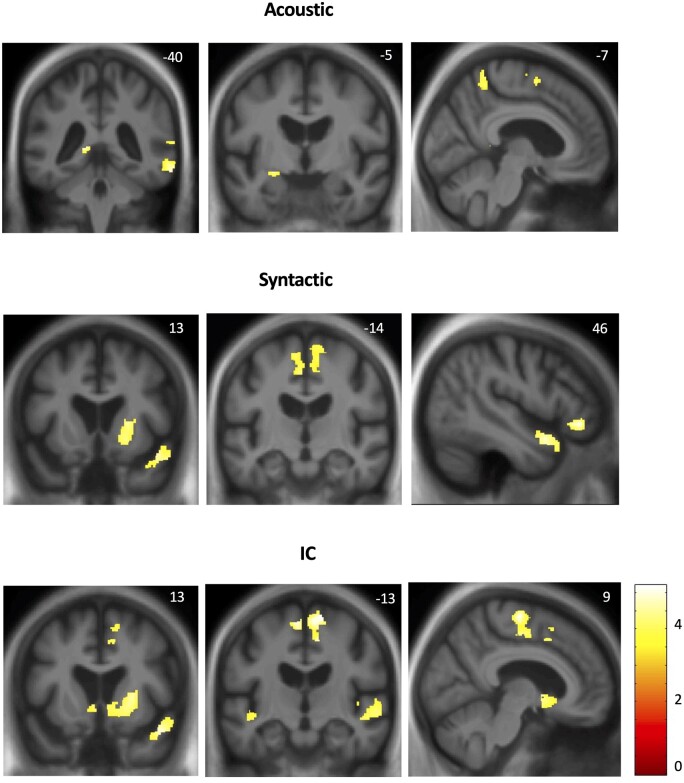
Significant grey matter associations of deviant detection accuracy and coupling between deviant detection accuracy and deviant note IC for the combined patient cohort are summarized in Table 3; statistical parametric maps of these associations are presented in Fig. 3. All associations here are reported thresholded at P < 0.05 after FWE correction for multiple voxel-wise comparisons within pre-specified anatomical regions of interest.
Table 3.
Neuroanatomical correlates of melodic deviant detection and surprise coding in the combined patient cohort
| Condition | Region | Side | Cluster (voxels) | Peak (mm) |
T score | PFWE | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Acoustic | Precuneus | R | 471 | 9 | −46 | 67 | 4.94 | 0.022 |
| L | 451 | −10 | −50 | 57 | 4.48 | 0.028 | ||
| Hippocampus | L | 94 | −14 | −40 | 4 | 4.66 | 0.032 | |
| Middle temporal gyrus | R | 1489 | 64 | −40 | −14 | 4.59 | 0.022 | |
| Supplementary motor area | L | 75 | −5 | 2 | 62 | 4.46 | 0.031 | |
| Amygdala | L | 124 | −27 | −6 | −12 | 4.19 | 0.039 | |
| Syntactic | Supplementary motor area | R | 982 | 4 | −2 | 50 | 5.13 | 0.004 |
| L | 1352 | −4 | −16 | 51 | 4.92 | 0.005 | ||
| Inferior frontal gyrus: pars orbitalis | R | 488 | 50 | 38 | −8 | 4.79 | 0.014 | |
| Putamen | R | 121 | 22 | 20 | −6 | 4.61 | 0.006 | |
| Temporal pole | R | 1807 | 46 | 10 | −20 | 4.51 | 0.015 | |
| IC correlation | Supplementary motor area | R | 1076 | 14 | −14 | 69 | 4.85 | 0.005 |
| L | 945 | −8 | −16 | 62 | 4.84 | 0.007 | ||
| Superior temporal gyrus | L | 1949 | −56 | −30 | 0 | 4.85 | 0.018 | |
| R | 2489 | 56 | −16 | −2 | 4.25 | 0.045 | ||
| Temporal pole | R | 2489 | 46 | 9 | −22 | 4.64 | 0.007 | |
| Putamen | R | 2883 | 22 | 20 | −4 | 4.57 | 0.002 | |
| Nucleus accumbens | L | 1657 | −4 | 10 | −3 | 4.11 | 0.038 | |
| R | 2883 | 9 | 4 | −8 | 4.08 | 0.018 | ||
The table presents the results of the voxel-based morphometry analysis. Shown are the locations of regional grey matter positively associated with accuracy of detection for different types of deviants and for the coupling between deviant detection accuracy and deviant note IC (individual Spearman rho) in familiar melodies, over the combined patient cohort (see also text and Fig. 3). Coordinates of local maxima are in standard MNI space. P values were all significant (P < 0.05) after family-wise error (FWE) correction for multiple voxel-wise comparisons within pre-specified anatomical regions of interest (see text and Supplementary Fig. 2).
Figure 3.
Neuroanatomical correlates of accurate detection of melodic deviants and coupling between detection and musical surprise value in the combined patient cohort. Statistical parametric maps (SPMs) show regional grey matter volume positively associated with acoustic (top panels) and syntactic (middle panels) deviant detection accuracy and coupling between deviant detection accuracy and deviant note IC (bottom panels) in familiar melodies, based on voxel-based morphometry of patients’ brain MR images. SPMs are thresholded for display purposes at P < 0.001 uncorrected over the whole brain; however, local maxima of areas shown were each significant at P < 0.05 after family-wise error correction for multiple voxel-wise comparisons within pre-specified anatomical regions of interest (see Table 3 and Supplementary Fig. 2); T-scores are coded on the colour bar. SPMs are overlaid on sections of the normalized study-specific T1-weighted mean brain MR image; the MNI coordinate (mm) of the plane of each section is indicated, and the right hemisphere is shown on the right in coronal sections.
Syntactic deviant detection accuracy correlated with regional grey matter in bilateral supplementary motor area and right pars orbitalis of inferior frontal gyrus, temporal pole and putamen. Acoustic deviant detection accuracy correlated with regional grey matter in a more postero-ventral, bi-hemispheric network including right precuneus and middle temporal gyrus, hippocampus and amygdala as well as left supplementary motor area. No associations of semantic deviant detection accuracy were identified at the prescribed significance threshold.
Coupling between deviant detection accuracy and deviant note IC (individual Spearman rho) correlated with regional grey matter in an extended bi-hemispheric network comprising bilateral supplementary motor area, superior temporal gyrus and nucleus accumbens and right temporal pole and putamen.
Discussion
Here, we have shown that canonical syndromes of FTD and Alzheimer’s disease have differentiated behavioural and autonomic responses to musically surprising events. Consistent with previous evidence,60,93–95 healthy older controls here showed a graded response profile with more accurate detection of ‘surprising’ deviations in fundamental acoustic structure and generic syntactic musical rules than deviations in the semantic structure of specific musical objects (melodies). Further, in healthy controls, both detection accuracy and autonomic reactivity correlated with the information-theoretic quantity of surprise (IC) in deviant musical events but were differentially modulated by the predictability (ENT) of the musical environment. In line with previous work,96,97 detection accuracy was enhanced in more predictable musical environments, whereas pupillary response was not affected by environmental predictability. Whereas the Alzheimer’s disease group detected musical deviants normally, the bvFTD and svPPA groups showed strikingly similar profiles of impaired syntactic and semantic deviant detection and impaired cognitive and autonomic sensitivity to deviant IC, relative both to healthy controls and other syndromic groups; while the nfvPPA group showed a generalized deficit of auditory deviant discriminability but retained sensitivity to IC. These syndromic signatures were evident after taking elementary pitch pattern perception and musical experience into account. Across the patient cohort, detection of musical and acoustic deviants and the linkage between deviant detection accuracy and surprise had separable neuroanatomical substrates in cortico-subcortical networks previously implicated in the perceptual, semantic and hedonic analysis of music and in tracking probabilistic information in musical sequences.
Behaviourally, impaired detection of musical ‘rule’ violations in bvFTD and svPPA accords with previous evidence that these syndromes impair psychological expectations about melodies,45,46,49 auditory scenes6 and other complex sensory signals.1,5,7–11 Such deficits are in turn likely to underpin the difficulties these patients experience in interpreting the often ambiguous or conflictual emotional and social signals of other people and in regulating their own socio-emotional behaviours.2,98–102 In predictive coding terms, such phenomena might reflect impaired matching of incoming signals to stored neural ‘templates’ (predictions established through past experience), inefficient updating of those predictions and/or degraded templates per se. While any of these may operate in bvFTD and svPPA, the correlation here between detection accuracy and melody recognition (familiarity d-prime) underlines how an ‘incorrect model’ of the current sensory environment precludes detection of incongruent events. In bvFTD and svPPA, environmental deviations are essentially rendered ‘unsurprising’, while false-alarm rates (most strikingly, in svPPA) are correspondingly low (Table 2): this pattern would follow if stored melody templates are degraded to the extent that most incoming facsimiles of the template (including aberrant ones) achieve a ‘match’. Loss of the normal dependence of deviant detection on surprise value (IC) and lack of any modulatory effect from environmental predictability (ENT) in bvFTD and svPPA are consistent with previous evidence that svPPA fundamentally impairs analysis of the IC of auditory sequences50,51 while bvFTD impairs stimulus salience coding,45,101 as determined by the interaction of event surprise and environmental predictability.103,104
Our finding of a generalized abnormality of musical and acoustic deviant discriminability in nfvPPA builds on emerging evidence for disordered auditory processing in this syndrome.50,66,105–107 In contrast to other dementia syndromes (and mirroring the profile in svPPA), nfvPPA was associated with an abnormally high false-alarm rate (Table 2), indicating that these patients tended to over-interpret variations from musical canonicity in melodies without deviant notes (for example, timbral or key changes associated with transcribing the melodies) as ‘errors’. This abnormally heightened ‘surprise’ sensitivity is consistent with a previous predictive-coding account of degraded speech processing in this syndrome,52 according to which patients with nfvPPA tend to make inflexible predictions about incoming auditory data. In patients’ daily lives, reduced predictive flexibility under dynamic listening conditions may contribute to a range of deficits, including impaired hearing even in quiet environments, impaired perception of less familiar accents and emotional prosody and reduced modulation of social signals, such as conversational laughter.106–109
The reduced coupling between pupillary response and deviant IC in the svPPA and bvFTD groups here extends the evidence for central autonomic dysregulation in these syndromes.63,110–115 The syndromic pupillary response profiles here, taken together with the behavioural data on detection accuracy, suggest that coding of musical salience was deficient in patients with bvFTD and svPPA. A mutual interplay between prediction formation and salience coding, and between cognitive and autonomic mechanisms, is essential to our normal experience of musical events.44,116,117 Peak pupillary responses in our nfvPPA group tracked deviant surprise value in relatively less (but not more) predictable musical environments, as would be anticipated if pupillary responses in this syndrome tend to signal relatively less salient events linearly but become saturated in more predictable environments.79
Neuroanatomically, detection of acoustic deviants correlated with grey matter in middle temporal gyrus, precuneus and hippocampus: key components of the so-called ‘default-mode’ network that governs the interface between self and environment, facilitates reallocation of attention and more particularly, has been shown to play a key role in auditory scene analysis, both in the healthy brain and in neurodegenerative disease.118,119 Noise bursts interrupting a melody potentially signal a fundamental change in the prevailing auditory environment, engaging neural mechanisms that decode the significance of environmental fluctuations in relation to the internal milieu and the neural record of continuous auditory experience. Connected brain regions mediate the preparation of responses to such salient, potentially behaviourally relevant events: semantic control processes that integrate incongruous sensory information with stored conceptual representations are mediated via middle temporal gurus120–122 while physiological arousal is mediated via limbic structures, including amygdala.123,124
Detection of syntactic deviants and coupling of detection to deviant surprise value had closely overlapping neuroanatomical correlates subsuming a generic fronto-striatal ‘prediction network’ previously proposed to test sensory hypotheses and minimize prediction error in diverse domains, including music.125 Our findings are consistent with a scheme in which the prediction network is hierarchically engaged with involvement of additional regions mediating different levels of musical surprise analysis. According to this scheme, superior temporal gyrus initially represents musical object structure (especially, pitch pattern) and IC.43,126–130 Temporal polar cortex and pars orbitalis of inferior frontal cortex together track and evaluate incoming musical patterns against stored templates and rules acquired implicitly through the individual’s cumulative past experience of music.55,131,132 The temporal pole hosts the canonical ‘hub’ of the semantic memory system, while pars orbitalis integrates semantic and affective signals across sensory modalities and mediates subjective experience of expectation violations in music.70,133,134 Both regions are implicated in the recognition of melodies49,65,91,135–138 and in processing violations of musical semantic representations.139 The conjoint involvement of dorsal and ventral striatum here underlines the critical role of striatal dopaminergic circuitry in coding musical expectation and surprise probabilistically,43,44 an operation integral to hedonic valuation of music.37,88,117 Nucleus accumbens tracks reward prediction errors in music, a prime mover of musical learning and behaviour.87 While we did not assess reward prediction explicitly in this study, ventral striatum is engaged in resolving musical uncertainty through the integration of cognitive and affective information.44,87 By employing highly familiar melodies, this study may have primed the implicit coding of musical reward potential by striatal circuitry.
A common correlate of musical and acoustic surprise processing was identified in supplementary motor cortex. This is a core effector region for predicting actions and preparing behavioural responses to salient and arousing events, in music, vocalizations and other cognitive domains.65,92,137,140–142 This region is intimately linked to the salience network90 and plays an essential role in processing auditory expectations, especially when these have been established through sensorimotor integration as is generally the case for music.92,143,144
This study suggests that major dementias have distinct profiles of sensory ‘surprise’ processing, as instantiated in music. This is a fundamental cognitive and pathophysiological signal that applies a unifying approach to understanding the pathophysiology of complex behavioural changes in these diseases, suggests musical ‘tools’ to measure such changes and might inform future experimental work. However, the study raises a number of issues. The findings should be corroborated in larger patient cohorts and other neurodegenerative pathologies, and the basis for individual variability—a substantial factor within syndromic profiles (see Fig. 1)—and the longitudinal evolution of deficits require elucidation, particularly if stratification of syndromic groups is to yield novel disease biomarkers. The musical paradigm could be elaborated in various ways, including manipulation of prior melody familiarity (the listener’s prior expectations about the musical environment). Accumulating evidence that music processing shares neurobiological circuitry with the processing of social and emotional signals, in health as well as neurodegenerative disease,42,53,54,145 lends credence to our proposal that music may be a pertinent model system for deconstructing the neural mechanisms that underpin the wider phenotypic repertoire of FTD and Alzheimer’s disease. This is an exciting prospect, given that we currently largely lack experimental paradigms to assess social cognition conveniently, flexibly and cross-linguistically in cognitively impaired people.
The predictive coding formulation we have adopted in this study goes beyond neuroanatomical convergence, in suggesting a generic pathophysiological framework that potentially extends across different scales of description (from local neural circuits to the whole brain) and modalities (cognitive, autonomic and neuroimaging). However, the validity and utility of the predictive coding framework will only be established through the application of physiological and computational techniques—such as magnetoencephalography and dynamic causal modelling—that can capture neural microcircuits and large-scale network dynamics directly. Our findings suggest that music is an attractive candidate target for the further application of such techniques and the extraction of novel pathophysiological metrics of neurodegenerative disease. The potential explanatory power of the predictive coding paradigm in deconstructing complex symptoms has gained considerable traction in clinical psychiatry.146–148 However, it will be crucial to demonstrate that deficits of musical predictive coding are relevant to the socio-emotional symptoms reported by patients and caregivers, if this paradigm is ultimately to yield novel psychophysiological tools to detect and measure disease activity and strategies to ameliorate the effects of neurodegenerative proteinopathies.
Supplementary material
Supplementary material is available at Brain Communications online.
Supplementary Material
Acknowledgements
The authors are grateful to all participants and caregivers.
Funding
This work was supported by the Alzheimer’s Society, the University College London Hospitals (UCLH) National Institute for Health Research (NIHR) Biomedical Research Centre and the Wellcome Trust. E.B. was supported by a Brain Research UK PhD Studentship. H.S. was funded by a Clinical Research Fellowship from the Leonard Wolfson Experimental Neurology Centre. J.C.S.J. was supported by an Association of British Neurologists Clinical Research Training Fellowship. M.C.R.K. was supported by a Wellcome Trust PhD Studentship. R.L.B. was supported by a Medical Research Council (MRC) Mental Health PhD Studentship. J.E.P.L. was funded by an Engineering and Physical Sciences Research Council (EPSRC) PhD Studentship. C.H. was funded by an Action on Hearing Loss–Dunhill Medical Trust Pauline Ashley Fellowship (grant PA23_Hardy). J.D.R. was supported by a Medical Research Council (MRC) Clinician Scientist Fellowship (grant MR/M008525/1).
Conflict of interest
The authors report no conflict of interest.
Glossary
- AUC =
area under the curve
- bvFTD =
behavioural variant frontotemporal dementia
- ENT =
entropy
- FTD =
frontotemporal dementia
- FWE =
family-wise error
- IC =
information-content
- MMSE =
Mini-Mental State Examination
- nfvPPA =
non-fluent-agrammatic variant primary progressive aphasia
- ROC =
receiver-operating-characteristic
- svPPA =
semantic variant primary progressive aphasia
- VBM =
voxel-based morphometry
References
- 1.Johnen A, Bertoux M.. Psychological and cognitive markers of behavioral variant frontotemporal dementia—a clinical neuropsychologist’s view on diagnostic criteria and beyond. Front Neurol. 2019;10:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivasathiaseelan H, Marshall CR, Agustus JL, et al. Frontotemporal dementia: a clinical review. Semin Neurol. 2019;39(2):251–263. [DOI] [PubMed] [Google Scholar]
- 3.Warren JD, Rohrer JD, Rossor MN.. Frontotemporal dementia. BMJ. 2013;347:f4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibañez A, Manes F.. Contextual social cognition and the behavioral variant of frontotemporal dementia. Neurology. 2012;78(17):1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu I, Piguet O, Diehl-Schmid J, et al. Facial emotion recognition performance differentiates between behavioral variant frontotemporal dementia and major depressive disorder. J Clin Psychiatry. 2018;79(1). [DOI] [PubMed] [Google Scholar]
- 6.Clark CN, Nicholas JM, Agustus JL, et al. Auditory conflict and congruence in frontotemporal dementia. Neuropsychologia. 2017;104:144–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark CN, Nicholas JM, Henley SMD, et al. Humour processing in frontotemporal lobar degeneration: A behavioural and neuroanatomical analysis. Cortex. 2015;69:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton MA, Weickert TW, Hodges JR, Piguet O, Hornberger M.. Impaired acquisition rates of probabilistic associative learning in frontotemporal dementia is associated with fronto-striatal atrophy. Neuroimage Clin. 2012;2:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleichgerrcht E, Torralva T, Roca M, et al. Decision making cognition in primary progressive aphasia. Behav Neurol. 2012;25(1):45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krueger CE, Bird AC, Growdon ME, Jang JY, Miller BL, Kramer JH.. Conflict monitoring in early frontotemporal dementia. Neurology. 2009;73(5):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumfor F, Ibañez A, Hutchings R, Hazelton JL, Hodges JR, Piguet O.. Beyond the face: How context modulates emotion processing in frontotemporal dementia subtypes. Brain. 2018;141(4):1172–1185. [DOI] [PubMed] [Google Scholar]
- 12.Bettcher BM, Giovannetti T, Macmullen L, Libon DJ.. Error detection and correction patterns in dementia: A breakdown of error monitoring processes and their neuropsychological correlates. J Int Neuropsychol Soc. 2008;14(2):199–208. [DOI] [PubMed] [Google Scholar]
- 13.Chenery HJ, Ingram JC, Murdoch BE.. The resolution of lexical ambiguity with reference to context in dementia of the Alzheimer’s type. Int J Lang Commun Disord. 1998;13(2):393–412. [DOI] [PubMed] [Google Scholar]
- 14.Daffner KR, Mesulam MM, Cohen LG, Scinto LF.. Mechanisms underlying diminished novelty-seeking behavior in patients with probable Alzheimer’s disease. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12(1):58–66. [PubMed] [Google Scholar]
- 15.Duclos H, Bejanin A, Eustache F, Desgranges B, Laisney M.. Role of context in affective theory of mind in Alzheimer’s disease. Neuropsychologia. 2018;119:363–372. [DOI] [PubMed] [Google Scholar]
- 16.Fittipaldi S, Ibanez A, Baez S, Manes F, Sedeno L, Garcia AM.. More than words: Social cognition across variants of primary progressive aphasia. Neurosci Biobehav Rev. 2019;100:263–284. [DOI] [PubMed] [Google Scholar]
- 17.Ho B-L, Lin S-F, Chou P-S, Hsu C-Y, Liou L-M, Lai C-L.. Impaired conflict monitoring in cognitive decline. Behav Brain Res. 2019;363:70–76. [DOI] [PubMed] [Google Scholar]
- 18.Lenoble Q, Corveleyn X, Szaffarczyk S, Pasquier F, Boucart M.. Attentional capture by incongruent object/background scenes in patients with Alzheimer disease. Cortex. 2018;107:4–12. [DOI] [PubMed] [Google Scholar]
- 19.Peraita H, Díaz C, Anllo-Vento L.. Processing of semantic relations in normal aging and Alzheimer’s disease. Arch Clin Neuropsychol. 2008;23(1):33–46. [DOI] [PubMed] [Google Scholar]
- 20.Rohrer JD, Warren JD.. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci. 2010;293(1-2):35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsiao F-J, Chen W-T, Wang P-N, Cheng C-H, Lin Y-Y.. Temporo-frontal functional connectivity during auditory change detection is altered in Alzheimer’s disease. Hum Brain Mapp. 2014;35(11):5565–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes LE, Rowe JB.. The impact of neurodegeneration on network connectivity: A study of change detection in frontotemporal dementia. J Cogn Neurosci. 2013;25(5):802–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito J, Kitagawa J.. Error processing in patients with Alzheimer’s disease. Pathophysiology. 2005;12(2):97–101. [DOI] [PubMed] [Google Scholar]
- 24.Jiang S, Yan C, Qiao Z, et al. Mismatch negativity as a potential neurobiological marker of early-stage Alzheimer disease and vascular dementia. Neurosci Lett. 2017;647:26–31. [DOI] [PubMed] [Google Scholar]
- 25.Laptinskaya D, Thurm F, Küster OC, et al. Auditory memory decay as reflected by a new mismatch negativity score is associated with episodic memory in older adults at risk of dementia. Front Aging Neurosci. 2018;10:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazaheri A, Segaert K, Olichney J, et al. EEG oscillations during word processing predict MCI conversion to Alzheimer’s disease. Neuroimage Clin. 2018;17:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruzzoli M, Pirulli C, Mazza V, Miniussi C, Brignani D.. The mismatch negativity as an index of cognitive decline for the early detection of Alzheimer’s disease. Sci Rep. 2016;6:33167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Firbank M, Kobeleva X, Cherry G, et al. Neural correlates of attention-executive dysfunction in lewy body dementia and Alzheimer’s disease. Hum Brain Mapp. 2016;37(3):1254–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hughes LE, Ghosh BCP, Rowe JB.. Reorganisation of brain networks in frontotemporal dementia and progressive supranuclear palsy. Neuroimage Clin. 2013;2:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Josef Golubic S, Aine CJ, Stephen JM, Adair JC, Knoefel JE, Supek S.. MEG biomarker of Alzheimer’s disease: Absence of a prefrontal generator during auditory sensory gating. Hum Brain Mapp. 2017;38(10):5180–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobeleva X, Firbank M, Peraza L, et al. Divergent functional connectivity during attentional processing in Lewy body dementia and Alzheimer’s disease. Cortex. 2017;92:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw AD, Hughes LE, Moran R, Coyle-Gilchrist I, Rittman T, Rowe JB.. In vivo assay of cortical microcircuitry in frontotemporal dementia: A platform for experimental medicine studies. Cereb Cortex. 2021;31(3):1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahnaou A, Moechars D, Raeymaekers L, et al. Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer’s disease pathology. Sci Rep. 2017;7(1):14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim B, Shin J, Kim Y, Choi JH.. Destruction of ERP responses to deviance in an auditory oddball paradigm in amyloid infusion mice with memory deficits. PLoS One. 2020;15(3):e0230277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichelt AC, Killcross S, Wilkinson LS, Humby T, Good MA.. Transgenic expression of the FTDP-17 tauV337M mutation in brain dissociates components of executive function in mice. Neurobiol Learn Mem. 2013;104:73–81. [DOI] [PubMed] [Google Scholar]
- 36.Koelsch S, Gunter T, Friederici AD, Schröger E.. Brain indices of music processing: ‘nonmusicians’ are musical. J Cogn Neurosci. 2000;12(3):520–541. [DOI] [PubMed] [Google Scholar]
- 37.Blood AJ, Zatorre RJ.. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci U S A. 2001;98(20):11818–11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huron DB.Sweet anticipation: Music and the psychology of expectation. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- 39.Feldman H, Friston KJ.. Attention, uncertainty, and free-energy. Front Hum Neurosci. 2010;4:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friston KJ, Daunizeau J, Kilner J, Kiebel SJ.. Action and behavior: A free-energy formulation. Biol Cybern. 2010;102(3):227–260. [DOI] [PubMed] [Google Scholar]
- 41.Pearce MT.Statistical learning and probabilistic prediction in music cognition: Mechanisms of stylistic enculturation. Ann N Y Acad Sci. 2018;1423(1):378–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benhamou E, Warren JD.. Chapter 4 – Music and the aging brain. In: Cuddy LL, Belleville S, Moussard A, eds. Disorders of music processing in dementia. London: Academic Press; 2020:107–149. [Google Scholar]
- 43.Omigie D, Pearce M, Lehongre K, et al. Intracranial recordings and computational modeling of music reveal the time course of prediction error signaling in frontal and temporal cortices. J Cogn Neurosci. 2019;31(6):855–873. [DOI] [PubMed] [Google Scholar]
- 44.Cheung VKM, Harrison PMC, Meyer L, Pearce MT, Haynes J-D, Koelsch S.. Uncertainty and surprise jointly predict musical pleasure and amygdala, hippocampus, and auditory cortex activity. Curr Biol. 2019;29(23):4084–4092.e4. [DOI] [PubMed] [Google Scholar]
- 45.Clark CN, Golden HL, McCallion O, et al. Music models aberrant rule decoding and reward valuation in dementia. Soc Cogn Affect Neurosci. 2018;13(2):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golden HL, Clark CN, Nicholas JM, et al. Music perception in dementia. J Alzheimer Dis. 2016;1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golden HL, Downey LE, Fletcher PD, et al. Identification of environmental sounds and melodies in syndromes of anterior temporal lobe degeneration. J Neurol Sci. 2015;352(1-2):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh S, Hornberger M, Piguet O, Hodges JR.. Neural basis of music knowledge: Evidence from the dementias. Brain. 2011;134(Pt 9):2523–2534. [DOI] [PubMed] [Google Scholar]
- 49.Johnson JK, Chang C-C, Brambati SM, et al. Music recognition in frontotemporal lobar degeneration and Alzheimer disease. Cogn Behav Neurol. 2011;24(2):74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hardy CJD, Agustus JL, Marshall CR, et al. Behavioural and neuroanatomical correlates of auditory speech analysis in primary progressive aphasias. [Internet]. Alzheimers Res Ther. 2017;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardy CJD, Agustus JL, Marshall CR, et al. Functional neuroanatomy of speech signal decoding in primary progressive aphasias. Neurobiol Aging. 2017;56:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cope TE, Sohoglu E, Sedley W, et al. Evidence for causal top-down frontal contributions to predictive processes in speech perception. Nat Commun. 2017;8(1):2154- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Omar R, Henley SMD, Bartlett JW, et al. The structural neuroanatomy of music emotion recognition: Evidence from frontotemporal lobar degeneration. Neuroimage. 2011;56(3):1814–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van't Hooft JJ, Pijnenburg YAL, Sikkes SAM, et al. Frontotemporal dementia, music perception and social cognition share neurobiological circuits: A meta-analysis. Brain Cogn. 2021;148:105660. [DOI] [PubMed] [Google Scholar]
- 55.Griffiths TD, Warren JD.. What is an auditory object? Nat Rev Neurosci. 2004;5(11):887–892. [DOI] [PubMed] [Google Scholar]
- 56.Alamia A, VanRullen R, Pasqualotto E, Mouraux A, Zenon A.. Pupil-linked arousal responds to unconscious surprisal. J Neurosci. 2019;39(27):5369–5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bradley MM.Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao H-I, Yoneya M, Kidani S, Kashino M, Furukawa S.. Human pupillary dilation response to deviant auditory stimuli: Effects of stimulus properties and voluntary attention. Front Neurosci. 2016;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang C-A, Munoz DP.. A circuit for pupil orienting responses: Implications for cognitive modulation of pupil size. Curr Opin Neurobiol. 2015;33:134–140. [DOI] [PubMed] [Google Scholar]
- 60.Zénon A.Eye pupil signals information gain. Proc R Soc B Biol Sci. 2019;286(1911):20191593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao S, Chait M, Dick F, Dayan P, Furukawa S, Liao H-I.. Pupil-linked phasic arousal evoked by violation but not emergence of regularity within rapid sound sequences. Nat Commun. 2019;10(1):4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearce MT, Ruiz MH, Kapasi S, Wiggins GA, Bhattacharya J.. Unsupervised statistical learning underpins computational, behavioural, and neural manifestations of musical expectation. Neuroimage. 2010;50(1):302–313. [DOI] [PubMed] [Google Scholar]
- 63.Fletcher PD, Nicholas JM, Shakespeare TJ, et al. Physiological phenotyping of dementias using emotional sounds. Alzheimer’s & Dementia: Diagnosis. Assessment Dis Monitoring. 2015;1(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groussard M, Chan TG, Coppalle R, Platel H.. Preservation of musical memory throughout the progression of Alzheimer’s disease? Toward a reconciliation of theoretical, clinical, and neuroimaging evidence. J Alzheimers Dis. 2019;68(3):857–883. [DOI] [PubMed] [Google Scholar]
- 65.Slattery CF, Agustus JL, Paterson RW, et al. The functional neuroanatomy of musical memory in Alzheimer’s disease. Cortex. 2019;115:357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goll JC, Crutch SJ, Loo JHY, et al. Non-verbal sound processing in the primary progressive aphasias. Brain. 2010;133(Pt 1):272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bianco R, Novembre G, Keller PE, et al. Neural networks for harmonic structure in music perception and action. Neuroimage. 2016;142:454–464. [DOI] [PubMed] [Google Scholar]
- 68.Cheung VKM, Meyer L, Friederici AD, Koelsch S.. The right inferior frontal gyrus processes nested non-local dependencies in music. Sci Rep. 2018;8(1):3822- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koelsch S, Jentschke S, Sammler D, Mietchen D.. Untangling syntactic and sensory processing: An ERP study of music perception. Psychophysiology. 2007;44(3):476–490. [DOI] [PubMed] [Google Scholar]
- 70.Lehne M, Rohrmeier M, Koelsch S.. Tension-related activity in the orbitofrontal cortex and amygdala: An fMRI study with music. Soc Cogn Affect Neurosci. 2014;9(10):1515–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sammler D, Koelsch S, Ball T, et al. Co-localizing linguistic and musical syntax with intracranial EEG. Neuroimage. 2013;64:134–146. [DOI] [PubMed] [Google Scholar]
- 72.Stewart L, Overath T, Warren JD, Foxton JM, Griffiths TD.. fMRI evidence for a cortical hierarchy of pitch pattern processing. PLoS One. 2008;3(1):e1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014;13(6):614–629. [DOI] [PubMed] [Google Scholar]
- 74.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rascovsky K, Grossman M.. Clinical diagnostic criteria and classification controversies in frontotemporal lobar degeneration. Int Rev Psychiatry. 2013;25(2):145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hailstone JC, Omar R, Henley SMD, Frost C, Kenward MG, Warren JD.. It's not what you play, it's how you play it: Timbre affects perception of emotion in music. Quarterly Journal of Experimental Psychology. 2009;62(11):2141–2155. 10.1080/17470210902765957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mathôt S, Fabius J, Van Heusden E, Van der Stigchel S.. Safe and sensible preprocessing and baseline correction of pupil-size data. Behav Res Methods. 2018;50(1):94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maris E, Oostenveld R.. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. [DOI] [PubMed] [Google Scholar]
- 79.Bianco R, Ptasczynski LE, Omigie D.. Pupil responses to pitch deviants reflect predictability of melodic sequences. Brain Cogn. 2020;138:103621. [DOI] [PubMed] [Google Scholar]
- 80.Pearce MT. The construction and evaluation of statistical models of melodic structure in music perception and composition. 2005. https://openaccess.city.ac.uk/id/eprint/8459/. Accessed 3 September 2020.
- 81.Cohen J, Cohen P, West SG, Aiken LS.. Applied multiple regression/correlation analysis for the behavioral sciences. Mahwah: Routledge; 2013. [Google Scholar]
- 82.Ridgway GR, Henley SMD, Rohrer JD, Scahill RI, Warren JD, Fox NC.. Ten simple rules for reporting voxel-based morphometry studies. Neuroimage. 2008;40(4):1429–1435. [DOI] [PubMed] [Google Scholar]
- 83.Tsai C-G, Chou T-L, Li C-W.. Roles of posterior parietal and dorsal premotor cortices in relative pitch processing: Comparing musical intervals to lexical tones. Neuropsychologia. 2018;119:118–127. [DOI] [PubMed] [Google Scholar]
- 84.Zatorre RJ, Halpern AR, Bouffard M.. Mental reversal of imagined melodies: A role for the posterior parietal cortex. J Cogn Neurosci. 2010;22(4):775–789. [DOI] [PubMed] [Google Scholar]
- 85.Agustus JL, Golden HL, Callaghan MF, et al. Melody processing characterizes functional neuroanatomy in the aging brain. Front Neurosci. 2018;12:815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Johnsrude IS, Penhune VB, Zatorre RJ.. Functional specificity in the right human auditory cortex for perceiving pitch direction. Brain. 2000;123 (Pt 1):155–163. [DOI] [PubMed] [Google Scholar]
- 87.Gold BP, Mas-Herrero E, Zeighami Y, Benovoy M, Dagher A, Zatorre RJ.. Musical reward prediction errors engage the nucleus accumbens and motivate learning. Proc Natl Acad Sci U S A. 2019;116(8):3310–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koelsch S.Brain correlates of music-evoked emotions. Nat Rev Neurosci. 2014;15(3):170–180. [DOI] [PubMed] [Google Scholar]
- 89.Zatorre RJ, Salimpoor VN.. From perception to pleasure: Music and its neural substrates. Proc Natl Acad Sci U S A. 2013;110 Suppl 2:10430–10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonnelle V, Ham TE, Leech R, et al. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109(12):4690–4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Freitas C, Manzato E, Burini A, Taylor MJ, Lerch JP, Anagnostou E.. Neural correlates of familiarity in music listening: A systematic review and a neuroimaging meta-analysis. Front Neurosci. 2018;12(686). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lima CF, Krishnan S, Scott SK.. Roles of supplementary motor areas in auditory processing and auditory imagery. Trends Neurosci. 2016;39(8):527–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo S, Koelsch S.. Effects of veridical expectations on syntax processing in music: Event-related potential evidence. Sci Rep. 2016;6:19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Miranda RA, Ullman MT.. Double dissociation between rules and memory in music: An event-related potential study. Neuroimage. 2007;38(2):331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tillmann B, Bigand E.. Musical structure processing after repeated listening: Schematic expectations resist veridical expectations. Mus Sci. 2010;14(2_suppl):33–47 [Google Scholar]
- 96.Southwell R, Baumann A, Gal C, Barascud N, Friston K, Chait M.. Is predictability salient? A study of attentional capture by auditory patterns. Philos Trans R Soc Lond B Biol Sci. 2017;372: 1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Southwell R, Chait M.. Enhanced deviant responses in patterned relative to random sound sequences. Cortex. 2018;109:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clark CN, Warren JD.. Emotional caricatures in frontotemporal dementia. Cortex. 2016;76:134–136. [DOI] [PubMed] [Google Scholar]
- 99.Downey LE, Mahoney CJ, Buckley AH, et al. White matter tract signatures of impaired social cognition in frontotemporal lobar degeneration. Neuroimage Clin. 2015;8:640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koenig P, Smith EE, Grossman M.. Semantic categorisation of novel objects in frontotemporal dementia. Cogn Neuropsychol. 2006;23(4):541–562. [DOI] [PubMed] [Google Scholar]
- 101.Perry DC, Datta S, Sturm VE, et al. Reward deficits in behavioural variant frontotemporal dementia include insensitivity to negative stimuli. Brain. 2017;140(12):3346–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van den Stock J, Stam D, De Winter F-L, et al. Moral processing deficit in behavioral variant frontotemporal dementia is associated with facial emotion recognition and brain changes in default mode and salience network areas. Brain Behav. 2017;7(12):e00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hansen NC, Pearce MT.. Predictive uncertainty in auditory sequence processing. Front Psychol. 2014;5:1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quiroga-Martinez DR, Hansen NC, Højlund A, Pearce MT, Brattico E, Vuust P.. Reduced prediction error responses in high-as compared to low-uncertainty musical contexts. Cortex. 2019;120:181–200. [DOI] [PubMed] [Google Scholar]
- 105.Grube M, Bruffaerts R, Schaeverbeke J, et al. Core auditory processing deficits in primary progressive aphasia. Brain. 2016;139(Pt 6):1817–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hardy CJD, Frost C, Sivasathiaseelan H, et al. Findings of Impaired Hearing in Patients With Nonfluent/Agrammatic Variant Primary Progressive Aphasia [Internet. JAMA Neurol. 2019;76(5):607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rohrer JD, Sauter D, Scott S, Rossor MN, Warren JD.. Receptive prosody in nonfluent primary progressive aphasias. Cortex. 2012;48(3):308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hailstone JC, Ridgway GR, Bartlett JW, Goll JC, Crutch SJ, Warren JD.. Accent processing in dementia. Neuropsychologia. 2012;50(9):2233–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pressman PS, Simpson M, Gola K, et al. Observing conversational laughter in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2017;88(5):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahmed RM, Ke YD, Vucic S, et al. Physiological changes in neurodegeneration — mechanistic insights and clinical utility. Nat Rev Neurol. 2018;14(5):259–271. [DOI] [PubMed] [Google Scholar]
- 111.Femminella GD, Rengo G, Komici K, et al. Autonomic dysfunction in Alzheimer’s disease: Tools for assessment and review of the literature. J Alzheimers Dis. 2014;42(2):369–377. [DOI] [PubMed] [Google Scholar]
- 112.Joshi A, Jimenez E, Mendez MF.. Pavlov’s orienting response in frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2017;29(4):351–356. [DOI] [PubMed] [Google Scholar]
- 113.Joshi A, Mendez MF, Kaiser N, Jimenez E, Mather M, Shapira JS.. Skin conductance levels may reflect emotional blunting in behavioral variant frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2014;26(3):227–232. [DOI] [PubMed] [Google Scholar]
- 114.Marshall CR, Hardy CJD, Russell LL, et al. The functional neuroanatomy of emotion processing in frontotemporal dementias. Brain. 2019;142(9):2873–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marshall CR, Hardy CJD, Russell LL, et al. Motor signatures of emotional reactivity in frontotemporal dementia. Sci Rep. 2018;8(1):1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koelsch S, Vuust P, Friston K.. Predictive processes and the peculiar case of music. Trends Cogn Sci. 2019;23(1):63–77. [DOI] [PubMed] [Google Scholar]
- 117.Salimpoor VN, Zald DH, Zatorre RJ, Dagher A, McIntosh AR.. Predictions and the brain: How musical sounds become rewarding. Trends Cogn Sci. 2015;19(2):86–91. [DOI] [PubMed] [Google Scholar]
- 118.Golden HL, Agustus JL, Goll JC, et al. Functional neuroanatomy of auditory scene analysis in Alzheimer’s disease. Neuroimage Clin. 2015;7:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goll JC, Kim LG, Ridgway GR, Hailstone JC, et al. Impairments of auditory scene analysis in Alzheimer’s disease. Brain. 2012;135(Pt 1):190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Davey J, Thompson HE, Hallam G, et al. Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. Neuroimage. 2016;137:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hickok G, Poeppel D.. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. [DOI] [PubMed] [Google Scholar]
- 122.Kocagoncu E, Clarke A, Devereux BJ, Tyler LK.. Decoding the cortical dynamics of sound-meaning mapping. J Neurosci. 2017;37(5):1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Critchley HD, Mathias CJ, Dolan RJ.. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–545. [DOI] [PubMed] [Google Scholar]
- 124.Sander D, Grafman J, Zalla T.. The human amygdala: An evolved system for relevance detection. Rev Neurosci. 2003;14:303–316. [DOI] [PubMed] [Google Scholar]
- 125.Siman-Tov T, Granot RY, Shany O, Singer N, Hendler T, Gordon CR.. Is there a prediction network? Meta-analytic evidence for a cortical-subcortical network likely subserving prediction. Neurosci Biobehav Rev. 2019;105:262–275. [DOI] [PubMed] [Google Scholar]
- 126.Patterson RD, Uppenkamp S, Johnsrude IS, Griffiths TD.. The processing of temporal pitch and melody information in auditory cortex. Neuron. 2002;36(4):767–776. [DOI] [PubMed] [Google Scholar]
- 127.Koelsch S.Neural substrates of processing syntax and semantics in music. Curr Opin Neurobiol. 2005;15(2):207–212. [DOI] [PubMed] [Google Scholar]
- 128.Lappe C, Steinsträter O, Pantev C.. Rhythmic and melodic deviations in musical sequences recruit different cortical areas for mismatch detection. Front Hum Neurosci. 2013;7:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Janata P.Neural basis of music perception. Handb Clin Neurol. 2015;129:187–205. [DOI] [PubMed] [Google Scholar]
- 130.Golden HL, Agustus JL, Nicholas JM, et al. Functional neuroanatomy of spatial sound processing in Alzheimer’s disease. Neurobiol Aging. 2016;39:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lappe C, Steinsträter O, Pantev C.. A beamformer analysis of MEG data reveals frontal generators of the musically elicited mismatch negativity. PLoS One. 2013;8(4):e61296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Warrier CM, Zatorre RJ.. Right temporal cortex is critical for utilization of melodic contextual cues in a pitch constancy task. Brain. 2004;127(Pt 7):1616–1625. [DOI] [PubMed] [Google Scholar]
- 133.Belyk M, Brown S, Lim J, Kotz SA.. Convergence of semantics and emotional expression within the IFG pars orbitalis. Neuroimage. 2017;156:240–248. [DOI] [PubMed] [Google Scholar]
- 134.Mikutta CA, Dürschmid S, Bean N, et al. Amygdala and orbitofrontal engagement in breach and resolution of expectancy: A case study. Psychomusicol. 2015;25(4):357–365. [Google Scholar]
- 135.Groussard M, Rauchs G, Landeau B, et al. The neural substrates of musical memory revealed by fMRI and two semantic tasks. Neuroimage. 2010;53(4):1301–1309. [DOI] [PubMed] [Google Scholar]
- 136.Herholz SC, Halpern AR, Zatorre RJ.. Neuronal correlates of perception, imagery, and memory for familiar tunes. J Cogn Neurosci. 2012;24(6):1382–1397. [DOI] [PubMed] [Google Scholar]
- 137.Jacobsen J-H, Stelzer J, Fritz TH, Chételat G, La Joie R, Turner R.. Why musical memory can be preserved in advanced Alzheimer’s disease. Brain. 2015;138(Pt 8):2438–2450. [DOI] [PubMed] [Google Scholar]
- 138.Sikka R, Cuddy LL, Johnsrude IS, Vanstone AD.. An fMRI comparison of neural activity associated with recognition of familiar melodies in younger and older adults. Front Neurosci. 2015;9:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Levitin DJ, Menon V.. Musical structure is processed in “language” areas of the brain: A possible role for Brodmann Area 47 in temporal coherence. NeuroImage. 2003;20(4):2142–2152. [DOI] [PubMed] [Google Scholar]
- 140.Gordon CL, Cobb PR, Balasubramaniam R.. Recruitment of the motor system during music listening: An ALE meta-analysis of fMRI data. PLoS One. 2018;13(11):e0207213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sammler D, Grosbras M-H, Anwander A, Bestelmeyer PEG, Belin P.. Dorsal and ventral pathways for prosody. Curr Biol. 2015;25(23):3079–3085. [DOI] [PubMed] [Google Scholar]
- 142.Warren JE, Sauter DA, Eisner F, et al. Positive emotions preferentially engage an auditory–motor “mirror” system. J Neurosci. 2006;26(50):13067–13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herrmann B, Henry MJ, Scharinger M, Obleser J.. Supplementary motor area activations predict individual differences in temporal-change sensitivity and its illusory distortions. Neuroimage. 2014;101:370–379. [DOI] [PubMed] [Google Scholar]
- 144.Jung W-M, Ryu Y, Park H-J, Lee H, Chae Y.. Brain activation during the expectations of sensory experience for cutaneous electrical stimulation. Neuroimage Clin. 2018;19:982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alcalá-López D, Smallwood J, Jefferies E, et al. Computing the social brain connectome across systems and states. Cereb Cortex. 2018;28(7):2207–2232. [DOI] [PubMed] [Google Scholar]
- 146.Adams RA, Huys QJM, Roiser JP.. Computational psychiatry: Towards a mathematically informed understanding of mental illness. J Neurol Neurosurg Psychiatry. 2016;87(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gonzalez-Gadea ML, Chennu S, Bekinschtein TA, et al. Predictive coding in autism spectrum disorder and attention deficit hyperactivity disorder. J Neurophysiol. 2015;114(5):2625–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lawson RP, Rees G, Friston KJ.. An aberrant precision account of autism. Front Hum Neurosci. 2014;8:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We are precluded by institutional ethics agreements from publishing the full dataset in the public domain. However, data and stimuli will be made available on reasonable request to Prof Warren, under appropriate data transfer agreements.