Abstract
Patients with extracranial tumors like lung, breast and skin cancers often develop brain metastases (BM) during the course of their diseases, and BM commonly represent the terminal stage of cancer progression. Recent insights in the immune biology of BM and the increasing focus of immunotherapy as a therapeutic option for cancer has prompted testing of promising biological immunotherapies, including immune cell-targeting, virotherapy, vaccines and different cell-based therapies. Here we review the pathobiology of BM progression and evaluate the potential of next generation immunotherapies for BM tumors. We also provide future perspectives on the development and implementation of such therapies brain metastatic cancer patients.
Keywords: Metastases, brain, immunotherapy, oncolytic virus, CAR-T, stem cells
Introduction
Metastases (See Glossary) is accountable for 90% of cancer related mortality (1). One of the most common metastases locations from solid tumors is the brain. Brain Metastases (BM) are particularly prevalent in lung cancer (~45% of BM), breast cancer (~15%), and melanoma (~10%) (2, 3). Among them, melanoma has the highest propensity to metastasize to brain; over 40% of patients with advanced melanoma will develop BM, and this percentage has been found to be increased up to the 75% after autopsy (4, 5). Notably, the incidence of BM varies widely between cancer types and among different subtypes. For instance, in the context of non-small cell lung cancer (NSCLC), adenocarcinoma more commonly spreads to the brain than squamous carcinoma, whereas in the case of breast cancer, the HER2-overexpressing subtype has a higher propensity to develop BM than other subtypes. The occurrence of BM in the clinic has been further increasing, probably due to effective treatments for primary tumors and extracranial metastases, leading to a steadily growing number of patients for which effective treatments are needed.
Nevertheless, current therapeutic options for BM, including surgical resection, stereotactic radiosurgery, whole brain radiotherapy and chemotherapy, have limited success in most patients (6, 7). There are several factors that hinder the efficacy of these treatments. The presence of multiple metastatic lesions at the time of diagnosis in most patients makes surgery an inadequate therapeutic option on its own (8), while the particularities of the brain tissue, shielded by a blood brain barrier (BBB), prevents the permeability of systemic therapies such as chemotherapy (9). These factors, coupled with the detrimental side effects associated with the use of radiotherapy in the brain (10, 11), urges exploration of novel tumor-specific therapies that overcome both current challenges and improve the clinical benefit of patients, while minimizing potential damages to the delicate brain tissue.
Recent advances in the understanding of the pathobiology of BM have evidenced the important role that the microenvironment plays not only in the establishment of the metastatic tumor, as has been traditionally proposed in the “seed and soil” hypothesis (12, 13), but also in the survival and growth of metastatic tumor cells in the brain. Thus, interaction of these cells with their surroundings allows for their adaptation to the brain microenvironment, characterized by an abundant supply of oxygen and glucose, and their contribution to the progression of BM. In this way, identification of the rate-limiting steps and main signaling pathways involved in this metastatic cascade has the potential for development of novel therapies that target key molecular mechanisms for cancer cell survival, proliferation, dormancy and recurrence within the brain. Particularly, the increasing awareness and knowledge of the involvement of the immune system in tumor growth, as well as of the unique immune microenvironment within the brain, has brought to the fore immunotherapy as a new and promising therapeutic regimen for BM, especially given its relative success in other extracranial tumors such as melanoma and lymphoma. Multiple immunotherapeutic approaches are being extensively studied and tested in clinical trials currently, with a goal of overcoming the limitations of current treatments and increasing the prognosis and survival rate of the patients. This review highlights the pathogenesis of BM, with a focus on the role of the brain microenvironment and how understanding its interaction with metastatic cells may lead to novel therapeutic options. Additionally, an overview of the current status and ongoing clinical research of immunotherapy for BM is presented, addressing the main obstacles and future perspectives for the application of this treatment in BM patients.
Targeting the Microenvironment of Brain Metastasis
Astrocytes
For effective metastases, cancer cells from primary tumor sites must undergo a complicated process of acquiring metastatic capability that allows them to spread to and invade distal organs (Figure 1). In the case of BM, these metastatic cells have to face the unique structure of BBB as well, which efficiently prohibits molecules and cells from entering into the brain parenchyma via circulation (14, 15). It also represents the biggest obstacle for drug delivery into brain for treating brain malignancies. Communication between invading tumor cells and brain microenvironment is viewed as an essential event during the pathological progression, however, little is known about the accurate control of this process. Anatomically, upon tumor cells’ successful extravasation, astrocytes are their first encounters within the brain microenvironment (16). Reactive astrocytes are intensively associated with BM, even a single invading tumor cell can induce reactive astrocyte response nearby, indicating that tumor cell-astrocyte communication occurs even at a single cellular level (Figure 2). These cells exert tumor killing by secreting FasL to trigger the execution of cell death pathway within tumor cells (17). However, some tumor cells evade such death induction by releasing Serpin protein that results in the cleavage of FasL, eventually blunting the astrocyte-FasL mediated tumor cell killing. Astrocytes also have the capability to downregulate PTEN protein levels in metastatic tumor cells via exosomal PTEN-targeting microRNAs (18). A better understanding of how astrocytes communicate with invading tumor cells within the TME and facilitate the metastatic progression is thus of great intrigue and high clinical relevance.
Figure 1. Metastatic cascade.
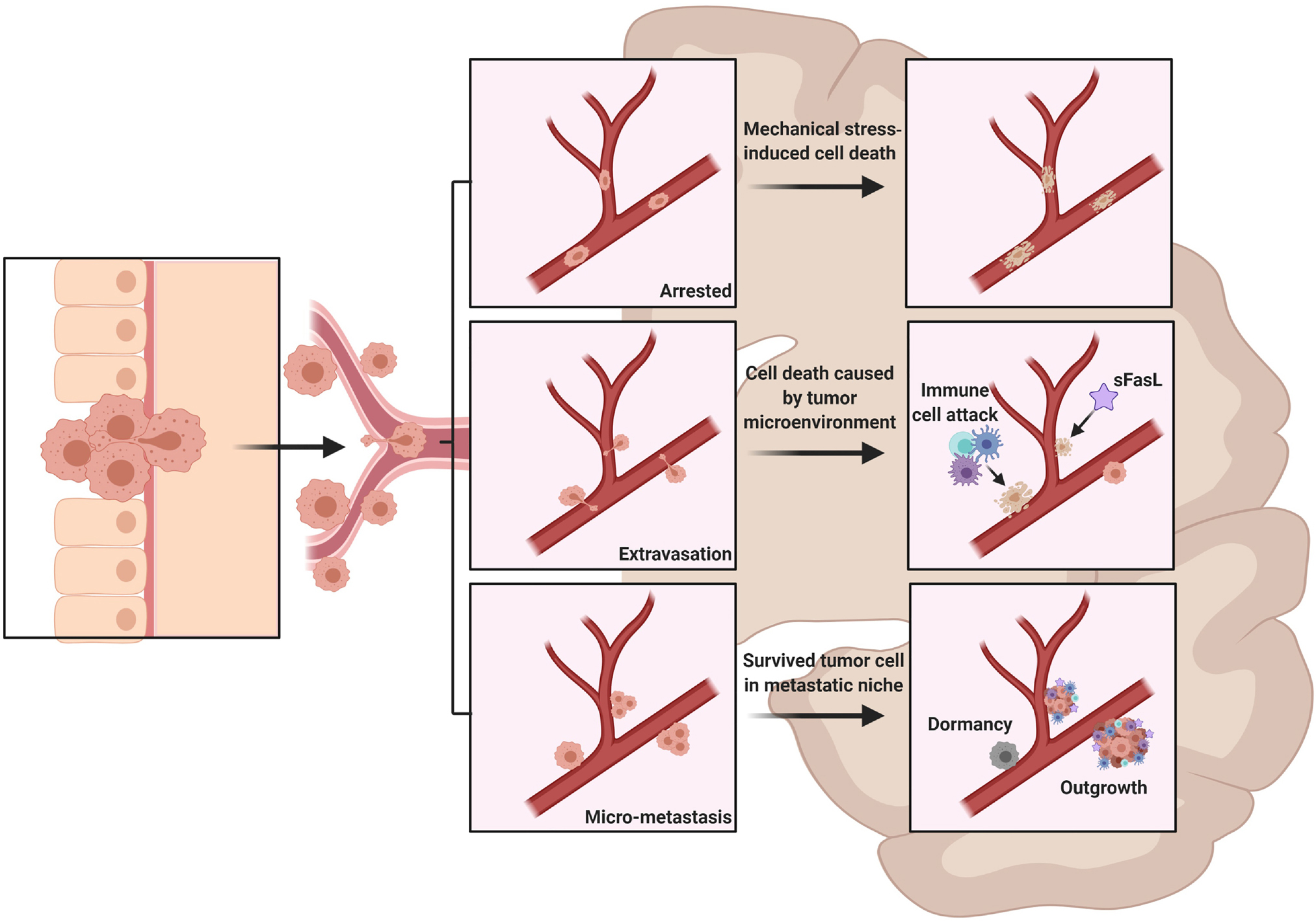
Cancer cells detach from the primary tumor site and spread to the brain through the systemic circulation. On their way, these cells may get arrested at brain capillary, leading to their death due to mechanical stress (A). Upon their arrival at the brain vasculature, the metastatic cells will have to undergo extravasation and invasion of the brain parenchyma, where they will need to survive the CNS microenvironment (B). The surviving cells will grow and form metastatic niches near blood vessels or, alternatively, they will enter a dormancy status and recur at some time (C).
Abbreviation: sFasL=soluble Fas Ligand
Figure 2. Immune-tumor interactions in the Brain Tumor Microenvironment.
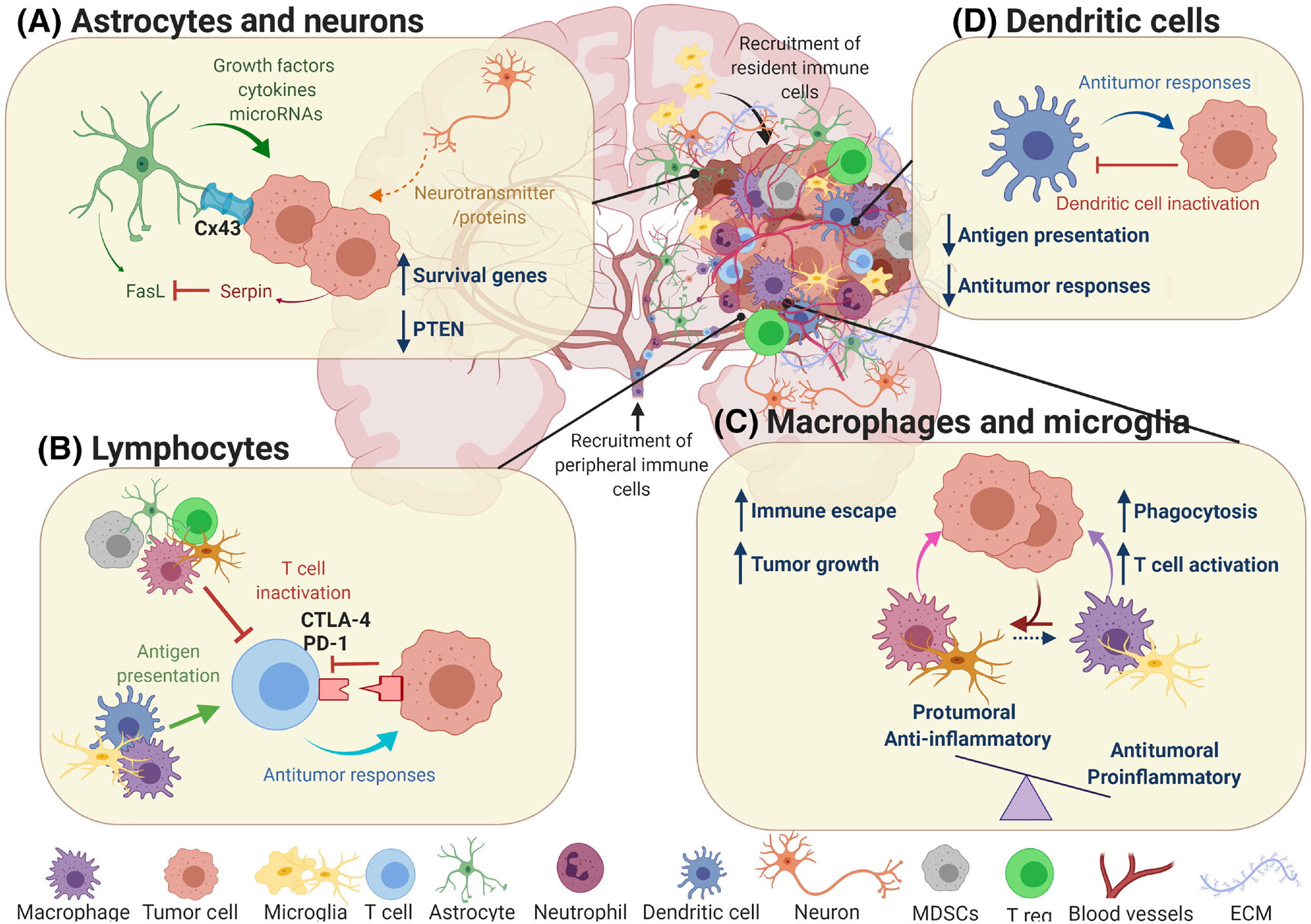
Once the metastasis is established, tumor cells will encounter different cell types in the brain parenchyma. Interaction between these cells will affect metastatic growth and progression in different ways. A) Astrocytes contribute to the survival of tumor cells and the establishment of brain metastases by providing various signaling molecules, mainly through gap junctions. Although astrocytes also release FasL to induce apoptosis in the metastatic cells, the production of serpin by metastatic cells counteract this anti-tumoral mechanism. There is increasing evidence that neurons also interact with tumor cells, mainly through the release of neurotransmitters and other proteins, although the exact consequences of these interactions remain unclear. B) Lymphocytes are among the immune cells recruited from the periphery to the tumor site in the brain. They can be activated by APCs (microglia, macrophages, dendritic cells) and mount an immune response against the tumor. However, the immunosuppressive microenvironment of brain metastases inhibits the effector functions of the lymphocytes.
C) Macrophages and microglia contribute to the metastatic cell death both directly (i.e. phagocytosis) or indirectly (T-cell activation). Nevertheless, interaction with tumor cells will favor the pro-tumoral phenotype of these cells, which support metastatic progression and the establishment of an immunosuppressive microenvironment. D) Dendritic cells are mainly involved in antigen presentation and activation of T-cells, as well as in the release of different molecules and factors that elicit an anti-tumoral response. These abilities become impaired upon interaction with tumor cells, rendering dendritic cells ineffective.
Abbreviations: APCs=Antigen Presenting Cells, DCs=Dendritic Cells, ECM=Extracellular Matrix, FasL=Fas Ligand, MDSCs= Myeloid-Derived Suppressor Cells, T reg.=Regulatory T Cells
The leading role of astrocytes in the formation and subsequent growth of BM makes them a promising target. Furthermore, reactive astrocytes interact with other immune cells l, modulating the immune response and contributing to the immunosuppressive TME that is characteristic of brain tumors. Therefore, targeting these cells may help attenuate immune escape. Blocking the crosstalk between astrocytes and tumor cells with gap junction inhibitors, meclofenamate or tonabersat led to an inhibition in the progression of BM in a mouse model of BM (19). This effect was greatly improved when combined with a chemotherapeutic agent, consistent with the findings that reactive astrocytes render BM chemo-resistant (19). Notch signaling is also involved in the interaction between reactive astrocytes and cancer stem-like cells (20). Blocking this signaling pathway with Compound E suppressed BM growth in mouse model of breast cancer BM (20).
The safety concerns that arise when targeting a general signaling pathway involved in the crosstalk between astrocytes and other cells, which may ultimately impair the normal function of the brain tissue, encourage the search of alternatives that target specifically protumoral, reactive astrocytes. STAT3 has been identified as a potential marker of these astrocytes, and its inhibition using Legasil resulted in an increase in the overall survival of lung cancer patients with BM (21). Moreover, STAT3 is an immune-regulator, and pSTAT3+ reactive astrocytes have been found to modulate both the innate and adaptive immune response towards an immunosuppressive state, so the combination of Legasil with other immunotherapy, such as the abovementioned ICBs, may help further boosting the antitumoral immune response (21).
Myeloid cells
The population of myeloid cells found in the TME of BM is quite heterogeneous, being composed of recruited monocytes, macrophages, MDSCs and granulocytes, as well as the resident myeloid cells of the CNS, such as microglia or perivascular macrophages (22).There is strong clinical evidence that microglia, the main immunogenic cell type within brain parenchyma, heavily infiltrate into metastatic tumor deposits and play a pivotal role in the progression of BM (23). Microglia have multidimensional activation states in brain diseases and injuries, such that these cells can have beneficial or detrimental roles depending on the context and timing (24). Close interactions between microglia and brain tumors have been reported in patient brain samples (25, 26). In BM mouse models, heterogeneous microglia, activated and non-activated, have been shown to accumulate proximal to invading tumor cells and infiltrate multiple BM deposits (27). However, whether microglia are tumor-suppressive or tumor-supportive remains elusive.
Despite the initial efforts of myeloid cells to combat the tumor, they eventually facilitate immune escape and metastatic growth (Figure 2) (28). This seems to be mainly due to the signaling processes between the TME and the immune cells in BM tumors, which impair their functions and contribute to skewing the myeloid population towards a pro-tumoral phenotype (29). Thus, one of the current therapeutic strategies is to target the myeloid compartment, either by depleting these cells from the TME or by re-polarizing them to a pro-inflammatory, anti-tumoral state, in order to promote an immune response against the tumor.
A molecule that has gained increasing interest due to its involvement in macrophages activation and phenotype switching is colony stimulating factor 1 (CSF1). Although the effects of targeting this protein have not been evaluated in the context of BM, inhibition of CSF1 receptor (CSF1-R) decreased the polarization of macrophages towards the anti-inflammatory phenotype, while also increasing the survival of the murine model of primary brain tumors, glioblastoma (GBM) (30), suggesting that this kind of approach might be beneficial for other brain malignancies. Likewise, blocking PI3K signaling pathway with Buparlisib resulted in a switch in the phenotype of macrophages and microglia towards an immunologically active form in a mouse model of breast cancer BM (31). Of note, a phase Ib clinical trial of this drug in patients with breast cancer BM showed only limited clinical efficacy (32). The effects of specifically depleting the anti-inflammatory, protumoral macrophages/microglia from the TME with clodronate liposomes in a mouse model of BM, lead to a reduction in the tumor burden (33, 34).
Another myeloid cell type highly involved in shielding the tumor from an effective immune response are MDSCs (Figure 2). Targeting these cells indirectly, using antiangiogenic agents that inhibit the VEGF-VEGFR interaction, reduces their immunosuppressive capacity, leading to an increase in overall survival in an intracranial melanoma mouse model when used alone or in combination with ICBs (35, 36). This combination also resulted in an increase in the number and anti-tumoral activity of dendritic cells (DCs), and may be able to repolarize macrophages back to an anti-tumoral phenotype (37).
T-Lymphocytes
Historically brain has been viewed as an immune privileged organ, but the concept of “immune privilege” has been greatly modified due to the discovery of meningeal lymphatic system in murine and human brain (38, 39). Recent studies have demonstrated that peripheral immune cells, such as CD4+ and CD8+ T cells, can cross the BBB, enter brain parenchyma, and home to BM spots (40–42). Plenty of evidence have shown that tumor infiltrating T lymphocytes (TILs) are recruited to metastatic tumor areas in the brain. Although the exact role of TILs in BM remains unclear, an increased TILs density has been previously associated with improved diagnoses and overall survival in BM patients (43–45), suggesting that these cells may be relevant for the defense mechanisms against tumor cells in the brain (Figure 2). This is consistent with the link found between higher TILs and a favorable prognosis in a number of primary tumors (46–49). However, TILs have also been reported to facilitate BM in breast cancer (50) and to be involved in immune evasion, as evidenced by the infiltration of regulatory T-cells, which have an immunosuppressive nature, in the TME of BM (51, 52). Moreover, alternative studies could not find any significant correlation between patient survival and TILs density (42, 53). Therefore, further research is needed to elucidate the functions of TILs in the context of BM.
Immunotherapies for Brain Metastases
The promising outcomes of immune based therapies in recent years, in several cancers has given insight into the potential of this type of therapy. This has encouraged further immune-therapeutic research to overcome some of the current limitations and enhance its effectiveness (28) (Figure 3). Nevertheless, BM represent an additional challenge to this form of treatment due to their physical location within the CNS and the presence of the BBB. Additionally, the immunological status of the CNS is unique and is characterized by an increased regulation of immune responses and inflammation and the presence of its own set of resident immunocompetent glial cells, namely astrocytes and microglia.
Figure 3. Current immunotherapies for the treatment of Brain Metastases.
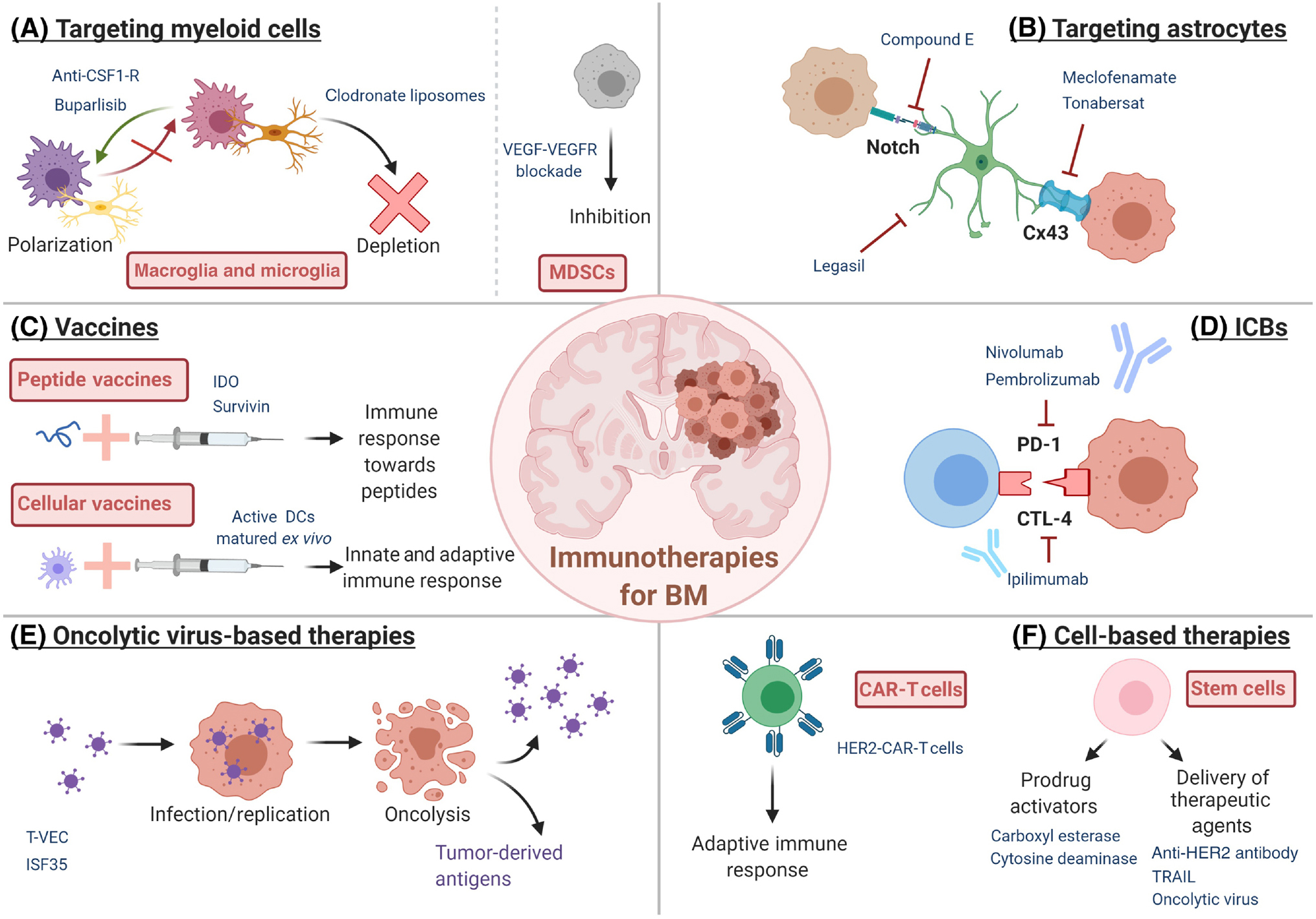
Immunotherapeutic strategies being tested in pre-clinical and clinical trials for the treatment of BM include targeting immune components of the TME, such as myeloid cells or astrocytes (A and B), both peptide-based and whole cells-based vaccines (C), immune-checkpoint blockade (D), virotherapy (E) and cell-based therapies (F). Abbreviations: BM=Brain Metastases, CAR=Chimeric Antigen Receptor, CSF1-R=Colony Stimulating Factor 1 Receptor, DCs=Dendritic Cells, HER2=Human Epidermal Growth Factor Receptor 2, IDO=indoleamine 2,3-dioxygenase, MDSCs=Myeloid-Derived Suppressor Cells, TRAIL=TNF-Related Apoptosis-Inducing Ligand, T-vec=Talimogene Laherparepvec
In recent years, the number of clinical trials for immunotherapies that include patients with BM has increasing substantially, thus providing a better understanding of the real potential of immunotherapy in the context of BM (Table 1). The immune profile differs between primary tumor and their matched BM (54–56), suggesting that immunotherapies that are effective for the primary tumor may not be as effective against the BM. The most promising immunotherapy that has been extensively tested in BM are immune checkpoint blockers (ICBs). Expression of immune checkpoint molecules, such as PD-1 or CTLA-4, together with their respective ligands, has also been found in a great proportion of BM patients (29, 44, 51). The expression of both PD-1 and PD-L1 in BM tumor microenvironment is different than in the primary tumor, and also varies depending on the primary tumor from which the BM is derived (41, 42, 55, 57). Therefore, differences in the efficacy of these immunotherapeutics are expected not only between primary tumors and BM, as remarked above, but also between BM of different origins, hindering the finding of a universal treatment that may be suitable for all BM. In accordance to this, most of the clinical trials testing ICBs in BM to date have been performed in melanoma patients, as this metastasis is one of most immunogenic BM, with a high expression of PD-L1 (42). Although modest, the results of these trials are promising; a recent phase II clinical trial using pembrolizumab, a monoclonal antibody against PD-1, as a treatment managed to achieve an overall survival of 17 months, with 48% of the patients still alive after 2 years (58). CTLA-4 is another immune checkpoint protein that is often targeted by ICBs. Several clinical trials have tested the efficacy and safety of ipilimumab, a monoclonal antibody against CTLA-4, in melanoma BM patients. When combined with fotemustine, a chloroethylating nitrosourea, the administration of ipilimumab led to complete regressions in some patients, and an overall survival of 3 years in 27.8% of the patients (59). Recently, combination therapy of both ipilimumab and nivolumab antibodies in a phase II clinical trial resulted in a clinical benefit rate of 58% in asymptomatic melanoma BM patients, although the outcome in symptomatic patients was considerably lower (60).
Table 1.
Ongoing clinical trials of Immunotherapies for Brain Metastasis.
| Intervention | Condition | Phase (study start) | Status | National Clinical Trial Number |
|---|---|---|---|---|
| Immune Checkpoint Inhibitors: | ||||
| Ipilimumab + SRS | Melanoma BM | II (04/2014) | Active, not recruiting | NCT02097732 |
| Nivolumab / Nivolumab + Ipilimumab | Melanoma BM | II (11/2014) | Active, not recruiting | NCT02374242 |
| Pembrolizumab + Bevacizumab | Melanoma and NSCLC BM | II (05/2016) | Active, recruiting | NCT02681549 |
| Nivolumab + SRS | Melanoma BM | I (06/2016) | Active, not recruiting | NCT02716948 |
| Pembrolizumab + MRI + PET/CT (+ SRS in Melanoma) | CNS Metastases | II (10/2016) | Active, recruiting | NCT02886585 |
| Pembrolizumab + SRS | Melanoma and NSCLC BM | I (10/2016) | Active, recruiting | NCT02858869 |
| Nivolumab + SRS / Nivolumab + WBRT / Nivolumab + Ipilimumab + SRS / Nivolumab + Ipilimumab + WBRT | NSCLC BM | I/II (12/2016) | Active, recruiting | NCT02696993 |
| Nivolumab + SRS | NSCLC and RCC BM | II (06/2017) | Active, recruiting | NCT02978404 |
| Atezolizumab + Bevacizumab / Atezolizumab + Bevacizumab + Cobimetinib | Melanoma BM | II (06/2017) | Active, recruiting | NCT03175432 |
| Pembrolizumab + Ferumoxytol MRI | NSCLC BM | II (11/2017) | Active, recruiting | NCT03325166 |
| Nivolumab + Ipilimumab | Melanoma BM | II (04/2018) | Active, recruiting | NCT03728465 |
| Atezolizumab + SRS | Triple-Negative Breast Cancer BM | II (05/2018) | Active, recruiting | NCT03483012 |
| Atezolizumab + Carboplatin + Pemetrexed | NSCLC BM | II (07/2018) | Active, recruiting | NCT03526900 |
| Pembrolizumab + SRS | Breast Cancer BM | I/II (11/2018) | Active, not recruiting | NCT03449238 |
| Nivolumab + SRS | Breast Cancer BM | I (01/2019) | Active, recruiting | NCT03807765 |
| Ipilimumab + Pembrolizumab | Melanoma BM | II (02/2019) | Active, not recruiting | NCT03873818 |
| Sintilimab + Bevacizumab | NSCLC BM | II (04/2019) | Active, recruiting | NCT04213170 |
| Optune + Ipilimumab + Nivolumab | Melanoma BM | II (07/2019) | Active, recruiting | NCT03903640 |
| Nivolumab + Ipilimumab + concurrent SRS / Nivolumab + Ipilimumab | Melanoma BM | II (08/2019) | Active, recruiting | NCT03340129 |
| Pembrolizumab + LITT | Melanoma, NSCLC and RCC BM | I (01/2020) | Active, recruiting | NCT04187872 |
| Camrelizumab + Pemetrexed or Carboplatin | NSCLC BM | II (01/2020) | Active, recruiting | NCT04211090 |
| Durvalumab + SRS | NSCLC BM | II (03/2020) | Active, recruiting | NCT03955198 |
| Camrelizumab + Local Treatment | NSCLC BM | II (05/2020) | Not yet recruiting | NCT04291092 |
| Neoadjuvant Nivolumab + Ipilimumab | BM | II (07/2020) | Not yet recruiting | NCT04434560 |
| Vaccines: | ||||
| DCs vaccine + HSCs + CTLs | Breast Cancer BM | II/III (12/2012) | Enrolling by invitation | NCT01782274 |
| DCs vaccine + HSCs + CTLs | Lung Cancer BM | I/II (12/2012) | Enrolling by invitation | NCT01782287 |
| mRNA tumor antigen pulsed DCs vaccine | BM | I (06/2016) | Active, not recruiting | NCT02808416 |
| DCs vaccine (DCVax-Direct) | Lung and Breast Cancer BM | I (11/2018) | Not yet recruiting | NCT03638765 |
| Anti-HER2/HER3 Dendritic Cell Vaccine + Cytokine Modulation Regimen + Pembrolizumab | Breast Cancer BM | II (06/2020) | Not yet recruiting | NCT04348747 |
| CAR T-cells: | ||||
| Anti-NY ESO-1 Murine TCR-Gene Engineered Lymphocytes | BM | I (08/2016) | Active, recruiting | NCT02774291 |
| HER2-CAR T cells | Breast Cancer BM | I (08/2018) | Active, recruiting | NCT03696030 |
| Oncolytic virus: | ||||
| PexaVec + Ipilimumab | Metastatic Solid Tumor (treated and stable BM accepted) | I (01/2017) | Active, recruiting | NCT02977156 |
| NG-350A | Metastatic Epithelial Tumor (locally treated BM accepted) | I (02/2019) | Active, recruiting | NCT03852511 |
| ASP9801 | Metastatic Solid Tumor (treated and stable BM accepted) | I (08/2019) | Active, recruiting | NCT03954067 |
| NG-641 | Metastatic Epithelial Tumor (locally treated BM accepted) | I (01/2020) | Active, recruiting | NCT04053283 |
| Immunomodulation, TAMs- & Astrocyte-targeted therapies: | ||||
| Meclofenamate | BM | N/A (04/2015) | Active, not recruiting | NCT02429570 |
| STAT3 Inhibitor WP1066 | GBM and Melanoma BM | I (07/2018) | Active, recruiting | NCT01904123 |
| CSF1R inhibitor TPX-0022 | Solid Tumor (asymptomatic BM accepted) | I (08/2019) | Active, recruiting | NCT03993873 |
| STAT3 Inhibitor WP1066 | Brain Tumor (including BM) | I (04/2020) | Not yet recruiting | NCT04334863 |
Abbreviations: BM=Brain Metastases, CAR= Chimeric Antigen Receptor ,CNS=Central Nervous System, CSF1R=Colony Stimulating Factor 1 Receptor, CTLs=Cytotoxic T Lymphocytes, DCs=Dendritic Cells, GBM=Glioblastoma, HER2/3=Human Epidermal Growth Factor Receptor 2/3,HSCs=Hematopoietic Stem Cells, LITT=Laser Interstitial Thermal Therapy, MRI=Magnetic Resonance Imaging, NSCLC=Non-Small-Cell Lung Carcinoma, PET/CT=Positron Emission Tomography/Computed Tomography, RCC=Renal Cell Carcinoma, SRS=Stereotactic Radiosurgery, TCR= T Cell Receptor , WBRT=Whole Brain Radiation Therapy
In addition to melanoma BM, ICBs have been tested in other malignancies such as NSCLC or renal cell carcinoma BM. Although the overall results are encouraging and suggest the potential of ICBs, alone or in combination, for the treatment of BM of various origins, further research is still needed to improve efficacy and safety in different types of patients (59). Indeed, several clinical trials including ICBs or a combination of them for BM are ongoing and, hopefully, will give more insights into how to increase the therapeutic benefit of this sort of treatment.
Oncolytic virus-based therapies
Oncolytic viruses have also become promising therapeutic agents for cancer, demonstrating significant effects against a number of solid tumors (61). Furthermore, these viruses can be genetically modified to express proteins, such as cytokines, that further enhance the immune response, as is the case of T-VEC. Additionally, oncolytic viruses can be combined with other immunotherapies, particularly ICBs, to achieve a synergistic effect. For instance, treatment of BM patients with oncolytic reovirus resulted not only in the infection and subsequent death of tumor cells, but also in an increase in both the number of TILs and the expression of PD-L1 (62), thus priming the metastatic lesions to anti-PD-1 agents like pembrolizumab or nivolumab. Treatment with oncolytic adenovirus genetically modified to express a CD40 agonist, which acts on and stimulates antigen-presenting cells, in a mouse model of melanoma BM was significantly more effective when combined with both anti-PD-1 and anti-CTLA-4 antibodies, leading to a 45% complete cure rate and an improvement in overall survival (63). Nevertheless, virotherapy in BM still needs to be explored more in detail, especially to overcome some of the current limitations that reduce its efficacy. These limitations include the delivery of the virus to the metastatic lesions in the brain, as intratumoral delivery is a complex procedure and intravenous administration leads to neutralization or sequestration of the virus by immune system, as well as suboptimal entry through the BBB, reducing the viral load that ultimately reaches the tumor (64). One way to improve this is through the use of carriers that shield the viral particles on their way to the tumor while also supporting their replication and amplification (65). For instance, mesenchymal stem cells (MSCs) can be loaded with oncolytic herpes simplex virus (oHSV) (66). Intra-arterial injection of oHSV loaded MSCs crossed the BBB and migrated towards metastatic lesions in the brain, where they released the virus, infecting nearby tumor cells. This resulted in an increase in viral infection and tumor cell death compared to the administration of purified oncolytic virus, as well as in an improved survival in a mouse model of melanoma BM (66).
Importantly, both ICBs and virotherapy have showed modest results on their own in the treatment of BM, while their efficacy is significantly enhanced when combined. This finding demonstrates that the right combination may help increase the therapeutic benefit of previously unsuccessful therapies, highlighting the importance of multidisciplinary treatments.
Cell based therapies
Adoptive chimeric receptor (CAR) T-cell therapy has shown encouraging results in different types of cancer and has been approved for the treatment of hematologic malignancies. In the context of BM, intraventricular administration of HER2-CAR T-cells in a breast cancer BM mouse model resulted in the development of an antitumoral immune response, tumor regression and prolonged overall survival (67). Based on the favorable results of this preclinical work, an ongoing phase I clinical trial is looking at the effects of intraventricular delivery of HER2-CAR T-cells in patients with recurrent BM (ClinicalTrials.gov Identifier: NCT03696030). Despite this promising research, there is still a significant shortage of studies testing CAR T-cell therapies in BM. Additionally, stem cells are gaining increasing popularity as a cell-based therapy for cancer. Stem cells home to tumors and are able to penetrate the BBB (68), making them the perfect cells to target BM, either by themselves or as carriers of other antitumor agents. Previous studies have shown that neural stem cells (NSC) genetically modified to express antibodies anti-HER2 demonstrated an improvement in overall survival after intracranial administration in a mouse model of breast cancer BM (69). Moreover, NSC engineered to secrete TRAIL suppresses tumor growth and increases survival in a mouse model of breast cancer BM (70). Alternatively, stem cells can be modified to express a suicide gene that induces the death of the tumor cells by the bystander effect. Intracardiac administration of neural stem cells expressing carboxyl esterase coupled with injection of CPT-11 led to a decrease in tumor size and increased survival in a mouse model of lung cancer BM (71). Similarly, NSC expressing cytosine deaminase, together with the prodrug 5-FC, were able to reduce tumor size in a mouse model of breast cancer BM (72).
Vaccine based therapies
The concept of cancer vaccines has been extensively researched in several solid tumors, including primary brain tumors such as gliomas. In the case of BM, vaccines have been far less studied, with clinical trials specific for brain metastatic patients starting only recently. Therefore, the only evidence of the efficacy of vaccines in brain metastatic lesions come from clinical studies that included few of these patients (73). A vaccine including two antigenic peptides, Indoleamine 2,3-dioxygenase (IDO) and survivin, proved to elicit immunity towards these peptides in a small number of melanoma BM patients included in the trial, although no real therapeutic benefit was observed (74). Based on individual cases of BM patients treated with DCs vaccines in clinical trials for various metastatic malignancies, it seems that cellular vaccines might be able to induce an effective antitumor immune response within the BM (73). Nonetheless, the results of the ongoing clinical trials will define the true potential of both types of cancer vaccines in the context of BM.
Concluding Remarks
The limited clinical outcome and overall ineffectiveness of current treatments highlight the urgent need of alternative tumor-specific therapies for BM. In order to achieve this, a better understanding of the pathophysiology of metastatic brain tumors is crucial, and this will come from depicting and dissecting the behaviors, molecular signatures and crosstalk of individual cells within the metastatic tumor niche (See Outstanding Questions) (75). Acknowledging the importance of the interaction between tumor cells and the different components of the brain microenvironment in the development and survival of BM is key to the identification of new mechanisms of BM progression and therefore discovering novel therapeutic targets. While most of these targets might be common for BM derived from different primary cancer sources, the peculiar characteristics of each of them should be taken into consideration when designing new therapeutic strategies. These differences may come from the metastatic cascade —for instance, brain metastatic tumor cells from lung cancer develop neuroagiogenesis for their survival and growth, while breast cancer or melanoma BM remodel and co-opt the vasculature (76)—, as well as from driver genetic mutations specific to each type of BM (77). Recent advances in single cell RNA sequencing and RNA scope have the potential to facilitate the identification of these novel targets. Additionally, the in-depth transcriptome analysis for individual microglia/macrophage and metastatic tumor cell at various pathological stages will help us reveal the heterogeneity of immune responses to invasive tumor cells with unprecedented scale, resolution and precision. Understanding the specific roles of different subpopulations of TME cells will allow us to tailor our strategies to precisely target either pro- or anti-tumor signaling pathways with better therapeutic efficiency. Lastly, characterization of the BBB in BM is key to the formulation of therapeutic agents that can successfully reach the tumor post- systemic treatment.
Outstanding Questions.
What are the molecular mechanisms behind tumor cell dormancy and why are there differences in the dormancy periods depending on the primary tumor?
What is the role of TILs in BM? Why are there contrasting results on whether or not they support the development of BM?
What additional molecular mechanisms control the crosstalk between tumor and immune cells, and between different populations of immune cells? How can they be targeted to avoid immune escape and promote an effective antitumoral response?
Why is there a huge variability in the outcome of immunotherapies among BM patients?
How should screening methods be designed to allow stratification of patients that could potentially benefit from a specific immunotherapeutic?
What combination strategies are ideal to maximize the clinical benefit of the patients while minimizing the risk of resistance and side effects?
Development and preclinical testing of new cancer therapies, as well as identification of potential new therapeutic targets, is heavily relying on in vivo animal models that can faithfully reproduce tumor growth and metastatic progression. Previous studies developed experimental breast cancer BM cell line, MDA231BrM2 and CN34-BrM2, which are obtained via two rounds of in vivo selection of human metastatic breast cells through intracardiac injection of human breast cancer cells. The first report about spontaneous BM mouse model reported that two metastatic variants, originating from human melanoma cell line WM239, give rise to spontaneous BM after orthotopic transplantation in mice and the removal of primary tumors (78). Such spontaneous metastases model authentically mimics all steps of metastatic cascade, such as primary tumor progression, metastatic tumor cell traveling via circulation, extravasation and successfully colonization within brain. However, the efficiency of building successful models is currently low and requires long periods of time for metastatic tumor spots to appear. Soon, we anticipate using humanized mice for xenograft tumor models, in order to better mimic BM clinical scenario and test potential immunotherapies.
In light of the unprecedented success achieved by immunotherapy in a variety of cancers and the intricate role that brain immunology plays in the establishment of BM, it is not surprising that this form of treatment has drawn the attention of the research community as a potential therapeutic option for BM. The current results from those prospective pre-clinical (Table 2) and clinical studies demonstrate relative safety and efficacy of immunotherapies in patients with BM. Nevertheless, there are still some additional challenges that immunotherapy has to overcome prior to its successful application in brain metastatic tumors, one of them being the immune response variability among BM patients, as only a selective population of patients will demonstrate good response. In the near future, a more stringent initial screening of BM patients with their immune profiles, such as PD-1(+) and PD-1(−), is needed in order to tailor the immunotherapy case-by-case and identify which patients may benefit from this treatment. Alternatively, a more personalized approach can be adopted, which could lead to therapies specifically designed for an individual patient. For example, cell-based vaccines which are created from patient derived tumor cells ensuring therefore their suitability and circumventing problems derived from BM inter-heterogeneity. Finally, the rapid evolution of tumor cells contributes to immune escape mechanisms and resistance to immunotherapy, which will ultimately render the treatment ineffective. Therefore, a rational design of the right combination of immunotherapeutics is required to maximize the clinical benefit of individual BM patients and reduce the occurrence of resistance. Current clinical trials already show the advantage of using combination therapies over single-agent therapies, pointing that future therapeutic options for BM should aim at multidisciplinary approaches that target multiple aspects of its immunology and pathobiology at the same time.
Table 2.
Recent pre-clinical studies of Immunotherapies for Brain Metastasis.
| Treatment | Mechanism of Action | Tumor Model | Results | Reference |
|---|---|---|---|---|
| Myeloid Cell-targeted therapies | ||||
| Nanoparticles containing DOX and MMC | Target and deliver chemotherapy to TAMs and tumor cells | Triple Negative Breast Cancer BM mouse model | - Inhibition of tumor growth - Reduction of TAM population - Increase in OS |
(83) |
| Buparlisib (BKM120) | Inhibition of PI3K | Breast Cancer BM mouse model | - Reduction of TAMs infiltration - Re-education of TAMs - Increase in OS |
(31) |
| Clodronate Liposomes | Selectively target and deplete anti-inflammatory TAMs | Breast Cancer BM mouse model | - Reduction of tumor growth | (33) |
| Axitinib + anti-CTLA-4 antibody | Blockade of VEGFR and CTLA-4 | Melanoma BM mouse model | - Reduction of MDSCs immunosuppression - Increase in antigen-presenting activity of DCs - Increase in OS |
(36) |
| Astrocyte-targeted therapies | ||||
| Meclofenamate + Tonabersat | Modulation of tumor-astrocyte gap junctions | NSCLC and Breast Cancer BM mouse model | - Reduction of metastatic burden - Increase in chemotherapy susceptibility |
(19) |
| Legasil | Inhibition of STAT3 | Melanoma BM mouse model Lung Cancer BM patients | - Reduction of metastatic burden - Increase in OS |
(21) |
| Oncolytic virus therapies | ||||
| ISF35 + anti-CTLA-4 and anti-PD-L1 antibodies | Adenovirus encoding CD40 agonist and immune checkpoint blockade | Melanoma BM mouse model | - Eradication of brain tumor -Increase in OS |
(63) |
| MSCs containing oncolytic herpes simplex viruses | Oncolysis of tumor cells and activation of immune system | Melanoma BM mouse model | - Reduction of metastatic burden - Increase in OS |
(66) |
| CAR T-cells and Stem cell therapies | ||||
| HER2-CAR T-cells | Target HER2+ tumor cells | Breast Cancer BM mouse model | - Tumor regression -Increase in OS |
(67) |
| EGFR-CAR NK cells + oHSV-1 | Target both EGFR+ and EGFR-tumor cells and activation of immune system | Breast Cancer BM mouse model | - Inhibition of tumor growth -Increase in OS |
(84) |
| NSCs secreting anti-HER2 antibodies | Target HER2+ tumor cells and inhibit PI3K-Akt signaling | Breast Cancer BM mouse model | - Inhibition of metastatic progression - Increase in OS |
(69) |
| NSCs secreting TRAIL | Induction of death receptor signalling in tumor cells | Breast Cancer BM mouse model | - Inhibition of tumor growth - Increase in OS |
(70) |
| NSCs expressing cytosine deaminase and IFN-β + 5-FC | Induction of apoptosis in tumor cells | Breast Cancer BM mouse model | - Inhibition of tumor growth - Increase in OS |
(85) |
Abbreviations: 5-FC=Flucytosine, BM=Brain Metastases, CAR=Chimeric Antigen Receptor, DCs=Dendritic Cells, DOX=Doxorubicin, EGFR= Epidermal Growth Factor Receptor, HER2= Human Epidermal Growth Factor Receptor 2, IFN-β=Interferon-β, MDSCs=Myeloid-Derived Suppressor Cells, MMC=Mitomycin, MSCs=Mesenchymal Stem Cells, NK=Natural Killer, NSCs=Neural Stem Cells, NSCLC=Non-Small-Cell Lung Carcinoma, oHSV-1=Oncolytic Herpes Simplex Virus-1, OS=Overall Survival, PI3K=Phosphoinositide 3-kinase,TAMs=Tumor-Associated Macrophages, TRAIL=TNF-Related Apoptosis-Inducing Ligand, VEGFR=Vascular Endothelial Growth Factor Receptor
Besides immunotherapy, new insights for treating metastatic brain tumors can come from other aspects of advances in neuroscience, such as the innovative therapeutic strategies of in vivo conversion of astrocytes to neurons (79, 80), which suggest the possibility to minimize BM progression by on-site conversion of brain tumor cells to non-tumor-generous brain resident cells. Preventive strategies that avoid or reduce the occurrence of BM are also critical for high-risk patients with primary tumors that tend to metastasize into the brain, and might be developed by determining and targeting the rate-limiting steps of the metastatic cascade that lead to the establishment of BM. Therefore, a better understanding of tumor cell dormancy is of huge interest. It should be noted that, depending on the primary tumor source, the corresponding BM will display varying dormancy periods (81, 82). Based on these unique features, the development of ways to control tumor cell dormancy and prevent these cells from exiting this state may have a huge impact not only on preventive strategies for metastatic brain tumors, but also on reducing the recurrence rate in already treated BM patients.
Highlights:
Brain microenvironment plays a key role in the development of BM. Interaction between the different cell populations within the brain and the metastatic tumor cells is crucial for the establishment of the metastatic niche and the progression of BMs.
Immune cells promptly infiltrate the TME and interact with metastatic tumor cells early in the formation of BMs. The ability of BMs to escape immune-surveillance hinders the initial efforts of the immune system to mount an effective immune response towards the tumor, resulting ultimately in an immunosuppressive TME that contributes to the growth of the metastases.
Recent understandings of the brain immunology and immune/tumor crosstalk have resulted in an increasing number of pre-clinical and clinical trials for immunotherapies in BM patients.
The success of immune-based therapies in other malignancies, together with the encouraging results cell and vaccine based immune-therapies in pre-clinical models suggests that immunotherapy could potentially fill the unmet need for treatment of BMs.
Acknowledgments
This work was supported by NIH grants R01-CA201148 (K.S.) and by DoD grant CA180698.
Glossary:
- Adoptive chimeric receptor (CAR) T-cell therapy
Adoptive chimeric receptor (CAR) T-cell therapy consists of generically modifying T-cells expressing CARs that can target and bind to antigens expressed on tumor cells. Once these CAR T-cells are transferred back to the patients, an adaptive immune response against the tumor is induced
- Blood Brain Barrier
Highly selective barrier that imposes a physical boundary between the CNS and the rest of the body, regulating the entry of ions, molecules and cells into the brain and spinal cord
- Brain Metastasis
Secondary brain tumor originated from cancer cells that have spread to the brain from primary tumor sites
- Cancer vaccine
Mostly composed of tumor-derived peptides or ex vivo maturated cells, mainly DCs. Once implanted in a patient, induces an immune response towards the tumor
- Carboxyl esterase
Enzyme that hydrolyzes the prodrug CPT-11 to an inhibitor of the topoisomerase 1
- Immunotherapy
Form of treatment that aims at boosting the immune system of the patient to recognize and attack tumor cells
- Immune Checkpoint Molecules
Main regulators of immune pathways. Interaction of immune checkpoint receptors such as PD-1 or CTLA-4 — expressed on T-cells and their respective ligands expressed on tumor cells and a number of immune cells within the TME leads to inactivation of the TCR signaling and T cells elimination
- Metastases
Spreading of cancer cells from primary tumor sites to distal organs
- Metastatic cascade
Term used to describe the transformation associated with acquired metastatic capability, the journey of tumor cells through blood vessels or lymphatic system and formation of new colonies that grow into metastatic tumors in a specific organ
- Oncolytic virus
Virus that is able to selectively replicate inside tumor cells while sparing normal, healthy cells, eventually causing immunogenic cell death and evoking strong immune response locally
- Stem Cells
Undifferentiated cells with the potential to self-renewal and to develop into multiple cell lineages. They are able to migrate to different tissues (“homing”) as part of normal homeostasis or in response of signals of damage, such as inflammatory cytokines released by the TME
- TRAIL
TNF-related apoptosis inducing ligand, a pro-apoptotic cytokine which induces cell death via the extrinsic apoptosis pathway upon binding to its receptors DR4/5
- T-vec
Talimogene laherparepvec, an oncolytic herpes simplex virus engineered to express human GM-CSF and approved for its clinical use in melanoma patients
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interests
K.S. owns equity in and is a member of the Board of Directors of AMASA Therapeutics, a company developing stem cell-based therapies for cancer. K.S.’s interests were reviewed and are managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. The other authors declare that they have no competing interests.
References:
- 1.Gavrilovic IT, Posner JB, Brain metastases: epidemiology and pathophysiology. J Neurooncol 75, 5–14 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Cagney DN et al. , Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol 19, 1511–1521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schouten LJ, Rutten J, Huveneers HA, Twijnstra A, Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94, 2698–2705 (2002). [DOI] [PubMed] [Google Scholar]
- 4.Bafaloukos D, Gogas H, The treatment of brain metastases in melanoma patients. Cancer Treat Rev 30, 515–520 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Flanigan JC, Jilaveanu LB, Chiang VL, Kluger HM, Advances in therapy for melanoma brain metastases. Clin Dermatol 31, 264–281 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Valiente M et al. , The Evolving Landscape of Brain Metastasis. Trends Cancer 4, 176–196 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall WA, Djalilian HR, Nussbaum ES, Cho KH, Long-term survival with metastatic cancer to the brain. Med Oncol 17, 279–286 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Moravan MJ et al. , Current multidisciplinary management of brain metastases. Cancer 126, 1390–1406 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Berghoff AS, Preusser M, Role of the blood-brain barrier in metastatic disease of the central nervous system. Handb Clin Neurol 149, 57–66 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Tallet AV et al. , Neurocognitive function impairment after whole brain radiotherapy for brain metastases: actual assessment. Radiat Oncol 7, 77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telera S et al. , Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. J Neurooncol 113, 313–325 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Seyfried TN, Huysentruyt LC, On the origin of cancer metastasis. Crit Rev Oncog 18, 43–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fidler IJ, The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3, 453–458 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Pardridge WM, The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2, 3–14 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolson GL et al. , Brain metastasis: role of trophic, autocrine, and paracrine factors in tumor invasion and colonization of the central nervous system. Curr Top Microbiol Immunol 213 (Pt 2), 89–115 (1996). [DOI] [PubMed] [Google Scholar]
- 16.Sofroniew MV, Vinters HV, Astrocytes: biology and pathology. Acta Neuropathol 119, 7–35 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valiente M et al. , Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L et al. , Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q et al. , Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533, 493–498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing F et al. , Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Molecular Medicine 5, 384–396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priego N et al. , STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis article. Nature Medicine 24, 1024–1035 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Schulz M, Salamero-Boix A, Niesel K, Alekseeva T, Sevenich L, Microenvironmental Regulation of Tumor Progression and Therapeutic Response in Brain Metastasis. Frontiers in Immunology 10, 1713–1713 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorger M, Felding-Habermann B, Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol 176, 2958–2971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Q, Barres BA, Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18, 225–242 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Graeber MB, Scheithauer BW, Kreutzberg GW, Microglia in brain tumors. Glia 40, 252–259 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Shinonaga M, Chang CC, Suzuki N, Sato M, Kuwabara T, Immunohistological evaluation of macrophage infiltrates in brain tumors. Correlation with peritumoral edema. J Neurosurg 68, 259–265 (1988). [DOI] [PubMed] [Google Scholar]
- 27.Fitzgerald DP et al. , Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin Exp Metastasis 25, 799–810 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruger S et al. , Advances in cancer immunotherapy 2019 - Latest trends. Journal of experimental & clinical cancer research : CR 38, 268–268 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farber SH et al. , Embracing rejection: Immunologic trends in brain metastasis. Oncoimmunology 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pyonteck SM et al. , CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature Medicine 19, 1264–1272 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blazquez R et al. , PI3K: A master regulator of brain metastasis-promoting macrophages/microglia. GLIA 66, 2438–2455 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Pistilli B et al. , Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res Treat 168, 357–364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andreou KE et al. , Anti-inflammatory Microglia/Macrophages As a Potential Therapeutic Target in Brain Metastasis. Frontiers in Oncology 7, 251–251 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooijen N, Hendrikx E, Liposomes for specific depletion of macrophages from organs and tissues. Methods in molecular biology (Clifton, N.J.) 605, 189–203 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Du Four S et al. , Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. OncoImmunology 4, e998107–e998107 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du Four S et al. , Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. American Journal of Cancer Research 6, 2514–2531 (2016). [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y et al. , Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proceedings of the National Academy of Sciences of the United States of America 109, 17561–17566 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louveau A et al. , Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Absinta M et al. , Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacobs JF et al. , Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol 11, 394–402 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kluger HM et al. , Characterization of PD-L1 Expression and Associated T-cell Infiltrates in Metastatic Melanoma Samples from Variable Anatomic Sites. Clin Cancer Res 21, 3052–3060 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harter PN et al. , Distribution and prognostic relevance of tumor-infiltrating lymphocytes (TILs) and PD-1/PD-L1 immune checkpoints in human brain metastases. Oncotarget 6, 40836–40849 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berghoff AS et al. , Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology 5, e1057388 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lorger M, Andreou T, Fife C, James F, Immune Checkpoint Blockade – How Does It Work in Brain Metastases? Frontiers in Molecular Neuroscience 12, 282–282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zakaria R et al. , T-Cell Densities in Brain Metastases Are Associated with Patient Survival Times and Diffusion Tensor MRI Changes. Cancer Res 78, 610–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuchs TL et al. , Assessment of Tumor-infiltrating Lymphocytes Using International TILs Working Group (ITWG) System Is a Strong Predictor of Overall Survival in Colorectal Carcinoma. The American Journal of Surgical Pathology 44, 536–544 (2020). [DOI] [PubMed] [Google Scholar]
- 47.Gooden MJM, De Bock GH, Leffers N, Daemen T, Nijman HW, The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. British journal of cancer 105, 93–103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loi S et al. , Tumor-infiltrating lymphocytes and prognosis: A pooled individual patient analysis of early-stage triple-negative breast cancers. Journal of Clinical Oncology 37, 559–569 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miksch RC et al. , Prognostic impact of tumor-infiltrating lymphocytes and neutrophils on survival of patients with upfront resection of pancreatic cancer. Cancers 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mustafa DAM et al. , T lymphocytes facilitate brain metastasis of breast cancer by inducing Guanylate-Binding Protein 1 expression. Acta Neuropathol 135, 581–599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.B C-D et al. , Tumor microenvironment differences between primary tumor and brain metastases. Journal of translational medicine 18, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sevenich L, Turning “Cold” into “Hot” tumors - Opportunities and challenges for radio-immunotherapy against primary and metastatic brain cancers. Frontiers in oncology 9, 163–163 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castaneda CA et al. , Impact of pathological features of brain metastases in prognosis. Biomarkers in Medicine 12, 475–485 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Fischer GM et al. , Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discovery 9, 628–645 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim R et al. , Differences in tumor microenvironments between primary lung tumors and brain metastases in lung cancer patients: Therapeutic implications for immune checkpoint inhibitors. BMC Cancer 19, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogiya R et al. , Comparison of immune microenvironments between primary tumors and brain metastases in patients with breast cancer. Oncotarget 8, 103671–103681 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.In GK et al. , Molecular profiling of melanoma brain metastases (MBM) compared to primary cutaneous melanoma (CM). Journal of Clinical Oncology 37, 9565–9565 (2019). [Google Scholar]
- 58.Kluger HM et al. , Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. Journal of Clinical Oncology 37, 52–60 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Giacomo AM et al. , Immunotherapy of brain metastases: Breaking a “dogma”. J Exp Clin Cancer Res 38, 419–419 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tawbi HA-H et al. , Efficacy and safety of the combination of nivolumab (NIVO) plus ipilimumab (IPI) in patients with symptomatic melanoma brain metastases (CheckMate 204). Journal of Clinical Oncology 37, 9501–9501 (2019). [Google Scholar]
- 61.Kaufman HL, Kohlhapp FJ, Zloza A, Oncolytic viruses: A new class of immunotherapy drugs. Nature Reviews Drug Discovery 14, 642–662 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samson A et al. , Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Science Translational Medicine 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh M et al. , Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain. Nature Communications 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Totsch SK et al. , Oncolytic herpes simplex virus immunotherapy for brain tumors: current pitfalls and emerging strategies to overcome therapeutic resistance. Oncogene 38, 6159–6171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez-Quintanilla J, Seah I, Chua M, Shah K, Oncolytic viruses: overcoming translational challenges. J Clin Invest 129, 1407–1418 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du W et al. , Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proceedings of the National Academy of Sciences of the United States of America 114, E6157–E6165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Priceman SJ et al. , Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2 + breast cancer metastasis to the brain. Clinical Cancer Research 24, 95–105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah K, Mesenchymal stem cells engineered for cancer therapy. Advanced drug delivery reviews 64, 739–748 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanojia D et al. , Neural Stem Cells Secreting Anti-HER2 Antibody Improve Survival in a Preclinical Model of HER2 Overexpressing Breast Cancer Brain Metastases. Stem Cells 33, 2985–2994 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bagci-Onder T, Du W, Figueiredo J-L, Martinez-Quintanilla J, Shah K, Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells. Brain : a journal of neurology 138, 1710–1721 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong SH et al. , Human neural stem cells expressing carboxyl esterase target and inhibit tumor growth of lung cancer brain metastases. Cancer Gene Therapy 20, 678–682 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Joo KM et al. , Human neural stem cells can target and deliver therapeutic genes to breast cancer brain metastases. Molecular Therapy 17, 570–575 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leibold AT, Monaco GN, Dey M, The role of the immune system in brain metastasis. Current neurobiology 10, 33–48 (2019). [PMC free article] [PubMed] [Google Scholar]
- 74.Nitschke NJ, Bjoern J, Iversen TZ, Andersen MH, Svane IM, Indoleamine 2,3-dioxygenase and survivin peptide vaccine combined with temozolomide in metastatic melanoma. Stem Cell Investigation 4, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Quail DF, Joyce JA, The Microenvironmental Landscape of Brain Tumors. Cancer Cell 31, 326–341 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Preusser M et al. , Brain metastases: pathobiology and emerging targeted therapies. Acta Neuropathol 123, 205–222 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Chamberlain MC, Baik CS, Gadi VK, Bhatia S, Chow LQM, Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro-oncology 19, i1–i24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cruz-Munoz W, Man S, Xu P, Kerbel RS, Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res 68, 4500–4505 (2008). [DOI] [PubMed] [Google Scholar]
- 79.Su Z, Niu W, Liu ML, Zou Y, Zhang CL, In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun 5, 3338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Torper O et al. , Generation of induced neurons via direct conversion in vivo. Proc Natl Acad Sci U S A 110, 7038–7043 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kienast Y et al. , Real-time imaging reveals the single steps of brain metastasis formation. Nature medicine 16, 116–122 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Giancotti FG, Mechanisms governing metastatic dormancy and reactivation. Cell 155, 750–764 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang T et al. , Multitargeted Nanoparticles Deliver Synergistic Drugs across the Blood–Brain Barrier to Brain Metastases of Triple Negative Breast Cancer Cells and Tumor-Associated Macrophages. Advanced Healthcare Materials 8, 1900543 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Chen X et al. , A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget 7, 27764–27777 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yi BR, Kim SU, Choi KC, Synergistic effect of therapeutic stem cells expressing cytosine deaminase and interferon-beta via apoptotic pathway in the metastatic mouse model of breast cancer. Oncotarget 7, 5985–5999 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


