Abstract
An extensive epidemiological literature indicates increased exposure to tobacco retail outlets (TROs) places never-smokers at greater risk for smoking uptake and current smokers at greater risk for increased consumption and smoking relapse. Yet research into the mechanisms underlying this effect has been limited. This preliminary study represents the first effort to examine the neurobiological consequences of exposure to personally-relevant tobacco retail outlets among both smokers (n = 17) and non-smokers (n = 17). Individuals carried a GPS tracker for two weeks. Traces were used to identify tobacco retail outlets and control outlets that fell inside and outside their ideographically-defined activity space. Participants underwent fMRI scanning during which they were presented with images of these storefronts, along with similar store images from a different county and rated their familiarity with these stores. A main effect of activity space was additive with a smoking status × store type interaction, resulting in smokers exhibiting greater neural activation to TROs falling inside activity space within the parahippocampus, precuneus, medial prefrontal cortex and dorsal anterior insula. A similar pattern was observed for familiarity ratings. Together, these preliminary findings suggest that the otherwise distinct neural systems involved in self-orientation/self-relevance and smoking motivation may act in concert and underlie tobacco retail outlet influence on smoking behavior. This study also offers a novel methodological framework for evaluating the influence of community features on neural activity that can be readily adapted to study other health behaviors.
INTRODUCTION
There is widespread interest in developing tobacco control policies with greater potential for large-scale impact [1]. One policy that has received particular attention in recent years focuses on changes and restrictions to the sales and marketing of tobacco products, particularly in the retail environment (i.e. tobacco retail outlets or TROs) where tobacco companies spend nearly 90% of the $8.6 billion dollar marketing budget [2]. There are approximately 375,000 TROs in the United States [3]. Tobacco products are sold at many locations necessary for daily living, such as grocery stores and gas stations. Mounting evidence indicates that TRO exposure is a significant risk factor for initiation of smoking among adolescents and young adults [4,5], urges to smoke among ongoing smokers [6] and relapse after quitting smoking [7,8]. Moreover, the geographic distribution of TROs is distinctly non-random, clustering in communities with lower socioeconomic status and larger minority populations [9]. Thus, TROs may contribute to the substantial health disparities observed in tobacco-related diseases [10,11]. Unfortunately, the scientific literature available to guide policy changes with regards to TROs is still relatively limited. Within the field of tobacco regulatory science, researchers have begun calling for the increased application of paradigms from neuroscience and other basic science fields to tobacco control issues [12,13]. These approaches have the potential to offer objective measures of potential policy impacts and can facilitate a deeper understanding of mediating mechanisms, providing a theoretical framework to guide optimal strategies.
The present study was designed as an initial attempt to characterize the neural responses of smokers and non-smokers to TROs. Such responses are likely multifaceted. They may be driven by basic cognitive processes - such as spatial memory or personal-relevance/familiarity. Numerous studies have documented the roles for hippocampal regions in object recognition, spatial memory, and navigation [14–16]. Midline regions (i.e. precuneus, medial prefrontal cortex) may also play a crucial role given their involvement in processing self-relevant information [17,18]. Neural activations to TROs could also be driven by the same appetitive conditioning processes studied in traditional drug cue reactivity research. These typically include subregions of the insula associated with drug use urges, as well as the sub-cortical regions involved in reward and emotion (i.e. striatum, amygdala) [19,20].
A growing literature suggests that personally-relevant smoking scenes, when integrated into a standard smoking cue reactivity paradigm, yield significantly larger effects on cue-provoked craving and also increase affective response, vividness ratings and physiological signals of arousal [21]. Subsequent work revealed this approach also enhances neural activation in some of the key brain regions noted above, including the hippocampus and insula). Moreover, these increased activations predicted smoking behavior in a subsequent laboratory task [22]. Accordingly, we sought to extend this work on personally-relevant smoking stimuli by developing a procedure for identifying personally-relevant TROs for examination in subsequent laboratory tasks. Participants carried a global positioning system (GPS) device for two weeks, during which their activity patterns were used to identify outlets that fell inside or outside of their personal activity space. Images of these storefronts were then used in subsequent neuroimaging and laboratory sessions aimed at characterizing neural and behavioral responses. In response to TROs that fell inside their personal activity space, we hypothesized that: (1) smokers would report greater familiarity and likelihood of tobacco purchase relative to all other trial types; (2) smokers would exhibit greater neural activation to personally-relevant TROs relative to all other trial types in brain regions previously shown to be activated by personally-relevant cues or involved in the processing of personally-relevant information (i.e. hippocampus, parahippocampus, precuneus, medial prefrontal cortex); and (3) smokers would exhibit greater neural activation to personally-relevant TROs relative to all other trial types in brain regions involved in smoking urge and drug cue reactivity (i.e. insula, striatum, amygdala). Identifying these neural signatures is a critical step in determining the cognitive mechanisms mediating TROs influence on smoking behavior that can in turn help guide the pursuit of optimal policy strategies for curtailing use.
MATERIALS AND METHODS
The first phase of this project involved developing and curating a database of local TROs using an established store-audit procedure[23]. These procedures are aimed at identifying all TROs within a target region and quantifying relevant features (e.g. store types, storefront advertising). In addition to standard audit procedures, all store exteriors were photographed during this stage. A total of 241 unique TROs were identified within Durham, NC (i.e. study county). In addition, we identified 100 retail outlets Durham, NC that did not sell tobacco to serve as store-type controls (e.g. clothing stores, bookstores). Control outlets were obtained from a database of all registered businesses and selected to match the geographic distribution of TROs (see supplement for additional details). Lastly, a set of 12 TROs and 12 control outlets were selected from Forsyth, NC (hereby termed “Different County”), which has similar geography and demographics to the study county. These served as an additional outside activity-space control, as well as a fixed (versus personally-tailored) stimulus set that could be shown during the MRI. Different County outlets were selected to be proportional to the different types of TRO and control outlets in the study county databases. All store exteriors were photographed using a standardized procedure. Store audits occurred between October, 2015 and January, 2016. Additional procedural details are included in the supplemental material.
In the second phase, smokers and non-smokers were recruited into a neuroimaging study beginning in February, 2016 and continuing through June, 2017. After carrying GPS devices for two weeks, GPS tracks were downloaded and cross-referenced with the TRO and control outlet databases to identify personally relevant (i.e. stores falling within activity space for a given participant) and not personally-relevant (i.e. outside of activity space) outlets. Images of these and other storefronts were presented during an MRI scan and laboratory session in order to characterize neural and behavioral responses to these specific outlets.
Participants
A total of 20 smokers and 17 non-smokers completed all study procedures. To be eligible for participation, smokers had to report smoking ≥ 5 cigarettes per day for ≥ 1 year, have a breath carbon monoxide (CO) level > 8 ppm or a urinary cotinine level ≥ 1000 ng/mL at the baseline visit, and report no interest in quitting for the duration of the experiment. Non-smokers had to report having smoked less than 100 cigarettes in their lifetime and have a breath CO level < 4 ppm at the baseline visit. All participants were between the ages of 18 and 55, right-handed, generally healthy and had to report living and (if employed) working in the study county. Additional exclusion criteria and details on enrollment and attrition are available in the supplemental material.
General Procedures
All participants completed an initial screening session consisting of informed consent, vital signs, confirmation of smoking status via breath CO (Vitalograph Inc., Lenexa, KS) and confirmation that participants were not currently intoxicated via breath alcohol readings (Intoximeters Inc., St. Louis, MO; required at all visits). A urine sample was obtained for drug screening, cotinine assessment (if smoker and CO ≤ 8) and pregnancy testing (if female). Next, participants completing a battery of questionnaires assessing demographics, medical and smoking history, and MRI safety. All participants completed a brief measure of alcohol dependence [24]. Smokers also completed measures of nicotine dependence (Fagerström Test for Cigarette Dependence [25]) and motivation to quit smoking (Contemplation Ladder [26]).
Eligible participants then attended a training session in which they completed a practice MRI task to familiarize them with the scanning environment and use of the scanner response boxes. Images presented during the practice task were fixed non-smoking locations and did not overlap with images used in the primary task. Afterwards, participants received training in the use of a GPS logger and completion of daily “Purchasing Behavior” surveys. Participants were provided with an i-Blue 747 Data Logger and GPS Receiver and instructed to carry it on their person at all times and charge it nightly. Devices were set to sample at 30 second intervals.a In the Purchasing Behavior surveys (completed each evening), smokers were asked to identify any locations where they purchased cigarettes or other tobacco products that day and the type(s) of product purchased. Non-smokers completed equivalent surveys focused on alcohol purchases. Device-return visits were scheduled at least 14 days after the initial session, after which activity traces were extracted and processed through an established pipeline to identify TROs and control outlets for viewing during the MRI and laboratory visits (procedures detailed below). Given our primary interest was in relative contact to different stores within individuals and not absolute store contact, if an insufficient number of store contacts were obtained within two weeks, participants were permitted to carry the device for an additional two weeks and tracks over all collection periods were combined.
Smokers were required to abstain from tobacco and all other nicotine products for 24 hours prior to MRI visits (verified by CO ≤ 5 ppm). MRI scanning was conducted on a 3T GE MR750 scanner that included localizers/shimming, resting-state and task-based functional scans, as well as a structural scan to be used in registration. Details on the scanning sequences are available in the Supplemental Material. During the MRI store reactivity task (described in detail below), participants were presented with images of both TROs and control outlets that fell inside and outside their individual activity space within the study county, as well as the standard set from a different county. They also rated images using a 1 (Not familiar at all) to 8 (Extremely familiar) scale in response to the following instructions “Each time a store photo appears on the screen, please rate how familiar you are with this specific store”. After the MRI, participants attended a final laboratory session during which they viewed one image of each storefront and rated it using a 1 (Not at all likely) to 8 (Extremely likely) scale in response to the following question “How likely would you be to purchase tobacco products from this specific store?”
GPS Tracking Procedures and Outlet Identification
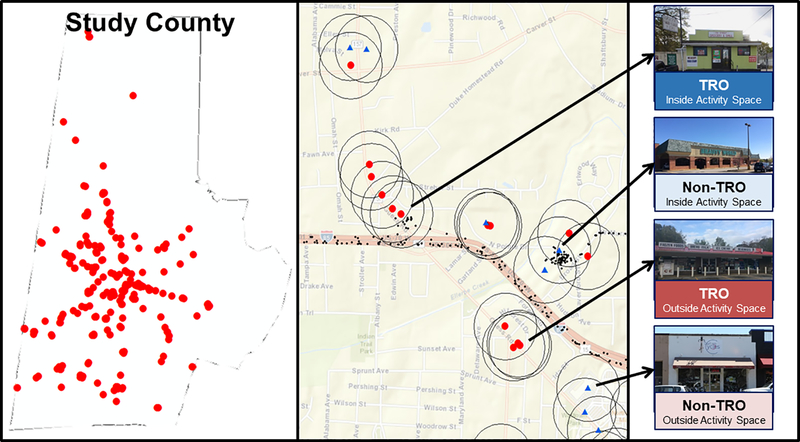
Custom Python scripts were used to characterize outlet interactions. Circular buffers of 200m, 500m and 1000m were drawn around each outlet and the total sum of points falling within these buffers during the entire tracking period was calculated for each outlet and buffer. The 12 outlets with the largest number of points falling within the 200m buffer were considered to fall “Inside Activity Space” for each participant. In the event of a tie, or on occasions where participants did not have 12 outlets from each category with at least 1 activity point within a 200m buffer, the 500m and 1000m buffers were used to determine activity space. For smokers, any TRO they reported purchasing tobacco from in the daily surveys was considered to fall “Inside Activity Space” regardless of whether this contact was captured by the GPS tracks. Stores were considered to be “Outside Activity Space” if no GPS points fell within the 500m buffer at any point during the tracking period. A random selection of 12 TROs and 12 control outlets that met criteria were selected for presentation during the MRI task. See Figure 1.
Figure 1.
Left - Image of study county with TRO locations overlaid; Center - Sample GPS tracks (small black points) used to identify TROs (red circles) and Control Outlets (blue triangles); Right – Example images of storefronts inside and outside activity space based on sample GPS tracks.
MRI Task
In the store reactivity task, two images of each storefront were presented for 4 seconds each in an event-related design. This resulted in 144 total trials divided evenly among 6 categories: (1) Different County control outlets (e.g., clothing store); (2) Different County TROs; (3) Outside Activity Space control outlets; (4) Outside Activity Space TROs; (5) Inside Activity Space control outlets; and (6) Inside Activity Space TROs. The task was divided into 4 runs, each with a duration of 306 seconds. Each store image was presented only a single time during the task. The inter-trial interval was jittered between 2 and 8 seconds in a pattern designed to minimize overlap in estimates of the hemodynamic response [27].
Data Processing and Analysis
BOLD signal was processed using FSL version 5.0.9. Brain extraction was performed on structural images using the FSL BET tool (see supplement for extraction and QA parameters). Functional images were corrected for interleaved slice acquisition; high-pass filtered (50s); spatially smoothed using an 8mm FWHM Gaussian kernel; and registered to an MNI 2mm space using 12DOF linear transformation for registering to subject-anatomical scans and a 12DOF nonlinear transformation for registration to standard space. Motion correction was done using MCFLIRT [28]. Volumes with extreme outlying values for motion (trial-by-trial root-mean-square [RMS] intensity differences ≥ 90, approximately 4 standard deviations from the grand mean) were removed using voxelwise confound regressors [29] and translation and rotation parameters were included to address residual motion variance. Each image presentation was modeled as a 4s boxcar function convolved with a double-gamma HRF. Fixation crosses presented during ITIs remained un-modeled to serve as an implicit baseline in analyses.
Primary analyses were conducted using an ROI-based approach to maximize power to detect effects in a priori regions. Detailed descriptions, images of all ROIs and code for generating them are presented in the Supplemental Material. Percent signal change was extracted from the second level (i.e. after collapsing across runs) using the FSL featquery tool. As the paradigm includes manipulations of both activity space and store type (i.e. tobacco versus control), we focused our analysis on brain regions highlighted in prior research that manipulated either stimulus familiarity or smoking relevance. Our primary focus was on regions involved in spatial perception, memory and self-relevance including: (1) Anterior Hippocampus; (2) Posterior Hippocampus; (3) Parahippocampus; (4) Precuneus; and (5) Medial Prefrontal Cortex. Given our strong a priori hypotheses for these regions based on the nature of our paradigm, Type I error was set to p < .05 for these primary ROIs. Secondary regions focused on those involved in smoking cue reactivity, including: (6) Ventral Anterior Insula; (7) Dorsal Anterior Insula; (8) Ventral Striatum; (9) Dorsal Striatum; and (10) Amygdala. Secondary regions were evaluated at the p < .01 level to minimize risk of Type I error.
Given the novelty of the paradigm, additional exploratory voxelwise analyses were conducted to identify other potential activations outside of hypothesized regions. Three specific contrasts were examined in this stage: (1) Overall Activity Space (Inside Activity Space TROs/Control Outlets > Outside Activity Space TROs/Control Outlets); (2) Overall Store Type (Outside/Inside Activity Space TROs > Outside/Inside Activity Space Control Outlets); and (3) Inside Activity Space Store Type (Inside Activity Space TRO > Inside Activity Space Control outlet). These contrasts were chosen as they reflect either the most robust task manipulations (1 and 2) or the contrast most central to our goals (3). For these exploratory whole-brain analyses, we used a stricter error correction procedure (Z = 3.1; cluster correction p < .05).
RESULTS
Data Quality Assurance and Participant Characteristics
A total of 20 smokers and 17 non-smokers completed all study procedures. Three smokers exhibited outlying RMS intensity difference values on > 5% of trials and were therefore excluded from analyses. Smokers and non-smokers did not differ on any key sample characteristics (all p’s > .1; Table 1). In addition, smokers and non-smokers did not differ in the average number of days their GPS tracks exhibited motion, indicating the tracker was carried on the participant for at least a portion of the day (Smokers: M = 13.1, SD 3.3; Non-Smokers: M = 13.9, SD = 3.6; F = 0.47, p = .497).
Table 1.
Sample Characteristics and Outlet Exposure
| Non-Smokers (N = 17) | Smokers (N = 17) | |
|---|---|---|
|
| ||
| Mean (SD) or % | Mean (SD) or % | |
| Demographic Characteristics | ||
| Age | 32.5 (12.2) | 37.1 (9.2) |
| Gender (% female) | 47.1% | 47.1% |
| Race | ||
| Black | 58.8% | 64.7% |
| White | 29.4% | 23.5% |
| Other | 11.8% | 11.8% |
| Ethnicity (% Hispanic) | 11.8% | 11.8% |
| Education (% ≤ HS Diploma) | 29.4% | 23.5% |
| Smoking and Drinking Characteristics | ||
| CPD | --- | 13.2 (5.4) |
| Years Smoking | --- | 19.5 (9.4) |
| FTCD | --- | 5.3 (1.9) |
| Contemplation Ladder | --- | 4.1 (2.9) |
| ADS | 1.2 (1.7) | 2.1 (2.4) |
| Total Store Exposure | ||
| # Unique TROs Encountered | 67.1 (33.2) | 88.6 (36.4) |
| # Unique Control Outlets Encountered | 31.2 (14.1) | 37.9 (16.8) |
| # of TRO Encounter Occurrences | 437.9 (327.2) | 521.2 (388.1) |
| # of Control Outlet Encounter Occurrences | 241.7 (215.1) | 194.2 (169.0) |
| Total Amount of TRO Exposure (# of points) | 5,242.8 (6,028.7) | 11,089.2 (15,321.0) |
| Total Amount of Control Outlet Exposure (# of points) | 6,013.5 (9,561.7) | 1,771.2 (1,639.1) |
| Inside Activity Space (Top 12) Store Exposure | ||
| # of TRO Encounters | 192.8 (149.2) | 167.5 (96.8) |
| # of Control Outlet Encounters | 168.3 (176.0) | 97.6 (76.2) |
| Total Amount of TRO Exposure (# of points) | 4,296.4 (6,066.7) | 9,294.2 (15,557.7) |
| Total Amount of Control Outlet Exposure (# of points) | 5,767.2 (9,525.6) | 1,284.8 (975.5) |
Note. HS = High School; CPD = Cigarettes Per Day; FTCD = Fagerström Test for Cigarette Dependence; ADS = Alcohol Dependence Scale (9-item). # Unique Outlets Encountered refers to the number of distinct stores of a given type each individual encountered (i.e. # of stores for which participants entered the 200m buffer). # Outlet Encounter Occurrences refers to the number of times an individual encountered any store of a given type over the 2-week data collection period. Once a participant entered the 200m buffer it was considered a single exposure until they exited the buffer and re-entered. Total Amount of Exposure refers to the total # of points (recorded by the GPS unit every 30 seconds) that fell within the 200m buffer for all stores of a given type.
Store Exposure Metrics
Data on store exposure is also included in Table 1. Exposure metrics are presented both for the full population of stores, as well as stores deemed “inside activity space” (i.e. 12 stores with the greatest exposure based on GPS point count or where tobacco purchases were made). Although the general pattern of findings was consistent with greater exposure to TROs among smokers and greater exposure to control outlets among non-smokers, none of these findings reached significance (all p’s > .05). To determine whether this effect could have been due to overall differences in activity between the groups or compliance with carrying GPS loggers, tracks were segmented by day for each participant and visually inspected for some evidence of activity that extended beyond a circumscribed area (presumably home residence) and thus indicated a participant had left their home on a given day. The pattern of findings was identical when exposure indices were standardized based on the number of days where evidence of movement was present in GPS traces.
Familiarity and Tobacco Purchase Likelihood Ratings
Prior to examining smoker and non-smoker differences in ratings across trial types, a series of trial-level analyses were conducted to assess reliability of ratings and covariation with GPS exposure metrics. For familiarity ratings, the two images of each individual outlet exhibited a strong positive correlation overall (r =.774, p < .0001). Effects were similar when each of the 6 task conditions were examined separately (r = .674−.816, all p’s < .0001). For stores inside activity space, the average familiarity rating of the two images of each store was positively correlated with the number of GPS points that fell inside the 200m buffer for that store (r = .161, p < .001) following log10 transformation of the GPS points. When broke down by store type, results were comparable for both Control Outlets (r = .126, p = .011) and TROs (r = .139, p < .005). Three participants had > 10% missing responses for familiarity ratings, but results did not differ when these participants were excluded (see Supplemental Material).
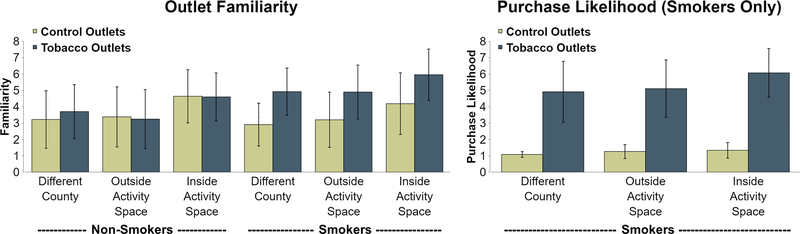
Primary analyses of familiarity data revealed main effects for Activity Space (F = 25.8, p < .0001) and Store Type (F = 40.1, p < .0001), as well as a Smoking Status × Store Type interaction (F = 32.0, p < .0001). For Activity Space, pairwise contrasts indicated significantly higher familiarity ratings for Inside Activity Space stores relative to both Different County (p < .0001) and Outside Activity Space stores (p < .0001), but no differences in familiarity ratings between Different County and Outside Activity Space stores (p = .972). Breakdown of the Smoking Status × Store Type interaction indicated smokers reported significantly greater familiarity with TROs relative to Control Outlets (p < .0001), whereas this effect was absent for non-smokers (p = .543). When further broken down across Activity Space, smokers reported substantially greater familiarity with TROs across each level of activity space (p’s < .001) while for non-smokers this difference in familiarity was comparatively smaller for Different County outlets (p = .013) and entirely absent for Outside Activity Space (p = .384) and Inside Activity Space (p = .880).
For tobacco purchase likelihood data, an extremely robust effect of store type was present (F = 461.9, p < .0001), indicating smokers were able to correctly identify TROs. When considering only TROs, a main effect of Activity Space was observed (F = 10.9, p < .001). As with familiarity data, there were no differences were seen between Different County TROs and Outside Activity Space TROs (p = .478), but participants indicated they were significantly more likely to purchase tobacco from Inside Activity Space TROs relative to both Different County TROs (p < .001) and Outside Activity Space TROs (p < .001). For Inside Activity Space TROs, tobacco purchase likelihood was significantly correlated with familiarity (r = .301, p < .0001), but was not related to outlet exposure (r = −.027, p = .585). Primary findings for both familiarity and purchasing likelihood are presented in Figure 2.
Figure 2.
Ratings of Familiarity and Purchasing Likelihood by Smoking Status, Activity Space and Store Type. Bars represent standard deviation. For familiarity (left & center), both smokers and non-smokers report greater familiarity with outlets inside activity space whereas only smokers show a store type effect and report greater familiarity with tobacco versus control outlets. Higher likelihood of purchasing tobacco (right; evaluated in smokers only) was found for outlets inside activity space and for tobacco versus control stores.
Neuroimaging Outcomes – Regions of Interest
Primary Regions (Anterior/Posterior Hippocampus, Parahippocampus, Precuneus, mPFC)
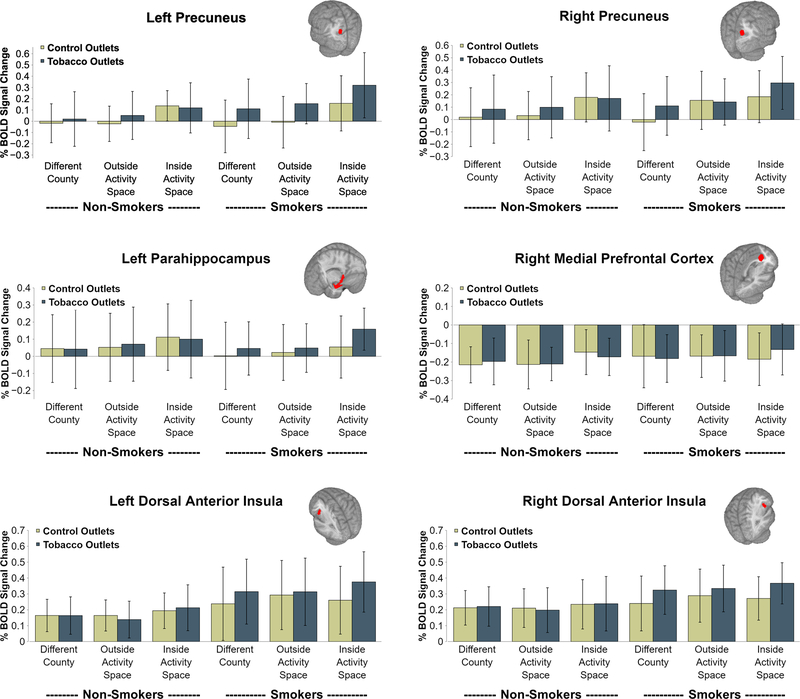
For both the left and right hemispheres across all 5 brain regions, main effects of activity space were observed (all p’s < .05). In each case, these effects were driven by greater activation to Inside Activity Space outlets relative to both Outside Activity Space outlets (right Posterior Hippocampus p < .1; all other p’s < .01) and Different County outlets (all p’s < .05). In addition, a significant Smoking Status × Store Type interaction was observed for left Precuneus (F = 14.8, p < .001), right Precuneus (F = 10.4, p = .002) and left Parahippocampus (F = 5.5, p = .020). In all cases these effects were driven by significantly greater activation to TROs relative to control outlets among smokers (all p’s < .01), with weak or absent Store Type effects for non-smokers (all p’s > .05). Lastly, for right medial Prefrontal Cortex a significant Smoking Status × Activity Space × Store Type interaction was found (F = 4.1, p = .018). This was driven by a significant Store Type effect indicating relatively greater activation to TROs relative to control outlets among Inside Activity Space outlets for smokers (p = .002), with no Store Type effect for other Activity Space levels for smokers or any Store Type effect for non-smokers (all p’s > .1). Notably, across all regions effects the effect of Activity Space was either interactive (in the case of the mPFC) or additive (in the case of the Precuneus and Parahippocampus) additive with the Smoking Status × Store Type effect, resulting in smokers but not non-smokers exhibiting greater activation to Inside Activity Space TROs relative to all other trial types (all p’s < .001). See Figure 3 for plots depicting findings for left and right Precuneus, left Parahippocampus and right medial Prefrontal Cortex. Detailed findings for all primary regions are available in the Supplementary Material.
Figure 3.
BOLD activation as a function of trial type and smoking status within regions of interest. Bars represent standard deviation. The pattern of findings was consistent with ratings data. Main effects of activity space were found across both groups, with greater brain activation to inside activity space outlets. Only smokers showed a store type effect, with greater brain activation to tobacco versus control outlets.
Secondary Regions (Dorsal/Ventral Anterior Insula, Dorsal/Ventral Striatum, Amygdala)
As with primary regions, a main effect of activity space was observed across multiple regions (left and right Amygdala, left dorsal Anterior Insula, left and right dorsal Striatum; all p’s < .005). Also as with primary regions, in each case this effect was driven by greater activation to Inside Activity Space TROs relative to both Outside TROs (all p’s < .01) and Different County outlets (all p’s < .05). The Smoking Status × Store Type effect was significant for only the left (F = 13.4, p < .001) and right (F = 12.3, p < .001) dorsal Anterior Insula. In both cases these findings paralleled results from primary regions, with significant Store Type effects present for smokers (p’s < .001) and absent for non-smokers (p’s > .8). Smokers again exhibited greater activation to Inside Activity Space TROs relative to all other trial types in the left dorsal Anterior Insula (p < .05). For the right dorsal Anterior Insula, Inside Activity Space TROs exhibited greater activation relative to control outlet trial types (all p’s < .01), but not other TRO trial types (p’s > .1).
Exploratory Voxelwise Analyses
Exploratory whole-brain analyses did not reveal any additional differences between smokers and non-smokers for the Inside Activity Space > Outside Activity Space contrast. However, effects did emerge for the TRO > Control Outlet contrast, as well as the more specific Inside Activity Space TRO > Inside Activity Space Control Outlet contrasts. For the former contrast, smokers exhibited greater BOLD activation in the right temporal occipital fusiform cortex (overlapping into cerebellum), the right inferior temporal gyrus and the left and right lateral occipital cortex relative to non-smokers. An effect was also found for the right precuneus, though this was partially (but not completely) overlapping with the a priori precuneus ROI. For the latter contrast, smokers exhibited greater BOLD activation in the right frontal pole, the left orbitofrontal cortex and the left precuneus (again partially overlapping with the ROI) relative to non-smokers. Across both contrasts, non-smokers exhibited greater activation than smokers only in the pre- and post-central gyrus. See Table 2 for cluster information and Supplementary Material for a visual depiction.
Table 2.
Cluster details from voxelwise analysis
| X | Y | Z | Area (# Voxels) | Zmax | |
|---|---|---|---|---|---|
| IT + OT > IN + ON | |||||
| SMK > NS | |||||
| R Precuneus | 15.8 | −63.2 | 25.1 | 321 | 4.21 |
| R Temporal Occipital Fusiform Cortex | 22.3 | −49.9 | −13.9 | 275 | 3.97 |
| R Inferior Temporal Gyrus | 63.1 | −51 | −12.2 | 195 | 3.92 |
| R Lateral Occipital Cortex | 40.4 | −80 | 32.4 | 192 | 4.07 |
| L Lateral Occipital Cortex | −28.4 | −83.9 | 29.4 | 183 | 4.10 |
| NS > SMK | |||||
| R Precentral Gyrus | 36.1 | −24 | 58.4 | 1097 | 4.58 |
|
| |||||
| IT > IN | |||||
| SMK > NS | |||||
| R Frontal pole | 31.3 | 52.3 | 13 | 207 | 3.84 |
| L Precuneus | −8.42 | −65.2 | 35.5 | 177 | 3.95 |
| L Orbitofrontal Cortex | −30 | 22 | −11.6 | 175 | 4.18 |
| NS > SMK | |||||
| R Precentral Gyrus | 24.5 | −23 | 64.7 | 183 | 4.03 |
Note. XYZ reflect coordinates for cluster center of gravity (original voxel space). SMK = Smoker; NS = Non-Smoker. IT = Inside Activity Space Tobacco Outlet; OT = Outside Activity Space Tobacco Outlet; IN = Inside Activity Space Control Outlet; ON = Outside Activity Space Control Outlet.
DISCUSSION
Results from this study indicate smokers exhibit differential neurobiological responses to personally-relevant TROs compared to TROs with less personal relevance and control outlets. These findings provide the first insight into the neural mechanisms that may underlie the widely-documented epidemiological association between tobacco retail outlet exposure and smoking behavior [30,31]. To our knowledge, this was both the first study to use GPS tracking to ideographically identify community-level features for use in a functional neuroimaging study, as well as the first to examine neural responses to exterior storefronts. Much of the research on TROs to date has entailed so-called ‘black-box’ epidemiologic approaches [32], in which risk factors are identified with only minimal heed to mechanisms or the potential implications for intervention. Limitations to this approach have long been recognized within the broader epidemiology literature, with calls made to increase the emphasis on mediating mechanisms, particularly across multiple levels of analysis [33,34]. Though a first step, this effort to unpack the epidemiological “black box” provides an opportunity to deepen our understanding of how community features can serve to influence behavior [35].
Neural findings spanned a number of brain regions, including those involved in spatial memory, self-relevance and smoking cue reactivity [36–38]. Across all regions, these findings were characterized by a main effect of Activity Space that was additive with a Smoking Status? Store Type interaction, resulting in the highest levels of activation occurring in response to Inside Activity Space TROs among smokers. The effect of activity space is consistent with prior research showing similar regions are recruited when individuals are shown familiar landmarks and asked to compare them based on spatial information and/or their own prior experiences [39]. One study found that the hippocampus is only involved in processing of recently learned locations, whereas processing of highly familiar locations evoked activation in the precuneus, posterior cingulate and parahippocampus [40]. This is generally consistent with findings from the present study, where tobacco retail outlets inside activity space (and thus presumably more familiar) evoked stronger activation in comparison to those outside activity space in these regions than in the hippocampus itself. Interestingly, numerous brain regions shown to be sensitive to smoking cues in previous work and meta-analyses (e.g. striatum, amygdala) [19,20,41] did not exhibit differential activation to TROs in the present study. However, differential activation was observed in the insula, which has perhaps received the most attention as a critical pathway for smoking urge and behavior [38,42,43]. It is intriguing that the general pattern of effects (main effect of activity space, smoking status × store type interaction) was present across both the regions involved in spatial memory and self-reference (i.e. mPFC, precuneus, parahippocampus) [16,41,44,45], as well as those involved in smoking cue reactivity [42,46]. Though speculative, the effects may reflect integration of cognitive and appetitive conditioning processes that ultimately serves to govern complex reward-seeking behavior like tobacco purchasing. Additional confirmatory work is needed before firm conclusions around this matter should be drawn.
Although methodologically novel, the study also has a number of limitations. Foremost among these is the small sample size, which not only precluded examination of individual differences among smokers but also limited potential for further exploratory analyses examining the influence of various store characteristics (e.g. type of retail outlet, amount and nature of storefront advertising). The heterogeneity of products sold at certain TROs include other appetitive stimuli (e.g. alcohol, food) and our focus on naturalistic and personally-relevant stimuli render identification of optimal controls challenging. In light of evidence indicating smokers exhibit a generally heightened salience to appetitive stimuli it is possible that effects are partially driven by other products commonly sold at these stores [47]. Replication of these effects across a larger geographic region is also critical. Exclusive reliance on GPS to measure exposure is itself a limitation (i.e. physical proximity does not guarantee awareness) and the nature of exposures could differ in important ways (e.g. actual store visits versus stores at frequently traversed intersections). Inclusion of two activity space control conditions (Outside Activity Space and Different County) generated an imbalanced task design, which conceivably could have amplified the salience of Inside Activity Space outlets. Scans were also conducted under deprivation to enhance the salience of smoking-related stimuli [48,49] and better mimic conditions following a quit attempt, so may not generalize to other contexts, including ongoing smoking. Lastly, methods for validating GPS tracker data are not yet well-established and it is likely that additional technological and analytical advances will improve our ability to characterize store exposure.
Overall, this project scaffolds off several different areas within the tobacco use literature, spanning multiple levels of analysis, to provide new insights into factors that may influence tobacco use. Future research can expand on this methodological framework to examine whether the neural responses observed here predict relapse following a quit attempt or uptake among non-smokers. It also opens the door to developing neurobiologically-informed tests of potential policy interventions, such as examining the impact of image manipulations designed to remove storefront advertising or by assessing the cumulative neurobiological effects of various policy options to alter TRO density [50]. A number of additional analyses relevant to both basic and applied topics (e.g. dissociating effects of familiarity and purchase likelihood, trial-level analysis examining specific store features) were not tenable in the present project due to the modest sample size, but should be considered for subsequent work using this paradigm. Larger followup studies should consider inclusion of a larger number of stores along a full continuum of exposure to allow for more rigorous examination of the effects of exposure. It is also important to acknowledge that the results presented here are preliminary. Replication in a larger sample and additional efforts to better understand the underlying cause of neural activations is needed before policy applications can be considered. Regardless, the ‘community neuroscience’ approach developed herein can be readily adapted for the examination of contextual influences on other health behaviors, such as neural response to frequently encountered fast food restaurants and its relationship to diet. Just as the epidemiological literature has been hindered by a limited understanding of mechanisms, so too has neuroscience research been hindered by limited integration of naturalistic scenarios. By integrating these two fields, we have the potential to build upon the strengths and overcome the weaknesses of each. This will provide new insights into how community-level features influence human motivation and behavior, potentially accelerating the development of novel clinical interventions and providing a framework to guide new tobacco control policy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Ms. Rebecca Pratt for her assistance with data collection, Sara Vandegrift for coordination of the audit effort, and members of the UNC Center for Health Promotion and Disease Prevention for their assistance with ground-truthing and auditing tobacco retail outlet audits.
FUNDING AND DISCLOSURE
This work was funded by a grant from the Duke Cancer Institute to FJM. Additional support was provided by a Career Development Award from the National Institute on Drug Abuse (K23 DA042898) and a Brain & Behavior Research Foundation Young Investigator Award to JAO. KMR has received fees for serving as an expert consultant for in litigation against cigarette manufacturers and e-cigarette companies. The authors declare no other conflicts of interest.
Footnotes
During post-study data quality assurance checks, it was noted that subset of GPS trackers had a “smart tracking” feature enabled during the data collection procedure, which caused periodic intensification of the sampling rate. Additional details and analyses are presented in the supplementary material. Findings indicate this technical problem had minimal impact on store exposure metrics or store selection.
Declaration of Competing of Interests: None
Clinical Trial Registration: N/A
REFERENCES
- 1.Thomas S, Fayter D, Misso K, Ogilvie D, Petticrew M, Sowden A, et al. Population tobacco control interventions and their effects on social inequalities in smoking: systematic review. Tobacco control. 2008;17(4):230–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Federal Trade Commission. (Federal Trade Commission, Washington, DC, 2019). [Google Scholar]
- 3.Rodriguez D, Carlos HA, Adachi-Mejia AM, Berke EM, Sargent JD. Predictors of tobacco outlet density nationwide: a geographic analysis. Tobacco Control. 2013;22(5):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johns M, Sacks R, Rane M, Kansagra SM. Exposure to Tobacco Retail Outlets and Smoking Initiation among New York City Adolescents. Journal of Urban Health. 2013;90(6):1091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantrell J, Pearson JL, Anesetti-Rothermel A, Xiao H, Kirchner TR, Vallone D. Tobacco Retail Outlet Density and Young Adult Tobacco Initiation. Nicotine & Tobacco Research 2016;18(2):130–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchner TR, Cantrell J, Anesetti-Rothermel A, Ganz O, Vallone DM, Abrams DB. Geospatial Exposure to Point-of-Sale Tobacco: Real-time craving and smoking-cessation outcomes. American Journal of Preventive Medicine. 2013;45(4):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitzel LR, Cromley EK, Li Y, Cao Y, Dela Mater R, Mazas CA, et al. The effect of tobacco outlet density and proximity on smoking cessation. American Journal of Public Health. 2011;101(2):315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pulakka A, Halonen JI, Kawachi I, Pentti J, Stenholm S, Jokela M, et al. Association between distance from home to tobacco outlet and smoking cessation and relapse. JAMA internal medicine. 2016;176(10):1512–19. [DOI] [PubMed] [Google Scholar]
- 9.Lee JGL, Sun DL, Schleicher NM, Ribisl KM, Luke DA, Henriksen L. Inequalities in tobacco outlet density by race, ethnicity and socioeconomic status, 2012, USA: results from the ASPiRE Study. Journal of Epidemiology and Community Health. 2017;71(5):487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagan P, King G, Lawrence D, Petrucci SA, Robinson RG, Banks D, et al. Eliminating Tobacco-Related Health Disparities: Directions for Future Research. American Journal of Public Health. 2004;94(2):211–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagan P, Moolchan ET, Lawrence D, Fernander A, Ponder PK. Identifying health disparities across the tobacco continuum. Addiction. 2007;102:5–29. [DOI] [PubMed] [Google Scholar]
- 12.Maynard OM, McClernon FJ, Oliver JA, Munafò MR. Using Neuroscience to Inform Tobacco Control Policy. Nicotine & Tobacco Research. 2019;21(6):739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler CD, Gipson CD, Kleykamp BA, Rupprecht LE, Harrell PT, Rees VW, et al. Basic science and public policy: informed regulation for nicotine and tobacco products. Nicotine and Tobacco Research. 2017;20(7):789–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wixted JT, Squire LR. The role of the human hippocampus in familiarity-based and recollection-based recognition memory. Behav Brain Res. 2010;215(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum RS, Ziegler M, Winocur G, Grady CL, Moscovitch M. "I have often walked down this street before": fMRI studies on the hippocampus and other structures during mental navigation of an old environment. Hippocampus. 2004;14(7):826–35. [DOI] [PubMed] [Google Scholar]
- 16.Aguirre GK, Detre JA, Alsop DC, D’Esposito M. The parahippocampus subserves topographical learning in man. Cereb Cortex. 1996;6(6):823–9. [DOI] [PubMed] [Google Scholar]
- 17.Moran JM, Heatherton TF, Kelley WM. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Soc Neurosci. 2009;4(3):197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98(7):4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav. 2012; 106(3):317–24. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33(7):1318–26. [DOI] [PubMed] [Google Scholar]
- 21.Conklin CA, Perkins KA, Robin N, McClemon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug and alcohol dependence. 2010; 111(1–2):58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClernon FJ, Conklin CA, Kozink RV, Adcock RA, Sweitzer MM, Addicott MA, et al. Hippocampal and insular response to smoking-related environments: neuroimaging evidence for drug-context effects in nicotine dependence. Neuropsychopharmacology. 2016;41(3):877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JGL, Henriksen L, Myers AE, Dauphinee AL, Ribisl KM. A systematic review of store audit methods for assessing tobacco marketing and products at the point of sale. Tobacco Control. 2014;23(2):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahler CW, Strong DR, Stuart GL, Moore TM, Ramsey SE. Item functioning of the alcohol dependence scale in a high-risk sample. Drug Alcohol Depend. 2003;72(2):183–92. [DOI] [PubMed] [Google Scholar]
- 25.Heatherton TF, Kozlowski LT, Frecker RC, FAGERSTROM KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–27. [DOI] [PubMed] [Google Scholar]
- 26.Biener L, Abrams DB. The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10(5):360. [DOI] [PubMed] [Google Scholar]
- 27.Dale AM. Optimal experimental design for event-related fMRI. Human brain mapping. 1999;8(2–3):109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–41. [DOI] [PubMed] [Google Scholar]
- 29.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cantrell J, Anesetti-Rothermel A, Pearson JL, Xiao H, Vallone D, Kirchner TR. The impact of the tobacco retail outlet environment on adult cessation and differences by neighborhood poverty. Addiction. 2015;110(1):152–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loomis BR, Kim AE, Busey AH, Farrelly MC, Willett JG, Juster HR. The density of tobacco retailers and its association with attitudes toward smoking, exposure to point-of-sale tobacco advertising, cigarette purchasing, and smoking among New York youth. Prev Med. 2012;55(5):468–74. [DOI] [PubMed] [Google Scholar]
- 32.Susser M, Susser E. Choosing a future for epidemiology: I. Eras and paradigms. American Journal of Public Health. 1996;86(5):668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Susser E. Eco-epidemiology: thinking outside the black box. Epidemiology. 2004;15(5):519–20. [DOI] [PubMed] [Google Scholar]
- 34.Hafeman DM, Schwartz S. Opening the Black Box: a motivation for the assessment of mediation. International Journal of Epidemiology. 2009;38(3):838–45. [DOI] [PubMed] [Google Scholar]
- 35.Weed DL. Beyond black box epidemiology. Am J Public Health. 1998;88(1):12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. [DOI] [PubMed] [Google Scholar]
- 37.Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12(10):388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G, et al. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry. 2010;67(8):722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirshhorn M, Grady C, Rosenbaum RS, Winocur G, Moscovitch M. Brain regions involved in the retrieval of spatial and episodic details associated with a familiar environment: an fMRI study. Neuropsychologia. 2012;50(13):3094–106. [DOI] [PubMed] [Google Scholar]
- 40.Hirshhorn M, Grady C, Rosenbaum RS, Winocur G, Moscovitch M. The hippocampus is involved in mental navigation for a recently learned, but not a highly familiar environment: a longitudinal fMRI study. Hippocampus. 2012;22(4):842–52. [DOI] [PubMed] [Google Scholar]
- 41.Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60(1):252–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janes AC, Krantz NL, Nickerson LD, Frederick BB, Lukas SE. Craving and Cue Reactivity in Nicotine-Dependent Tobacco Smokers Is Associated With Different Insula Networks. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Courtney KE, Ghahremani DG, London ED, Ray LA. The association between cue-reactivity in the precuneus and level of dependence on nicotine and alcohol. Drug and alcohol dependence. 2014;141:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aminoff EM, Kveraga K, Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn Sci. 2013;17(8):379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janes AC, Ross RS, Farmer S, Frederick BB, Nickerson LD, Lukas SE, et al. Memory retrieval of smoking-related images induce greater insula activation as revealed by an fMRI-based delayed matching to sample task. Addict Biol. 2015;20(2):349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliver JA, Jentink KG, Drobes DJ, Evans DE. Smokers exhibit biased neural processing of smoking and affective images. Health Psychol. 2016;35(8):866–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson SJ, Sayette MA. Neuroimaging craving: urge intensity matters. Addiction. 2015;110(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl). 2009;204(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luke DA, Hammond RA, Combs T, Sorg A, Kasman M, Mack-Crane A, et al. Tobacco Town: Computational Modeling of Policy Options to Reduce Tobacco Retailer Density. Am J Public Health. 2017;107(5):740–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.