Abstract
Rationale:
Reducing nicotine content in cigarettes to ≤ 2.4 mg per g of tobacco [mg/g] reduces smoking behavior and toxicant exposure among adult daily smokers. However, cigarettes with similar nicotine content could support continued experimentation and smoking progression among young adults who smoke infrequently.
Objectives:
This study evaluated the threshold for nicotine in cigarettes that produces reactions associated with smoking progression in a sample of young adults who smoke infrequently.
Methods:
Young adults (n=87, 18-25 years, 49% female) using tobacco products ≤15 days per month completed three counterbalanced, double-blinded sessions, each measuring Positive and Negative subjective reactions to fixed doses of smoke from investigational cigarettes containing one of three different nicotine contents: normal (NNC; 15.8 mg/g); very low (VLNC; 0.4 mg/g); and intermediate (INC; 2.4 mg/g). In a final session, participants chose one of the cigarettes to self-administer.
Results:
Post-cigarette breath carbon monoxide was greater for VLNC than for NNC (p<.001). Positive reactions were greater for NNC than INC (p<.001), and for INC than VLNC (p=.001). Negative reactions were greater for NNC than INC and VLNC (both p<.001); INC and VLNC did not differ. Cigarette choices did not differ from an even distribution (43% NNC, 25% INC, 32% VLNC), but choice for NNC or INC was associated with higher ratio of Positive to Negative reactions during the NNC and INC fixed-dose sessions, respectively (p<.001).
Conclusions:
Reducing nicotine content will likely lower the abuse liability of cigarettes for most young, low-frequency smokers. Additional work is needed to determine if compensatory smoking may lead to increased toxicant exposure, and if a subset of individuals choosing lower nicotine cigarettes may continue to smoke regardless of nicotine content.
Keywords: nicotine, tobacco, policy, reduction, prevention, vulnerability
INTRODUCTION
The 2009 Family Smoking Prevention and Tobacco Control Act gave the Food and Drug Administration (FDA) the authority to reduce nicotine content in cigarettes if such regulatory action is deemed likely to improve the health of the population (U.S. Congress 2009). Evidence suggests that a nicotine reduction policy would reduce smoking, nicotine dependence, and toxicant exposure among daily, adult smoker (Benowitz et al. 2007; Donny et al. 2015; Donny, Houtsmuller, and Stitzer 2007; Hatsukami et al. 2010; Hatsukami et al. 2013; Hatsukami et al. 2019; Hatsukami et al. 2018). In a landmark 2015 multisite study (Donny et al. 2015), smokers assigned to smoke cigarettes with ≤ 2.4 mg of nicotine per gram of tobacco (mg/g) for 6 weeks reduced their smoking behavior and exhibited decreased exposure to toxicants relative to a control group that smoked normal nicotine content cigarettes (i.e. 15.8 mg/g).
Despite the growing literature addressing nicotine reduction among adult, dependent smokers (Berman and Glasser 2019; Denlinger-Apte et al. 2019; Higgins et al. 2017; Mercincavage et al. 2018; Tidey et al. 2019), far less is known about the potential impact of reducing the nicotine content in cigarettes among young adult infrequent smokers (i.e. ≤15 days per month). According to data from Wave 3 of the Population Assessment of Tobacco and Health (PATH) survey, 45.5% percent of cigarette smokers between the ages of 18-24 smoke 15 or fewer days per month (unpublished analysis), and this age range is a critical developmental period for increasing their smoking behavior (U.S. Department of Health and Human Services 2014). Furthermore, given differences in both age-related biology and history of nicotine exposure, it is unknown whether young adult, low-frequency smokers will respond to varying levels of nicotine in the same way as adult, daily smokers (Gellner, Belluzzi, and Leslie 2016; Levin et al. 2007; Levin et al. 2003; Schassburger et al. 2016; Shram, Li, and Le 2008).
Unlike daily cigarette smokers—whose smoking is driven largely by the need to maintain steady levels of nicotine in order to avoid withdrawal (Cassidy 2019; Baker, Brandon, and Chassin 2004)—evidence suggests that smoking behaviors of low-frequency smokers are driven by environmental factors (e.g. social setting, use of other drugs and/or alcohol) (Shiffman, Dunbar, and Ferguson 2015; Shiffman et al. 2014; Shiffman et al. 2012) to a much greater degree than by withdrawal alleviation (Cassidy 2019; Shiffman et al. 2015). As such, it is possible that a modest reduction in nicotine content that is sufficient to reduce smoking among adult, dependent smokers (e.g., 2.4 mg/g) may not reduce the utility of smoking among segments of the young adult population. Furthermore, given that lower nicotine contents may reduce some aversive effects that mitigate continued use among experimenting smokers (Cassidy et al. 2018; Rios-Bedoya et al. 2009; Wang et al. 1995; Zabor et al. 2013), it is possible that a modest reduction in nicotine content could actually be preferable to NNC cigarettes for some smokers in this group. Thus, understanding the impact of varying nicotine content on both positive and negative reactions to cigarettes in this population is critical to fully inform regulatory policy.
Several recent studies have begun to examine the effects of younger age or low-frequency smoking on reactions to reduced nicotine content cigarettes (Cassidy 2019; Cassidy et al. 2018; Cassidy et al. 2019; Davis et al. 2019; Shiffman, Kurland, et al. 2018). These studies generally suggest that young adult daily smokers would benefit from a reduced nicotine content standard (Cassidy et al. 2019); as would older non-daily smokers (Shiffman, Kurland, et al. 2018). However, it is worth emphasizing that no study has specifically evaluated these questions in a sample of young adults who smoke infrequently.
In the current research, we evaluated acute reactions to cigarette smoke with varying nicotine contents among 18-25-year-old individuals who use tobacco 15 or fewer days per month in a cross-sectional design. Nicotine contents were selected as the highest (NNC) and lowest (VLNC) levels evaluated in a previous multisite trial (Donny et al. 2015), as well as an intermediate nicotine content (INC) cigarette of 2.4 mg/g, corresponding to the upper limit of the range reducing smoking behavior among adult, daily smokers (Donny et al. 2015). We hypothesized that INC cigarettes would produce fewer aversive effects than NNC cigarettes, but greater positive effects than VLNC cigarettes, and that this increased ratio of positive to negative effects would correspond to greater preference for the INC cigarette during a final choice session.
METHODS
Inclusion/Exclusion Criteria and Screening
All procedures were approved by the Duke University Medical Center IRB. Participants were recruited from the community via online ads and flyers from 2017-2019. Following an initial phone screening, eligible participants attended an in-person screening session, where written informed consent was obtained, and further eligibility was determined. Eligible participants were between the ages of 18-25; generally healthy as determined by review of brief medical history, current medications, and vital signs by the study physician; used tobacco products between 1-15 days/month for at least 6 months; smoked at least 5 cigarettes/month; had a breath alcohol value = 0.000 (required at all visits); and had their smoking status corroborated by two individuals familiar with their smoking habits. Corroborators provided information by phone about their frequency of interaction with the participant, as well as estimated frequency in which the participant smokes cigarettes or uses other tobacco products (e.g., cigars or e-cigarettes). No corroborator PHI was collected, and they were not consented into the study as per our IRB-approved protocol. Participants were excluded if they self-reported any unstable medical or psychiatric condition; had a positive urine drug screen (excluding THC); made a serious quit attempt in the past 3 months or had plans to quit smoking in the next 2 months; currently used products to support tobacco cessation; had current or recent alcohol or drug abuse problems; were unable to attend all required experimental sessions; had blood pressure >160/100 mmHg or resting heart rate > 115 bpm; and/or were pregnant, trying to become pregnant, or breastfeeding.
Study Design and Procedures
Eligible participants completed a training session, followed by three fixed-dose (FD) sessions, and a final choice session. During the training session, participants were familiarized with the FD session procedures and completed the controlled puff volume apparatus (CPVA) (Levin 1989) procedures using their usual brand cigarette. Procedures during each FD session were the same as the training session, but with a different research cigarette (see below) used during each session. During the final choice session, participants were re-exposed to each study cigarette and then instructed to choose the one cigarette they would most like to smoke during the session. A minimum of four days was required to elapse between each session. Participants were asked to abstain from smoking for 24 hours prior to each session, confirmed with expired breath carbon monoxide (CO) < 6 ppm. Individuals who reported past 24-hr tobacco use or who did not meet CO abstinence criteria were required to reschedule.
Research Cigarettes
The study utilized SPECTRUM research cigarettes made available through the NIDA drug supply. Cigarettes were marked to indicate menthol or non-menthol (participants received their preference) but were otherwise devoid of markings indicating condition. Three different cigarettes with the following characteristics were used (nicotine content approximate and as characterized by both NIDA and CDC; see supplementary information for additional details): Normal nicotine content (NNC) cigarettes (15.8 mg/g nicotine content); Intermediate nicotine content (INC) cigarettes (2.4 mg/g nicotine content); Very low nicotine content (VLNC) cigarettes: (0.4 mg/g nicotine content). Nicotine condition was randomized and counterbalanced across the three FD sessions; participants and staff were blinded.
Fixed Dose Sessions
At the start of each session breath CO was assessed using Covita Micro+ Smokyerlyzer, and urine samples were collected for cotinine analysis. Total cotinine was quantitatively analyzed via ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). Participants were then seated in a quiet room to complete questionnaires, after which baseline measures of vital signs (heart rate, blood pressure) were collected. Participants then took a series of 7, 30 mL puffs of cigarette smoke from the CPVA with an inter-puff interval of 30 seconds in order to replicate smoking a single cigarette. CO, vitals, and subjective reactions were assessed following the dosing trial.
Choice session
Participants were again required to be 24 hours abstinent from smoking prior to the choice session. During the choice session, participants first underwent 3 sampling trials to become refamiliarized with the effects of the 3 cigarettes. Cigarettes were presented in boxes marked with FD session labels (A, B, C). Participants sampled two 30-mL puffs from each of the three cigarettes using the CPVA device in A, B, C order, with 15 minutes between each cigarette. Thirty minutes after the last sampling trial, participants chose the cigarette “they most desired to smoke” and then self-administered that cigarette ad lib. Breath CO and vitals were assessed pre- and post-smoking.
Outcome measures
Physiological effects.
As noted above, heart rate, blood pressure, and breath CO were assessed pre- and post- cigarette administration at each session, including pre- and post- ad lib smoking of the cigarette chosen by participants during the choice session. Pre- to post- changes in CO were quantified as CO boost to provide an index of smoke inhalation. Urinary cotinine, collected at the start of each session, was assessed as a confirmation of pre-session nicotine exposure.
Subjective effects.
After each FD administration, participants were asked to rate their “impressions of the cigarette you just smoked” on a 10-point scale ranging from 1 (Not at All) to 11 (Extremely). Questionnaire items were derived from the 8-item Early Smoking Experiences questionnaire (Pomerleau, Pomerleau, and Namenek 1998), which has been widely used to assess subjective responses to cigarette smoking (Hu, Davies, and Kandel 2006; Perkins et al. 2008; Rios-Bedoya et al. 2009). Prior research has demonstrated good construct and predictive validity of this measure (Haberstick et al. 2011; Rodriguez and Audrain-McGovern 2004). Factor analysis has found that items tend to load onto two primary factors of Positive (Pleasant sensations, Relaxation, Pleasurable rush or buzz) and Negative (Unpleasant sensations, Nausea, Coughing, and Difficulty Inhaling) reactions, with the single item Dizziness not loading clearly onto either factor (Perkins et al. 2008; Pomerleau, Pomerleau, and Namanek 1998; Bidwell et al. 2012; Rodriguez and Audrain-McGovern 2004). Accordingly, we considered Positive, Negative, and Dizzy reactions as separate primary outcomes. Ratio of Positive to Negative reactions (P/N) was also calculated to index the relative balance of these effects. Six additional items that have been shown to be sensitive to acute nicotine administration (Liking, Satisfying, Want More, Feel the effects, How much nicotine, and Irritation) were also included (Perkins et al. 2008).
Statistical Analyses
Acute reactions during FD sessions were analyzed using repeated measures ANOVA. Vitals and CO were evaluated in Time (pre versus post) by Condition (NNC, INC, or VLNC) models. Cotinine and subjective reactions to cigarettes were analyzed with the single Condition factor. Follow-up analyses examined pairwise comparisons to further explore the pattern of Condition effects. A Bonferroni correction was applied within each model to control for multiple post-hoc comparisons, resulting in a significance threshold of p<0.008 for models including both pre and post-cigarette assessments, and p<0.017 for models with only a single assessment per condition. Effect sizes for F tests are reported as partial ηp2. Effect sizes for pairwise comparisons are reported using Hedge’s gav (Lakens 2013), calculated using SPSS statistical software with square root of the average variance of measures as standardizer.
For the choice session, overall frequency of choices for each cigarette was compared with the null hypothesis of an even distribution of choices across cigarettes using chi-square goodness of fit tests. Additional analyses examined associations between cigarette choice, subjective reactions, and other participant characteristics using ANOVA, with cigarette choice (NNC, INC, or VLNC) entered as a between-subjects factor. A Bonferroni correction was applied within each model to account for 9 post-hoc pairwise comparisons, resulting in an alpha threshold of p< 0.005. Effect sizes for pairwise comparisons are reported using Hedge’s g or Hedge’s gav for between and within-subjects comparisons, respectively.
RESULTS
Participant characteristics
A total of 181 individuals completed screening, and 98 were enrolled in the study (see Figure 1). Nine eligible participants withdrew prior to completion (7 prior to randomization); 2 additional participants had baseline and/or pre-experimental session cotinine levels > 10 SD above the sample mean and were excluded from analyses, resulting in a final sample of 87 participants.
Fig 1.
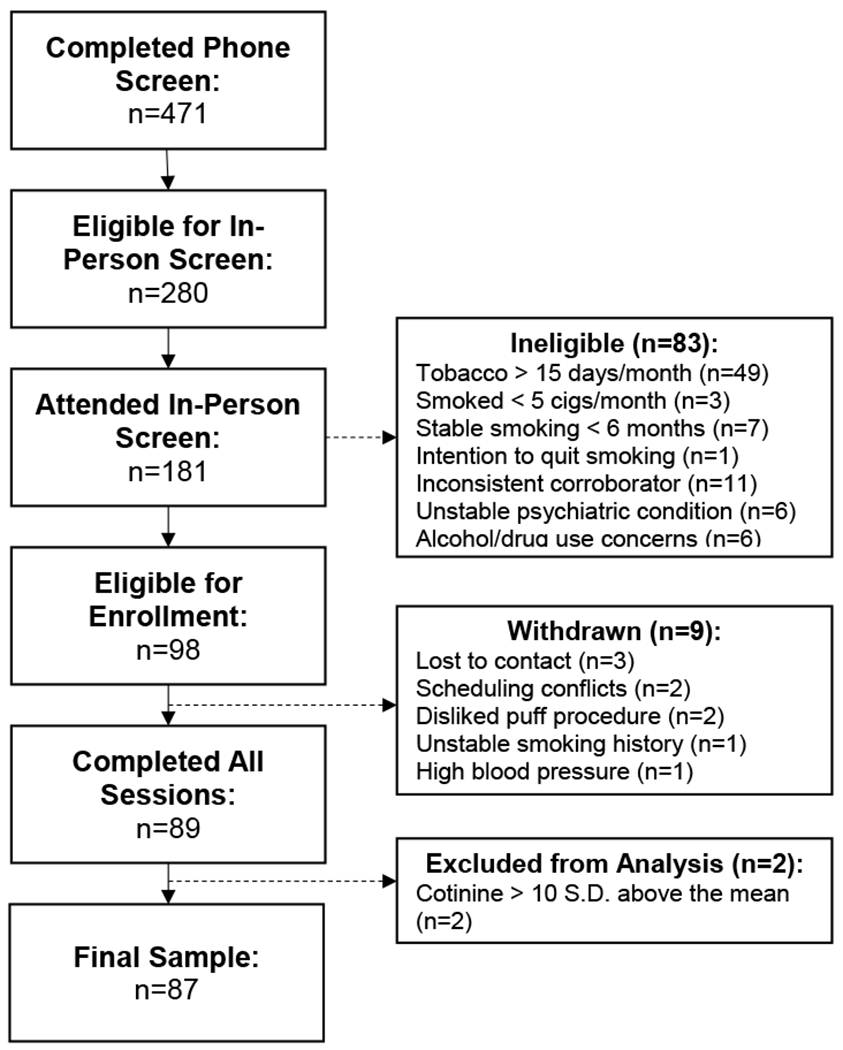
Flow of participants from completion of initial telephone screening to inclusion in final sample for analysis.
The final sample was 49% female, with mean age of 21.1 (SD=1.6). Reported race was 72% white, 2% black, 14% Asian, and 11% more than one race. (Note that numbers total 99% due to rounding.) Fifteen percent were of Hispanic ethnicity. Participants smoked an average of 8.0 (Min=2; Max=15; SD=3.3) days and 15.2 (Min=5; Max=100; SD=11.9) cigarettes per month. Average age of smoking initiation was 17.2 (SD=2.1) and they had smoked at their current rate for an average of 11.3 (SD=9.4) months. Thirty-five percent were menthol smokers. Seventy-one percent reported use of other tobacco products in the past 30 days, with an average of 4.5 days of use among those reporting any use (SD=3.5). (See supplementary information for additional details on other tobacco product use.) Baseline CO level was 3.9 ppm (SD=3.6) at screening, and mean nicotine dependence score as measured by the Hooked on Nicotine Checklist (HONC) (Wheeler et al. 2004) was 1.5 (SD=1.6), indicating low dependence. Mean urinary cotinine level was 49.8 ng/ml (n=63) at screening, 33.2 (n=67), 36.8 (n=66), and 39.8 (n=69) ng/ml for the 3 FD sessions in order of completion, respectively, and 36.1 ng/ml (n=68) for the choice session. Pre-session cotinine levels did not differ across sessions or by condition.
FD sessions: CO and Physiological Measures
Across sessions, CO levels robustly increased from pre- to post-cigarette administration, F(1,86)=318.8, p<.001, ηp2 = .79 (Figure 2). Main effects of Condition, F(2,172)=8.6, p<.001, ηp2 = .09, and a Time x Condition interaction, F(2,172)=10.0, p<.001, ηp2= .10, were observed. This interaction was due to a significant difference between conditions post-exposure, such that post-cigarette CO was greater in the VLNC condition compared with the NNC condition, t(86)=4.4, p<.001, Hedge’s gav = .40. Post-cigarette CO in the INC condition was intermediate to the VLNC and NNC conditions, but direct comparisons did not survive Bonferroni correction, t(86)= −2.7, p=.009, Hedge’s gav = .22, and t(86)=2.3, p<.05, Hedge’s gav = .17, for VLNC and NNC, respectively. There were no pre-exposure differences between conditions.
Fig 2.
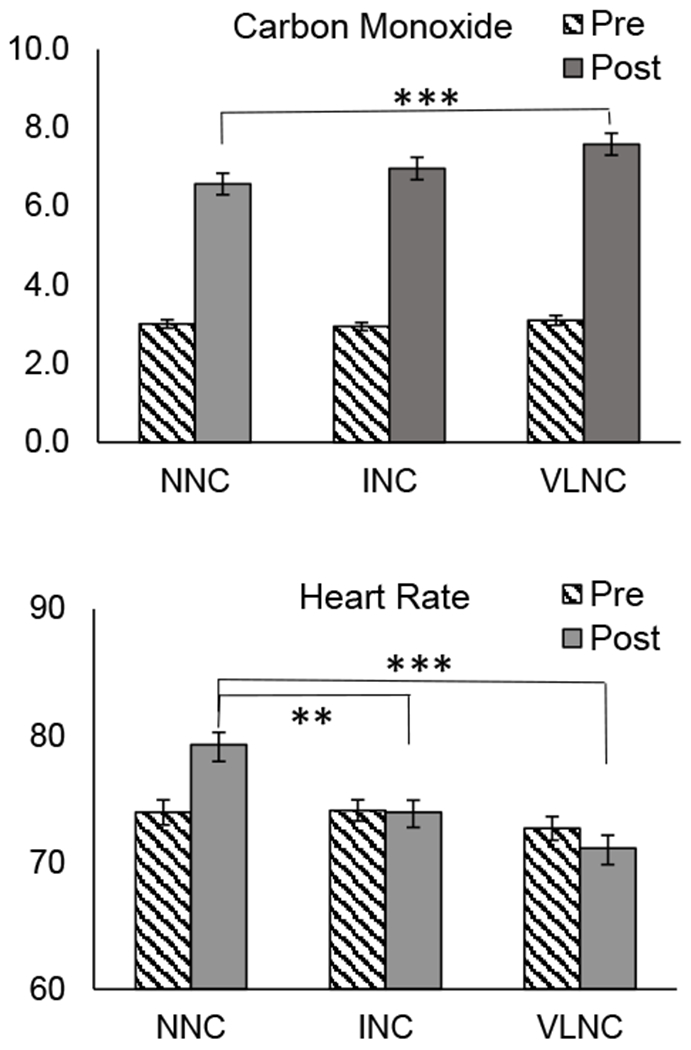
Expired breath carbon monoxide, ppm (top panel), and heart rate, bpm (bottom panel), taken before (striped bars) and after (solid bars) administration of cigarettes with normal nicotine content (NNC), intermediate nicotine content (INC), and very low nicotine content (VLNC) during fixed dose sessions. Error bars represent standard error of the mean. ***Indicates statistical significance of p < .001.
Heart rate did not increase from pre to post exposure, but a main effect of Condition, F(2,170)=7.4, p<.001, ηp2 = .08, and a Time x Condition, F(2,170)=11.1, p<.001, ηp2 = .12, interaction were observed (Figure 2). Follow-up comparisons indicated no pre-exposure differences in heart rate, whereas a higher post-cigarette heart rate were observed in the NNC condition compared with the INC, t(86)=3.6, p<.001, Hedge’s gav = .39, and VLNC, t(86)=5.3, p<.001, Hedge’s gav = .56, conditions. No difference was observed between INC and VLNC, t(86)=1.8, p=.08. Similar Condition, and Time x Condition effects were observed for systolic and diastolic blood pressure (see supplementary information, eFigure 1).
FD sessions: Subjective Reactions
Strong effects of Condition were observed for subjective reactions following smoke administration (Figure 3), including Positive reactions, F(2,172)=21.4, p<.001, ηp2 = .20; Negative reactions, F(2,172)=11.9, p<.001, ηp2 = .08; and Dizziness, F(2,172)=34.3, p<.001, ηp2 = .29. Pairwise comparisons indicated that ratings for all three measures were significantly higher in the NNC condition compared with both the INC and VLNC conditions (all p’s < .001), whereas ratings for the INC cigarette were higher than the VLNC cigarette only for Positive reactions, t(86)=3.4, p=.001 (see eTable 1 for effect sizes). However, P/N ratio did not differ between cigarette conditions. A similar pattern was observed for additional items (see supplementary information, eFigure 2 and eTable 2).
Fig 3.
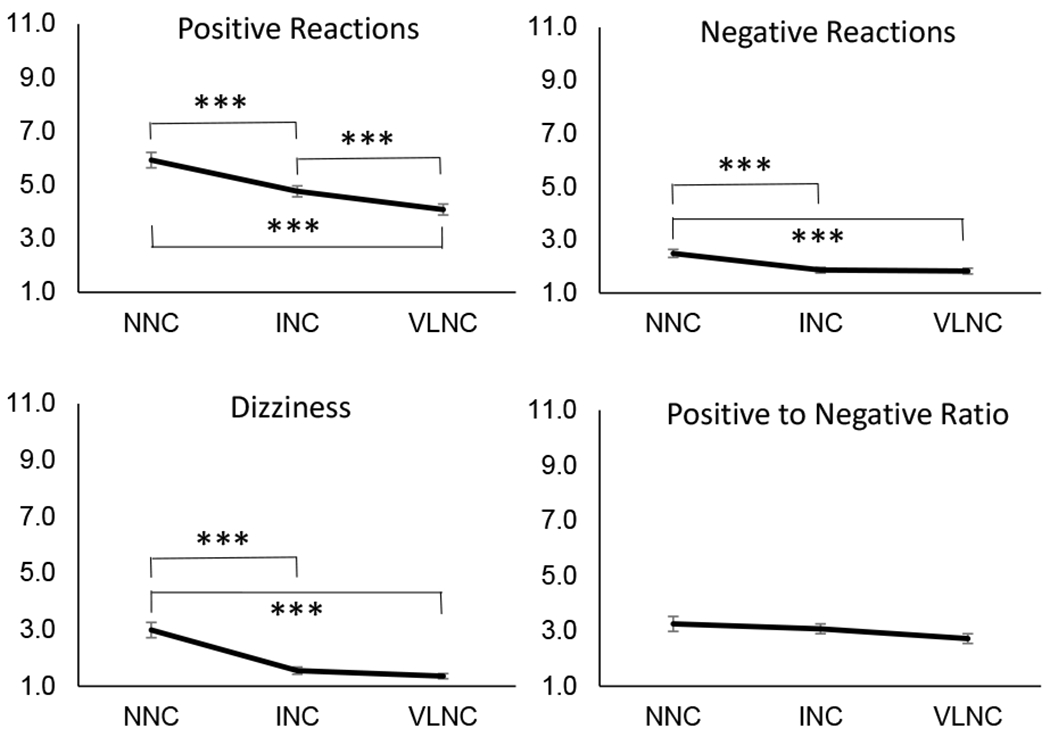
Ratings of subjective reactions assessed following admininstration of cigarettes with normal nicotine content (NNC), intermediate nicotine content (INC), and very low nicotine content (VLNC) during fixed dose sessions. All items were assessed on a 10-point scale ranging from 1 to 11. Positive and Negative reactions are composite scales representing average of 3 items and 5 items, respectively. Positive to Negative ratio was calculated as Positive reactions composite scale divided by Negative reactions composite scale. Error bars represent standard error of the mean. ***Indicates statistical significance of p < .001.
Cigarette choice
During the choice session, 43% of participants chose the NNC cigarette, 25% chose the INC cigarette, and 32% chose the VLNC cigarette. There were no significant differences in the frequency of choices across doses (i.e., observed frequency of choices did not differ significantly from an even distribution) (Chi-square=3.9, p=.14). Participants took an average of 12.6 (SD=5.0) puffs during the choice session; number of puffs and CO boost did not differ by cigarette choice.
We next examined whether the P/N ratio during each FD session was associated with cigarette choice during the choice session. The effect of cigarette condition on P/N ratio varied as a function of cigarette choice, as evidenced by significant Choice X Condition interaction, F(4,168)=5.2, p<.001, ηp2 = .11 (Figure 4). In particular, participants who chose the NNC cigarette reported a significantly greater P/N ratio for NNC compared with both INC, t(36)=3.1, p<.005, Hedge’s gav = .54, and VLNC, t(36)=3.0, p<.005, Hedge’s gav = .71. For those who chose the INC cigarette, P/N ratio was greater for INC than for VLNC, t(21)=3.6, p<.005, Hedge’s gav = .854. The comparison with NNC was in the predicted direction but did not survive correction, t(21)=2.9, p<.01, Hedge’s gav = .85. No differences were observed for VLNC choosers.
Fig 4.
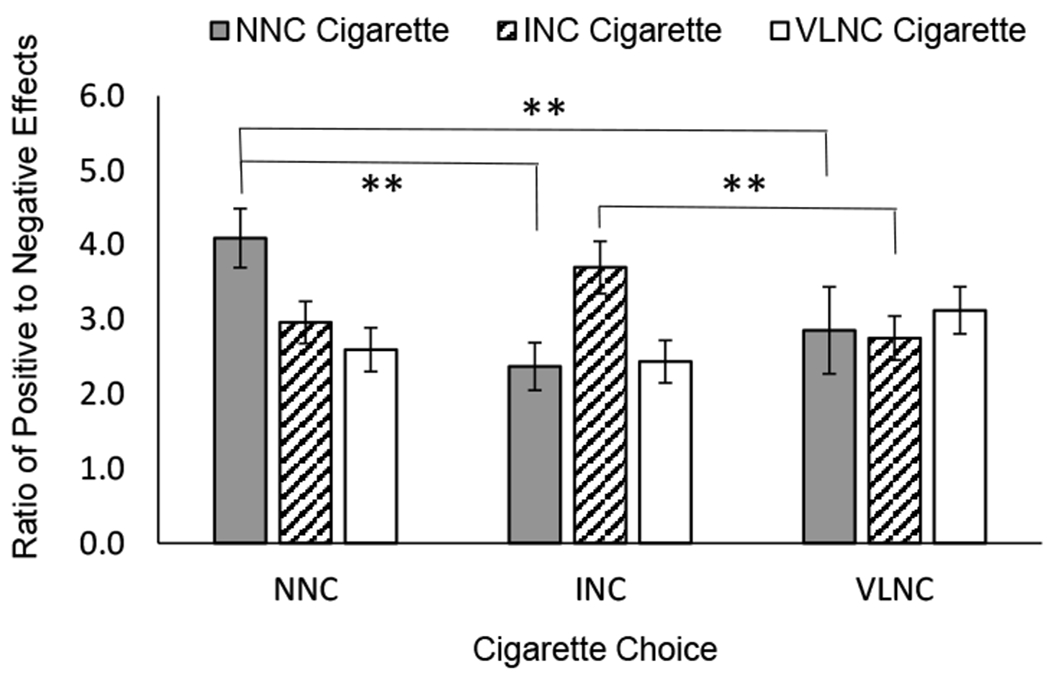
Subjective reactions, characterized as ratio of Positive to Negative Effects, to administration of normal nicotine content (NNC; black bars), intermediate nicotine content (INC; striped bars), and very low nicotine content (VLNC; white bars) during fixed dose sessions, plotted by cigarette choice during the final choice session. Error bars represent standard error of the mean. **Indicates statistical significance of p < .005. Positive to Negative ratio was significantly greater for the NNC cigarette relative to INC and VLNC cigarettes among those who went on to choose the NNC cigarette. Positive to Negative ratio was significantly greater for the INC cigarette relative to VLNC cigarettes among those who went on to choose the INC cigarette. Positive to Negative ratio did not differ between fixed dose cigarettes among those who chose the VLNC cigarette.
Exploratory analyses on predictors of subjective effects and choice
Cigarette choice was not associated with any demographic or smoking history variables (eTable 3). However, choice for lower nicotine content cigarettes was associated with lower average cotinine levels across experimental sessions (significant linear effect, F1,80 = 4.3, p<.05), and lower average CO boost across FD sessions, (significant linear effect, F1,84 = 5.4, p<.05 (Figure 5). Pairwise comparisons indicated that individuals choosing the VLNC cigarette had lower average cotinine levels, t(540.30)= −2.1, p<.05, and lower CO boost, t(61)= −2.3, p<.05, relative to NNC choosers. INC choosers had cotinine and CO boost levels that were intermediate to the other two groups, but did not differ significantly.
Fig 5.
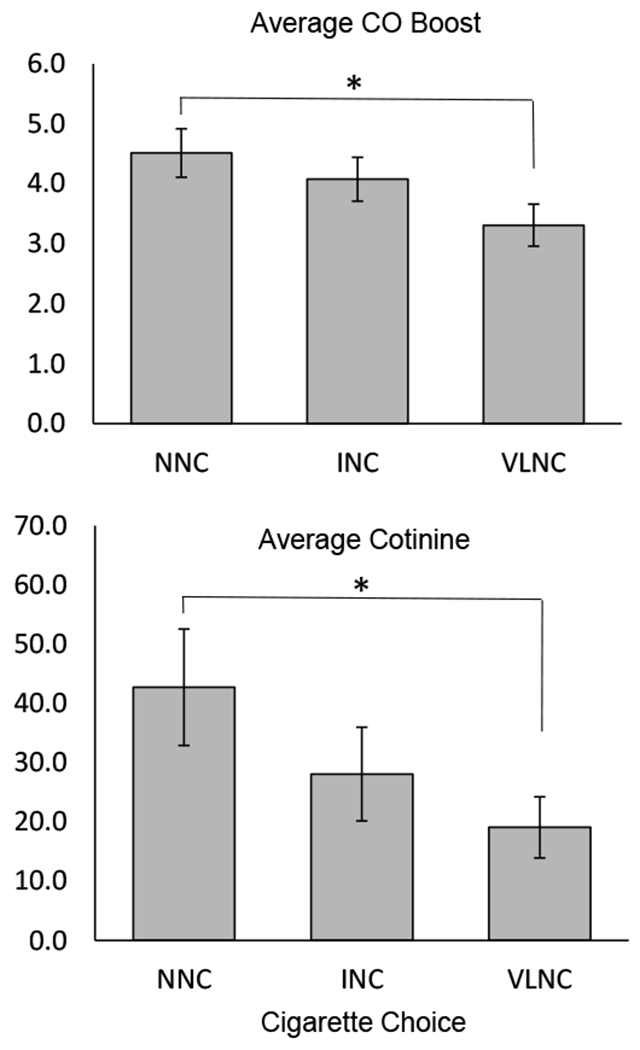
Physiological indices of smoking among those who chose to smoke the cigarette with normal nicotine content (NNC), intermediate nicotine content (INC), or very low nicotine content (VLNC) during the final choice session. (Top panel) Average boost in expired breath carbon monoxide (CO), ppm, during fixed dose sessions, calculated as difference in CO from pre- to post-cigarette administration. (Bottom panel) Average urinary cotinine, ng/mL, assessed at the start of experimental sessions. All error bars represent standard error of the mean. *Indicates statistical significance of p < 0.05. Participants who chose the NNC cigarette had significantly greater average CO boost and greater average pre-session cotinine than those who chose the VLNC cigarette, whereas no difference was observed among those who chose the INC cigarette.
DISCUSSION
We assessed, under double-blind conditions, subjective and physiological reactions to fixed doses of cigarette smoke with varying nicotine content among young adults who use tobacco products 15 or fewer days per month. After completing the three FD sessions, participants completed a final session during which they chose a cigarette to smoke. Dose response effects of nicotine content were observed for subjective reactions with participants rating NNC cigarettes as more pleasant, more unpleasant, and resulting in greater dizziness than lower nicotine content cigarettes. The largest proportion of the sample chose to smoke NNC cigarettes, though the frequency of choices did not differ significantly across doses.
Overall, results from each of our primary outcome measures (subjective fixed dose reactions, physiological fixed dose reactions, and cigarette choice) yield a somewhat mixed picture with regard to the potential benefits and/or risks of VLNC cigarettes for young adult, low frequency smokers. With respect to subjective reactions, we observed that our sample rated both Positive and Negative effects of VLNC and INC cigarettes lower than they did NNC, although the overall ratio of Positive to Negative effects did not differ between conditions. To the extent that Positive effects influence reinforcement and drive continued smoking (Hu, Davies, and Kandel 2006; Pomerleau 1995; Sartor et al. 2010), these data suggest that low-frequency young adult smokers would find VLNC less appealing than NNC and would likely decrease their use of cigarettes were a VLNC product standard mandated in cigarettes. In terms of Negative reactions, the literature is mixed as to whether aversive reactions are protective against continued use (Agrawal et al. 2014; Urban 2010; Sartor et al. 2010; Zabor et al. 2013). Some evidence suggests that intensity of subjective reactions, rather than Positive or Negative valence, is most predictive of progression to smoking (Pomerleau, 1995), further supporting the conclusion that INC and VLNC cigarettes are both likely to have lower abuse liability than NNC. Alternatively, if aversive effects serve as a deterrent to continued smoking, then it is possible that lower nicotine levels could be tolerated by some individuals who otherwise might find smoking prohibitively unpleasant. Interestingly, direct comparisons between INC and VLNC cigarettes indicated that Positive, but not Negative, effects were greater for INC. This suggests that selecting the lowest possible nicotine content could minimize abuse potential by achieving the lowest possible positive effects without further reducing the negative side effect profile beyond that of an intermediate level of nicotine.
In light of these subjective reactions data, it is interesting that more than half of the sample chose INC or VLNC cigarettes during the choice session. Given the limited exposure to each cigarette condition prior to the choice session, it is possible that these responses were made at random. However, it is important to note that choice appeared to be influenced by subjective reactions during FD sessions. In particular, individuals in the NNC and INC choice groups exhibited a higher P/N ratio for the cigarette they chose compared to the cigarettes they did not, strengthening the conclusion that cigarette choice, to some extent, reflected actual preference. Importantly, the hypothesis that our sample of younger, low frequency smokers might actually prefer INC over the NNC cigarettes, given fewer unpleasant effects, was not supported by the choice data. However, there also was no clear preference for the NNC cigarettes during the choice session, and more than half of smokers chose to self-administer lower nicotine options. Although it is unclear if such preferences would emerge following repeated use in a naturalistic setting, these data raise the possibility that not all young, low-frequency smokers will experience the same degree of benefit of reduced smoking in response to a nicotine reduction policy. In particular, if some individuals continue to smoke for reasons that are not clearly linked to nicotine administration, additional public health measures may be needed to fully curb combusted cigarette use in this group (e.g. public health campaigns, targeted warning labels).
A third major finding of this study is that, despite the CPVA procedure during FD sessions, post-cigarette CO levels were greater in the VLNC condition than for NNC, raising concerns that compensatory smoking could increase toxicant exposure. However, it is important to note that whereas compensation typically involves changes in frequency of smoking or puff volume, neither of these were possible under the controlled laboratory conditions imposed here. Given that the CVPA procedure prevented a compensatory increase in puff volume, these data suggest that individuals were likely titrating their depth or duration of inhalation in response to nicotine content (Zacny et al. 1987). Importantly, it is unclear based on these data whether this titration reflects an increased inhalation under VLNC conditions, or a limiting of inhalation during the NNC condition. The CVPA procedure could have delivered higher puff volumes than some participants were accustomed to, thereby contributing to decreased inhalation to offset aversive effects with higher nicotine content. It is also important to note that while previous studies conducted with adults have found some evidence of compensation following a single laboratory administration of VLNC versus NNC cigarettes (Strasser et al. 2007), these effects have not been sustained following repeated use (Denlinger-Apte et al. 2020; Donny et al. 2015; Donny, Houtsmuller, and Stitzer 2007; Macqueen et al. 2012). As such, further research examining topography and CO boost following repeated administration would be necessary to determine whether any meaningful compensation that could contribute to increased toxicant exposure would be observed in this population.
Whereas previous research has examined subjective and behavioral reactions to VLNC cigarettes among adolescent daily smokers (Cassidy et al. 2019; Colby et al. 2019) and, separately, in adult, intermittent smokers (Shiffman, Kurland, et al. 2018; Shiffman, Mao, et al. 2018), this is the first study to examine these questions among low-frequency, young adult smokers. This study included several strengths, including focus on a unique, understudied population and well-powered within-subjects experimental design. However, several limitations should be noted. First, it is possible that aspects of the experimental design may have affected the reactions and preferences for each cigarette condition. In particular, the CPVA procedure allowed for standardization of smoke exposure, but may have prevented participants from titrating their intake to preferred levels as they would under naturalistic conditions. As such, some participants may have experienced greater aversive effects and shifted their preference away from the NNC cigarette under these laboratory, fixed-dose conditions. The finding that individuals choosing the VLNC cigarette had lower average cotinine and CO boost compared with NNC choosers provides some support for this interpretation, suggesting that some smokers within our sample may have been accustomed to a lower puff volume and nicotine intake. It is also notable that the INC and VLNC cigarettes had slightly lower tar levels than NNC (1.5 mg difference). Although this difference is well within the range of variability found in commercial cigarettes, and is far lower than studies manipulating tar levels to investigate its effects (Donny et al. 2015), it is possible that this could have contributed to lower aversive effects. It is also possible that the brief sampling procedure at the start of the choice session, intended to refamiliarize participants with each cigarette, could have contributed to satiety among these low frequency smokers, prompting some individuals to shift their preference to a lower nicotine cigarette.
Relatedly, given participants’ limited exposure to the study cigarettes, it is unclear how responses might change with repeated use. Although provision of experimental cigarettes for an extended period of ad lib use raises ethical concerns in a sample of low-frequency smokers, examining subjective responses and use characteristics (e.g., cigarette preference, puff topography) following repeated sampling would provide additional information about potential impact of a VLNC cigarette standard. An additional important limitation is that we were unable, despite specific and significant efforts, to recruit a sample representing the racial make-up of our surrounding region, potentially limiting generalizability. Moreover, it is unclear whether our findings will generalize to adolescents who may be initiating smoking at younger ages than those in our sample. Finally, while our sample size was large for testing within-subject comparisons, our ability to make inferences about individual differences that might contribute to variability in patterns of responses was limited.
Despite these limitations, our results provide additional support for a VLNC product standard in combusted cigarettes, and suggest that reducing nicotine content will lower the positive subjective effects—and likely the abuse liability—of cigarettes for the majority of young adult, low frequency smokers. These benefits are likely to be strengthened by choosing a threshold well below 2.4 mg/g in order to achieve the greatest reduction in positive effects. Although a majority of participants chose lower nicotine content cigarettes during the choice session, there was no clear preference for any one cigarette condition; as such these data do not provide evidence to suggest that the general population of young, low-frequency smokers would be at increased risk of continued smoking if the FDA were to enact a new, VLNC cigarette standard. Moreover, even if VLNCs sustain some experimentation within this population, reducing nicotine to non-addictive levels may still prevent a transition to dependent smoking. Additional work is needed to determine if a subset of individuals choosing lower nicotine content cigarettes may continue to smoke regardless of nicotine content, and to ensure that compensatory smoking does not occur with longer-term use.
Supplementary Material
Funding/Conflicts of Interest:
This research was supported by NIH grants R01 DA042532 (FJM), K01 DA043413 (LRP) and K23 DA039294 (MMS). The authors do not have any conflicts of interest to disclose.
REFERENCES
- Agrawal A, Madden PA, Bucholz KK, Heath AC, & Lynskey MT (2014). Initial reactions to tobacco and cannabis smoking: a twin study. Addiction, 109(4):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, & Chassin L (2004). Motivational influences on cigarette smoking. Annu Rev Psychol, 55, 463–491. doi: 10.1146/annurev.psych.55.090902.142054 [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, & Jacob P 3rd. (2007). Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev, 16(11), 2479–2485. doi: 10.1158/1055-9965.EPI-07-0393 [DOI] [PubMed] [Google Scholar]
- Berman ML, & Glasser AM (2019). Nicotine reduction in cigarettes: Literature review and gap analysis. Nicotine Tob Res, 21(Suppl 1), S133–S144. doi: 10.1093/ntr/ntz162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, Garrett ME, McClernon FJ, Fuemmeler BF, Williams RB, Ashley-Koch AE, & Kollins SH (2012). A preliminary analysis of interactions between genotype, restrospective ADHD symptoms, and initial reactions to smoking in a sample of young adults. Nicotine Tob Res, 14(2), 229–33. doi: 10.1093/ntr/ntr125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RN, Colby SM, Tidey JW, Jackson KM, Cioe PA, Krishnan-Sarin S, & Hatsukami D (2018). Adolescent smokers’ response to reducing the nicotine content of cigarettes: Acute effects on withdrawal symptoms and subjective evaluations. Drug Alcohol Depend, 188, 153–160. doi: 10.1016/j.drugalcdep.2018.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RN, Miller ME, Tidey JW, Diguiseppi G, Denlinger-Apte R, Colby SM (2019). The impact of nicotine dose on the reinforcing effects of cigarettes in adolescents. Tob Regul Sci, 5(2), 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RN, Tidey JW, Cao Q, Colby SM, McClernon FJ, Koopmeiners JS, et al. , & Donny EC. (2019). Age Moderates Smokers’ Subjective Response to Very-Low Nicotine Content Cigarettes: Evidence from a Randomized Controlled Trial. Nicotine Tob Res, 21(7), 962–969. doi: 10.1093/ntr/nty079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SM, Cassidy RN, Denlinger-Apte R, Smith TT, Pacek LR, McClernon FJ, & Tidey JW (2019). Anticipated Effects of Nicotine Reduction on Youth Smoking Initiation and Maintenance. Nicotine Tob Res, 21(Suppl 1), S46–S48. doi: 10.1093/ntr/ntz101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Parker MA, Villanti AC, Streck JM, Priest JS, Tidey JW, et al. , Higgins ST. (2019). Examining Age as a Potential Moderator of Response to Reduced Nicotine Content Cigarettes in Vulnerable Populations. Nicotine Tob Res, 21(Suppl 1), S49–S55. doi: 10.1093/ntr/ntz134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger-Apte RL, Donny EC, Lindgren BR, Rubin N, Goodwin C, DeAtley T, et al. , & Tidey JW. (2020). Smoking Topography Characteristics During a 6-Week Trial of Very Low Nicotine Content Cigarettes in Smokers With Serious Mental Illness. Nicotine Tob Res, 22(8), 1414–1418. doi: 10.1093/ntr/ntz198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger-Apte RL, Kotlyar M, Koopmeiners JS, Tidey JW, Luo X, Benowitz NL, et al. , & Hatsukami DK. (2019). Effects of Very Low Nicotine Content Cigarettes on Smoking Behavior and Biomarkers of Exposure in Menthol and Non-menthol Smokers. Nicotine Tob Res, 21(Suppl 1), S63–S72. doi: 10.1093/ntr/ntz160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. , & Hatsukami DK. (2015). Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med, 373(14), 1340–1349. doi: 10.1056/NEJMsa1502403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, & Stitzer ML (2007). Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction, 102(2), 324–334. doi: 10.1111/j.1360-0443.2006.01670.x [DOI] [PubMed] [Google Scholar]
- Gellner CA, Belluzzi JD, & Leslie FM (2016). Self-administration of nicotine and cigarette smoke extract in adolescent and adult rats. Neuropharmacology, 109, 247–253. doi: 10.1016/j.neuropharm.2016.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Ehringer MA, Lessem JM, Hopfer CJ, & Hewitt JK (2011). Dizziness and the genetic influences on subjective experiences to initial cigarette use. Addiction, 106(2), 391–399. doi: 10.1111/j.1360-0443.2010.03133.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, Roper-Batker AN, Mackowick KM, et al. , & Donny E. (2013). Dose-response effects of spectrum research cigarettes. Nicotine Tob Res, 15(6), 1113–1121. doi: 10.1093/ntr/nts247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, et al. , & Hecht SS. (2010). Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction, 105(2), 343–355. doi: 10.1111/j.1360-0443.2009.02780.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Luo X, Heskin AK, Tang MK, Carmella SG, Jensen J, et al. , & Hecht SS. (2019). Effects of immediate versus gradual nicotine reduction in cigarettes on biomarkers of biological effects. Addiction, 114(10), 1824–1833. doi: 10.1111/add.14695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Luo X, Jensen JA, al’Absi M, Allen SS, Carmella SG, et al. , & Donny EC. (2018). Effect of Immediate vs Gradual Reduction in Nicotine Content of Cigarettes on Biomarkers of Smoke Exposure: A Randomized Clinical Trial. JAMA, 320(9), 880–891. doi: 10.1001/jama.2018.11473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Sigmon SC, Tidey JW, Gaalema DE, Hughes JR, et al. , & Tursi L (2017). Addiction Potential of Cigarettes With Reduced Nicotine Content in Populations With Psychiatric Disorders and Other Vulnerabilities to Tobacco Addiction. JAMA Psychiatry, 74(10), 1056–1064. doi: 10.1001/jamapsychiatry.2017.2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Davies M, & Kandel DB (2006). Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health, 96(2), 299–308. doi: 10.2105/AJPH.2004.057232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D 2013. ’Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs’, Front Psychol, 4: 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, & Slotkin TA (2007). Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol, 29(4), 458–465. doi: 10.1016/j.ntt.2007.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, & Swartzwelder HS (2003). Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology (Berl), 169(2), 141–149. doi: 10.1007/s00213-003-1486-y [DOI] [PubMed] [Google Scholar]
- Levin ED, Rose JE, Behm F (1989). Controlling puff volume without disrupting smoking topography. Behav Res Methods Instrum Comput, 21(3), 383–386. [Google Scholar]
- Macqueen DA, Heckman BW, Blank MD, Janse Van Rensburg K, Evans DE, & Drobes DJ (2012). Transient compensatory smoking in response to placebo cigarettes. Psychopharmacology (Berl), 223(1), 47–54. doi: 10.1007/s00213-012-2685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercincavage M, Lochbuehler K, Wileyto EP, Benowitz NL, Tyndale RF, Lerman C, & Strasser AA (2018). Association of Reduced Nicotine Content Cigarettes With Smoking Behaviors and Biomarkers of Exposure Among Slow and Fast Nicotine Metabolizers: A Nonrandomized Clinical Trial. JAMA Netw Open, 1(4), e181346. doi: 10.1001/jamanetworkopen.2018.1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Coddington S, & Karelitz JL (2008). Association of retrospective early smoking experiences with prospective sensitivity to nicotine via nasal spray in nonsmokers. Nicotine Tob Res, 10(8), 1335–1345. doi: 10.1080/14622200802238886 [DOI] [PubMed] [Google Scholar]
- Pomerleau OF (1995). Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet, 25(2), 161–177. doi: 10.1007/bf02196925 [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, & Namenek RJ (1998). Early experiences with tobacco among women smokers, ex-smokers, and never-smokers. Addiction, 93(4), 595–599 [DOI] [PubMed] [Google Scholar]
- Rios-Bedoya CF, Pomerleau CS, Neuman RJ, & Pomerleau OF (2009). Using MIMIC models to examine the relationship between current smoking and early smoking experiences. Nicotine Tob Res, 11(9), 1035–1041. doi: 10.1093/ntr/ntp093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez D, & Audrain-McGovern J (2004). Construct validity analysis of the early smoking experience questionnaire for adolescents. Addict Behav, 29(5), 1053–1057. doi: 10.1016/j.addbeh.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Sartor CE, Lessov-Schlaggar CN, Scherrer JF, Bucholz KK, Madden PA, Pergadia ML, et al. (2010). Initial response to cigarettes predicts rate of progression to regular smoking: findings from an offspring-of-twins design. Addict Behav, 35(8), 771–778. doi: 10.1016/j.addbeh.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schassburger RL, Pitzer EM, Smith TT, Rupprecht LE, Thiels E, Donny EC, & Sved AF (2016). Adolescent Rats Self-Administer Less Nicotine Than Adults at Low Doses. Nicotine Tob Res, 18(9), 1861–1868. doi: 10.1093/ntr/ntw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, & Ferguson SG (2015). Stimulus control in intermittent and daily smokers. Psychol Addict Behav, 29(4), 847–855. doi: 10.1037/adb0000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Li X, Scholl SM, Tindle HA, Anderson SJ, & Ferguson SG (2014). Smoking patterns and stimulus control in intermittent and daily smokers. PLoS One, 9(3), e89911. doi: 10.1371/journal.pone.0089911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Scholl SM, & Tindle HA (2012). Smoking motives of daily and non-daily smokers: a profile analysis. Drug Alcohol Depend, 126(3), 362–368. doi: 10.1016/j.drugalcdep.2012.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Tindle HA, & Ferguson SG (2015). Nondaily smokers’ experience of craving on days they do not smoke. J Abnorm Psychol, 124(3), 648–659. doi: 10.1037/abn0000063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Kurland BF, Scholl SM, & Mao JM (2018). Nondaily Smokers’ Changes in Cigarette Consumption With Very Low-Nicotine-Content Cigarettes: A Randomized Double-blind Clinical Trial. JAMA Psychiatry, 75(10), 995–1002. doi: 10.1001/jamapsychiatry.2018.1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Mao JM, Kurland BF, & Scholl SM (2018). Do non-daily smokers compensate for reduced cigarette consumption when smoking very-low-nicotine-content cigarettes? Psychopharmacology (Berl), 235(12), 3435–3441. doi: 10.1007/s00213-018-5056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Li Z, & Le AD (2008). Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology (Berl), 197(1), 45–58. doi: 10.1007/s00213-007-1003-9 [DOI] [PubMed] [Google Scholar]
- Strasser AA, Lerman C, Sanborn PM, Pickworth WB, & Feldman EA (2007). New lower nicotine cigarettes can produce compensatory smoking and increased carbon monoxide exposure. Drug Alcohol Depend, 86(2-3), 294–300. doi: 10.1016/j.drugalcdep.2006.06.017 [DOI] [PubMed] [Google Scholar]
- Tidey JW, Colby SM, Denlinger-Apte RL, Goodwin C, Cioe PA, Cassidy RN, et al. , & Donny EC. (2019). Effects of 6-Week Use of Very Low Nicotine Content Cigarettes in Smokers With Serious Mental Illness. Nicotine Tob Res, 21(Suppl 1), S38–S45. doi: 10.1093/ntr/ntz133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban R (2010). Early smoking experience in adolescents. Addictive behaviors, 35(6): 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Congress. (2009). Family smoking prevention and tobacco control federal reform act. In Pub. L No. 111$31. [Google Scholar]
- U.S. Department of Health and Human Services. (2014). The health consequences of smoking: 50 years of progress. A report of the Surgeon General. In. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. [Google Scholar]
- Wang MQ, Fitzhugh EC, Trucks J, Cowdery J, & Perko M (1995). Physiological sensations of initial smoking in the development of regular smoking behavior. Percept Mot Skills, 80(3 Pt 2), 1131–1134. doi: 10.2466/pms.1995.80.3c.1131 [DOI] [PubMed] [Google Scholar]
- Wheeler KC, Fletcher KE, Wellman RJ, & Difranza JR (2004). Screening adolescents for nicotine dependence: the Hooked On Nicotine Checklist. J Adolesc Health, 35(3), 225–230. doi: 10.1016/j.jadohealth.2003.10.004 [DOI] [PubMed] [Google Scholar]
- Zabor EC, Li Y, Thornton LM, Shuman MR, Bulik CM, Lichtenstein P, et al. , & Furberg H (2013). Initial reactions to tobacco use and risk of future regular use. Nicotine Tob Res, 15(2), 509–517. doi: 10.1093/ntr/nts180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Stitzer ML, Brown FJ, Yingling JE, & Griffiths RR (1987). Human cigarette smoking: effects of puff and inhalation parameters on smoke exposure. J Pharmacol Exp Ther, 240(2), 554–564. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


