Abstract
Summary
This systematic review and meta-analysis suggests that fracture liaison service (FLS) is associated with a significantly lower probability of subsequent fractures and mortality although the latter was only found in studies comparing outcomes before and after the introduction of an FLS.
Introduction
To systematically review and evaluate the impact of fracture liaison services (FLSs) on subsequent fractures and mortality using meta-analysis.
Methods
A literature search was performed within PubMed and Embase to identify original articles published between January 1, 2010, and April 30, 2020, reporting the effect of FLSs on subsequent fractures and/or mortality. Only studies comparing FLS to no-FLS were included. A meta-analysis using random-effects models was conducted. The quality of studies was appraised after combining and modifying criteria of existing quality assessment tools.
Results
The search retrieved 955 published studies, of which 16 studies fulfilled the inclusion criteria. Twelve studies compared outcomes before (pre-FLS) and after (post-FLS) FLS implementation, two studies compared outcomes between hospitals with and without FLS, and two other studies performed both comparisons. In total, 18 comparisons of FLS and no-FLS care were reported. Follow-up time varied from 6 months to 4 years. Sixteen comparisons reported on subsequent fractures and 12 on mortality. The quality assessment revealed methodological issues in several criteria. Excluding studies with very high selection bias, the meta-analysis of nine comparisons (in eight papers) revealed that the FLS care was associated with a significantly lower probability of subsequent fractures (odds ratio: 0.70, 95% CI: 0.52–0.93, P=0.01). In studies with a follow-up > 2 years, a significantly lower probability of subsequent fractures was captured for FLS care (odds ratio: 0.57, 95% CI: 0.34–0.94, P=0.03), while in studies ≤ 2 years, there was no difference in the odds of subsequent fractures. No significant difference in the odds of mortality was observed (odds ratio: 0.73, 95% CI: 0.49–1.09, P=0.12) in the meta-analysis of eight comparisons (in seven papers). However, a significantly lower probability of mortality was identified in the six pre-post FLS comparisons (odds ratio: 0.65, 95% CI: 0.44–0.95, P=0.03), but not in studies comparing hospitals with and without FLS. No difference was observed in mortality stratified by follow-up time.
Conclusion
This systematic review and meta-analysis suggests that FLS care is associated with a significantly lower probability of subsequent fractures and mortality although the latter was only found in studies comparing outcomes before and after the introduction of an FLS. The quality assessment revealed that some important methodological issues were unmet in the currently available studies. Recommendations to guide researchers to design high-quality studies for evaluation of FLS outcomes in the future were provided.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00198-021-05911-9.
Keywords: Fracture liaison service, Meta-analysis, Mortality, Subsequent fracture
Introduction
Osteoporotic fractures are associated with increased subsequent fracture risk, morbidity, and excess mortality, placing a large medical and economic burden on healthcare systems [1]. Subsequent fracture risk is not constant, but fluctuates over time, and is the highest immediately after initial fractures [2]. One-quarter of all subsequent fractures occur within 1 year after a first fracture, and one in two occur within 5 years [3]. Additionally, the majority of deaths following fractures occur within the first year, thereafter the excess mortality gradually declines [4]. Mortality risk in the first 5 years is increased approximately twofold in women and two- to threefold in men [5]. Of note, the absolute impact on mortality is higher for non-hip non-vertebral (NHNV) fractures, since these account for three-quarters of the number of fractures in the population [6].
Despite the availability of various effective pharmacologic interventions and well-established guidelines for fracture prevention, the majority of patients sustaining a fragility fracture do not receive anti-osteoporosis drugs (AOD) [1]. This treatment gap is more pronounced in men than in women, and worsened in recent years [7]. The magnitude of the treatment gap is reported to be highly variable throughout Europe, ranging between 25 and 95% [8]. An Australian study showed that even less than 20% of postmenopausal women with a fracture received specific treatment for osteoporosis in primary care [9]. The low prescription rate of AOD is attributed to inadequate clinical management, including inadequate communication between physicians, disconnected care between healthcare settings, and knowledge gaps by both patients and physicians [10, 11]. These factors represent missed opportunities to actively manage osteoporosis and the prevention of subsequent fractures [12].
In response to this care gap, the International Osteoporosis Foundation (IOF) launched the Capture the Fracture (CTF) Campaign in 2012 to facilitate the implementation of coordinator-based, multi-disciplinary models of care for secondary fracture prevention. Fracture liaison services (FLSs) are nowadays widely advocated as the most appropriate approach to cover all aspects of secondary fracture prevention, including patient identification, education, risk evaluation, treatment, and long-term monitoring. Until November 2020, more than 550 FLSs (registered in CTF) have been implemented, leading to an increasing number of studies investigating the effectiveness of FLS. A previous review [13] including studies reporting the impact of FLS on subsequent fractures up to 2016 concluded that the observed reduction in subsequent fracture risk after the introduction of a FLS should be further quantified in better-designed studies. Especially the follow-up duration and the comparability of groups with or without FLS care were the main methodological issues. As new studies have been conducted recently, and considering the fact that FLS could also have an impact on mortality, it is worthwhile to update the search, summarize results, and critically appraise studies. This systematic review and meta-analysis was therefore designed to summarize the effectiveness of FLS on subsequent fractures and mortality.
Methods
A systematic literature search was undertaken according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline to identify eligible studies comparing FLS to no-FLS care with subsequent fractures and/or mortality as outcomes [14].
Literature search
The search was conducted in PubMed and Embase (Ovid) and restricted to English articles published between January 1, 2010, and April 30, 2020. The search strategy was designed to retrieve records addressing the following PICO research question: population (patients with a fracture), intervention (FLS care), comparator (no-FLS care), and outcome (subsequent fractures and/or mortality). Details on the complete search strategy based on the PICO criteria are provided in Supplementary 1.
Study selection
After removing duplicates, titles and abstracts were screened by one reviewer (NL). Then, full-text screening was performed for eligible studies by two independent reviewers (NL, RB), and discrepancies were resolved by consensus with the consultation of additional reviewers (MH and JB). Finally, reference lists and citations of included articles were manually screened for additional relevant studies using Web of Science.
Studies were included if they reported the effectiveness of FLS care in terms of subsequent fractures and/or mortality compared to no-FLS care. Therefore, studies comparing the outcomes of FLS to historical data (post-FLS vs. pre-FLS) or studies comparing the outcomes of a hospital with FLS to a hospital without FLS were included. Studies comparing FLS attenders to non-attenders were excluded. Of note, during study selection, alternative names for FLS included fracture prevention service, orthogeriatric service/care or active osteoporosis care, etc. Non-original articles (e.g., editorials, review) and abstracts were excluded.
Data extraction
Study characteristics were extracted including publication characteristics (author, year of publication), study design (e.g., experimental or (type of) semi-experimental design, prospective or retrospective data collection), population characteristics (country, inclusion and exclusion criteria for FLS and no-FLS populations, number of participants in each group, percentage of female participants, follow-up time, attendance proportion of FLS care), and outcomes (cumulative incidence of subsequent fractures and mortality, and corresponding P-value). Initiation of anti-osteoporosis treatment and bone mineral density (BMD) measurement were extracted as secondary outcomes when reported within the selected studies.
Study quality
Currently available quality assessment/risk of bias tools (such as ROBINS-I, Newcastle–Ottawa scale, and NIH tool) [15–17] did not address all potential methodological issues which we pre-identified. Therefore, concepts and items of the available checklists were combined and adjusted forming our quality assessment checklist, which better aligned to our needs. Overall, ten criteria were identified covering the traditional four domains (selection of participants and completeness of follow-up, exposure to post-fracture care, outcome, and statistical accuracy and analyses) for both intervention (FLS) and control (no-FLS) group. Supplementary 2 shows the checklist and indicates the source of the criteria.
Specifically, patients’ selection was considered a key methodological issue in the study of evaluating the outcomes of FLS. All patients with a fracture should be included in the analysis regardless of whether they attended FLS clinic. Failing this principle could result in spurious associations due to large prognostic dissimilarity between groups. Besides, osteoporotic fracture is more prevalent in the geriatric population. In such population, competition between risk of subsequent fracture and risk of death is particularly high, which would hinder or modify the chance that the event of interest (subsequent fractures) occurs.
Each of the final ten criteria was scored using “Yes” (fulfilled the requirement), “No” (not fulfilled the requirement), “Part” (partially fulfilled the requirement), or “Not reported.” To estimate a total quality score, we assigned a score of 1 for “Yes,” 0.5 for “Part,” and 0 for “No.” Two researchers (NL and MO) independently evaluated the eligible studies; discrepancies were resolved by consensus through discussion.
Meta-analysis
A meta-analysis was performed to synthesize the results of included studies. Pooled results of subsequent fractures and mortality between the FLS and the no-FLS group were reported as odds ratio (OR) with associated 95% confidence interval (CI). Of note, in the meta-analysis, crude events data (how many patients had subsequent fracture/mortality) rather than cumulative incidence of subsequent fracture/mortality were entered, and the OR were calculated based on these data. Statistical heterogeneity was assessed using the I2 test. A fixed-effects model was used in case of small heterogeneity (I2<50%), and a random-effects model was applied if the analysis showed to have high heterogeneity (I2≥50%) [18]. In addition, subgroup analysis by study design (pre-post-FLS vs. hospitals with or without FLS care) and by follow-up time (follow-up ≤ 1 year vs. 1 year < follow-up ≤ 2 years vs. follow-up > 2 years) were conducted.
Of note, studies that did not include all patients with a fracture in both FLS and no-FLS cohorts (only inclusion of FLS attenders, or patient selection by consent procedure for both groups) were regarded as very high selection bias and were excluded from the main meta-analysis. However, to investigate the impact of studies with selection bias, these studies (patients’ selection by consent) were additionally included into the model in the sensitivity analysis.
Given the number of studies included in the main meta-analysis for both subsequent fractures and mortality was less than ten, investigation of publication bias through computation of funnel plot is not meaningful.
All statistical analyses were performed in Review Manager (RevMan 5.4; The Nordic Cochrane Centre, Cochrane Collaboration, Copenhagen, Denmark, 2020).
Results
Study selection
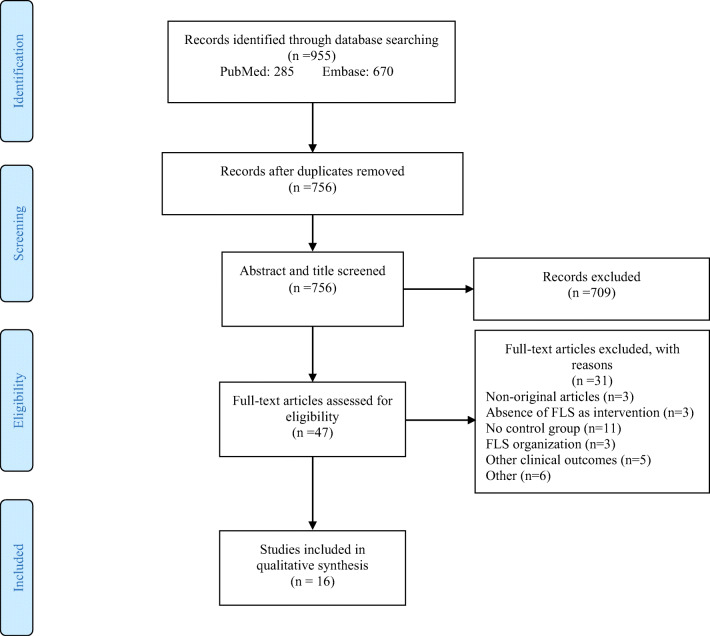
From the initial search, 955 records were retrieved (Fig. 1), of which 199 duplicates were removed. Following screening of titles and abstracts, 709 of the remaining 756 studies were excluded since they did not meet inclusion criteria. Upon review of the full text of the remaining 47 studies, 31 articles were excluded for reasons such as non-original articles (n=3), related to FLS organization (n=3), capturing other clinical outcomes (n=5), no control group (n=11), the intervention was not FLS care (n=3), and other reasons (n=6). In total 16 articles were thus eligible for inclusion.
Fig. 1.
PRISMA flowchart of the study selection process
Characteristics of included studies
The characteristics of the included studies are reported in Table 1. Most studies (n=8) were conducted in Europe (the Netherlands, Sweden, Italy, UK, Ireland, and Spain), followed by Australia (n=3) and Asia (n=3), and the remaining two studies were performed in Canada and the USA. All studies were designed as cohort studies. Data for the FLS cohort were prospectively collected in ten [12, 19–22, 24–26, 28, 30] and retrospectively collected in six studies [1, 23, 27, 29, 31, 32]. The mean and median duration of follow-up for both FLS and no-FLS groups was 1.8 (2) years, varying from 6 months to 4 years. Of note, Inderjeeth et al. [12] presented the outcomes at 3 and 12 months. Considering 3-month follow-up was quite short, we reported the result of 12 months in our study. The sample size of individual studies varied from 47 to 33,152, and all studies included both genders, with 66 to 89% women.
Table 1.
Characteristics of included studies that assessed the effect of fracture liaison service care
| References (year) | Country | Data collection (FLS ) | Follow-up (both groups) | Comparator | Inclusion and exclusion criteria (both group) | Number of participants | Female | Attendance proportion % (FLS) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inclusion | Exclusion | No-FLS | FLS | No-FLS (%) | FLS (%) | ||||||
| Pre-FLS vs. post-FLS | |||||||||||
| Huntjens et al. (2011) [19] | The Netherlands | Prospective | 2 years | Pre-FLS vs. post-FLS | Patients ≥ 50 years presenting with a NVF at ED | Patients with a pathological, a clinical vertebral, or a skull fracture. For FLS group, patients were also excluded if they were selected to the no-FLS group | 1920 | 1335 | 74.6 | 72.5 | 68 |
| Ruggiero et al. (2015) [20] | Italy | Prospective | 1 year | Pre-FPS vs. post-FPS | Patients ≥ 65 years with proximal hip fracture at orthopedic or traumatology department | NR | 172 | 210 | 78.5 | 71.9 | NR |
| Amphansap et al. (2016) [21] | Thailand | Prospective | 1 year | Pre-FLS vs. post-FLS | Patients ≥50 years with hip fracture due to low energy trauma | Patients with a fracture due to high energy trauma, secondary osteoporosis, and bone tumors | 120 | 75 | 73 | 84 | NR |
| Axelsson et al. (2016) [22] | Sweden | Prospective | 344 days | Pre-FLS vs. post-FLS | Patients ≥50 years with a hip, vertebra, shoulder, wrist, or pelvis fracture at the ED or orthopedic department | Patients with pathological fractures or who deceased prior to DXA referral | 2713 | 2616 | 73 | 74 | NR |
| Hawley et al. (2016) [23] | UK | Retrospective | 2 years | Pre- vs. post-FLS (OG) | Patients ≥ 60 years with a primary hip fracture | NR | NR | 33,152 | NR | 75 | NR |
| Bachour et al. (2017) [1] | Lebanon | Retrospective | 2 years | Pre-FLS vs. post-FLS | Patients ≥50 years with a MTF at ED | NR | 100 | 98 | 69 | 80 | 82 |
| Davidson et al. (2017) [24] | Australia | Prospective | 3 years | Pre-FLS vs. post-FLS | Patients ≥45 years with a MTF (femur, tibia and fibula, ankle, pelvis, humerus, and wrist) | Patients with a pathological fracture (vertebral, clavicle, and rib) or if they were deceased | 47 | 93 | 80.9 | 75.3 | NR |
| Henderson et al. (2017) [25] | Ireland | Prospective | 1 year | Pre-OG vs. post-OG | Patients with hip fracture (fractured neck of femur) | NR | 248 | 206 | 66 | 73 | NR |
| Singh et al. (2019) [26] | Canada | Prospective | 6 months | Pre-FLS vs. post-FLS | Patients ≥50 years with a MTF (wrist, humerus, pelvis, hip, or vertebrae) at orthopedic department | Patients with a significant trauma or an underlying disease other than osteoporosis that leads to increased bone fragility, and patients had cognitive dysfunction or insufficient English language skills | 65 | 130 | 85 | 84 | NR |
| Wasfie et al. (2019) [27] | USA | Retrospective | 2 years | Pre-FLS vs. post-FLS | Patients with a vertebral compression fracture with follow-up at the neurosurgery department | NR | 150 | 215 | 69 | 71 | NR |
| González-Quevedo et al. (2020) [28] | Spain | Prospective | 1 year | Pre-FLS vs. post-FLS | Patients ≥60 years with a hip fracture | Patients with pathological fractures | 357 | 367 | 80 | 79 | 86 |
| Shin et al. (2020) [29] | Korea | Retrospective | 4 years | Pre- vs. post-active osteoporosis care | Patients ≥60 years with DRF caused by minor trauma | Patients with high energy trauma, multiple fractures, or injuries caused by motor vehicle accident or fall | 205 | 852 | 80.9 | 85.6 | NR |
| Hospital with FLS vs. hospital without FLS | |||||||||||
| Huntjens et al. (2014) [30] | The Netherlands | Prospective | 2 years | Without FLS vs. with FLS | Patients ≥50 years with a NVF | Patients with pathological or vertebral fractures | 1910 | 1412 | 70 | 73 | 68 |
| Nakayama et al. (2016) [31] | Australia | Retrospective | 3 years | Without FLS vs. with FLS | Patients ≥50 years with MTF at ED | Patients without MTF and patients diagnosed as having a fracture but their imaging reported no fracture | 416 | 515 | 73.6 | 75.3 | 20 |
| Pre-FLS vs. post-FLS and hospital with FLS vs. hospital without FLS | |||||||||||
| (a) Inderjeeth et al. (2018) [12] | Australia | Prospective | 3 months and 12 months | Pre-FLS vs. post-FLS | Patients ≥50 years with MTF at ED | Patients without MTF but with fractures of the hands, feet or skull, and patients in high-level residential aged care facilities | 105 | 241 | 72 | 82 | 69 |
| (b) Inderjeeth et al. (2018) [12] | Australia | Prospective | 3 months and 12 months | Without FLS vs. with FLS | Patients ≥50 years with MTF at ED | Patients without MTF but with fractures of the hands, feet or skull, and patients in high-level residential aged care facilities | 55 | 241 | 89 | 82 | 69 |
| (a) Axelsson et al. (2020) [32] | Sweden | Retrospective | 2.2 years | Pre-FLS vs. post-FLS | Patients ≥50 years with a major osteoporotic fracture | Patients with malignancies and obvious high-energy fractures | 4828 | 10,621 | 76 | 77 | NR |
| (b) Axelsson et al. (2020) [32] | Sweden | Retrospective | 2.2 years | Without FLS vs. with FLS | Patients ≥50 years with a major osteoporotic fracture | Patients with malignancies and obvious high-energy fractures | 5634 | 15,449 | 76 | 76 | NR |
BMD bone mineral density, FLS fracture liaison service, NVF non-vertebral fracture, FPS fracture prevention service, ED emergency department, MTF minimal trauma fracture, DRF distal radius fracture, OG orthogeriatric service, DXA dual-energy X-ray absorptiometry, NR not reported, vs. versus
Twelve studies [1, 19–29] compared the outcomes of FLS to historical data (post-FLS vs. pre-FLS). Two studies [30, 31] compared the outcomes of the FLS with data from a hospital without FLS, and two other studies [12, 32] performed both comparisons (pre-FLS vs. post-FLS, hospital with FLS vs. hospital without FLS).
When stratified by FLS outcome, 14 studies (16 comparisons) [1, 12, 19, 21–24, 26–32] reported subsequent fractures, and eleven studies (12 comparisons) [1, 19–22, 24–26, 28, 30, 32] reported mortality. Interestingly, Hawley et al. [23] reported the results from a post-hip care model in 11 hospitals, where each hospital was analyzed separately and acted as its own control in a before-and-after time series design. However, given specific data for both FLS and no-FLS cohorts were not available, this study was therefore excluded from the meta-analysis. In addition, within selected studies, eight studies [1, 12, 20–22, 24, 26, 29] reported BMD measurement, and nine studies [1, 12, 20–22, 24, 28, 29, 32] reported initiation of anti-osteoporosis treatment as secondary outcome.
When stratified by type of secondary fracture prevention care, 13 studies reported the outcomes of a typical FLS clinic. In these studies, case finding was conducted by an FLS coordinator such as a fracture nurse, secretaries at the emergency department (ED), or a physician champion, followed by BMD assessment, patients’ education, and treatment initiation. The remaining three studies provided care to patients with fractures in the context of orthogeriatric care/service (OG), fracture prevention service (FPS), and active osteoporosis care, which resemble the model of FLS care and were regarded as FLS care [20, 25, 29].
The proportion of patients who attend the FLS defined as the number of patients actually attending the FLS divided by the total number of patients eligible or invited for the FLS (and thus assuming all patients with fractures are invited), which were available in six studies [1, 12, 19, 28, 30, 31] varying from 20 [31] to 86% [28]. The other ten studies did not report the proportion of FLS attenders.
Quality assessment and recommendations
Table 4 presents the results of quality assessment of the included studies. The average score was 5.4 out of 10 (range 3–8.5). Only 50% of studies fulfilled more than half of the criteria, and room for improvement was thus identified for most studies. For patients’ selection, most studies (n=11) made the comparison between all patients in both FLS and no-FLS groups. However, five studies [1, 12, 21, 24, 26] did not include all patients with fractures in the FLS or no-FLS cohort and were regarded with very high selection bias. Specifically, one study [21] compared FLS attenders to all patients with fractures in the no-FLS cohort, and four other studies [1, 12, 24, 26] only included and compared consenting subjects in both FLS and no-FLS groups. In addition, the quality was especially suboptimal for other criteria including “analyses of outcomes account for competing risk of death,” “sample size is described based on power calculation,” “loss to follow-up rate ≤20% in FLS/no-FLS group,” and “at least 50% eligible patients attend FLS.” Recommendations for each criterion were formulated given that they are the most important methodological issues for studies evaluating the outcomes of FLS (Table 4). Except for criteria mentioned in Table 4, the length of follow-up duration was also crucial to capture the effect of FLS care on subsequent fracture and mortality, and future studies should consider a longer duration of follow-up (at least 2 years).
Table 4.
Quality of included studies assessed using self-designed tool
| Quality criteria | Reference | Author’s recommendations | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [19] | [20] | [21] | [22] | [23] | [1] | [24] | [25] | [26] | [27] | [28] | [29] | [30] | [31] | [12] | [32] | |||
| Selection and completeness of follow-up | Patient baseline characteristics with no/minor significant differences between FLS and no-FLS group | No | Yes | Yes | Yes | No | Yes | Yes | NR | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Participants in two groups should be carefully selected with no/minor significant differences in characteristics to avoid selection bias |
| All patients were included and analyzed in both FLS and no-FLS cohorts | Yes | Yes | No | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | All patients should be included and analyzed regardless of whether they were seen in the FLS clinic | |
| Inclusion/exclusion criteria are clearly described for FLS and no-FLS group | Yes | Part | Part | Part | Part | Part | Yes | Part | Yes | Part | Yes | Yes | Yes | Yes | Yes | Yes | Inclusion/exclusion criteria should be clearly described for completeness of reporting reason | |
| At least 50% eligible patients attend FLS | Yes | NR | NR | NR | NR | Yes | NR | NR | NR | NR | Yes | NR | Yes | No | Yes | NR | The proportion of FLS attending is expected to be at least 50% to provide confidence of the results | |
| Loss to follow-up ≤20% in FLS and no-FLS group | Yes | Yes | Part | NR | NR | NR | NR | NR | Part | NR | Yes | NR | NR | NR | Yes | NR | The loss of follow-up for both groups is expected to be less than 20% to guarantee statistical power for the results | |
| Exposure | Clear description of care for FLS and no-FLS group | Yes | Part | Part | Yes | No | Part | No | Part | Part | Part | Yes | Part | Part | Part | Part | Part | Fracture care including BMD testing, treatment, education, long-term adherence, etc. should be clearly described for both groups |
| Outcome | Outcomes assessed in FLS and no-FLS groups using similar method | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | The same statistical methods should be used in both groups to assess the outcomes |
| Statistical accuracy and analyses | Analyses of outcomes accounted for relevant confounders | Yes | No | No | Yes | Yes | No | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | Yes | Relevant confounders should be fully adjusted using statistical models, such as multivariable cox regression model |
| Sample size is based on power calculation | No | No | No | Yes | No | No | No | No | Yes | No | No | No | No | No | Yes | No | To avoid insufficient statistical power for the results, sample size should be based on power calculation | |
| Analyses of outcomes account for competing risk of death | No | No | No | No | Yes | No | Yes | No | No | No | No | No | No | Yes | No | Yes | Competing risk analysis should be included in studies designed to evaluate risk of subsequent fracture | |
| Total score | 7 | 5 | 3.5 | 6.5 | 4.5 | 4 | 5 | 3 | 6 | 4 | 8 | 4.5 | 5.5 | 5.5 | 8.5 | 6.5 | ||
Yes, fully fulfilled the criteria; No, not fulfilled the criteria; Part, partially fulfilled the criteria
NR not reported, BMD bone mineral density, FLS fracture liaison service
Subsequent fracture
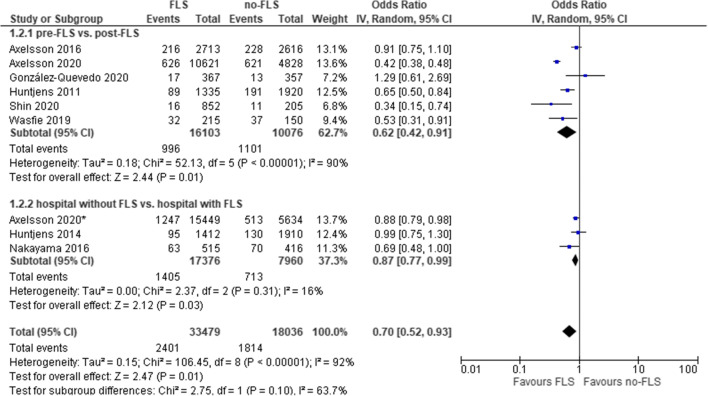
As shown in Table 2, 10 out of 16 comparisons reported that the reduction of subsequent fractures in the FLS group was significant. Excluding five studies with very high selection bias, the mean cumulative incidence of subsequent fractures was 7.7% (SD 3.9%) and 10.9% (SD 6.5%) (median 6.7% and 9.1%) in the FLS versus no-FLS group. Of note, since Wasfie et al. [27] included patients with vertebral fractures (VFs) that were treated with vertebral augmentation, we did not use the data of VFs and only reported the data of other fractures (hip, ribs, and extremities) in our study. The result of meta-analysis on subsequent fractures of nine comparisons (eight studies) is presented in Fig. 2. Overall, FLS care was associated with a significantly lower probability of subsequent fractures (OR: 0.70, 95% CI: 0.52–0.93, P=0.01; heterogeneity: I2=92%).
Table 2.
Results from cohort studies reporting cumulative incidence of subsequent fracture
| Comparison | Cumulative incidence of subsequent fracture | P-value | |
|---|---|---|---|
| No-FLS | FLS | ||
| Pre-FLS vs. post-FLS | |||
| Huntjens et al. [19] | 9.9% | 6.7% | P=0.001* |
| Amphansap et al. [21] | 30.0% | 0.0% | P<0.0001* |
| Axelsson et al. [22] | 8.4% | 8.3% | P=0.85 |
| Hawley et al. [23] | NA | 4.2% | NA |
| Bachour et al. [1] | 18.0% | 8.2% | P=0.004* |
| Davidson et al. [24] | 19.1% | 10.5% | P=0.013* |
| Singh et al. [26] | 1.8% | 3.0% | P=0.667 |
| Wasfie et al. [27] | 25.0% | 15.0% | P=0.01* |
| González-Quevedo et al. [28] | 3.6% | 4.6% | P=0.50 |
| Shin et al. [29] | 5.4% | 1.9% | P=0.004* |
| Hospital with FLS vs. hospital without FLS | |||
| Huntjens et al. [30] | 6.8% | 6.7% | Time-dependent** |
| Nakayama et al. [31] | 16.8% | 12.2% | NR |
| Pre-FLS vs. post-FLS and hospital with FLS vs. hospital without FLS | |||
| (a) Inderjeeth et al. [12] | 18.3% | 8.1% | P<0.05* |
| (b) Inderjeeth et al. [12] | 17.3% | 8.1% | NS |
| (a) Axelsson et al. [32] | 12.9% | 5.9% | P<0.001* |
| (b) Axelsson et al. [32] | 9.0%# | 8.0%# | NR |
NA not applicable, NR not reported, NS not significant, FLS fracture liaison service, vs. versus
*Statistical significant P<0.05
**Significantly lower subsequent fracture from fifteen months onward
(a) Study compared pre-FLS to post-FLS care
(b) Study compared hospitals with and without FLS
#Calculated based on available data
Fig. 2.
FLS versus no-FLS for subsequent fracture: overall and subgroup analysis by study design. CI, confidence interval; IV, inverse variance; FLS, fracture liaison service. Asterisk indicates comparison between hospitals with and without FLS
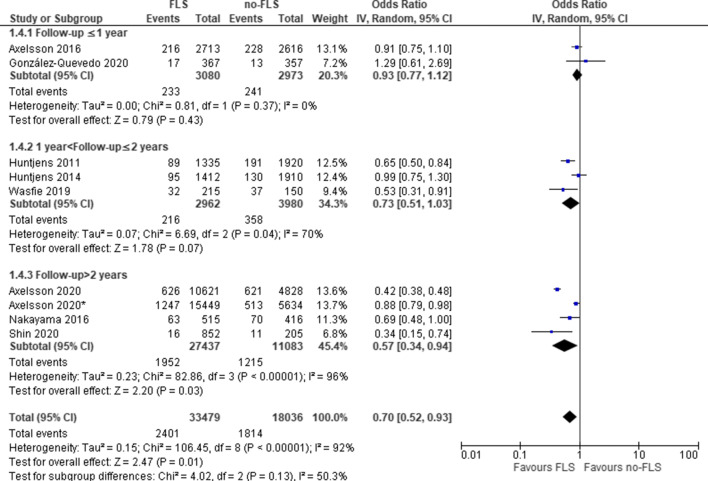
The first subgroup analysis by study design (Fig. 2) revealed that the OR of subsequent fractures in post versus pre-FLS group was 0.62 (95% CI: 0.42–0.91, P=0.01; heterogeneity: I2=90%) and the OR for hospitals with versus without FLS care was 0.87 (95% CI: 0.77–0.99, P=0.03; heterogeneity: I2=16%), both indicating a significant lower probability of subsequent fractures with FLS. The second subgroup analysis by follow-up duration (Fig. 3) revealed that in studies with a follow-up > 2 years, a significantly lower probability of subsequent fractures was captured for FLS care (odds ratio: 0.57, 95% CI: 0.34–0.94, P=0.03), while in studies ≤ 2 years, there was no difference in the odds of subsequent fractures.
Fig. 3.
FLS versus no-FLS for subsequent fracture: subgroup analysis by follow-up duration. CI, confidence interval; IV, inverse variance; FLS, fracture liaison service. Asterisk indicates comparison between hospitals with and without FLS
Sensitivity analyses (Supplementary 3, Figure 1) including studies with very high selection bias also indicated that the FLS care was associated with a lower probability of subsequent fractures (OR: 0.70, 95% CI: 0.54–0.91, P=0.007). Subgroup analyses by study design remained overall similar.
Mortality
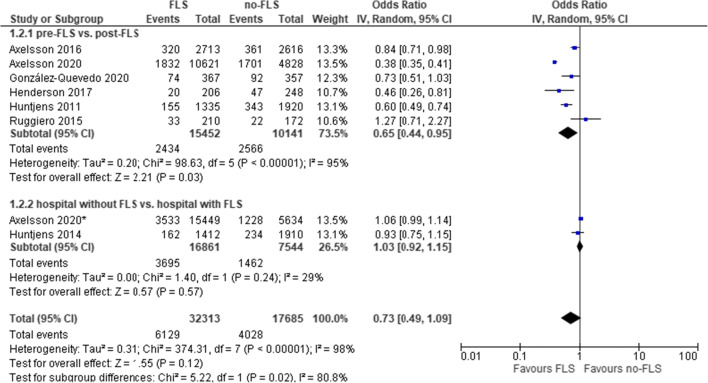
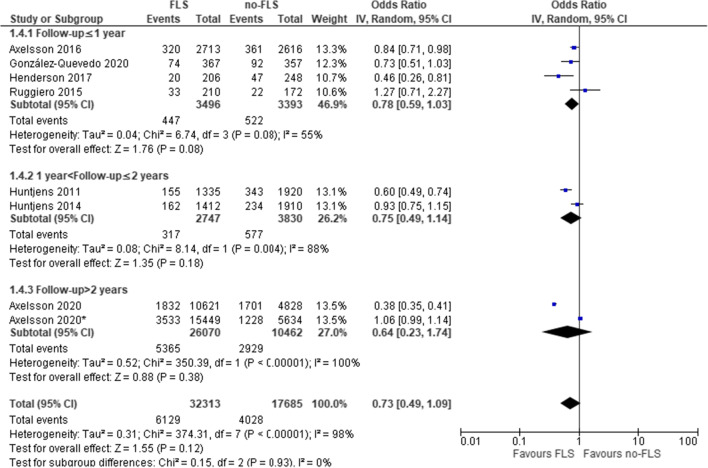
As shown in Tables 3,4 out of 12 comparisons indicated a significantly lower cumulative mortality incidence in the FLS group. Excluding five studies with very high selection bias, the mean cumulative incidence of mortality was 15.1% (SD 4.7%) and 22.8% (SD 7.8%) (median 13.8% and 18.4%) in the FLS versus no-FLS group. The result of meta-analysis on mortality of eight comparisons (seven studies) is presented in Fig. 4. Overall, FLS care was not significantly associated with lower mortality (OR: 0.73, 95% CI: 0.49–1.09, P=0.12; heterogeneity: I2=98%).
Table 3.
Results from cohort studies reporting cumulative incidence of mortality
| Comparison | Cumulative incidence of mortality | P-value | |
|---|---|---|---|
| No-FLS | FLS | ||
| Pre-FLS vs. post-FLS | |||
| Huntjens et al. [19] | 17.9% | 11.6% | P<0.001* |
| Ruggiero et al. [20] | 12.7% | 15.7% | P=0.50 |
| Amphansap et al. [21] | 9.2% | 10.7% | P=0.731 |
| Axelsson et al. [22] | 13.3% | 12.2% | P=0.24 |
| Hawley et al. [23] | NA | 29.8% | NA |
| Bachour et al. [1] | 16.0% | 16.3% | P=0.95 |
| Davidson et al. [24] | 12.2% | 20.6% | P=0.035* |
| Henderson et al. [25] | 19.0% | 9.7% | P<0.001* |
| González-Quevedo et al. [28] | 25.8% | 20.2% | P=0.07 |
| Hospital with FLS vs. hospital without FLS | |||
| Huntjens et al. [30] | 12.3% | 11.5% | P<0.05* |
| Pre-FLS vs. post-FLS and hospital with FLS vs. hospital without FLS | |||
| (a) Axelsson et al. [32] | 35.2% | 17.2% | P=0.11 |
| (b) Axelsson et al. [32] | 21.8%# | 22.9%# | NR |
NA not applicable, NR not reported, FLS fracture liaison service, vs. versus
*Statistical significant P<0.05
(a) Study compared pre-FLS to post-FLS care
(b) Study compared hospitals with and without FLS
#Calculated based on available data
Fig. 4.
FLS versus no-FLS for mortality: overall and subgroup analysis by study design. CI, confidence interval; IV, inverse variance; FLS, fracture liaison service. Asterisk indicates comparison between hospitals with and without FLS
The first subgroup analysis by study design (Fig. 4) revealed a lower probability of mortality in the pre- versus post-FLS studies (OR: 0.65, 95% CI: 0.44–0.95, P=0.03; heterogeneity: I2=95%) but not for studies that compared two different hospitals (OR: 1.03, 95% CI: 0.92–1.15, P=0.57; heterogeneity: I2=29%). In the second subgroup analysis by follow-up duration (Fig. 5), we found no significant influence by duration of follow-up.
Fig. 5.
FLS versus no-FLS for mortality: subgroup analysis by follow-up duration. CI, confidence interval; IV, inverse variance; FLS, fracture liaison service. Asterisk indicates comparison between hospitals with and without FL
Sensitivity analyses (Supplementary 3, Figure 2) including studies with very high selection bias also indicated that the FLS care was not associated with a lower probability of mortality (OR: 0.81, 95% CI: 0.56–1.17, P=0.27). Subgroup analyses showed that the reduced probability of mortality in pre-post studies was not significant (OR: 0.76, 95% CI: 0.52–1.10, P=0.15).
Secondary outcomes
Within selected studies, nine studies (11 comparisons) [1, 12, 20–22, 24, 28, 29, 32] reported the initiation of anti-osteoporosis treatment, and 9 out of 11 comparisons showed a significantly higher treatment proportion in post-FLS group. In addition, of the eight studies (9 comparisons) reported BMD measurement [1, 12, 20–22, 26, 29], and 8 out of 9 comparisons indicated that FLS was associated with a significant increase of BMD measurement proportion (Supplementary 4).
Discussion
This systematic review and meta-analysis was performed to evaluate and summarize the evidence regarding the effectiveness of the FLS on subsequent fractures and mortality. The pooled overall results indicated that FLS care is associated with a significantly lower probability of subsequent fractures (30%) and mortality although the latter was only found in studies comparing outcomes before and after the introduction of an FLS. Overall, the effects of FLS care on both outcomes were larger in studies with a pre-post design compared to studies addressing hospitals with and without an FLS. Since only two studies were available for the analysis of mortality in hospitals with or without FLS, this may be insufficient to capture a significant impact. It is difficult to conclude that these study designs provide the most valid estimates. Each study design has some potential limitations. For the pre-post study design, changes in patients’ lifestyles or the effectiveness of healthcare could happen over time. For (two) hospitals’ study design, bias could result from differences in content of care and patients groups regarding lifestyle, comorbidities, or other confounders. Of note, high heterogeneity was revealed, especially for pre-post comparisons, even when the random-effects model and subgroup analysis were applied, which may limit the reliability of the analysis and could be recognized as a limitation.
Subgroup analysis by follow-up duration revealed that studies with relatively longer follow-up duration (more than 2 years) were associated with significantly lower probability of subsequent fractures; however, it was not the case for mortality. The potential reason could be that the impact of the FLS intervention on mortality may require a longer follow-up time to capture, while the studies included in the meta-analysis for mortality had a relatively short follow-up time (the longest was 2.2 years). Therefore, future studies should consider a follow-up duration of at least 2 years to adequately capture the effect of FLS care on subsequent fractures and mortality.
For quality appraisal, several methodology issues were identified among the included studies. Firstly, given it was difficult to design randomized controlled trials (RCTs) to evaluate the outcomes of FLS, some patients’ characteristics could be considered potential confounders and available for adjustment through statistical methods (e.g., the multivariable cox regression model). However, due to the retrospective nature of some studies, several potential variables such as family fracture history, smoking/alcohol consumption, and physical activity that might impact the results were unable to be taken into account. Besides, avoiding selection bias during patients’ enrollment is crucial to guarantee the comparability of two cohorts. As indicated by Huntjens et al. [19, 30], patients who were unable or not willing to visit the FLS should be included in the FLS group and in all analyses although the level of health is not known in non-attenders and the effect of FLS care can only be achieved in the attenders. Sensitivity analysis additionally included studies with very high selection bias suggesting that these studies had no impact on overall results of meta-analysis; however, the impact on subgroups (by study design) was revealed. Future studies should avoid selection bias in the process of designing a study. Moreover, we recommend that some other criteria including “sample size is based on power calculation,” “loss to follow-up ≤20%,” and “at least 50% eligible patients attend the FLS” should be taken into account in future studies to provide sufficient statistical power.
Furthermore, when analyzing subsequent fracture risk, competing mortality risk may be an important methodological issue, which may particularly be the case in the geriatric population. Ignoring the competing risk of subsequent fractures and mortality could bias the results of studies on FLS care. Berry et al. [33] performed a simulation study comparing standard survival analysis versus a competing risk approach in a study of second hip fracture, indicating that standard survival analysis overestimated the 5-year risk of second hip fracture by 37% and the 10-year risk by 75% compared with competing risk estimates. Out of the 16 included studies, four reported a competing risk survival regression analysis [23, 24, 31, 32] (Supplementary 5). Three studies [23, 31, 32] used the method of Fine and Gray [34], which deals with the competing risk of mortality by retaining participants in the risk set with a diminishing weight when they die, rather than simply censoring them at the time of death [31]. Similar results were identified in three studies before and after accounting for competing risk of mortality, which allowed to evaluate (partly) the effect of competing risk (of mortality) on subsequent fractures. However, considering especially major fractures are associated with excess mortality [4], competing risk analyses should be taken into account in future studies to accurately estimate cumulative incidence of subsequent fracture.
The findings of this systematic review and meta-analysis is partially consistent with the study of Wu et al. [35], which included studies up to February 2017 suggesting that FLS programs improved outcomes of osteoporosis-related fractures, with significant increases in BMD testing, treatment initiation, and adherence to treatment and reductions in re-fracture incidence. Given more outcomes of interest were investigated and a wider search strategy was applied, more studies (n=159, including studies before CTF) were included in this previous study. By contrast, our study had a specific focus on effectiveness defined as subsequent fractures and mortality, and restricted inclusion of studies comparing FLS to no-FLS. Further, more precise meta-analyses (exclude studies with selection bias) were conducted. Besides, subgroup and sensitivity analysis could also add value to our review. Compared to other previous reviews [13, 36], our study provides a quality assessment, recommendations for patients’ selection, outcome measurement, and statistical analysis provided for future studies, which would guide researchers to design high-quality studies and further help to reduce inter-study heterogeneity, thereby facilitating inter-study comparisons.
This systematic review and meta-analysis has certain limitations. First, we did not conduct a systematic literature search for additional outcomes (initiation of anti-osteoporosis treatment and BMD measurement) since they were not the outcomes of interest in this review. The results of secondary outcomes should thus be interpreted with caution. Second, the quality assessment tool used in our study was generated through combining and modifying available quality assessment tools to fit several methodological issues, and each criterion was treated equally in scoring, the inter-validity of this tool was not verified.
Conclusion
This systematic review and meta-analysis suggests that FLS care is associated with a significantly lower probability of subsequent fractures and mortality although the latter was only found in studies comparing outcomes before and after the introduction of an FLS. The quality assessment revealed that some important methodological issues were unmet in the currently available studies. We therefore provided recommendations to guide researchers to design high-quality studies for evaluation of FLS outcomes in the future.
Supplementary information
(DOCX 78 kb)
Acknowledgements
Nannan Li is funded by the China Scholarship Council (grant number 201909110080).
Availability of data and material
All data analyzed as part of this study are included in this published article (and its supplementary information files).
Declarations
Conflict of interest
Mickaël Hiligsmann has received research grants through institution from Amgen, Radius Health, and Theramex; Joop P. van den Bergh has received research funding from Amgen, Eli Lilly, and UCB; Annelies Boonen, Robin de Bot, Sandrine P.G. Bours, Caroline E. Wyers, and Marsha M. van Oostwaard declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bachour F, Rizkallah M, Sebaaly A, Barakat A, Razzouk H, el Hage R, Nasr R, el Khoury M, Maalouf G. Fracture liaison service: report on the first successful experience from the Middle East. Arch Osteoporos. 2017;12(1):4–9. doi: 10.1007/s11657-017-0372-x. [DOI] [PubMed] [Google Scholar]
- 2.Van Geel TACM, Huntjens KMB, Van Den Bergh JPW, Dinant GJ, Geusens PP. Timing of subsequent fractures after an initial fracture. Curr Osteoporos Rep. 2010;8(3):118–122. doi: 10.1007/s11914-010-0023-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Geel TACM, Van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis. 2009;68(1):99–102. doi: 10.1136/ard.2008.092775. [DOI] [PubMed] [Google Scholar]
- 4.Tran T, Bliuc D, Hansen L, Abrahamsen B, van den Bergh J, Eisman JA, van Geel T, Geusens P, Vestergaard P, Nguyen TV, Center JR. Persistence of excess mortality following individual nonhip fractures: a relative survival analysis. J Clin Endocrinol Metab. 2018;103(9):3205–3214. doi: 10.1210/jc.2017-02656. [DOI] [PubMed] [Google Scholar]
- 5.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. The Lancet 353(9156):878–82. 10.1016/s0140-6736(98)09075-8 [DOI] [PubMed]
- 6.Tran T, Bliuc D, van Geel T, Adachi JD, Berger C, van den Bergh J, Eisman JA, Geusens P, Goltzman D, Hanley DA, Josse RG (2017) Population‐wide impact of non‐hip non‐vertebral fractures on mortality. J Bone Min Res 32(9):1802–10. 10.1002/jbmr.3118 [DOI] [PubMed]
- 7.Skjødt MK, Khalid S, Ernst M, Rubin KH, Martinez-Laguna D, Delmestri A, Javaid MK, Cooper C, Libanati C, Toth E, Abrahamsen B, Prieto-Alhambra D. Secular trends in the initiation of therapy in secondary fracture prevention in Europe: a multi-national cohort study including data from Denmark, Catalonia, and the United Kingdom. Osteoporos Int. 2020;31(8):1535–1544. doi: 10.1007/s00198-020-05358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon DH, Johnston SS, Boytsov NN, McMorrow D, Lane JM, Krohn KD. Osteoporosis medication use after hip fracture in U.S. patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929–1937. doi: 10.1002/jbmr.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisman J, Clapham S, Kehoe L. Osteoporosis prevalence and levels of treatment in primary care: the Australian bonecare study. J Bone Miner Res. 2004;19(12):1969–1975. doi: 10.1359/JBMR.040905. [DOI] [PubMed] [Google Scholar]
- 10.Geusens P, Bours SPG, Wyers CE, van den Bergh JP. Fracture liaison programs. Best Pract Res Clin Rheumatol. 2019;33(2):278–289. doi: 10.1016/j.berh.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Inderjeeth CA, Glennon D, Petta A. Study of osteoporosis awareness, investigation and treatment of patients discharged from a tertiary public teaching hospital. Intern Med J. 2006;36(9):547–551. doi: 10.1111/j.1445-5994.2006.01146.x. [DOI] [PubMed] [Google Scholar]
- 12.Inderjeeth CA, Raymond WD, Briggs AM, Geelhoed E, Oldham D, Mountain D. Implementation of the Western Australian Osteoporosis Model of Care: a fracture liaison service utilising emergency department information systems to identify patients with fragility fracture to improve current practice and reduce re-fracture rates: a 12. Osteoporos Int. 2018;29(8):1759–1770. doi: 10.1007/s00198-018-4526-5. [DOI] [PubMed] [Google Scholar]
- 13.Soiza RL, Donaldson AIC, Myint PK. Fracture liaison services: do they reduce fracture rates? Ther Adv Vaccines. 2018;9(6):259–261. doi: 10.1177/https. [DOI] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7). 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed]
- 15.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:1–7. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos PTM. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 17.National Institutes of Health. Quality assessment tool for observational cohort and cross-sectional studies. Published 2014. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 18.Grant J, Hunter A. Measuring inconsistency in knowledgebases. J Intell Inf Syst. 2006;27(2):159–184. doi: 10.1007/s10844-006-2974-4. [DOI] [Google Scholar]
- 19.Huntjens KMB, Van Geel TCM, Geusens PP, et al. Impact of guideline implementation by a fracture nurse on subsequent fractures and mortality in patients presenting with non-vertebral fractures. Injury. 2011;42(SUPPL. 4):S39–S43. doi: 10.1016/S0020-1383(11)70011-0. [DOI] [PubMed] [Google Scholar]
- 20.Ruggiero C, Zampi E, Rinonapoli G, et al. Fracture prevention service to bridge the osteoporosis care gap. Clin Interv Aging. 2015;10:1035–1042. doi: 10.2147/CIA.S76695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amphansap T, Stitkitti N, Dumrongwanich P. Evaluation of Police General Hospital’s fracture liaison service (PGH’s FLS): the first study of a fracture liaison service in Thailand. Osteoporos Sarcopenia. 2016;2(4):238–243. doi: 10.1016/j.afos.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Axelsson KF, Jacobsson R, Lund D, Lorentzon M. Effectiveness of a minimal resource fracture liaison service. Osteoporos Int. 2016;27(11):3165–3175. doi: 10.1007/s00198-016-3643-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawley S, Kassim Javaid M, Prieto-Alhambra D, et al. Clinical effectiveness of orthogeriatric and fracture liaison service models of care for hip fracture patients: population-based longitudinal study. Age Ageing. 2016;45(2):236–242. doi: 10.1093/ageing/afv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson E, Seal A, Doyle Z, Fielding K, McGirr J. Prevention of osteoporotic refractures in regional Australia. Aust J Rural Health. 2017;25(6):362–368. doi: 10.1111/ajr.12355. [DOI] [PubMed] [Google Scholar]
- 25.Henderson CY, Shanahan E, Butler A, Lenehan B, O’Connor M, Lyons D, Ryan JP. Dedicated orthogeriatric service reduces hip fracture mortality. Ir J Med Sci. 2017;186(1):179–184. doi: 10.1007/s11845-016-1453-3. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Whitehurst DG, Funnell L et al (2019) Breaking the cycle of recurrent fracture: implementing the first fracture liaison service (FLS) in British Columbia, Canada. Arch Osteoporos 14(1). 10.1007/s11657-019-0662-6 [DOI] [PubMed]
- 27.Wasfie T, Jackson A, Brock C, Galovska S, McCullough JR, Burgess JA. Does a fracture liaison service program minimize recurrent fragility fractures in the elderly with osteoporotic vertebral compression fractures? Am J Surg. 2019;217(3):557–560. doi: 10.1016/j.amjsurg.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 28.González-Quevedo D, Bautista-Enrique D, Pérez-del-Río V, Bravo-Bardají M, García-de-Quevedo D, Tamimi I. Fracture liaison service and mortality in elderly hip fracture patients: a prospective cohort study. Osteoporos Int. 2020;31(1):77–84. doi: 10.1007/s00198-019-05153-w. [DOI] [PubMed] [Google Scholar]
- 29.Shin YH, Hong WK, Kim J, Gong HS. Osteoporosis care after distal radius fracture reduces subsequent hip or spine fractures: a 4-year longitudinal study. Osteoporos Int. 2020;31(8):1471–1476. doi: 10.1007/s00198-020-05410-3. [DOI] [PubMed] [Google Scholar]
- 30.Huntjens KMB, Van Geel TACM, Van Den Bergh JPW, et al. Fracture liaison service: impact on subsequent nonvertebral fracture incidence and mortality. J Bone Jointt Surg Ser A. 2014;96(4):1–8. doi: 10.2106/JBJS.L.00223. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama A, Major G, Holliday E, Attia J, Bogduk N. Evidence of effectiveness of a fracture liaison service to reduce the re-fracture rate. Osteoporos Int. 2016;27(3):873–879. doi: 10.1007/s00198-015-3443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Axelsson KF, Johansson H, Lundh D, Möller M, Lorentzon M. Association between recurrent fracture risk and implementation of fracture liaison services in four Swedish hospitals: a cohort study. J Bone Miner Res. 2020;35(7):1216–1223. doi: 10.1002/jbmr.3990. [DOI] [PubMed] [Google Scholar]
- 33.Stark S, Landsbaum A, Palmer JL, Somerville EK, Morris JC (2010) Competing risk of death: an important consideration in studies of older adults. 58(4):235–246. 10.1111/j.1532-5415.2010.02767.x
- 34.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 35.Wu CH, Te Tu S, Chang YF, et al. Fracture liaison services improve outcomes of patients with osteoporosis-related fractures: a systematic literature review and meta-analysis. Bone. 2018;111(138):92–100. doi: 10.1016/j.bone.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Briot K. Fracture liaison services. Curr Opin Rheumatol. 2017;29(4):416–421. doi: 10.1097/BOR.0000000000000401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 78 kb)
Data Availability Statement
All data analyzed as part of this study are included in this published article (and its supplementary information files).