Abstract
Establishing a causal link between neural function and behavioral output has remained a challenging problem. Commonly used perturbation techniques enable unprecedented control over intrinsic activity patterns and can effectively identify crucial circuit elements important for specific behaviors. However, these approaches may severely disrupt activity, precluding an investigation into the behavioral relevance of moment-to-moment neural dynamics within a specified brain region. Here we discuss the application of mild focal cooling to slow down intrinsic neural circuit activity while preserving its overall structure. Using network modeling and examples from multiple species, we highlight the power and versatility of focal cooling for understanding how neural dynamics control behavior and argue for its wider adoption within the systems neuroscience community.
Banerjee et al. demonstrate the power and versatility of focally cooling neural circuits as a means of testing the link between brain dynamics and behavior, supporting this view using examples from multiple species as well as network simulations.
The need for cooling
Neuroscientists have long attempted to link brain function to behavior by studying the effects of focal perturbations to neural activity. Even as early as the 1870s, electrical stimulation of motor cortical areas was shown to drive specific muscle movements (Fritsch and Hitzig, 1870). Conversely, lesion studies have been instrumental in understanding the behavioral relevance of specific brain regions (Broca, 1861). More recent developments, such as optogenetics (Fenno et al., 2011) and pharmacogenetics (Sternson and Roth, 2014), have allowed specific neuronal cell types to be selectively manipulated to infer their contribution to brain function. These approaches continue to be enormously important for modern neuroscience, and methodological advances enable the perturbation of neural activity with ever-increasing levels of control (Deubner et al., 2019).
One potential disadvantage of standard perturbation approaches, however, is that they can disrupt the behavior they were designed to investigate. Often these methods result in fundamental changes to ongoing intrinsic neural dynamics (Bernard, 2020; Li et al., 2019; Panzeri et al., 2017; Wolff and Olveczky, 2018; Yoshihara and Yoshihara, 2018), preventing a more nuanced analysis of the relationship between neural dynamics and behavior. Neural population activity can be conceptualized as a single point in a multidimensional space, where each dimension corresponds to the firing rate of one neuron. Each point in this space represents the instantaneous “state” of the joint population activity. For illustration, we depict this in a dimensionality-reduced state space (Cunningham and Yu, 2014). Over time, the point moves around to create a neural trajectory (Figure 1A). There exists a moment-by-moment mapping between this ensemble neural activity and behavior, with trial-by-trial variability in neural dynamics during repetition of the same behavior determining the range of possible trajectories. Using this framework, we illustrate that standard methods for manipulating neural systems can strongly perturb both network dynamics and the resulting behavior (Figure 1B) by showing a neural trajectory abruptly deviating from its canonical path (Jazayeri and Afraz, 2017; Shenoy and Kao, 2021).
Figure 1. Two Approaches for Perturbing Neural Activity and Behavior.
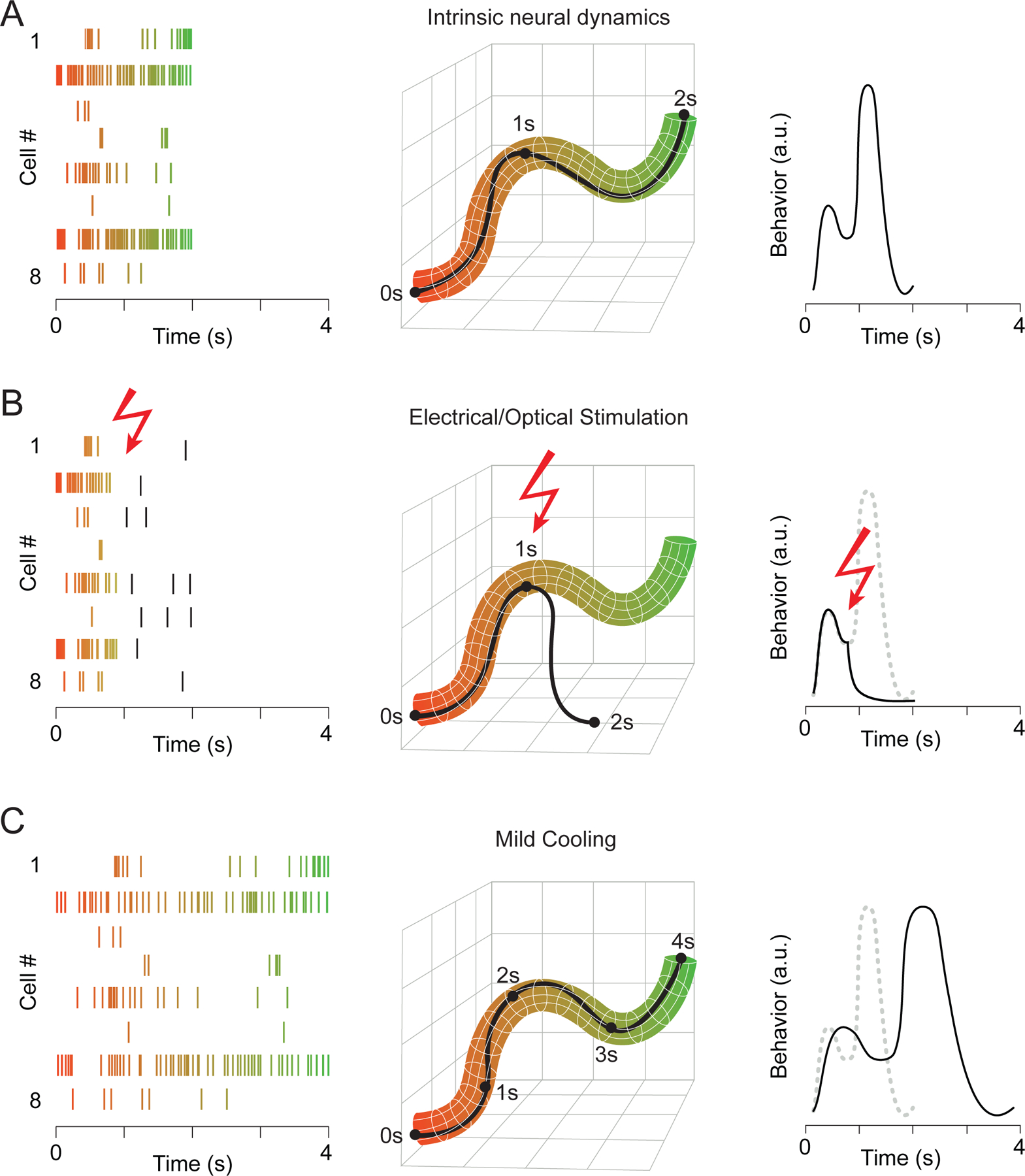
(A) Spike raster plot of eight simultaneously recorded neurons (left). The proportion of the trial is color-coded, transitioning from orange to green throughout its duration. Dimensionality-reduced neural activity plotted in state-space (middle). The black line describes the example trial at left. Since individual trials are not identical in this network, the standard network structure is determined by the correlated population activity, and its thickness reflects trial-by-trial variability. In this case, a causal relationship exists between the joint spiking activity of the network and a measurable behavioral output (right).
(B) Neural activity from (A) is interrupted in the middle of the trial, which causes the network to deviate from the normal activity patterns, leading to a concomitant behavioral disruption.
(C) A manipulation that changes the temporal dynamics of neural activity while preserving the overall structure of the population activity can maintain but temporally distort behavioral output.
To better understand the exact causal mapping between neural activity and behavior, we require a method that can avoid some of the difficulties of pushing dynamic systems from their operating regimes in extreme ways, like large-scale silencing. Instead, if we can simply affect the speed of neural activity without disrupting its overall structure, then behavior may be preserved, albeit at a different time scale (Figure 1C). If neural activity of a particular brain area controls behavioral output, then changing the speed of neural dynamics should lead to a concomitant change in behavior. Here we explore the idea that mild cooling can be used as a highly effective perturbation technique; we build an intuition about the effects of cooling on neural circuit activity by providing examples from various systems; and we point out the advantages and limitations of using focal cooling compared to other existing approaches.
Cooling an isolated neural circuit
Before examining how focal cooling can accurately assess the behavioral relevance of identified brain regions embedded within a larger neural network, we will first discuss the impact of temperature changes on a single, isolated neural circuit: the stomatogastric ganglion (STG). The STG contains approximately 30 neurons that can generate rhythmic behavior even after being dissected away from the rest of the nervous system, including a cyclic pyloric rhythm that regulates stomach musculature (Marder et al., 1998). Three cell types within this rhythm-generating circuit (PD, LP, and PY) are sequentially active, as revealed by intracellular recordings and visualized by plotting the joint activity of these three cell types in state space (Figure 2) (Tang et al., 2010). Using this preparation, temperature can be manipulated by simply superfusing the ganglion with cooled or warmed saline solutions. As with other invertebrate central pattern generators (Calabrese and Arbas, 1984; Katz et al., 2004), the pyloric rhythm is robust over a wide temperature range (Robertson and Money, 2012). For instance, lowering the temperature from 23°C to 15°C causes the rhythmic cycles to slow their tempo while maintaining the relative network timing (Figure 2A). In fact, when we linearly stretch voltage traces recorded at 23°C to match the timescale of those recorded at 15°C, some minor changes become apparent (e.g., slight increase in spikes per burst at the warmer temperature), but the phase relationship of neural activity across neurons remains strikingly similar (Figure 2B). Therefore, cooling can slow down neural dynamics without disrupting them. Importantly, similar temperature sensitivity of the pyloric rhythm can be seen in vivo despite the presence of a considerably more complex network and neurochemical environment (Soofi et al., 2014). Taken together, these results demonstrate that cooling a simple neural circuit can selectively alter the speed of neural activity while preserving its overall structure.
Figure 2. Temperature-induced Manipulation of Neural Dynamics in an Isolated Circuit.
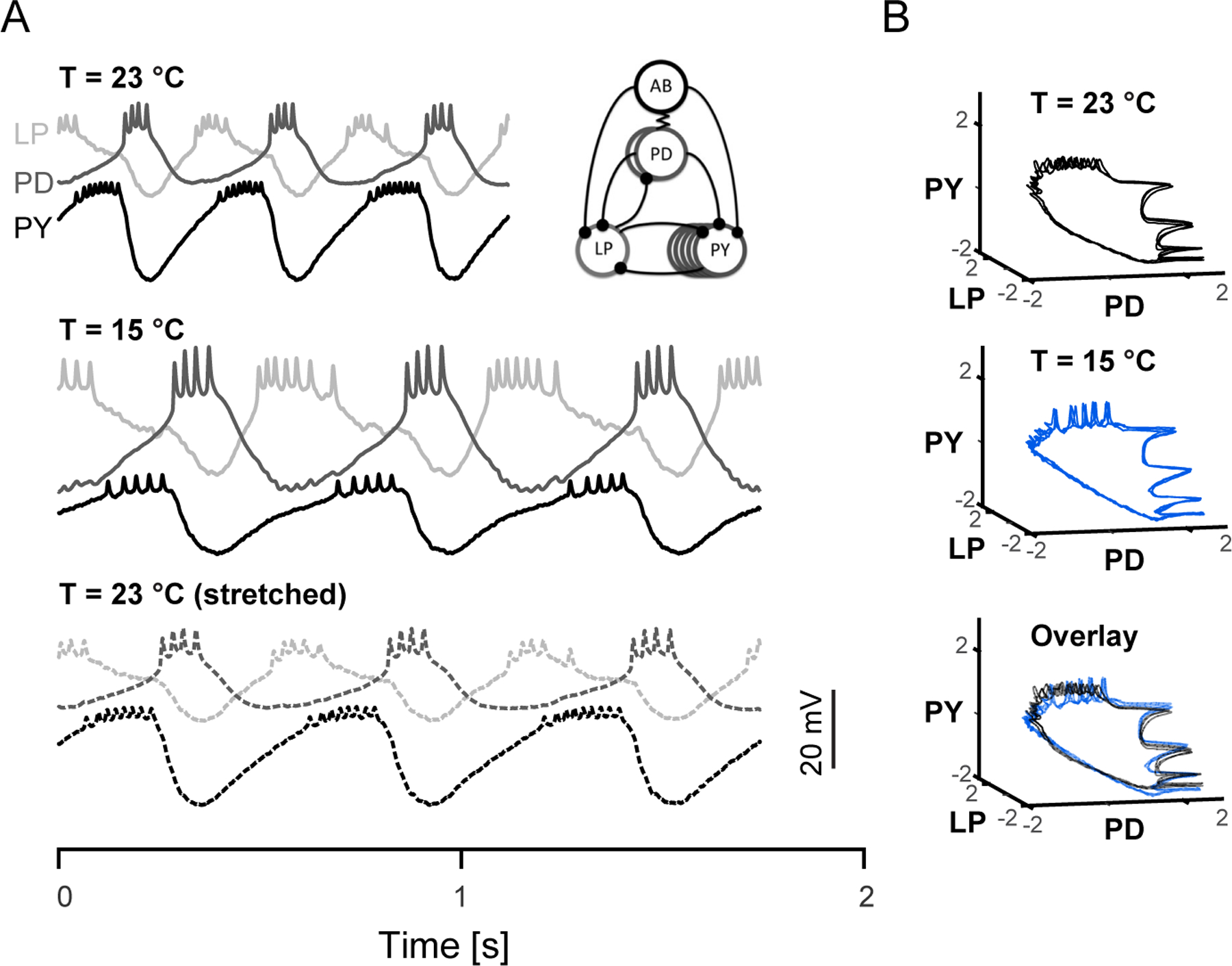
(A) Simultaneous intracellular recordings from three interconnected neurons (LP, PD and PY) from the crab somatogastric ganglion. Activity recorded from three complete pyloric rhythm cycles are plotted at 23°C (top) and 15°C (middle). Artificially stretched traces at 23°C (bottom) closely match profile of 15°C traces. Inset: simplified diagram of pyloric circuit. Replotted data from (Tang et al., 2010).
(B) Data from (A) depicted in a state-space plot (z-scored) forms a simple 3D trajectory at 23°C (top) and 15°C (middle). Both trajectories (23°C black, 15°C blue) occupy similar space when plotted together (bottom).
Biophysical substrates of cooling
Before examining how focal cooling can be used as a tool to identify behaviorally relevant dynamics, we examine the cellular and synaptic mechanisms leading to temperature-driven network changes. Brain function is broadly temperature dependent, including both pre- and postsynaptic cellular processes (Kalmbach and Waters, 2012; Volgushev et al., 2000a; Volgushev et al., 2000b). For instance, Strong cooling (ΔT: ~ −30°C) can block spiking (Coomber et al., 2011; Lomber, 1999; Michalski et al., 1993), likely due to a strong depolarization of neurons leading to sodium channel inactivation (Jasper et al., 1970). As a result, this manipulation has provided systems neuroscientists with a means of rapidly and reversibly inactivating brain regions. At more moderate cooling levels (ΔT: < −10°C), however, cellular processes remain largely intact but are slowed. For instance, cooling can slow down spontaneous membrane potential oscillations, as in the case of the inferior olivary nucleus, where the frequency of large amplitude rhythms generated by a confluence of voltage-gated conductances (Lampl and Yarom, 1997) can be halved by lowering the temperature by approximately 5°C (Figure 3).
Figure 3. Biophysical Mechanisms of Cooling.
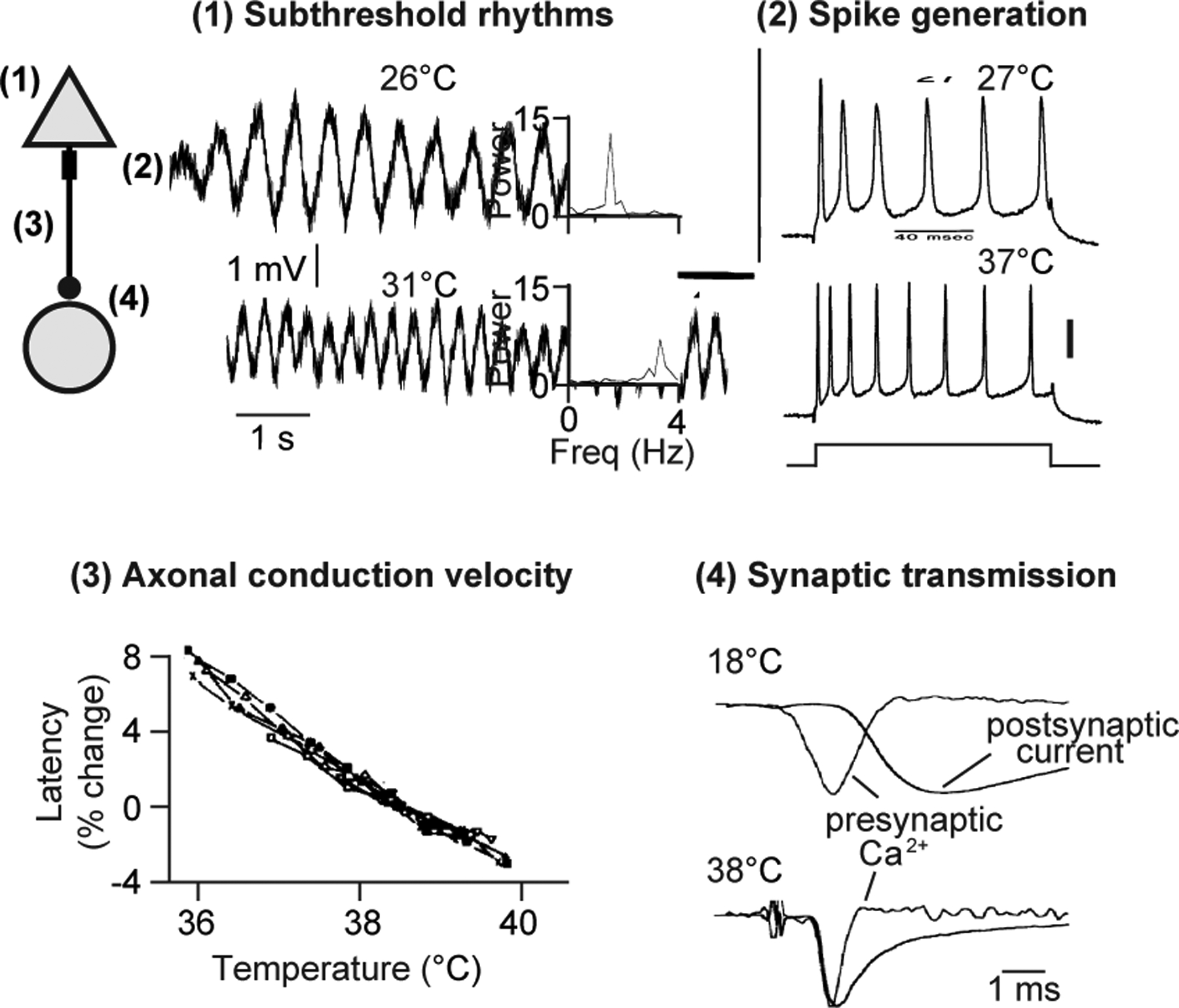
At left, a schematic of a synaptically connected pair of neurons with numbers indicating four sites at which temperature-sensitive processes are occurring as illustrated in the accompanying examples: (1) Frequency of subthreshold membrane potentials from a guinea pig inferior olivary neuron decreases with cooling, as indicated with power spectra at right. Adapted from (Lampl and Yarom, 1997).
(2) Evoked spikes of a guinea pig CA1 pyramidal neuron are slower and wider at lower temperatures (Injected current: 120 ms, 0.85 nA). Scale bar: 20 mV. Adapted from (Thompson et al., 1985).
(3) Relationship between brain temperature and conduction latency in 8 unmyelinated axons from the Dutch belted rabbit. Adapted from (Swadlow et al., 1981).
(4) Difference in timing between presynaptic calcium current and postsynaptic current (i.e., synaptic delay) at two different temperatures. Adapted from (Sabatini and Regehr, 1996).
Distinct aspects of neural circuit function are often variably affected by temperature. Such a dependency is quantified as the Q10 coefficient, or the rate of change of the time constant underlying a linearly varying biological process resulting from altering the temperature by 10°C. For instance, the aforementioned olivary rhythm changes considerably over a small temperature range with a Q10 of ~4. Other cellular processes are also highly temperature sensitive, such as synaptic transmission (Hodgkin and Katz, 1949; Sabatini and Regehr, 1996; Volgushev et al., 2000a). The time between an action potential arriving at the axon terminal and the onset of the postsynaptic current (i.e., synaptic delay) can be ~100 μs at mammalian body temperatures but expand by nearly an order of magnitude when cooled (Figure 3), likely due to kinetics of presynaptic calcium channels involved in neurotransmitter release (Nobile et al., 1990). Mild cooling can also widen action potentials and lower the rate of repetitive firing (Figure 3) (Thompson et al., 1985; Volgushev et al., 2000b) (AP width Q10: ~ 2, AP rate Q10: ~ 2). Some other processes are considerably less dependent on temperature, including axonal conduction velocity (Figure 3), which changes by approximately 3%/°C (Swadlow et al., 1981), or a Q10 of 1.3.
Given the heterogeneous Q10 values that exist within neural circuits, it is surprising that nervous system function can remain robust across a range of temperatures (Hamaguchi et al., 2016; Tang et al., 2010). For instance, the STG maintains a rhythmicity from approximately 0.7 to 2.9 Hz, with a network-level Q10 of 2.3, despite being the product of multiple conductances that each exhibit different relationships with temperature, including synaptic currents (Q10 = 2.3), input conductance (Q10 = 1.6), and voltage-gated currents, such as IA (Q10 = 3) (Tang et al., 2010). Such temperature robustness could reflect evolutionary adaptations that enable animals to withstand environmental temperature variations (Donahue et al., 2009) and may also be crucial for maintaining network function during physiologically challenging conditions (Hoffstaetter et al., 2018).
It should be noted that increasing brain temperature can also affect neural function. These effects are especially relevant for modern neuroscience, given that substantial heating can result from commonly employed methods, such as 2-photon imaging (Podgorski and Ranganathan, 2016) and optogenetic light delivery protocols (Owen et al., 2019). Importantly, heating can result in devastating side effects, such as seizure generation (Kasahara et al., 2018) or lasting tissue damage (Yarmolenko et al., 2011), while mild focal cooling appears to avoid such complications (Ibayashi et al., 2021). Additionally, the relationship between heating and cellular function may be considerably more complex than that observed with cooling (Owen et al., 2019), which may lead to difficulties in interpretation for studies in which focal heating is used. Therefore, we maintain a focus on mild cooling for the remainder of this manuscript.
Control of neural dynamics and behavior in the songbird
We now explore how focal cooling can be used to pinpoint the link between neural dynamics and behavior. For our first example, we will focus on the song control pathway of the zebra finch. Male zebra finches vocally imitate their courtship song; once learned, the structure of the song is highly stereotyped with timing that varies by less than 3% across renditions (Egger et al., 2020; Glaze and Troyer, 2006; Sossinka and Böhner, 1980). Song production is controlled by a series of nuclei located throughout the brain (Nottebohm et al., 1982; Nottebohm et al., 1976). Premotor neurons within a forebrain nucleus, HVC (proper name) (Figure 4A), collectively display a sequence of activity during song production (Egger et al., 2020; Lynch et al., 2016; Picardo et al., 2016), with each neuron producing a high-frequency burst of action potentials at a single time point during the song (Figure 4B) (Hahnloser et al., 2002; Kozhevnikov and Fee, 2007). Remarkably, small variations in burst timing of premotor neurons precisely mirror the small variations in overall song duration (Figure 4C). Hence, the population activity of HVC premotor neurons forms a sequential pattern that unfolds at slightly different speeds across song renditions, corresponding to minute changes in behavioral timing under normal conditions.
Figure 4. Change to Neural Dynamics and Behavior from Cooling Zebra Finch HVC.
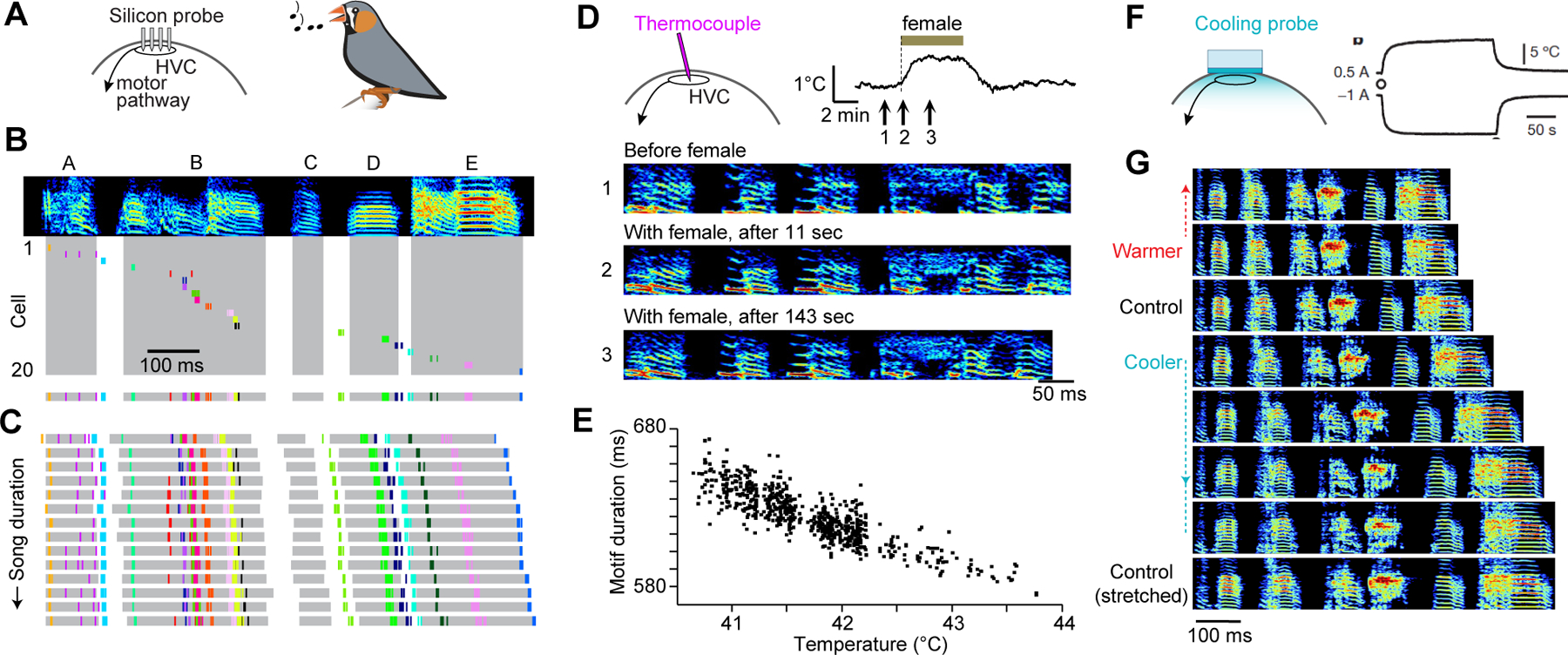
(A) Schematic of recording array in the forebrain nucleus HVC of the zebra finch.
(B) Spectrogram of a zebra finch song motif consisting of syllables A through E (top). Spike times of 20 simultaneously recorded HVC premotor neurons in which activity from each neuron separated vertically (middle) or collapsed into a single row (bottom). Syllables shaded in grey. Sonograms here and below display frequencies from 0.5 to 8.0 kHz. Adapted from (Egger et al., 2020).
(C) Spike times of the same neurons as in (B) and syllable durations during 14 song renditions, sorted by increasing duration. Adapted from (Egger et al., 2020).
(D) Context-dependent increase in brain temperature upon exposure to a female (top). Numbers correspond to spectrograms from a male zebra finch singing in isolation (undirected song) or to a female (directed song) (bottom). Adapted from (Aronov and Fee, 2012).
(E) In the context of naturally occurring brain temperature variability, song motif duration is inversely proportional to HVC temperature. Adapted from (Aronov and Fee, 2012).
(F) HVC temperature can be selectively controlled using a chronically implanted Peltier device. Measuring temperature levels 0.5 mm below the surface of the cooling probe demonstrates that applied current (0.5A and −1.0A) leads to heating and cooling, respectively. Adapted from (Long and Fee, 2008).
(G) Representative sonograms at a range of different experimentally determined HVC temperatures. For reference, a spectrogram of a control song linearly stretched to duration of motif produced at coldest temperature (below). Adapted from (Long and Fee, 2008).
Several factors determine the speed of neural dynamics and thereby song production; for instance, undirected song – performed in isolation – had long been shown to be slower than singing in the presence of a female (Sossinka and Böhner, 1980). Aronov and Fee (Aronov and Fee, 2012) tested the hypothesis that this context-specific change in timing could be explained by rapid changes in brain temperature (Figure 4D). To measure this, they inserted a thermocouple in either HVC or in a forebrain location outside the song system. Brain temperature in both locations increased by up to 1°C (leading to a ~ 3% increase in song speed) in less than a minute following the presentation of a female bird (Figure 4D). Song duration was shown to strongly vary as a result of natural changes in brain temperature (Figure 4E), consistent with the idea that brain temperature can alter the speed with which the network traverses its premotor neural trajectory.
Because the presence of a female increases temperature across the male bird’s entire brain non-specifically, the change in song tempo cannot be causally attributed to any of the individual nuclei within the song control pathway (Aronov and Fee, 2012). Such whole brain temperature changes affecting song timing have long been described in ectothermic animals; in field crickets, for instance, the chirp rate is so sensitive to ambient temperature that the inter-chirp interval can be used as an accurate “thermometer” (i.e., Dolbear’s law) (Dolbear, 1897). Subsequent studies have used focal temperature manipulations – administered to specific parts of the nervous system – to pinpoint song production circuitry in both insects (Pires and Hoy, 1992) and frogs (Yamaguchi et al., 2008). To address the relative contributions of various nuclei to zebra finch singing behavior, a head-mounted Peltier probe was used to selectively manipulate the temperature of HVC (Figure 4F). Cooling HVC led to a monotonic change in song length (Figure 4G), while cooling a downstream region – the robust nucleus of arcopallium – showed no such effect (Long and Fee, 2008). This result demonstrates that song production continues even when HVC temperatures are lowered to levels significantly less than what is naturally observed in zebra finches and other songbirds (Goldin et al., 2013; Zhang et al., 2017). Indeed, the behavioral impact of cooling HVC alone matched that of widespread natural temperature variations (~ 3%/°C, Q10: ~ 1.3), suggesting that HVC is largely orchestrating the temporal structure for song production. Furthermore, recent work has indicated that the timing of HVC activity sequences is largely determined by the kinetics of axonal conduction within HVC (Egger et al., 2020). Consistent with this view, axonal transmission exhibits a similar temperature sensitivity with singing behavior (Long and Fee, 2008; Swadlow et al., 1981). Because slowing down the natural premotor dynamics in HVC leads to a dilation of song timing, the songbird provides a clear example of using cooling to link neural activity to a complex learned behavior (Figure 4G).
Cooling alters speed of neural dynamics in a recurrent neural network model
Using cooling to manipulate neural dynamics in songbirds has demonstrated that this tool can be used to study neural control of behavior. One caveat is that the neurons within the zebra finch HVC are highly specialized, responding in a sparse and precise manner during song production, while brain regions in other systems may have more complex temporal activity patterns. To explore the generality of cooling in neural circuits, we developed a toy model (800 neurons) where the network activity at every moment controls a simple ‘behavioral’ output (Figure 5A). In this example, behavior is represented by two distinct rhythms chosen to independently assess the impact of activity perturbations at these two timescales. Neurons in the recurrent network produced a variety of complex firing rate patterns (Figure 5B), and a simplified version of network activity can be visualized by plotting the first two principal components of network activity.
Figure 5. Manipulations in a Recurrent Neural Network.
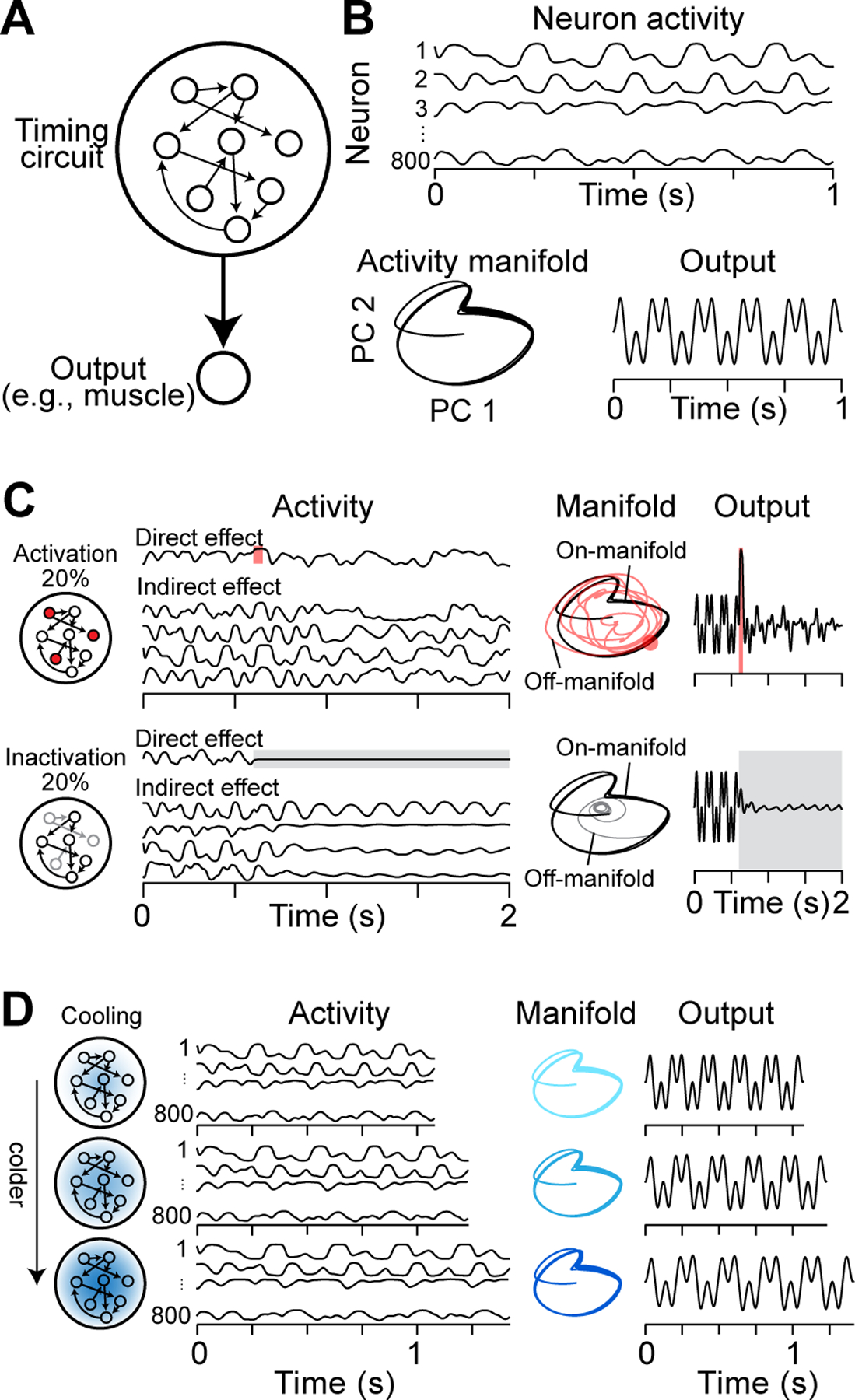
(A) Schematic of our timing circuit model which consists of N = 800 rate-based neurons forming an all-to-all recurrent neural network with random synaptic weights drawn from a normal distribution scaled by a factor with g set to 1.5 (Sussillo and Abbott, 2009). Additionally, all neurons synapse onto a single output unit. Recurrent and output weights are updated using the FORCE algorithm (Sussillo and Abbott, 2009) to learn a desired output pattern (Laje and Buonomano, 2013).
(B) Activity of 4 example neurons after learning (top). Network activity structure visualized using the first two principal components (PC; bottom left) and a learned output signal (bottom right).
(C) Simulated large-scale manipulations and their effects on neuronal activity (left), network dynamics (center), and output (right). Shown here are responses to activation (top row, shaded red area) and persistent (bottom row, shaded grey area) inactivation of 20% of neurons within the simulated network.
(D) Simulated cooling manipulation through three monotonically increasing levels of focal cooling. Mild cooling was modeled by augmenting network dynamics by a factor Q: . Here, τ = 10ms, Wrec is the recurrent weight matrix, and and are vectors of length N describing the internal dynamics and firing rates of each neuron. Temperature dependence of Q was modeled using Q10 = 2. Note that this simplified rate model does not account for axonal conduction delays (Egger et al., 2020; Swadlow et al., 1981).
Both activation and inactivation of a fraction of neurons in our recurrent neural network model resulted in changes to the activity patterns of all neurons. As a result, the trajectory of the neural population deviated significantly from its intrinsic activity pattern and resulted in qualitative changes in behavioral output (Figure 5C). In contrast, cooling the network (i.e., changing the timescales of biophysical properties of the model neurons) left activity patterns of individual neurons largely intact (Figure 5D). Since cooling-induced changes were consistent between neurons, the resultant neural activity maintained the same structure – albeit at a different speed – and consequently behavioral output was slowed down equally for both the fast and the slow rhythms. This result generalizes to other choices of ‘behavioral’ output and is conceptually similar to the stretching of zebra finch song at all timescales upon cooling HVC (Figure 4). In summary, modeling suggests that cooling can manipulate the timing of neural dynamics in recurrent neural networks, extending its utility to neural circuits with complex and diverse activity profiles. In the next section, we highlight some examples of how focal cooling can be used to infer behaviorally relevant neural dynamics in cortical circuits.
Using cooling to infer the organization of hierarchical neural networks
In the zebra finch model system, it is now widely accepted the timing of singing behavior appears to be primarily controlled by a single defined brain region, but see (Andalman et al., 2011; Goldin et al., 2013; Hamaguchi et al., 2016). However, a hierarchical relationship may exist in other systems in which different circuits can be spatially segregated and control different aspects of behavioral timing. For example, in the midshipman fish – a species that forms a variety of vocal behaviors (Figure 6A) – the duration of the vocal display and the production of each vocal element appears to be controlled in distinct brain regions (Figure 6B) (Bass, 2014; Chagnaud et al., 2011). To understand how focal cooling can disentangle the relationship between neural dynamics and behavior in such a scenario, we again turn to a simplified model. We consider two timing circuits: The first (‘Circuit 1’) represents a recurrent neural network (i.e., Figure 5), which is trained to produce a simple tonic signal that sets the duration of the behavior without directly specifying the rhythm. The second (‘Circuit 2’) acts as a pattern generator; specifically, a single neuron that produces high-frequency bursts at characteristic intervals in response to an input signal (e.g., a current injection). The timing circuits are arranged hierarchically, with the output of Circuit 1 providing input to the pattern generating Circuit 2 (Figure 6C). If the output of Circuit 1 is ‘On’, Circuit 2 can produce burst spiking at its own characteristic frequency.
Figure 6. Use of Cooling to Infer Hierarchical Organization of Timing Circuits.
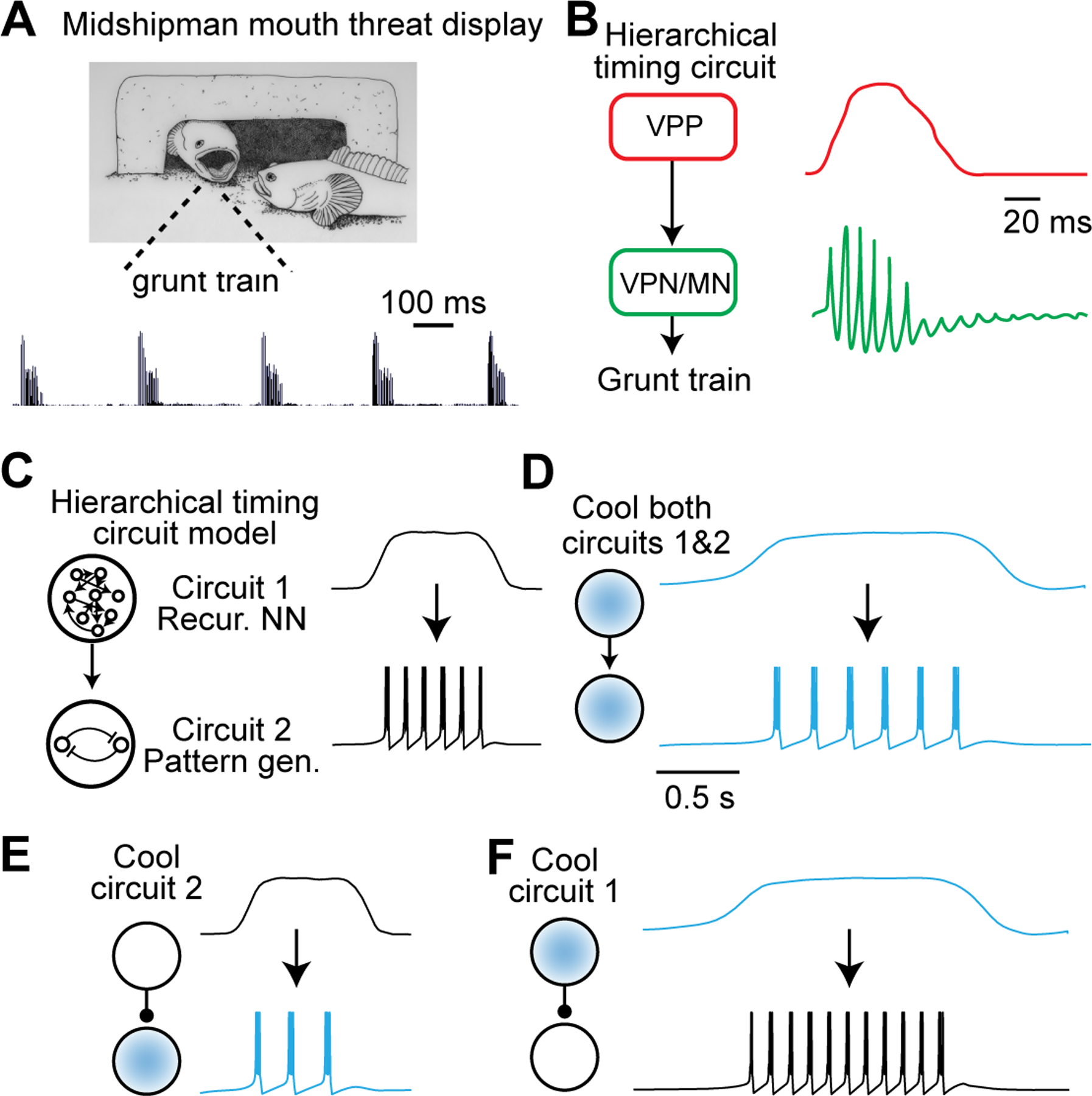
(A) Picture of a male plainfin midshipman fish and accompanying sound waveform indicating a grunt train. Adapted from (Brantley and Bass, 1994).
(B) Simplified diagram of part of the vocal control circuit in the male plainfin midshipman fish. VPP: vocal prepacemaker nucleus; VPN: vocal pacemaker nucleus; MN: vocal motoneurons. Right: Cartoon of intracellular membrane potential at different timescales in VPP (top) and VPN neurons (bottom). Adapted from (Chagnaud et al., 2011).
(C) Simple model of a hierarchically organized timing circuit. A recurrent neural network (top) produces an output that switches between a low and a persistent high level, which serves as input drive to a pattern-generating circuit (bottom) that produces bursts during the time the input is at a high level. For simplicity, we modeled the pattern-generating circuit (Izhikevich, 2003) with parameters a = 0.02, b = 0.2, c = −50mV and d = 2.0. Temperature dependence was modeled by scaling the time derivatives of the membrane potential v and the internal state variable u by a factor Q with Q10 = 2.
(D)–(F) Cooling both the recurrent neural network (upstream circuit) and the pattern generator (downstream) leads to a decrease in interburst interval and an expansion of the burst epoch (D). Cooling either the upstream or downstream network individually can selectively affect one of these two variables (i.e., interburst interval or burst epoch) without changing the other (E and F).
Cooling both timing circuits to the same degree results in coordinated stretching of both signals at all timescales (Figure 6D): The bursting neuron produces the same number of bursts after cooling but at a lower frequency, at longer intervals, and for a longer overall duration. In contrast, cooling Circuit 2 alone does not affect the overall duration of the bursting episode, but instead causes bursts to be produced at longer intervals, resulting in a smaller total number of bursts (Figure 6E). Cooling Circuit 1 alone stretches the activity patterns of individual recurrent neural network units (similar to Figure 5) resulting in a longer tonic output signal (Figure 6F, blue trace). Because the burst generating mechanism is not affected, elongating the behavioral duration leads to an increased total number of bursts (Figure 6F). Our simple model shows that focal cooling can decouple behavioral timescales by slowing neural dynamics in one circuit at a time and simultaneously monitoring behavioral output. It may therefore be possible to infer the position of the cooled circuit within a hierarchical network structure.
Recent experiments with Alston’s singing mice (Scotinomys teguina) – a highly vocal rodent native to the cloud forests of Central America – provide a compelling example of the application of focal cooling for characterizing hierarchically organized networks (Okobi et al., 2019). The advertisement song of the singing mice consists of a highly stereotyped series of notes, each associated with an exhalation, that evolve over many seconds (Campbell et al., 2010). To elucidate the neural circuit mechanisms that are relevant for these vocal performances, we used intracortical microstimulation to identify cortical sites whose activation led to reliable flexion of vocally relevant musculature (Figure 7A). Our search pinpointed an area in anterolateral motor cortex corresponding to a region identified in other rodents as relevant for orofacial motor control (Komiyama et al., 2010; Mercer Lindsay et al., 2019), which we subsequently refer to as the Orofacial Motor Cortex (OMC). Electrical stimulation of OMC during singing led to either truncation or reversible pauses, suggesting an important role for OMC in song production. However, electrical microstimulation may activate neural circuits nonspecifically (Histed et al., 2009) by impacting population activity in antidromically-activated upstream regions as well as a variety of downstream areas. Moreover, the truncation of the song precludes any further characterization of motor control, complicating further interpretation (Otchy et al., 2015).
Figure 7. Temporal and Spatial Segregation of Function Revealed by Cooling.
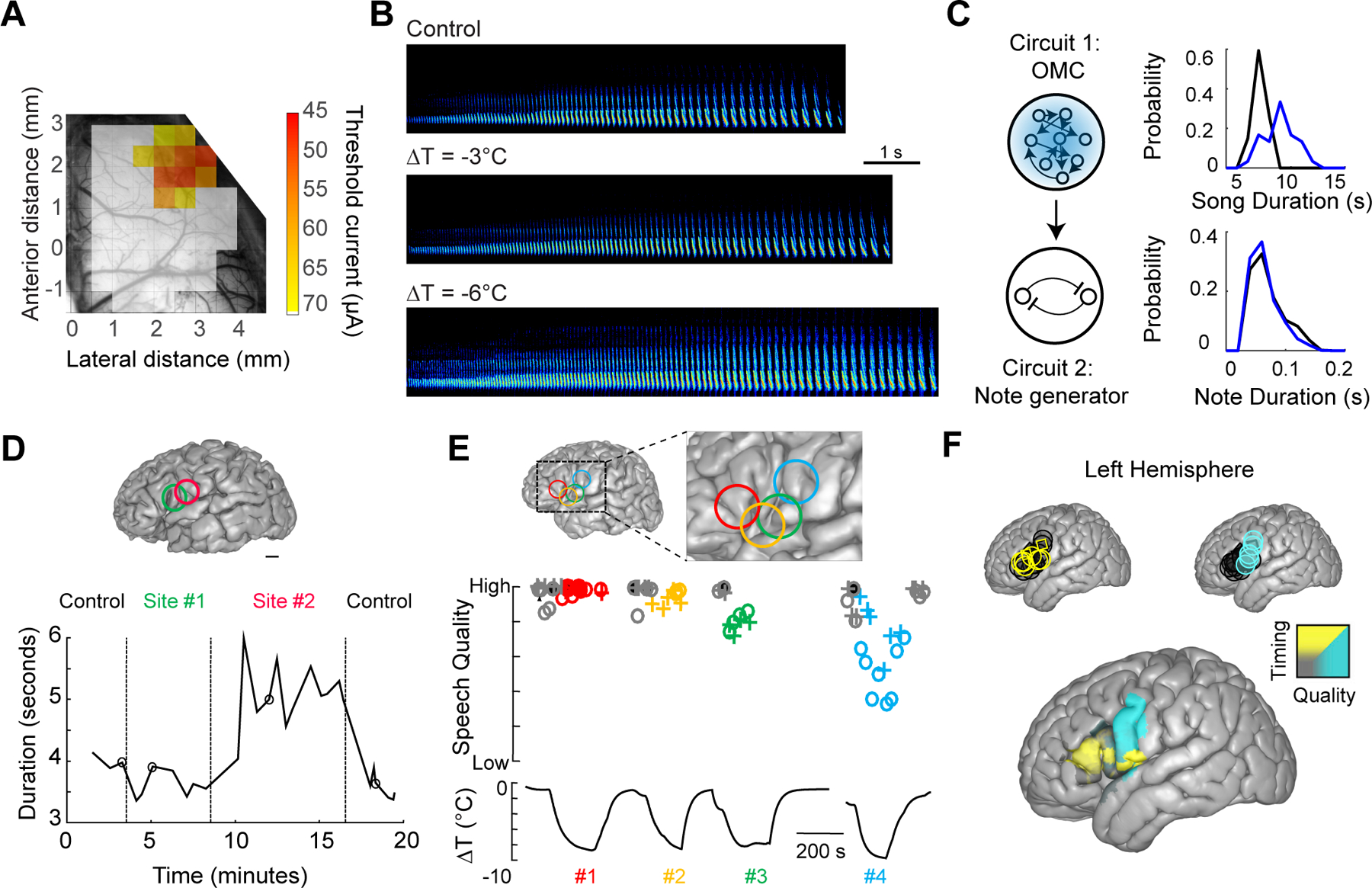
(A) Anatomical location of the orofacial motor cortex (OMC) in the singing mouse (S. teguina) as determined by intracortical microstimulation. Adapted from (Okobi et al., 2019).
(B) Spectrograms of example S. teguina songs during control and cooling sessions. Adapted from (Okobi et al., 2019).
(C) A schematic depicting a hierarchical song control network in S. teguina with an upstream OMC region coupled with a subcortical note generating circuit. Cooling OMC increases song duration without modifying note duration, supporting this model of a separation of timescales. Modified from (Okobi et al., 2019).
(D) Intraoperative mild cooling of human cortical surface can affect speech timing. Surface rendering of brain structure indicating two cooling sites. The duration of the speech task (i.e., ‘Monday, Tuesday, Wednesday, Thursday, Friday’) is significantly expanded when Site #2 is cooled with no effect of cooling Site #1 nearby. Adapted from (Long et al., 2016).
(E) Intraoperative cooling can affect speech quality. Brain surface rending with four cooling sites (top). While cooling each site, participants were asked to recite the days of the week (‘o’ symbols) or a string of numbers (‘+’ symbols) (middle). The estimated temperature change at each site (bottom). Site #4 had a strong relationship between temperature and speech quality. Adapted from (Long et al., 2016).
(F) Brain regions that affect speech quality and timing are spatially segregated in the human brain. Significant timing (upper left, yellow) and quality sites (upper right, blue) are provided as well as a higher spatial resolution map of cooling-induced changes. Adapted from (Long et al., 2016).
To further test OMC’s role in vocal behavior, we applied our cooling method during the production of the advertisement song. OMC cooling did not eliminate singing behavior, but it clearly changed song structure (Figure 7B), lengthening song duration without stretching out individual notes (Figure 7C). This evidence is consistent with our hierarchical model of behavioral control (Figure 6F) and reminiscent of the organization of song control in midshipman fish (Figures 6A and B). Unlike HVC in songbirds, which dictates the fine temporal structure of notes, OMC does not appear to be the song pattern generator but instead regulates activity in a downstream circuit that directly dictates note patterning (Figure 7C). Therefore, mild cooling of a brain region can be an effective circuit manipulation to infer its role within a hierarchically organized network.
Using cooling to manipulate cognitive variables
Thus far, we have considered the application of cooling to behaviorally relevant brain regions acting either as direct pattern generators (e.g., crab STG or zebra finch HVC) or as a higher-order control region in the motor hierarchy (e.g., singing mouse OMC). Can cooling also be used to gain insight into higher level functions that are less directly related to motor output? In recent years, mild focal cooling has also been used to localize important dynamics underlying a range of processes including those related to cognition. In the hippocampus, for instance, theta rhythms have been proposed to support cognitive processes such as memory formation and spatial navigation (Buzsaki and Tingley, 2018). Recently, a cooling probe was inserted into the medial septum to lower the frequency of hippocampal theta rhythms by approximately 10% (Petersen and Buzsaki, 2020). Spike sequences expanded as a function of temperature, but the relative phase of these events with respect to the theta cycle was maintained. From this work, cooling emerges as a potentially transformative means of manipulating neural oscillations (e.g., Figure 3), which are pervasive across the brain and have been proposed to have central roles in cognitive and behavioral neuroscience (Buzsaki, 2006) but have been notoriously difficult to experimentally manipulate with other methods.
In another study, bilaterally cooling the medial prefrontal cortex delayed behavioral responses in a motor timing task, a result that was not observed when a similar cooling manipulation was performed in the motor cortex (Xu et al., 2014). Population dynamics in the striatum and the accompanying decision-making processes were also slowed through focal temperature changes without affecting other aspects of motor timing (Monteiro et al., 2020), highlighting a role for the striatum as an important population clock for estimating elapsed time (Gouvea et al., 2015). Similarly, cooling may also be effective in testing evidence accumulation models in the brain; focal cooling is expected to slow down the rate of accumulated spikes per unit time and may thereby lead to longer reaction times in behavioral tasks (Brunton et al., 2013; Jazayeri and Shadlen, 2015; Roitman and Shadlen, 2002). These examples demonstrate the generality of focal cooling as a strategy for testing specific hypotheses about neural circuit function.
Given the utility of focal cooling in model systems, we have recently taken the first steps towards applying this perturbation approach towards investigating the link between brain function and behavior in human participants. Although cooling was previously employed as a therapeutic approach for addressing conditions such as hypoxia or epilepsy (Csernyus et al., 2020), the application of focal cooling to understand brain function has been considerably more limited. Recently, however, focal cooling has been adapted in a clinical setting (Bakken et al., 2003; Katlowitz et al., 2017; Long et al., 2016), enabling neurosurgeons to localize regions relevant for speech production during intercranial procedures targeted to remove tumors or epileptic tissue. Such awake functional mapping is typically performed through electrical stimulation (Ojemann et al., 1989; Penfield and Boldrey, 1937), which has the potential side-effect of seizure initiation (Piccioni and Fanzio, 2008). In contrast, mild cooling (ΔT = −6 to −8°C at a depth of 2 mm, or approximately cortical Layer 5) is a reversible and safe (Ibayashi et al., 2021) means of changing the processing speed of neural circuits in a spatially specific manner. In previous work, mild focal cooling was used while participants performed overlearned speech sequences (Figure 7 D and E), either counting (‘21’, ‘22’, ‘23’, ‘24’, ‘25’) or reciting the days of the week (‘Monday’, ‘Tuesday’, ‘Wednesday’, ‘Thursday’, ‘Friday’). Cooling the inferior frontal gyrus – including what is traditionally considered Broca’s region – led to a slowing down of speech sequences (Figure 7 D and F), while the same manipulation in the primary motor cortex resulted in a measurable decrease in speech quality. Uncovering this functional dissociation would not have been possible with electrical stimulation, a perturbation that often leads to speech arrest, where the patient is temporarily unable to produce words (Ojemann et al., 1989).
Technical considerations and Outlook
Given the utility of focal cooling for probing the relationship between neural dynamics and behavior, it is also important to discuss the limitations of this technique. First, surface cooling is spatially more restricted compared to cooling of deep structures with a typical length constant of ~1.5 mm (Aronov and Fee, 2011; Long and Fee, 2008). For brain regions smaller than the spatial length scale of cooling, the amount of cooling is likely to be homogenous across the entire structure. However, for laminar structures such as the cerebral cortex, placing a cooling device on the cortical surface will lead to strong yet nonuniform cooling, exponentially decaying from superficial to deeper layers. In such cases, a careful consideration of the spatial extent of temperature spread is necessary to interpret the results of focal cooling. Second, cooling deeper targets requires the implantation of a penetrating cooling probe (typically < 0.5 mm), which may damage important neural tissue and can lead to nonselective cooling of neighboring structures (Aubert et al., 2004; Hamaguchi et al., 2016; Long and Fee, 2008; Petersen and Buzsaki, 2020). Third, cooling is considerably slower than optogenetic or electrical stimulation (~1 ms); neural tissue typically takes 2–5 minutes to reach a steady-state temperature, which eliminates the possibility of selectively targeting behavioral epochs that unfold on a faster time scale (e.g., grasping as part of a more elaborate food retrieval task) (Guo et al., 2015). Fourth, unlike many genetically encoded methods for manipulating neural circuits, focal cooling does not yet feature cell type specificity. That said, membrane-bound actuators such as ferritin may offer the possibility of cell-autonomous temperature changes through DC magnetic fields (i.e., the magneto-caloric effect) (Barbic, 2019), although the extent to which this manipulation can preserve network structure remains unclear. Fifth, the utility of focal cooling – like most perturbations – is limited to cases in which intrinsic behavioral variability is small. As a result, cooling may be most effective in highly trained behavioral paradigms that require stereotyped motor readouts such as saccades, specific arm movements, or licking (Gouvea et al., 2015; Shenoy et al., 2013; Sparks and Barton, 1993; Svoboda and Li, 2018) since intrinsic variability of motor output after training is restricted in such tasks.
Despite these limitations, focal cooling has important strengths that warrant its wider adoption. First, as demonstrated above, cooling allows the experimenter to parametrically vary the speed of population activity without disrupting its structure. Thus, in contrast to methods that add or subtract large numbers of spikes, cooling maintains population activity on the manifold of endogenous patterns. Additionally, because the biophysical mechanisms of spike generation and conduction are largely conserved (e.g., Figure 3), the effect of mild cooling on neuronal dynamics may generalize across different circuits as well as different species. Furthermore, the application of this technique does not require specific genetic lines or viral tools, thereby expanding its utility to most model systems that are not traditionally used in the laboratory. By embracing this diversity, focal cooling presents an opportunity to lead the field towards important new insights concerning the link between brain function and behavior.
ACKNOWLEDGEMENTS
We thank Florin Albeanu, Shaul Druckmann, Matthew Smear, Stephen Shea, and members of the Long lab for helpful comments on earlier versions of this manuscript. M.A.L. would like to acknowledge funding support from the NIH (R01 NS113071, R01 NS075044) and the Simons Collaboration on the Global Brain. A.B would like to acknowledge support from the Simons Foundation Society of Fellows. We thank Katelyn Wadland (Marder lab) and Julia Kuhl for help with figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Andalman AS, Foerster JN, and Fee MS (2011). Control of vocal and respiratory patterns in birdsong: dissection of forebrain and brainstem mechanisms using temperature. PLoS One 6, e25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, and Fee MS (2011). Analyzing the dynamics of brain circuits with temperature: design and implementation of a miniature thermoelectric device. J Neurosci Methods 197, 32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, and Fee MS (2012). Natural changes in brain temperature underlie variations in song tempo during a mating behavior. PLoS One 7, e47856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert B, Barate R, Boutigny D, Gaillard JM, Hicheur A, Karyotakis Y, Lees JP, Robbe P, Tisserand V, Zghiche A, et al. (2004). Limits on the decay-rate difference of neutral B mesons and on CP, T, and CPT violation in B(0–0)B oscillations. Phys Rev Lett 92, 181801. [DOI] [PubMed] [Google Scholar]
- Bakken HE, Kawasaki H, Oya H, Greenlee JD, and Howard MA 3rd (2003). A device for cooling localized regions of human cerebral cortex. Technical note. J Neurosurg 99, 604–608. [DOI] [PubMed] [Google Scholar]
- Barbic M (2019). Possible magneto-mechanical and magneto-thermal mechanisms of ion channel activation in magnetogenetics. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AH (2014). Central pattern generator for vocalization: is there a vertebrate morphotype? Curr Opin Neurobiol 28, 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C (2020). Optogenetics: Keep Interpretations Light. eNeuro 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley RK, and Bass AH (1994). Alternative male spawning tactics and acoustic signaling in the plainfin midshipman fish, Porichthys notatus. . Ethology 96, 213–232. [Google Scholar]
- Broca MP (1861). Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bulletin de la Société Anatomique 6, 330–357. [Google Scholar]
- Brunton BW, Botvinick MM, and Brody CD (2013). Rats and humans can optimally accumulate evidence for decision-making. Science 340, 95–98. [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2006). Rhythms of the Brain (Oxford University Press; ). [Google Scholar]
- Buzsaki G, and Tingley D (2018). Space and Time: The Hippocampus as a Sequence Generator. Trends Cogn Sci 22, 853–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese RL, and Arbas EA (1984). Rate modification in the heartbeat central pattern generator of the medicinal leech. J Comp Physiol A 155, 783–794. [Google Scholar]
- Campbell P, Pasch B, Pino JL, Crino OL, Phillips M, and Phelps SM (2010). Geographic variation in the songs of neotropical singing mice: testing the relative importance of drift and local adaptation. Evolution 64, 1955–1972. [DOI] [PubMed] [Google Scholar]
- Chagnaud BP, Baker R, and Bass AH (2011). Vocalization frequency and duration are coded in separate hindbrain nuclei. Nat Commun 2, 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber B, Edwards D, Jones SJ, Shackleton TM, Goldschmidt J, Wallace MN, and Palmer AR (2011). Cortical inactivation by cooling in small animals. Front Syst Neurosci 5, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernyus B, Szabo A, Zatonyi A, Hodovan R, Lazar C, Fekete Z, Eross L, and Pongracz A (2020). Recent antiepileptic and neuroprotective applications of brain cooling. Seizure 82, 80–90. [DOI] [PubMed] [Google Scholar]
- Cunningham JP, and Yu BM (2014). Dimensionality reduction for large-scale neural recordings. Nat Neurosci 17, 1500–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubner J, Coulon P, and Diester I (2019). Optogenetic approaches to study the mammalian brain. Curr Opin Struct Biol 57, 157–163. [DOI] [PubMed] [Google Scholar]
- Dolbear A (1897). The cricket as a thermometer. The American Naturalist 31 970–971. [Google Scholar]
- Donahue MJ, Nichols A, Santamaria CA, League-Pike PE, Krediet CJ, Perez KO, and Shulman MJ (2009). Predation Risk, Prey Abundance, and the Vertical Distribution of Three Brachyuran Crabs on Gulf of Maine Shores. Journal of Crustacean Biology 29, 523–531. [Google Scholar]
- Egger R, Tupikov Y, Elmaleh M, Katlowitz KA, Benezra SE, Picardo MA, Moll F, Kornfeld J, Jin DZ, and Long MA (2020). Local Axonal Conduction Shapes the Spatiotemporal Properties of Neural Sequences. Cell 183, 537–548 e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, and Deisseroth K (2011). The development and application of optogenetics. Annu Rev Neurosci 34, 389–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch GT, and Hitzig E (1870). Über die elektrische Erregbarkeit des Grosshirns. Arch Anat Physiol Med Wiss 37, 300–332. [Google Scholar]
- Glaze CM, and Troyer TW (2006). Temporal structure in zebra finch song: implications for motor coding. J Neurosci 26, 991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin MA, Alonso LM, Alliende JA, Goller F, and Mindlin GB (2013). Temperature induced syllable breaking unveils nonlinearly interacting timescales in birdsong motor pathway. PLoS One 8, e67814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea TS, Monteiro T, Motiwala A, Soares S, Machens C, and Paton JJ (2015). Striatal dynamics explain duration judgments. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JZ, Graves AR, Guo WW, Zheng J, Lee A, Rodriguez-Gonzalez J, Li N, Macklin JJ, Phillips JW, Mensh BD, et al. (2015). Cortex commands the performance of skilled movement. Elife 4, e10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, and Fee MS (2002). An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419, 65–70. [DOI] [PubMed] [Google Scholar]
- Hamaguchi K, Tanaka M, and Mooney R (2016). A Distributed Recurrent Network Contributes to Temporally Precise Vocalizations. Neuron 91, 680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histed MH, Bonin V, and Reid RC (2009). Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63, 508–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, and Katz B (1949). The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol 109, 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaetter LJ, Mastrotto M, Merriman DK, Dib-Hajj SD, Waxman SG, Bagriantsev SN, and Gracheva EO (2018). Somatosensory Neurons Enter a State of Altered Excitability during Hibernation. Curr Biol 28, 2998–3004 e2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibayashi K, Cardenas AR, Oya H, Kawasaki H, Kovach CK, Howard MA 3rd, Long MA, and Greenlee JDW (2021). Focal Cortical Surface Cooling is a Novel and Safe Method for Intraoperative Functional Brain Mapping. World Neurosurg 147, e118–e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhikevich EM (2003). Simple model of spiking neurons. IEEE Trans Neural Netw 14, 1569–1572. [DOI] [PubMed] [Google Scholar]
- Jasper HH, Shacter DG, and Montplaisir J (1970). The effect of local cooling upon spontaneous and evoked electrical activity of cerebral cortex. Can J Physiol Pharmacol 48, 640–652. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, and Afraz A (2017). Navigating the Neural Space in Search of the Neural Code. Neuron 93, 1003–1014. [DOI] [PubMed] [Google Scholar]
- Jazayeri M, and Shadlen MN (2015). A Neural Mechanism for Sensing and Reproducing a Time Interval. Curr Biol 25, 2599–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach AS, and Waters J (2012). Brain surface temperature under a craniotomy. J Neurophysiol 108, 3138–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara Y, Ikegaya Y, and Koyama R (2018). Neonatal Seizure Models to Study Epileptogenesis. Front Pharmacol 9, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katlowitz KA, Oya H, Howard MA 3rd, Greenlee JDW, and Long MA (2017). Paradoxical vocal changes in a trained singer by focally cooling the right superior temporal gyrus. Cortex 89, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Sakurai A, Clemens S, and Davis D (2004). Cycle period of a network oscillator is independent of membrane potential and spiking activity in individual central pattern generator neurons. J Neurophysiol 92, 1904–1917. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O’Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, and Svoboda K (2010). Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature 464, 1182–1186. [DOI] [PubMed] [Google Scholar]
- Kozhevnikov AA, and Fee MS (2007). Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol 97, 4271–4283. [DOI] [PubMed] [Google Scholar]
- Laje R, and Buonomano DV (2013). Robust timing and motor patterns by taming chaos in recurrent neural networks. Nat Neurosci 16, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampl I, and Yarom Y (1997). Subthreshold oscillations and resonant behavior: two manifestations of the same mechanism. Neuroscience 78, 325–341. [DOI] [PubMed] [Google Scholar]
- Li N, Chen S, Guo ZV, Chen H, Huo Y, Inagaki HK, Chen G, Davis C, Hansel D, Guo C, et al. (2019). Spatiotemporal constraints on optogenetic inactivation in cortical circuits. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG (1999). The advantages and limitations of permanent or reversible deactivation techniques in the assessment of neural function. J Neurosci Methods 86, 109–117. [DOI] [PubMed] [Google Scholar]
- Long MA, and Fee MS (2008). Using temperature to analyse temporal dynamics in the songbird motor pathway. Nature 456, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Katlowitz KA, Svirsky MA, Clary RC, Byun TM, Majaj N, Oya H, Howard MA 3rd, and Greenlee JDW (2016). Functional Segregation of Cortical Regions Underlying Speech Timing and Articulation. Neuron 89, 1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GF, Okubo TS, Hanuschkin A, Hahnloser RH, and Fee MS (2016). Rhythmic Continuous-Time Coding in the Songbird Analog of Vocal Motor Cortex. Neuron 90, 877–892. [DOI] [PubMed] [Google Scholar]
- Marder E, Manor Y, Nadim F, Bartos M, and Nusbaum MP (1998). Frequency control of a slow oscillatory network by a fast rhythmic input: pyloric to gastric mill interactions in the crab stomatogastric nervous system. Ann N Y Acad Sci 860, 226–238. [DOI] [PubMed] [Google Scholar]
- Mercer Lindsay N, Knutsen PM, Lozada AF, Gibbs D, Karten HJ, and Kleinfeld D (2019). Orofacial Movements Involve Parallel Corticobulbar Projections from Motor Cortex to Trigeminal Premotor Nuclei. Neuron 104, 765–780 e763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski A, Wimborne BM, and Henry GH (1993). The effect of reversible cooling of cat’s primary visual cortex on the responses of area 21a neurons. J Physiol 466, 133–156. [PMC free article] [PubMed] [Google Scholar]
- Monteiro T, Rodrigues FS, Pexirra M, Cruz BF, Gonçalves AI, Rueda-Orozco PE, and Paton JJ (2020). Using temperature to analyse the neural basis of a latent temporal decision. bioRxiv, 2020.2008.2024.251827. [DOI] [PubMed] [Google Scholar]
- Nobile M, Carbone E, Lux HD, and Zucker H (1990). Temperature sensitivity of Ca currents in chick sensory neurones. Pflugers Arch 415, 658–663. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, and Paton JA (1982). Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol 207, 344–357. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, and Leonard CM (1976). Central control of song in the canary, Serinus canarius. J Comp Neurol 165, 457–486. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, and Berger M (1989). Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg 71, 316–326. [DOI] [PubMed] [Google Scholar]
- Okobi DE Jr., Banerjee A, Matheson AMM, Phelps SM, and Long MA (2019). Motor cortical control of vocal interaction in neotropical singing mice. Science 363, 983–988. [DOI] [PubMed] [Google Scholar]
- Otchy TM, Wolff SB, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SM, and Olveczky BP (2015). Acute off-target effects of neural circuit manipulations. Nature 528, 358–363. [DOI] [PubMed] [Google Scholar]
- Owen SF, Liu MH, and Kreitzer AC (2019). Thermal constraints on in vivo optogenetic manipulations. Nat Neurosci 22, 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S, Harvey CD, Piasini E, Latham PE, and Fellin T (2017). Cracking the Neural Code for Sensory Perception by Combining Statistics, Intervention, and Behavior. Neuron 93, 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, and Boldrey E (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. . Brain 60, 389–443. [Google Scholar]
- Petersen PC, and Buzsaki G (2020). Cooling of Medial Septum Reveals Theta Phase Lag Coordination of Hippocampal Cell Assemblies. Neuron 107, 731–744 e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardo MA, Merel J, Katlowitz KA, Vallentin D, Okobi DE, Benezra SE, Clary RC, Pnevmatikakis EA, Paninski L, and Long MA (2016). Population-Level Representation of a Temporal Sequence Underlying Song Production in the Zebra Finch. Neuron 90, 866–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioni F, and Fanzio M (2008). Management of anesthesia in awake craniotomy. Minerva Anestesiol 74, 393–408. [PubMed] [Google Scholar]
- Pires A, and Hoy RR (1992). Temperature coupling in cricket acoustic communication. II. Localization of temperature effects on song production and recognition networks in Gryllus firmus. J Comp Physiol A 171, 79–92. [DOI] [PubMed] [Google Scholar]
- Podgorski K, and Ranganathan G (2016). Brain heating induced by near-infrared lasers during multiphoton microscopy. J Neurophysiol 116, 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RM, and Money TG (2012). Temperature and neuronal circuit function: compensation, tuning and tolerance. Curr Opin Neurobiol 22, 724–734. [DOI] [PubMed] [Google Scholar]
- Roitman JD, and Shadlen MN (2002). Response of neurons in the lateral intraparietal area during a combined visual discrimination reaction time task. J Neurosci 22, 9475–9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, and Regehr WG (1996). Timing of neurotransmission at fast synapses in the mammalian brain. Nature 384, 170–172. [DOI] [PubMed] [Google Scholar]
- Shenoy KV, and Kao JC (2021). Measurement, manipulation and modeling of brain-wide neural population dynamics. Nat Commun 12, 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy KV, Sahani M, and Churchland MM (2013). Cortical control of arm movements: a dynamical systems perspective. Annu Rev Neurosci 36, 337–359. [DOI] [PubMed] [Google Scholar]
- Soofi W, Goeritz ML, Kispersky TJ, Prinz AA, Marder E, and Stein W (2014). Phase maintenance in a rhythmic motor pattern during temperature changes in vivo. J Neurophysiol 111, 2603–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossinka R, and Böhner J (1980). Song types in the zebra finch Poephilia guttata castanotis. Z Tierpsychol 53, 123–132. [Google Scholar]
- Sparks DL, and Barton EJ (1993). Neural control of saccadic eye movements. Curr Opin Neurobiol 3, 966–972. [DOI] [PubMed] [Google Scholar]
- Sternson SM, and Roth BL (2014). Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 37, 387–407. [DOI] [PubMed] [Google Scholar]
- Sussillo D, and Abbott LF (2009). Generating coherent patterns of activity from chaotic neural networks. Neuron 63, 544–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda K, and Li N (2018). Neural mechanisms of movement planning: motor cortex and beyond. Curr Opin Neurobiol 49, 33–41. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG, and Weyand TG (1981). Effects of variations in temperature on impulse conduction along nonmyelinated axons in the mammalian brain. Exp Neurol 71, 383–389. [DOI] [PubMed] [Google Scholar]
- Tang LS, Goeritz ML, Caplan JS, Taylor AL, Fisek M, and Marder E (2010). Precise temperature compensation of phase in a rhythmic motor pattern. PLoS Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Masukawa LM, and Prince DA (1985). Temperature dependence of intrinsic membrane properties and synaptic potentials in hippocampal CA1 neurons in vitro. J Neurosci 5, 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, and Eysel UT (2000a). Synaptic transmission in the neocortex during reversible cooling. Neuroscience 98, 9–22. [DOI] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Yousef T, and Eysel UT (2000b). Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol 522 Pt 1, 59–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SB, and Olveczky BP (2018). The promise and perils of causal circuit manipulations. Curr Opin Neurobiol 49, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Zhang SY, Dan Y, and Poo MM (2014). Representation of interval timing by temporally scalable firing patterns in rat prefrontal cortex. Proc Natl Acad Sci U S A 111, 480–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Gooler D, Herrold A, Patel S, and Pong WW (2008). Temperature-dependent regulation of vocal pattern generator. J Neurophysiol 100, 3134–3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, and Dewhirst MW (2011). Thresholds for thermal damage to normal tissues: an update. Int J Hyperthermia 27, 320–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, and Yoshihara M (2018). ‘Necessary and sufficient’ in biology is not necessarily necessary - confusions and erroneous conclusions resulting from misapplied logic in the field of biology, especially neuroscience. J Neurogenet 32, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YS, Wittenbach JD, Jin DZ, and Kozhevnikov AA (2017). Temperature Manipulation in Songbird Brain Implicates the Premotor Nucleus HVC in Birdsong Syntax. J Neurosci 37, 2600–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]


