Abstract
Objectives:
Chimpanzees (Pan troglodytes) are notable for exhibiting high levels of male-to-female aggression. Much of this aggression from adult males serves sexually coercive functions. Despite being smaller and lower-ranking than adult males, adolescent males also engage in regular aggression against adult females. Here, we test whether the primary function of this aggression is sexual coercion, as in adult males, or, alternatively, whether adolescent males use aggression to establish social dominance over females.
Materials and Methods:
We analyzed 1771 copulations and 1812 instances of male-initiated aggression between adolescent males (aged nine through 14 years) and adult females across 21 years of observation of the Kanyawara chimpanzee community in Kibale National Park, Uganda.
Results:
Our test of the sexual coercion hypothesis revealed that adolescent males did not selectively target cycling females for aggression, nor did aggression against cycling females predict rates of copulation with those females. Our test of the social dominance hypothesis showed that males succeeded in dominating all adult females before, or soon after, dominating their first adult male. Additionally, we found that adolescent males dominated females approximately in the order of the females' own ranks, from the bottom to the top of the female hierarchy.
Discussion:
Our data illustrate that the establishment of social dominance was more important than sexual coercion in explaining patterns of adolescent male aggression toward females. In comparison, evidence for sexual coercion was clear and compelling in adult males. These findings highlight that the primary function of male-to-female aggression differs between adolescent and adult males.
Keywords: adolescence, dominance rank, intersexual aggression, mating behavior, sexual coercion
1 ∣. INTRODUCTION
In many mammals, fighting success is important in determining access to females, and males have evolved armaments, such as antlers, elongated canines, and sexually dimorphic musculature to increase their formidability (Clutton-Brock, 2016). Although aggressive mating competition occurs primarily among males, in some species, females are also frequent victims of male aggression (Muller & Wrangham, 2009). Such aggression often functions as sexual coercion, increasing the probability that a female will mate with the aggressor (direct coercion), or decreasing the probability that she will mate with other males (indirect coercion), at a cost to the female (Clutton-Brock & Parker, 1995; Muller et al., 2009a; Smuts & Smuts, 1993). Direct coercion involves the use of force to overcome female resistance to mating, and includes harassment, intimidation, and forced copulation (e.g., orangutans [Pongo pygmaeus]: Fox, 2002; Knott, 2009; reviewed by Clutton-Brock & Parker, 1995). Indirect coercion involves the use of force to constrain female promiscuity, and includes herding, punishment, and sequestration (e.g., hamadryas baboons [Papio hamadryas hamadryas]: Swedell & Schreier, 2009; reviewed by Muller et al., 2009a). Both forms of coercion reflect sexual conflict (i.e., antagonistic strategies for optimizing fitness in the two sexes), as males employ force to override or constrain female mating preferences (Arnqvist & Rowe, 2005; Muller, 2017; Palombit, 2014; Smuts & Smuts, 1993; Watson-Capps, 2009).
In a variety of species (e.g., dolphins (Tursiops spp.): Scott et al., 2005; hamadryas baboons: Kummer, 1968; Swedell & Schreier, 2009; mountain gorillas [Gorilla beringei beringei]: Robbins, 2003, 2009; humans [Homo sapiens]: Flinn, 1988; reviewed by Muller, 2017; but see black-handed spider monkeys [Ateles geoffroyi]: Campbell, 2003), females with the greatest likelihood of conception experience the most male aggression, indicating that males strategically attempt to control female sexuality (reviewed by Muller et al., 2009b). Among primates, evidence for sexual coercion as a long-term conditioning strategy (i.e., manipulation of the female's future behavior: Wrangham & Muller, 2009) is most prominent in hamadryas baboons (Swedell & Schreier, 2009), chimpanzees (Pan troglodytes schweinfurthii: Muller et al., 2011; Feldblum et al., 2014), and humans (Wilson & Daly, 2009). Feldblum et al. (2014) utilized genetic data to assess paternity in a study of male chimpanzees and showed that there are fitness benefits associated with selectively targeting cycling females with aggression (reviewed by Muller, 2017).
Female-directed aggression can also serve functions other than sexual coercion (reviewed by Muller et al., 2009a). Males may direct aggression toward females to establish dominance over them, to compete for food, to signal their fighting ability to same-sex competitors (Kitchen et al., 2009), or to police conflicts among females (e.g., Ren et al., 1991; Sterck et al., 1997; Watts, 1997; Watts et al., 2000). For young males in particular, the process of dominating females may provide practice with the skills needed to coerce those females later. Alternatively, the process of dominating females could help males prepare for competition with fully grown adult males (Pereira, 1988; Pusey, 1990), similar to the sparring behavior observed in many young animals (e.g., American bison [Bison bison]: Rothstein & Griswold, 1991; bighorn sheep [Ovis canadensis]: Hass & Jenni, 1993; red-necked wallabies [Macropus rufogriseus banksianus]: Watson, 1993; pronghorn [Antilocapra americana]: Miller & Byers, 1998; reviewed by Fagen, 1981). In general, dominance rank can have important fitness consequences because a male's position in the dominance hierarchy affects his access to food resources and his success in mating competition (reviewed by Cowlishaw & Dunbar, 1991; Clutton-Brock, 2016; Alberts, 2019).
In this article, we describe patterns of aggression among adolescent male chimpanzees (P. t. schweinfurthii), who range from 9 to 14 years of age, are not fully grown, and are sexually, but not socially, mature (Pusey, 1990). Previous observations of wild eastern chimpanzees (P. t. schweinfurthii: Pusey, 1978; Goodall, 1986; Pusey, 1990; Nishida, 2003; Muller et al., 2009a; Reddy & Mitani, 2020) and captive western chimpanzees (P. t. verus: Adang, 1984, 1985, 1986) have shown that adolescent males often direct aggression toward females. Adolescent males are also invested in obtaining copulations with females (e.g., Hayaki, 1985; Muller et al., 2020; Pusey, 1990; Watts, 2015). However, only one study has directly tested whether adolescent males use their aggression to sexually coerce females, finding evidence for sexual coercion by adolescent and young adult males based on 17 months of data from Ngogo in Kibale National Park, Uganda (Reddy et al., 2021). Here, we utilize 21 years of data on a different chimpanzee community within the same park to test whether female-directed aggression by adolescent males is consistent with sexual coercion, or, alternatively, whether adolescent males use aggression to establish social dominance over females. We do not test other noncoercive functions of aggression because previous research suggests that they are less relevant for Kanyawara males than sexual coercion and the establishment of dominance (Muller et al., 2009a).
In at least five communities of eastern chimpanzees, adult males (ages 15 years and older) are known to exhibit high rates of aggression against females (e.g., Goodall, 1986; Matsumoto-Oda & Oda, 1998; Muller, 2002; Muller & Mitani, 2005; Newton-Fisher, 2006; Watts, 1998). Adult male chimpanzees are almost invariably higher-ranking than all adult females, and are rarely challenged by them (Bygott, 1974; Goodall, 1986; Nishida, 1979). Most aggression against females by adult male chimpanzees appears to represent indirect, rather than direct, coercion. Direct coercion is usually unnecessary, because female chimpanzees rarely resist copulation attempts, even from young or low-ranking males. Thus, coercion in chimpanzees typically serves an indirect function, constraining females' ability to mate with multiple males (Goodall, 1986; Muller et al., 2009a, 2011).
Studies of four eastern chimpanzee communities (Kanyawara: Muller et al., 2007, 2009a, 2011; Kasekela: Feldblum et al., 2014; Ngogo: Reddy et al., 2021; Sonso: Kaburu & Newton-Fisher, 2015) have demonstrated that adult males achieve mating or paternity advantages with the females toward whom they direct the most aggression (reviewed by Muller, 2017). Importantly, adult males direct more aggression toward females with sexual swellings than those who are pregnant or lactating, supporting a reproductive function to this aggression (Muller et al., 2009a). Females have been shown to incur significant costs from adult male aggression in the form of physical injuries (Muller et al., 2009a; cf. western chimpanzees, Novak & Hatch, 2009) and heightened glucocorticoid levels (Emery Thompson et al., 2010, 2020; Muller et al., 2007). Despite these costs, females preferentially approach coercive males for copulations on the days that they are most fertile, suggesting that females perceive the alternative of avoiding these males, or mating more promiscuously, to be even more costly (Muller et al., 2011).
In contrast to the four eastern chimpanzee communities in which sexual coercion has been documented, aggression was not correlated with mating success in the Mahale M-group. Instead, males appeared to trade grooming for mating access (Kaburu & Newton-Fisher, 2015). Additionally, a study of western chimpanzees found little evidence for sexual coercion (Stumpf & Boesch, 2010), though the authors primarily focused on direct coercion. At present, a sound comparison of sexual coercion between eastern and western chimpanzees is limited by differences in research methods across study sites (reviewed by Muller et al., 2011).
Adolescent males are sexually mature and capable of eliciting submissive responses from females (Pusey, 1990). Consequently, in chimpanzee populations where adult males employ sexual coercion, a coercive function of female-directed aggression by adolescent males is also plausible. Alternatively, adolescent male aggression toward females could be primarily motivated by dominance striving. Research on eastern chimpanzee populations in Gombe National Park, Tanzania and the Mahale Mountains National Park, Tanzania clearly documents struggles for dominance between the sexes. Early in the juvenile period (ages five through 8 years), male chimpanzees start to provoke females by waving branches or throwing sticks in their direction, though females typically ignore these behaviors (Nishida, 2003; Pusey, 1990). Ignoring aggressive advances becomes harder as males grow larger and more intimidating. During the first half of adolescence (ages nine through 11 years), males employ more serious threats than before, including charging displays, which often result in either female retaliation or submission (Nishida, 2003; Pusey, 1990). By the second half of adolescence (ages 12 through 14 years), male aggression intensifies beyond threats and displays to include overt attacks (e.g., slaps, kicks, and bites), prompting females to react more fearfully (Pusey, 1990).
Extensive research on captive western chimpanzees in the Arnhem Zoo illustrates the same pattern of female-directed aggression across male development as in Gombe and Mahale (Adang, 1984, 1985, 1986; de Waal & Hoekstra, 1980). In both captivity (Adang, 1984, 1985) and the wild (Nishida, 2003), female responses influenced male persistence, with adolescents typically escalating their aggression if a female reacted by retaliating or submitting, but ending their harassment sooner if she ignored them. Nishida (2003) concluded that adolescent male aggression toward females at Mahale initiated the process of dominance striving against members of the opposite sex, as a precursor to challenging mature males for status in the adult male hierarchy.
Despite a robust literature on the use of sexually coercive aggression by adult male chimpanzees, the ontogeny of such aggression remains largely unexplored (but see Reddy et al., 2021). In this article, we analyze 21 years of aggression and copulation data from the Kanyawara community of chimpanzees in Kibale National Park, Uganda to test predictions from two central hypotheses.
If adolescent males direct aggression against adult females in order to increase their relative mating access (H1, sexual coercion hypothesis), we predict that, like adult males, (1a) adolescent males will direct higher rates of aggression toward cycling than non-cycling females; and (1b) adolescent males will engage in higher rates of copulation with the individual cycling females toward whom they direct the most aggression (Table 1). Alternatively, adolescent males may direct aggression toward adult females primarily to dominate them (H2, social dominance hypothesis). Following this hypothesis, we predict that (2a) males will direct less aggression toward females after they have successfully dominated them. If female-directed aggression by adolescent males functions to dominate females as part of a male strategy of rising in rank (Muller et al., 2009a; Nishida, 2003), we predict that (2b) adolescent males will tend to dominate all females before successfully dominating any adult male, and (2c) the majority of aggression that a male initiates prior to adulthood will be directed toward adult females, rather than adult males.
TABLE 1.
Summary of hypotheses and predictions
| Hypothesis | Prediction for adolescent males |
Support? |
|---|---|---|
| H1: Sexual coercion hypothesis | 1a: More aggression toward cycling than non-cycling females | No |
| 1b: More copulations with females targeted most frequently with aggression by male | Weak | |
| H2: Social dominance hypothesis | 2a: Less aggression directed toward females after dominating them | No |
| 2b: Dominate all females before dominating any adult male | Yes | |
| 2c: Majority of aggression toward adult females, rather than adult males, prior to adulthood | Yes | |
| H3: Dominance-contingent coercion hypothesis | 3a: Female cycling status predicts male aggression more strongly after dominance | No |
| 3b: Male aggression predicts copulations more strongly after dominance | No | |
| H4: Female-rank-based aggression hypothesis (complements other hypotheses) | 4a: Initiate aggression against low- before high-ranking females | Yes |
| 4b: Dominate low- before high-ranking females | Yes |
Pusey (1978, 1990) suggested that male chimpanzees are better able to sexually coerce females after having dominated them. Accordingly, we hypothesize that adolescent males will show stronger evidence for sexual coercion after formally dominating a female (H3, dominance-contingent coercion hypothesis). Following this hypothesis, we predict that (3a) a female's cycling status will predict rates of adolescent male aggression more strongly after she has been formally dominated; and (3b) rates of adolescent male aggression toward individual cycling females will predict rates of copulation with those females more strongly after they have been dominated.
Finally, we hypothesize that female rank influences the pattern of female-directed aggression by adolescent males (H4, female-rank-based aggression hypothesis). This question has not yet been directly explored in wild chimpanzees, but in his study of captive western chimpanzees, Adang (1986) found that adolescent males tended to establish dominance relationships with females by starting from the bottom of the female hierarchy. Additionally, recent research from Gombe shows that while females did not compete for rank once established in the female hierarchy, they did compete for their entry ranks, which varied depending on how successful they were against resident females (Foerster et al., 2016). This suggests that female rank reflects at least some level of fighting ability, such that low-ranking females are generally less capable of winning agonistic encounters than higher-ranking females. Based on these previous studies, we predict that (4a) adolescent males will initiate aggression against low-ranking females before high-ranking females; and (4b) adolescent males will dominate low-ranking females before high-ranking females. Hypotheses are summarized in Table 1.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Study population and data collection
This study is based on long-term behavioral observations of the Kanyawara community of wild chimpanzees (P. t. schweinfurthii) in Kibale National Park, Uganda. This population has been continuously observed since 1987, so all individuals had already been habituated to human presence before the period of data collection for this study. Here, we include data spanning 21 years, from January 1996 to December 2016. Data were collected by university-based researchers and Ugandan field assistants employed by the Kibale Chimpanzee Project. Working in teams of two to four, observers recorded instances of aggression and copulations during attempted or completed full-day party-level follows of chimpanzees.
Because chimpanzees can flexibly join and separate from sub-groups, or “parties” (Goodall, 1986; Nishida, 1968; Sugiyama, 1968), throughout the day, observers noted which individuals were present in the social party every 15 min. Both aggression and copulation data were recorded via all-occurrence sampling and included the individuals involved, the sequence of events, and the duration of the interaction. A total of 1771 copulations and 1812 instances of male-initiated aggression were observed between adolescent males and adult females. In addition, 6134 copulations and 6177 instances of male-initiated aggression were observed between adult males and adult females. All field observations were conducted in accordance with the ethical standards and approval of the Uganda Wildlife Authority, the Uganda National Council for Science and Technology, and the Institutional Animal Care and Use Committees of Harvard University, Tufts University, and the University of New Mexico.
During the study, the community size ranged from 40 to 54 chimpanzees. Observations include a total of 15 adolescent males, 21 adult males (10 of which transitioned from adolescence during the study period), and 42 adult females. Our data span 44,665 h of observation of social parties in which at least one adolescent male was present. Males were considered adolescents from ages nine through 14 years, and adults starting at 15 years, the mean age at which males began to consistently and successfully challenge adult males. We included adult males in our analyses of sexual coercion to better contextualize adolescent male behavior. A female was considered an adult from the date of her first maximally tumescent sexual swelling onward if natal, and from the date of her entry into the community if an immigrant (all immigrants exhibited sexual swellings on or soon after their first observation, suggesting that they were sexually mature upon dispersal). We considered females to have 'maximal' sexual swellings if the sex skin was fully tumescent, with a tense and shiny appearance.
One male (“MX”) in our sample occasionally engaged in agonistic interactions with adult females during adolescence and young adulthood. However, MX lost both of his feet to snare injuries by early adolescence and was a common target of aggression, unusually low-ranking for his age, and relatively solitary when not with his mother. We therefore present data on his dominance relationships with females separately.
2.2 ∣. Definition of aggression and calculation of dominance ranks
Aggression took several forms, including physical attacks, chases, and charging displays directed toward a target, as defined by Muller (2002). Each targeted aggressive interaction was coded for the initiator (male, female, mutual, or unclear) and outcome (win, loss, tie, or unclear). We classified an interaction as a win for the aggressor if the victim produced a pant-grunt vocalization (i.e., a formal signal of subordinacy: Bygott, 1979) or responded submissively to an act of targeted aggression by fleeing, screaming, pant-barking, or whimpering. We scored an interaction as a tie if the initial target of aggression retaliated, and both individuals fled screaming, or neither fled screaming.
For the calculation of dominance ranks, we used the Elo-rating method, which assigns relative ranks for all individuals in the hierarchy at any point in time, based on the actual sequence of interactions (Albers & de Vries, 2001; Neumann et al., 2011). For the female dominance hierarchy, we followed Glickman and Doan's (2010) recommendation regarding sample size by excluding females who were involved in <9 interactions (N = 9/42 adult females) from rankings in the hierarchy. All females who were adults at the beginning of the study period were assigned a starting score of zero. Natal females were given a starting score at adulthood that considered all of their agonistic interactions with adult females during the previous 5 years, and immigrant females were assigned a starting score that considered their interactions with adult females during their first 6 months in the community. This resulted in a mean starting score of – 112 for natal females (N = 14) and – 101 for immigrant females (N = 8). We set the constant k in the Elo-rating equation to 20 (higher values of k resulted in rank changes that were less consistent with observed dyadic dominance relationships), and we applied the rating method to 1457 agonistic interactions observed between adult females. We then assigned ordinal ranks according to the relative order of females' Elo scores and standardized the ranks based on the number of females in the hierarchy, such that ranks ranged from 0 (lowest-ranked) to 1. We considered females with ranks below 0.5 as low-ranking, and females with ranks equal to or above 0.5 as high-ranking. Due to insufficient observations of female–female agonism prior to 2004, we were unable to calculate female ranks until that year. This resulted in the exclusion of 16 intersexual dyads across four males from our two social dominance analyses involving female rank.
To assess male–male dominance relationships, we calculated ranks from 12,091 agonistic interactions among adolescent and adult males during the study period (plus 354 interactions from 1993 to 1995, which allowed us to more accurately estimate the relative ranks of males at the beginning of the study period). For males who were already adolescents or adults in 1993, we used a starting score of zero. For males who matured into adolescents after 1993, we assigned a starting score corresponding to one point below the lowest-ranking adolescent male (excluding MX) on the date of their first interaction after reaching 9 years of age.
2.3 ∣. Sexual coercion analyses (H1)
To test whether adolescent males preferentially targeted females for aggression in reproductive contexts, we calculated rates of male-initiated aggression separately for female cycling and non-cycling periods. A non-cycling period for an adult female began on the day that she gave birth and ended on the day before she resumed cycling (after experiencing lactational amenorrhea). A cycling period began on the first day of maximal sexual swelling that followed lactational amenorrhea (or on the day of a female's first-ever maximal swelling if nulliparous) and ended when the female became pregnant. The date of pregnancy for a female was determined by back-calculating 230 days before the birthdate of an infant and assigning conception to the end of the most proximate sexual swelling phase (Emery Thompson, 2005). In some cases, we were able to narrow down conception windows more tightly with hormonal evidence.
We used a generalized linear mixed model (GLMM) to determine whether adolescent males directed higher rates of aggression toward cycling than non-cycling females (prediction 2a). We designated the number of male-initiated instances of aggression per dyad as the outcome variable, hours spent together as an offset, female cycling status (yes/no) as a predictor, female parity (nulliparous/parous) as a control, and male ID and female ID as random effects. We included parity as a control in this and the following models because male chimpanzees exhibit more mating interest in and direct more aggression toward parous females (Muller et al., 2006, 2007, 2011; Tutin, 1979; Wrangham, 2002). The unit of analysis for this model was the dyad, entered more than once if a female's cycling status or parity changed. To help contextualize adolescent male behavior, we ran this same model separately for adult males.
To test the second prediction of the sexual coercion hypothesis, we used a GLMM to determine whether aggression directed by an adolescent male toward each cycling female predicted the rate of copulation between the male and female. Due to a large number of cases where the cycling aggression rate was zero, we included both a binary predictor of whether any cycling aggression occurred within the dyad, and a second continuous predictor for the rate of aggression. We designated the number of copulations as the outcome variable. The rest of the model contained hours spent together in the same party (when the female was swollen) as an offset, female parity (nulliparous/parous) as a control, and male ID and female ID as random effects. The unit of analysis was the dyad, entered twice if a female's parity changed. Because previous reports indicate that male aggression throughout the full cycling period is predictive of mating success (e.g., Feldblum et al., 2014; Muller et al., 2011), we calculated cycling aggression rates relative to the number of hours in which the dyad was observed in the same party and the female was cycling (either swollen or not). Because copulations occur almost exclusively when females have maximal swellings, the offset variable was limited to the hours when the male and female were in the same party and the female had a maximal swelling. As above, we also ran this model for adult males.
2.4 ∣. Social dominance analyses (H2)
We considered a male to have formally dominated a female on the date that the male was first observed to receive a pant-grunt from her or to have initiated aggression against her and won. Approximately half of the male–female dyads (N = 57/115; 49.6%) in our social dominance analyses contained a period of uncertainty in dominance status, during which the female still achieved one or more wins against the male after losing or pant-grunting to him for the first time. In order to ensure that the male had decisively dominated the female in these 57 dyads, we designated the date of dominance as the date of the first male-initiated male win or female pant-grunt after which there were no subsequent female wins. We ran an alternative set of analyses to explore the possibility that a liminal period of time was required for the dominance status of a dyad to be considered definitive, following this date of dominance. Accordingly, we added 6 months to each date of dominance in all analyses involving dominance status, and found that the results were qualitatively the same. To be conservative in our assessment of dominance status, we only included dyads that were observed together for an average of at least 100 h per year and contained a combined sample of at least five pant-grunts and/or acts of aggression. This resulted in averages of 18.4 ± 5.9 (SE) pant-grunts and 42.3 ± 9.0 instances of aggression in either direction per male–female dyad, across 13 males for which we had sufficient data on intersexual dominance during adolescence.
To determine whether males were less aggressive toward females after successfully dominating them (prediction 2a), we ran a GLMM containing an outcome variable of aggression count, an offset term for hours that the male and female were observed together, a categorical predictor indicating whether the period occurred during the 12 months before or after the interaction that marked a male's dominance over a female, and random effects for male ID and female ID. The unit of analysis for this model was the dyad, with most dyads (N = 92/99) entered twice to represent the year before and the year after dominance. For all analyses involving rates of aggression, we included dyads or dyad-years (depending on the analysis) in which the male and female were observed in the same party for at least 100 h.
The final two predictions of the social dominance hypothesis required contrasts of intersexual and intrasexual dominance. For the first of these predictions, we determined whether there was any overlap between the dates of the last female dominated and the first male dominated by each male. For the last prediction, we tested whether the majority of all adult-focused aggression initiated by each adolescent male was directed toward females rather than males.
We also include a section at the end of the results containing descriptive statistics from several additional social dominance analyses. Specifically, we quantified the average time required for a male to establish dominance over a female, conducted a comparison between MX (the male missing both feet) and the rest of the males in the study, and explored third party interventions in conflicts resulting from adolescent male-initiated aggression against females.
2.5 ∣. Dominance-contingent coercion analyses (H3)
To determine whether the dominance status of male–female dyads influenced the relationship between female cycling status and the rate of male aggression toward the female (prediction 3a), we ran the same GLMM as for prediction 1a, but incorporated two additional predictors. The first was a categorical indication of whether the male had dominated the female (yes/no), and the second was an interaction between this variable and female cycling status. To examine whether dominance status influenced the relationship between rates of male aggression toward females and rates of copulation with those females (prediction 3b), we ran the same GLMM as for prediction 1b, but added a categorical predictor of whether the male had dominated the female (yes/no), and an interaction between this variable and cycling aggression rate. The unit of analysis for these models was the dyad, entered more than once if a dyad's dominance relationship or a female's cycling status or parity changed.
2.6 ∣. Female-rank-based aggression analyses (H4)
To test whether males initiated aggression against low-ranking before high-ranking females (prediction 4a), we determined whether male age was positively correlated with the average rank of female targets. This allowed us to determine whether males were generally more aggressive toward lower-ranking females early in adolescence and higher-ranking females later in adolescence. Males typically targeted most of the females in the community at least once during each year of adolescence. Thus, in order to more accurately quantify males' allocation of aggression toward particular females, we weighted female rank by the proportion of a male's overall aggression received by each female (corrected for the amount of time each dyad was observed together). Finally, we tested whether males dominated low-ranking before high-ranking females (prediction 4b) by determining whether the order in which males dominated females was positively correlated with average female rank.
2.7 ∣. Maternal relatedness
Before testing our hypotheses, we investigated whether aggression and sexual behavior differed between maternally related versus non-maternally related male–female dyads. Using a GLMM containing both male and female ID as random effects, we determined that adolescent males directed substantially less aggression toward their mothers and maternal sisters than toward other females (β = −2.068 ± 0.261 (SE), X2 = −7.9, p < 0.001, N = 171 dyads during periods in which the male had a living mother or adult maternal sister in the community; Table S1, Figure 1). Adolescent males were 17.3 times less aggressive toward their maternal relatives when comparing medians, and 8.9 times less aggressive when comparing means. Sexual behavior within maternally related dyads was also rare (i.e., an adolescent male was observed copulating with a maternally related female in only two of 14 such dyads). Therefore, we excluded dyads with mothers and maternal sisters from all analyses. After removing maternal relatives, our study contained 225 adolescent male-adult female dyads and 455 adult male-adult female dyads.
FIGURE 1.
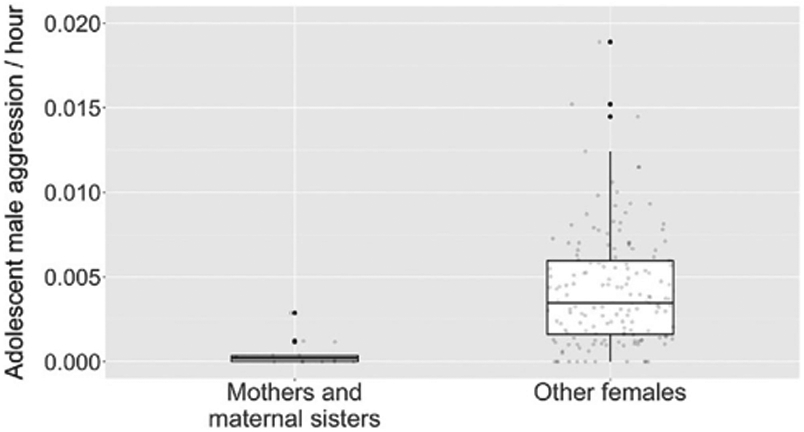
Rates of adolescent male-initiated aggression directed toward adult females who were either maternally related to the male (“Mothers and maternal sisters”) or not (“Other females”). The plot contains 171 male–female dyads across 13 adolescent males and 30 adult females
2.8 ∣. Model selection and validation
We used R 3.6.3 (R Core Team, 2020) for all analyses. We ran our models with the lme4 package (Bates et al., 2015) and executed likelihood ratio tests with the lmtest package (Zeileis & Hothorn, 2002). Because our data were overdispersed, we used a negative binomial distribution and log link function to run all of our GLMMs. We inspected residuals and Q–Q plots to check model assumptions and used likelihood ratio tests for model validation. Variance inflation factors ranged from one to three, indicating that our models were not influenced by multicollinearity. We used z-scores to standardize continuous predictor variables before running models. Whether we included both male ID and female ID as random effects, or either one individually, our model results did not qualitatively differ. For all correlational analyses, we visually inspected the data to determine that our results were not driven by particular males.
3 ∣. RESULTS
3.1 ∣. Sexual coercion (H1)
Confirming prior reports on sexual coercion at Kanyawara, adult males directed higher rates of aggression toward cycling than non-cycling females (β = 0.792 ± 0.059, X2 = 13.3, p < 0.001, N = 772; Table S2a). However, adolescent males did not (β = −0.082 ± 0.112, X2 = −0.7, p = 0.462, N = 366; Table S2b). Whereas adult males copulated most frequently with the females toward whom they were most aggressive (β = 0.258 ± 0.058, X2 = 4.4, p < 0.001, N = 357; Table S3a), aggression by adolescent males did not significantly predict their rates of copulating with their victims (β = 0.106 ± 0.056, X2 = 1.9, p = 0.057, N = 138; Table S3b).
This difference was magnified when we considered aggression against females with maximal sexual swellings, that is, those most likely to be mating. While adult males were more aggressive to these females than to those who were not cycling or cycling but not maximally swollen, adolescent males were less aggressive to them (Figure 2). Aggression toward maximally swollen females was a strong predictor of copulation success for adult males (β = 0.200 ± 0.054, X2 = 3.7, p < 0.001, N = 357; Table S4a), but did not predict the copulation success of adolescent males (β = 0.003 ± 0.068, X2 = 0.04, P = 0.970, N = 138; Table S4b). Overall, we found a sharp distinction between adult and adolescent males, with only adult males using female-directed aggression to sexually coerce females.
FIGURE 2.
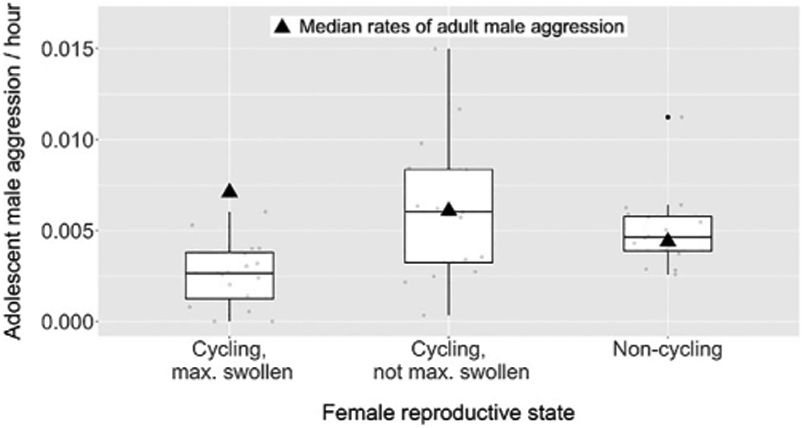
Rates of adolescent male-initiated aggression (box plots) directed toward adult females who were (1) cycling and maximally swollen, (2) cycling but not maximally swollen, or (3) non-cycling. The plot contains 16 females represented in at least three dyads with adolescent males in each reproductive state, and 20 females represented in at least three dyads with adult males in each reproductive state. Rates of adolescent male aggression were significantly lower against cycling, maximally swollen females than against cycling but not maximally swollen females (paired Wilcoxon signed-rank test: V = 598, p < 0.001, N = 95 dyads) or non-cycling females (V = 608, p < 0.001, N = 88). Rates of adolescent male aggression did not differ between the latter two reproductive states (V = 2392, p = 0.535, N = 101)
Nevertheless, among adolescent males, female-directed aggression was a near-significant predictor of copulation rates in the model containing cycling aggression (i.e., p = 0.057). Therefore, we further explored adolescent male behavior by considering nulliparous and parous females separately and comparing copulation rates of individual males with cycling females toward whom they directed relatively more or less aggression (above or below the median for that male; Figure S1). Most adolescent males (N = 9/10) copulated more often with the cycling, nulliparous females that they targeted for aggression than with those toward whom they were less aggressive (Figure S1a). Several adolescent males (N = 5/8) showed a pattern like that of adults, whereby they succeeded in copulating at higher rates with the cycling, parous females that they had targeted (Figure S1b). Still, the models described above did not clearly support a sexually coercive function to adolescent male aggression.
3.2 ∣. Social dominance (H2)
Males were not less aggressive toward females in the year after they dominated them compared to the previous year (β = 0.034 ± 0.135, X2 = 0.3, p = 0.802, N = 191; Table S5). With the exception of MX, the nine adolescent males who became adults over the course of the study dominated all adult females (with whom they were observed for an average of at least 100 h per year and with whom they interacted at least five times) by a mean age of 15.7 years (range: 14.1–18.4 years; Figure S2). Six of the males dominated all adult females before dominating any adult male (Figure S2). Furthermore, these six males experienced a substantial delay (473 ± 138 (SE) days) between dominating all adult females and dominating their first adult male. The remaining three males had surpassed only one adult male (i.e., the lowest-ranking, other than MX) in rank before achieving dominance over all adult females (Figure S2).
Our test of the final prediction of the social dominance hypothesis showed that all adolescent males directed a majority of their aggression toward adult females rather than adult males. Specifically, out of 1870 total instances of aggression initiated by adolescent males against adults of either sex, 1812 (95.9% ± 2.0% (SE)) were directed toward adult females, and only 58 were directed toward adult males. In their agonistic encounters with adult females, juvenile males were more likely to be victims than aggressors (Figure 3). However, by 10 years of age, males initiated more than half of the male–female aggression in which they engaged, and by the start of adulthood, they initiated nearly all such aggression (Figure 3).
FIGURE 3.
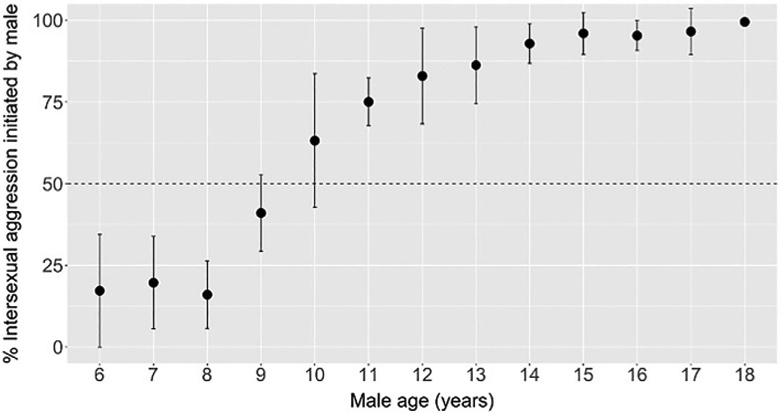
The percentage of intersexual aggression between males and adult females that was initiated by the male, rather than the female. Each point represents the mean percentage of male-initiated aggression at a given male age. The plot contains 94 male-years across 15 males. Error bars represent one standard deviation above and below the mean, and the horizontal, dashed line at 50% indicates when males and females are equally likely to initiate aggression against each other. The x-axis ranges from 6 years (the youngest male age at which there was a sufficient sample size of intersexual aggression to perform this analysis) through 18 years (the oldest male age by which all females had submitted to a male)
3.3 ∣. Dominance-contingent coercion (H3)
We hypothesized that adolescents would be more effective at sexually coercing females after formally dominating them. Our analyses, which considered interactions between cycling status and the male–female dominance relationship, did not support this hypothesis. A female's cycling status did not predict rates of adolescent male aggression more strongly after she had been formally dominated (interaction between cycling status and dominance status: β = −0.227 ± 0.217, X2 = −1.0, p = 0.294, N = 366; Table S6). Likewise, adolescent male aggression did not predict rates of copulation more strongly after a female had been dominated (interaction between aggression rate and dominance status: β = 0.074 ± 0.109, X2 = 0.7, p = 0.500, N = 123; Table S7).
3.4 ∣. Female-rank-based aggression (H4)
As males aged, they initiated aggression against females with increasingly high rank (Pearson's r = 0.66, p < 0.001, N = 52 male-years; Figure 4). During the first half of a male's period of adolescence, his female targets were primarily low-ranking (i.e., those below 0.5 in the female hierarchy), whereas during his last year of adolescence and the beginning of adulthood, they were primarily high-ranking (Figure 4).
FIGURE 4.
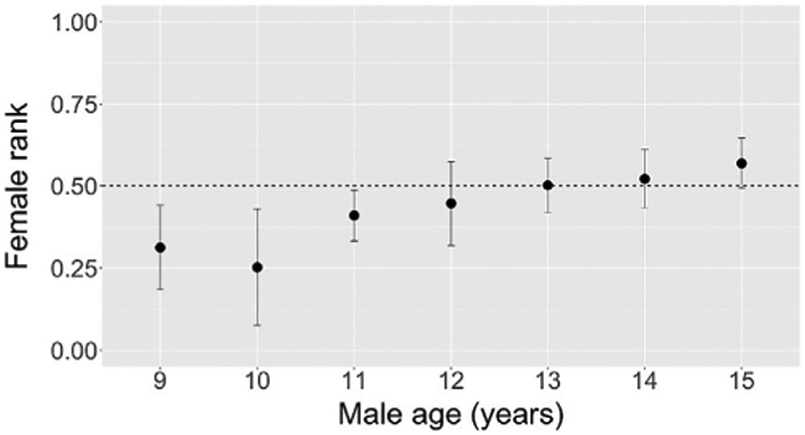
Ranks of females against whom males initiated aggression at ages nine through 15 years. Each point represents the mean rank of females who were aggressive targets of a male at a given age (yearly average). The plot contains 52 male-years across 10 males. Error bars represent one standard deviation above and below the mean. The x-axis range of 9–15 years spans from the earliest age that any male dominated a female to the age of male adulthood, by which time most males had dominated all females
Adolescent males dominated low-ranking females before high-ranking females (female rank vs. the order in which males dominated females, r = 0.64, p < 0.001, N = 94 dyads; Figure 5). Similarly, male age on the date that a female formally submitted predicted her rank (r = 0.53, p < 0.001, N = 99 dyads; Figure 6). It was not until the second half of adolescence that males tended to dominate females ranked in the top half of the hierarchy. The same patterns hold, though not as strongly, when female age replaces female rank in the analyses (see Figures S3, S4, S5).
FIGURE 5.
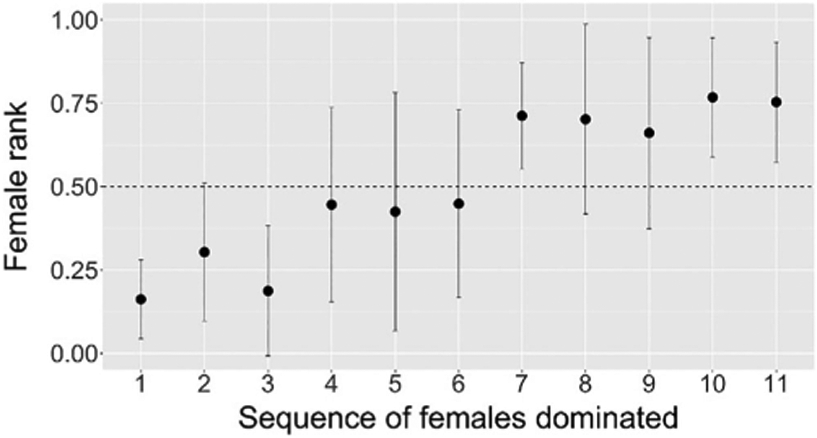
Adolescent males dominated low-ranking females earlier than high-ranking females. Points represent the rank of the first female who formally submitted to each male, averaged across males, then the rank of the second female, and so forth. The plot contains 94 male–female dyads across 10 males. Error bars represent one standard deviation above and below the mean
FIGURE 6.
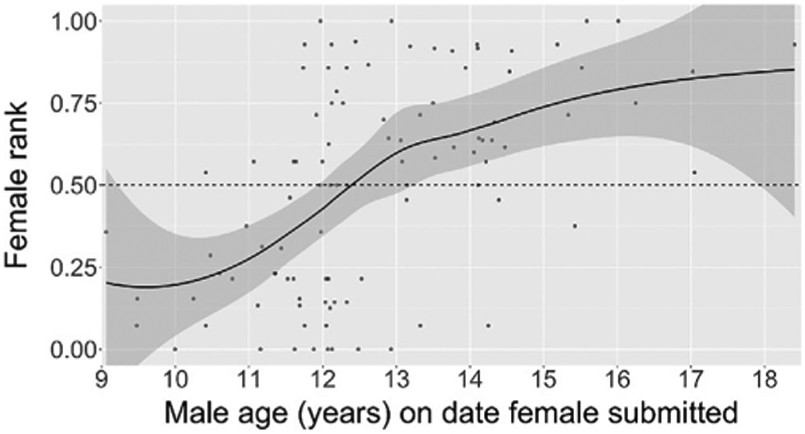
Female rank by male age on the date that the female submitted to the male. Each point represents a male–female dyad. The plot contains 99 dyads across 10 males and 18 adult females, and a LOESS curve fitted to the points (the shaded area indicates the 95% confidence interval)
3.5 ∣. Additional social dominance analyses
On average, we observed 9.2 acts of aggression from a male toward a given female prior to her submission. Some males successfully dominated a female with their first recorded instance of aggression against her, but this occurred for only 10 of 105 dyads (i.e., 9.5%). In most cases, males initiated multiple acts of aggression against females before dominating them (N = 75/105 dyads; 71.4%). In fact, there were five dyads (including three unique males) in which the male initiated 40 or more instances of aggression against the female prior to dominating her. These five dyads involved the four highest-ranking females. An average of 2.7 years elapsed between a male's first recorded initiation of aggression against a female and his achievement of dominance over her. For 20.0% of dyads (N = 21/105), more than 5 years separated these milestones.
In contrast to the other nine males who completed adolescence during the study, MX (the male missing both feet) never outranked all of the females in the community and never surpassed an adult male in rank. By the time that MX was nearly 19 years old (at the end of the study period), only three low-ranking adult females had submitted to him, while the remaining 11 females had not.
Third party interventions to impartially break up male–female conflicts resulting from adolescent male-initiated aggression or to aid the targeted female were uncommon (N = 157/1812 instances of adolescent male-initiated aggression; 8.7%). Interventions were initiated by a maternal relative of the female target in 11 cases (7.0%), an unrelated adult male in 83 cases (52.9%), an unrelated adult female in 50 cases (31.8%), and an unrelated adolescent male in 16 cases (10.2%; percentages sum to more than 100% because several interventions involved support from more than one individual).
4 ∣. DISCUSSION
Our test of the social dominance hypothesis (H2) suggested that adolescent males used aggression against females to increase their dominance rank. Across maturation, males succeeded in dominating all adult females before, or soon after, dominating their first adult male. Our analyses involving female rank (H4) additionally showed that males directed aggression toward females approximately in the order of the females' own ranks, from the bottom to the top of the female hierarchy. Females also submitted to males in the same general order, from low to high female rank. However, males did not reduce the aggression that they directed toward females after dominating them, suggesting additional functions for female-directed aggression during late adolescence. One such function could be continued practice intimidating females, facilitating a transition to the utilization of aggression for sexual coercion in late adolescence and early adulthood.
Our social dominance analyses revealed that males persistently competed for status with females during adolescence. In more than half of the male–female dyads in our study, the male initiated multiple instances of aggression against the female before she submitted to him. The most extreme cases (i.e., 40+ acts of observed aggression prior to submission) showed that the most difficult females to dominate were those occupying the top positions in the female hierarchy. On average, 2.7 years of effort were required before a male's aggression toward a female resulted in her formal submission. These findings build on previous studies of intersexual aggression in chimpanzees (e.g., Adang, 1986; Nishida, 2003; Pusey, 1990; Reddy & Mitani, 2020) by quantifying the aggressive effort needed for adolescent males to dominate the females of the community, and by detailing the extent of variation in this process across dyads. Additionally, our inclusion of female rank revealed that males were mostly unable to dominate females in the top half of the hierarchy until late adolescence, suggesting that the highest-ranking females possess the greatest fighting ability. This finding accords with previous research on captive western chimpanzees, which showed that adolescent males started from the bottom of the female hierarchy when dominating the females of the group (Adang, 1986).
Why do male chimpanzees engage in the arduous process of dominating females during adolescence? Why not wait to challenge females until fully mature, when winning against them would be more certain? One possibility is that establishing dominance over females and routinely attacking them throughout adolescence (as evident in our finding of sustained male aggression even after dominating females) allows males to practice intimidating females so that they may coerce them more effectively in early adulthood. Or, as suggested by Pusey (1990), the process of dominating females could be an effective way for adolescent male chimpanzees to evaluate and hone their competitive skills in relatively low-cost interactions, before ultimately challenging adult males. Similarly, Adang (1984, 1985) proposed that immature male chimpanzees harass adult females to learn about the competitive aspects of their social environment (reviewed and further supported by Boose & White, 2017, in a study of immature captive bonobos, Pan paniscus). Like chimpanzees, adolescent male mountain gorillas exhibit a propensity for aggressively targeting females before establishing dominance over them (Watts & Pusey, 1993), but further research is needed on intersexual aggression prior to adulthood in this species to facilitate more complete comparisons with chimpanzees.
In contrast to our findings of support for the social dominance hypothesis, we found only limited evidence for a sexually coercive function to adolescent male aggression (H1). Both predictions of the sexual coercion hypothesis were largely unsupported. In addition, we did not find any evidence that adolescent males were more effective at coercing females after having dominated them (H3), which could be due to an overall lack of coercive aggression during adolescence. In comparison to adolescent males, evidence for sexual coercion was clear and compelling in adult males. This finding reaffirms, with a larger dataset, results from earlier studies at Kanyawara, which showed that adult males exhibited sexually coercive aggression (Muller et al., 2007, 2009a, 2011). Recently, Reddy et al. (2021) reported that adolescent males were capable of sexually coercing females at Ngogo. However, the authors treated age as a continuous variable and focused on adolescence through early adulthood (ages nine through 20 years). Consequently, it is unclear how effective adolescent males were at sexual coercion, compared to adult males as a comprehensive age class.
While our formal tests of the two predictions of the sexual coercion hypothesis did not show evidence for coercion by adolescents, additional analyses indicated that some adolescent males mated more frequently with the cycling females toward whom they were more aggressive. This was especially true for nulliparous females, indicating that males may first practice employing sexual coercion through their interactions with these less attractive and lower-ranking females. Young adult males who have not yet achieved high rank might similarly focus on coercing nulliparous females. More generally, increases in the effectiveness of sexual coercion may take place gradually across male maturation, rather than abruptly upon reaching adulthood. Reddy et al. (2021) found support for this idea in their study of adolescent and young adult male chimpanzees at Ngogo, such that female-directed aggression predicted male mating success more strongly with each unit increase in male age. This study also emphasized the relatively high frequency with which adolescent males mated with nulliparous, rather than parous, females (see also Watts, 2015). In the same way, adolescent males in our study copulated at relatively high rates with nulliparous females. This likely reflects efforts to minimize competition with adult males, who are more interested in mating with parous females (Muller et al., 2006).
Several studies have shown that competition with adult males reduces adolescent males' ability to mate with the most fecund females (Hasegawa & Hiraiwa-Hasegawa, 1983; Muller et al., 2020; Pusey, 1990; Watts, 2015). For example, in the M-group chimpanzees of the Mahale Mountains National Park, even the presence of adult males in the social party deterred adolescent males from mating with maximally swollen females (Hayaki, 1985). Takahata et al. (1999) suggested that adult males similarly suppress adolescent male copulation rates in chimpanzees' close genetic relatives, bonobos. Additional research is required to determine the extent to which adult males tend to limit adolescent male success with sexual coercion of particular females at Kanyawara. Overall, our data suggest that the ability to sexually coerce females is constrained during adolescence as compared to adulthood. This result is consistent with previous reports that indirect coercion is employed most effectively by high-ranking males, who are most capable of monopolizing females (Feldblum et al., 2014; Muller et al., 2009a). In summary, we cannot dismiss a role for sexual coercion in the aggressive behavior of adolescent males at Kanyawara, but, in our data, any such role was weaker than that of intersexual status competition.
Evidence is mounting that sexual coercion in many species, including humans, is most effectively employed by males with greater sexual experience and mating opportunities, rather than males who may be unattractive to females or unable to compete through conventional means (e.g., Kanin, 1985; Lalumière et al., 1996, 2005; reviewed by van Schaik et al., 2004; Emery Thompson, 2009; Emery Thompson & Alvarado, 2012; Muller, 2017). In keeping with this generalization, it was not the low-ranking adolescents with limited sexual access to females who most clearly exhibited coercion in our study community of chimpanzees, but rather the higher-ranking adult males with the most mating opportunities. Future research on other chimpanzee communities and other species could help develop a more complete understanding of sexual coercion by similarly exploring patterns of male aggression against females prior to male adulthood.
In our study, adolescent males rarely targeted their mothers or maternal sisters with aggression, in comparison to non-maternally related females, toward whom adolescents directed more aggression by approximately an order of magnitude. These findings accord with Adang's (1984, 1985, 1986) observations of intersexual aggression in captive western chimpanzees, which showed that juvenile and adolescent males seldom directed aggression toward their mothers, preferring instead to target females outside of their maternal sub-group. Given that males risk incurring inclusive fitness costs by competing with their maternal relatives, it is not surprising that mothers and maternal sisters were largely exempt from adolescent males' struggle for dominance over members of the opposite sex. Low rates of aggression from adolescent males toward their mothers might reflect an attempt to retain one's mother as a source of agonistic support during this age period. For instance, Reddy and Sandel (2020) recently suggested that adolescent male chimpanzees rely on their mothers for agonistic support during conflicts. Mothers similarly provide varying forms of support to maturing and even fully adult sons in bonobos (Surbeck et al., 2011), humans (Chapais, 2008), and killer whales (Orcinus orca: Brent et al., 2015). Such support may be of limited value in chimpanzees, however, as data from seven study sites found no effect of having a mother alive in the group on paternity success in males aged 10 years or older (Surbeck et al., 2019).
In sum, we found strong evidence that adolescent male aggression toward females functioned to establish social dominance over them, as part of a male strategy of rising in rank by first dominating the lowest-ranking adults in the community. By frequently challenging females during adolescence, males likely learn how much persistence and force is required to ultimately outrank members of the opposite sex, preparing them to challenge even more intimidating adult males during late adolescence and early adulthood. In contrast to our findings for social dominance, we found only limited evidence that adolescent male aggression toward females functioned as sexual coercion. While some adolescent males attempted to sexually coerce females, they were much less successful than adult males, who exhibited clear evidence for sexual coercion. Our study highlights that the inability to effectively compete with larger, more powerful adult males can considerably shape males' behavioral tactics during adolescence.
Supplementary Material
ACKNOWLEDGMENTS
For field data collection, we thank Daniel Akaruhanga, Seezi Atwijuze, Fred Baguma, the late John Barwogeza, Richard Karamagi, Christopher Katongole, James Kyomuhendo, Francis Mugurusi, the late Donor Muhangyi, the late Christopher Muruuli, Solomon Musana, Japan Musunguzi, Denis Sebugwawo, John Sunday, Peter Tuhairwe, and Wilberforce Tweheyo. We thank Emily Otali for research oversight, Edgar Mugenyi, Christine Abbe, and Jovia Mahoro for field data entry, and the Uganda Wildlife Authority and Makerere University Biological Field Station for sponsoring long-term research in Kibale National Park. For helpful comments on the manuscript, we thank Siobhán Mattison and the anonymous editorial board member and reviewers. Research was supported by the National Science Foundation Graduate Research Fellowship Grant No. DGE-0237002; National Science Foundation Grants No. 1355014, 9807448, 0416125, and NCS-FO-1926352; the National Institutes of Health (National Institute on Aging/Office of Research on Women's Health - R01-AG049395); the Wenner-Gren Foundation; the Leakey Foundation; Harvard University; and the University of New Mexico.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
REFERENCES
- Adang OMJ (1984). Teasing in young chimpanzees. Behaviour, 88, 98–121. 10.1163/156853984X00506 [DOI] [Google Scholar]
- Adang OMJ (1985). Exploratory aggression in chimpanzees. Behaviour, 95, 138–162. 10.1163/156853985X00091 [DOI] [Google Scholar]
- Adang OMJ (1986). Exploring the social environment: A developmental study of teasing in chimpanzees. Ethology, 73, 136–160. 10.1111/j.1439-0310.1986.tb01005.x [DOI] [Google Scholar]
- Albers PCH, & de Vries H (2001). Elo-rating as a tool in the sequential estimation of dominance strengths. Animal Behaviour, 61, 489–495. 10.1006/anbe.2000.1571 [DOI] [Google Scholar]
- Alberts SC (2019). Social influences on survival and reproduction: Insights from a long-term study of wild baboons. Journal of Animal Ecology, 88, 47–66. 10.1111/1365-2656.12887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist G, & Rowe L (2005). Sexual Conflict. Princeton University Press. [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Boose K, & White F (2017). Harassment of adults by immatures in bonobos (Pan paniscus): Testing the exploratory aggression and rank improvement hypotheses. Primates, 58, 493–504. 10.1007/s10329-017-0616-9 [DOI] [PubMed] [Google Scholar]
- Brent LJN, Franks DW, Foster EA, Balcomb KC, Cant MA, & Croft DP (2015). Ecological knowledge, leadership, and the evolution of menopause in killer whales. Current Biology, 25, 746–750. 10.1016/j.cub.2015.01.037 [DOI] [PubMed] [Google Scholar]
- Bygott JD (1974). Agonistic behaviour in wild male chimpanzees (Doctoral dissertation). University of Cambridge, England. [Google Scholar]
- Bygott JD (1979). Agonistic behavior, dominance, and social structure in wild chimpanzees of the Gombe National Park. In Hamburg DA & McCown ER (Eds.), The great apes (pp. 405–427). Benjamin/Cummings. [Google Scholar]
- Campbell CJ (2003). Female-directed aggression in free-ranging Ateles geoffroyi. International Journal of Primatology, 24, 223–237. 10.1023/A:1023036830192 [DOI] [Google Scholar]
- Chapais B (2008). Primeval kinship: How pair-bonding gave birth to human society. Harvard University Press. [Google Scholar]
- Clutton-Brock TH (2016). Mammal societies. Wiley Blackwell. [Google Scholar]
- Clutton-Brock TH, & Parker GA (1995). Sexual coercion in animal societies. Animal Behaviour, 49, 1345–1365. 10.1006/anbe.1995.0166 [DOI] [Google Scholar]
- Cowlishaw G, & Dunbar RIM (1991). Dominance rank and mating success in male primates. Animal Behaviour, 41, 1045–1056. 10.1016/S0003-3472(05)80642-6 [DOI] [Google Scholar]
- de Waal FBM, & Hoekstra JA (1980). Contexts and predictability of aggression in chimpanzees. Animal Behaviour, 28, 929–937. 10.1016/S0003-3472(80)80155-2 [DOI] [Google Scholar]
- Emery Thompson M (2005). Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): Methodological considerations and the role of hormones in sex and conception. American Journal of Primatology, 67, 137–158. 10.1002/ajp.20174 [DOI] [PubMed] [Google Scholar]
- Emery Thompson M (2009). Human rape: Revising evolutionary perspectives. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 346–374). Harvard University Press. [Google Scholar]
- Emery Thompson M, & Alvarado LC (2012). Sexual conflict and sexual coercion in comparative evolutionary perspective. In Shackelford TK & Goetz AT (Eds.), Oxford handbook of sexual conflict in humans (pp. 100–121). Oxford University Press. [Google Scholar]
- Emery Thompson M, Fox SA, Berghänel A, Sabbi KH, Phillips-Garcia S, Enigk DK, Otali E, Machanda ZP, Wrangham RW, & Muller MN (2020). Wild chimpanzees exhibit humanlike aging of glucocorticoid regulation. Proceedings of the National Academy of Sciences, 117, 8424–8430. 10.1073/pnas.1920593117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery Thompson M, Muller MN, Kahlenberg S, & Wrangham RW (2010). Dynamics of social and energetic stress in wild female chimpanzees. Hormones and Behavior, 58, 440–449. 10.1016/j.yhbeh.2010.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagen R (1981). Animal play behavior. Oxford University Press. [Google Scholar]
- Feldblum JT, Wroblewski EE, Rudicell RS, Hahn BH, Paiva T, Cetinkaya-Rundel M, Pusey AE, & Gilby IC (2014). Sexually coercive male chimpanzees sire more offspring. Current Biology, 24, 2855–2860. 10.1016/j.cub.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn MV (1988). Mate guarding in a Caribbean village. Ethology and Sociobiology, 9, 1–28. 10.1016/0162-3095(88)90002-7 [DOI] [Google Scholar]
- Foerster S, Franz M, Murray CM, Gilby IC, Feldblum JT, Walker KK, & Pusey AE (2016). Chimpanzee females queue but males compete for social status. Scientific Reports, 6, 35404. 10.1038/srep35404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox EA (2002). Female tactics to reduce sexual harassment in the Sumatran orangutan (Pongo pygmaeus abelii). Behavioral Ecology and Sociobiology, 52, 93–101. 10.1007/s00265-002-0495-x [DOI] [Google Scholar]
- Glickman ME & Doan T (2010). The USCF rating system. http://www.glicko.net/ratings/rating.system.pdf [Google Scholar]
- Goodall J (1986). The chimpanzees of Gombe: Patterns of behavior. Harvard University Press. [Google Scholar]
- Hasegawa T, & Hiraiwa-Hasegawa M (1983). Opportunistic and restrictive matings among wild chimpanzees in the Mahale Mountains, Tanzania. Journal of Ethology, 1, 75–85. 10.1007/BF02347833 [DOI] [Google Scholar]
- Hass CC, & Jenni DA (1993). Social play among juvenile bighorn sheep: Structure, development, and relationship to adult behavior. Ethology, 93, 105–116. 10.1111/j.1439-0310.1993.tb00982.x [DOI] [Google Scholar]
- Hayaki H (1985). Copulation of adolescent male chimpanzees, with special reference to the influence of adult males, in the Mahale National Park, Tanzania. Folia Primatologica, 44, 148–160. 10.1159/000156209 [DOI] [Google Scholar]
- Kaburu SSK, & Newton-Fisher NE (2015). Trading or coercion? Variation in male mating strategies between two communities of east African chimpanzees. Behavioral Ecology and Sociobiology, 69, 1039–1052. 10.1007/s00265-015-1917-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanin EJ (1985). Date rapists: Differential sexual socialization and relative deprivation. Archives of Sexual Behavior, 14, 219–231. 10.1007/BF01542105 [DOI] [PubMed] [Google Scholar]
- Kitchen DM, Beehner JC, Bergman TJ, Cheney DL, Crockford C, Engh AL, Fischer J, Seyfarth RM, & Wittig RM (2009). The causes and consequences of male aggression directed at female chacma baboons. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 128–156). Harvard University Press. [Google Scholar]
- Knott CD (2009). Orangutans: Sexual coercion without sexual violence. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 81–111). Harvard University Press. [Google Scholar]
- Kummer H (1968). Social organization of Hamadryas Baboons. A field study. University of Chicago Press. [Google Scholar]
- Lalumière ML, Chalmers LJ, Quinsey VL, & Seto MC (1996). A test of the mate deprivation hypothesis of sexual coercion. Ethology and Sociobiology, 17, 299–318. 10.1016/S0162-3095(96)00076-3 [DOI] [Google Scholar]
- Lalumière ML, Harris GT, Quinsey VL, & Rice ME (2005). The causes of rape: Understanding individual differences in male propensity for sexual aggression. American Psychological Association. [Google Scholar]
- Matsumoto-Oda A, & Oda R (1998). Changes in the activity budget of cycling female chimpanzees. American Journal of Primatology, 46, 157–166. [DOI] [PubMed] [Google Scholar]
- Miller MN, & Byers JA (1998). Sparring as play in young pronghorn males. In Bekoff M & Byers JA (Eds.), Animal play: Evolutionary, comparative and ecological perspectives (pp. 141–160). Cambridge University Press. [Google Scholar]
- Muller MN (2002). Agonistic relations among Kanyawara chimpanzees. In Boesch C, Hohmann G, & Marchant LF (Eds.), Behavioural diversity in chimpanzees and bonobos (pp. 112–124). Cambridge University Press. [Google Scholar]
- Muller MN (2017). Sexual coercion in chimpanzees and humans. In Muller MN, Wrangham RW, & Pilbeam DR (Eds.), Chimpanzees and human evolution (pp. 572–601). Harvard University Press. [Google Scholar]
- Muller MN, Blurton Jones NG, Colchero F, Emery Thompson M, Enigk DK, Feldblum JT, Hahn BH, Langergraber KE, Scully EJ, Vigilant L, Walker KK, Wrangham RW, Wroblewski EE, & Pusey AE (2020). Sexual dimorphism in chimpanzee (Pan troglodytes schweinfurthii) and human age-specific fertility. Journal of Human Evolution., 144, 102795. 10.1016/j.jhevol.2020.102795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Emery Thompson M, Kahlenberg SM, & Wrangham RW (2011). Sexual coercion by male chimpanzees shows that female choice may be more apparent than real. Behavioral Ecology and Sociobiology, 65, 921–933. 10.1007/s00265-010-1093-y [DOI] [Google Scholar]
- Muller MN, Emery Thompson M, & Wrangham RW (2006). Male chimpanzees prefer mating with old females. Current Biology, 16, 2234–2238. 10.1016/j.cub.2006.09.042 [DOI] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, Emery Thompson M, & Wrangham RW (2007). Male coercion and the costs of promiscuous mating for female chimpanzees. Proceedings of the Royal Society B, 274, 1009–1014. 10.1098/rspb.2006.0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MN, Kahlenberg SM, & Wrangham RW (2009a). Male aggression against females and sexual coercion in chimpanzees. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 184–217). Harvard University Press. [Google Scholar]
- Muller MN, Kahlenberg SM, & Wrangham RW (2009b). Male aggression and sexual coercion of females in primates. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 3–22). Harvard University Press. [Google Scholar]
- Muller MN, & Mitani JC (2005). Conflict and cooperation in wild chimpanzees. Advances in the Study of Behavior, 35, 275–331. 10.1016/S0065-3454(05)35007-8 [DOI] [Google Scholar]
- Muller MN, & Wrangham RW (2009). Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females. Harvard University Press. [Google Scholar]
- Neumann C, Duboscq J, Dubuc C, Ginting A, Maulana Irwan A, Agil M, Widdig A, & Engelhardt A (2011). Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour, 82, 911–921. 10.1016/j.anbehav.2011.07.016 [DOI] [Google Scholar]
- Newton-Fisher NE (2006). Female coalitions against male aggression in wild chimpanzees of the Budongo Forest. International Journal of Primatology, 27, 1589–1599. 10.1007/s10764-006-9087-3 [DOI] [Google Scholar]
- Nishida T (1968). The social group of wild chimpanzees in the Mahali Mountains. Primates, 9, 167–224. 10.1007/BF01730971 [DOI] [Google Scholar]
- Nishida T (1979). The social structure of chimpanzees of the Mahale mountains. In Hamburg DA & McCown ER (Eds.), The great apes (pp. 73–121). Benjamin/Cummings. [Google Scholar]
- Nishida T (2003). Harassment of mature female chimpanzees by young males in the Mahale Mountains. International Journal of Primatology, 24, 503–514. 10.1023/A:1023870229247 [DOI] [Google Scholar]
- Novak SA, & Hatch MA (2009). Intimate wounds: Craniofacial trauma in women and female chimpanzees. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 322–345). Harvard University Press. [Google Scholar]
- Palombit RA (2014). Sexual conflict in nonhuman primates. Advances in the Study of Behavior, 46, 191–280. 10.1016/B978-0-12-800286-5.00005-5 [DOI] [Google Scholar]
- Pereira ME (1988). Effects of age and sex on intra-group spacing behaviour in juvenile savannah baboons, Papio cynocephalus cynocephalus. Animal Behaviour, 36, 184–204. 10.1016/S0003-3472(88)80262-8 [DOI] [Google Scholar]
- Pusey AE (1978). The physical and social development of wild adolescent chimpanzees (Pan troglodytes schweinfurthii) (Doctoral dissertation). Stanford University, CA. [Google Scholar]
- Pusey AE (1990). Behavioural changes at adolescence in in chimpanzees. Behaviour, 115, 203–246. 10.1163/156853990X00581 [DOI] [Google Scholar]
- R Core Team. (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Reddy RB, Langergraber KE, Sandel AA, Vigilant L, & Mitani JC (2021). The development of affiliative and coercive reproductive tactics in male chimpanzees. Proceedings of the Royal Society B, 288, 20202679. 10.1098/rspb.2020.2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RB, & Mitani JC (2020). Adolescent and young adult male chimpanzees form affiliative, yet aggressive, relationships with females. Journal of Human Evolution, 144, 102813. 10.1016/j.jhevol.2020.102813 [DOI] [PubMed] [Google Scholar]
- Reddy RB, & Sandel AA (2020). Social relationships between chimpanzee sons and mothers endure but change during adolescence and adulthood. Behavioral Ecology and Sociobiology, 74, 150. 10.1007/s00265-020-02937-7 [DOI] [Google Scholar]
- Ren R, Yan K, Su Y, Qi H, Liang B, Bao W, & de Waal FBM (1991). The reconciliation behavior of golden monkeys (Rhinopithecus roxellanae roxellanae) in small breeding groups. Primates, 32, 321–327. 10.1007/BF02382673 [DOI] [Google Scholar]
- Robbins MM (2003). Behavioral aspects of sexual selection in mountain gorillas. In Jones CB (Ed.), Sexual selection and reproductive competition in primates: New perspectives and directions (pp. 477–501). American Society of Primatologists. [Google Scholar]
- Robbins MM (2009). Male aggression against females in mountain gorillas: Courtship or coercion? In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 112–127). Harvard University Press. [Google Scholar]
- Rothstein A, & Griswold JG (1991). Age and sex preferences for social partners by juvenile bison bulls, Bison bison. Animal Behaviour, 41, 227–237. 10.1016/S0003-3472(05)80474-9 [DOI] [Google Scholar]
- Scott EM, Mann J, Watson-Capps JJ, Sargeant BL, & Connor RC (2005). Aggression in bottlenose dolphins: Evidence for sexual coercion, male-male competition, and female tolerance through analysis of tooth-rake marks and behaviour. Behaviour, 142, 21–44. 10.1163/1568539053627712 [DOI] [Google Scholar]
- Smuts BB, & Smuts RW (1993). Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Advances in the Study of Behavior, 22, 1–63. 10.1016/S0065-3454(08)60404-0 [DOI] [Google Scholar]
- Sterck EHM, Watts DP, & van Schaik CP (1997). The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology, 41, 291–309. 10.1007/s002650050390 [DOI] [Google Scholar]
- Stumpf RM, & Boesch C (2010). Male aggression and sexual coercion in wild west African chimpanzees, Pan troglodytes verus. Animal Behaviour, 79, 333–342. 10.1016/j.anbehav.2009.11.008 [DOI] [Google Scholar]
- Sugiyama Y (1968). Social organization of chimpanzees in the Budongo Forest, Uganda. Primates, 9, 225–258. 10.1007/BF01730972 [DOI] [Google Scholar]
- Surbeck M, Boesch C, Crockford C, Emery Thompson M, Furuichi T, Fruth B, Hohmann G, Ishizuka S, Machanda Z, Muller MN, Pusey A, Sakamaki T, Tokuyama N, Walker K, Wrangham R, Wroblewski E, Zuberbühler K, Vigilant L, & Langergraber K (2019). Males with a mother living in their group have higher paternity success in bonobos but not chimpanzees. Current Biology, 29, R354–R355. 10.1016/j.cub.2019.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surbeck M, Mundry R, & Hohmann G (2011). Mothers matter! Maternal support, dominance status and mating success in male bonobos (Pan paniscus). In Proceedings of the Royal Society B (Vol. 278, pp. 590–598). 10.1098/rspb.2010.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedell L, & Schreier A (2009). Male aggression toward females in hamadryas baboons: Conditioning, coercion, and control. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 244–268). Harvard University Press. [Google Scholar]
- Takahata Y, Ihobe H, & Idani G (1999). Do bonobos copulate more frequently and promiscuously than chimpanzees? Human Evolution, 14, 159–167. 10.1007/BF02440153 [DOI] [Google Scholar]
- Tutin CEG (1979). Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Behavioral Ecology and Sociobiology, 6, 29–38. 10.1007/BF00293242 [DOI] [Google Scholar]
- van Schaik CP, Pradhan GR, & van Noordwijk MA (2004). Mating conflict in primates: Infanticide, sexual harassment and female sexuality. In Kappeler PM & van Schaik CP (Eds.), Sexual selection in primates: New and comparative perspectives (pp. 131–150). Cambridge University Press. [Google Scholar]
- Watson DM (1993). The play associations of red-necked wallabies (Macropus rufogriseus banksianus) and relation to other social contexts. Ethology, 94, 1–20. 10.1111/j.1439-0310.1993.tb00543.x [DOI] [Google Scholar]
- Watson-Capps JJ (2009). Evolution of sexual coercion with respect to sexual selection and sexual conflict theory. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 23–41). Harvard University Press. [Google Scholar]
- Watts DP (1997). Agonistic interventions in wild mountain gorilla groups. Behaviour, 134, 23–57. 10.1163/156853997X00269 [DOI] [Google Scholar]
- Watts DP (1998). Coalitionary mate guarding by male chimpanzees at Ngogo, Kibale National Park, Uganda. Behavioral Ecology and Sociobiology, 44, 43–55. 10.1007/s002650050513 [DOI] [Google Scholar]
- Watts DP (2015). Mating behavior of adolescent male chimpanzees (Pan troglodytes) at Ngogo, Kibale National Park, Uganda. Primates, 56, 163–172. 10.1007/s10329-014-0453-z [DOI] [PubMed] [Google Scholar]
- Watts DP, Colmenares F, & Arnold K (2000). Redirection, consolation, and male policing: How targets of aggression interact with bystanders. In Aureli F & de Waal FBM (Eds.), Natural conflict resolution (pp. 281–301). University of California Press. [Google Scholar]
- Watts DP, & Pusey AE (1993). Behavior of juvenile and adolescent great apes. In Pereira ME & Fairbanks LF (Eds.), Juvenile primates: Life history, development and behavior (pp. 148–167). Oxford University Press. [Google Scholar]
- Wilson M, & Daly M (2009). Coercive violence by human males against their female partners. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 271–291). Harvard University Press. [Google Scholar]
- Wrangham RW (2002). The cost of sexual attraction: Is there a tradeoff in female Pan between sex appeal and received coercion? In Boesch C, Hohmann G, & Marchant LF (Eds.), Behavioural diversity in chimpanzees and bonobos (pp. 204–215). Cambridge University Press. [Google Scholar]
- Wrangham RW, & Muller MN (2009). Sexual coercion in humans and other primates: The road ahead. In Muller MN & Wrangham RW (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 451–468). Harvard University Press. [Google Scholar]
- Zeileis A & Hothorn T (2002). Diagnostic checking in regression relationships. R News, 2, 7–10. https://CRAN.R-project.org/doc/Rnews/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.


