Abstract
Unhealthy alcohol use, smoking, and depressive symptoms are risk factors for cardiovascular disease (CVD). Little is known about their co-occurrence – termed a syndemic, defined as the synergistic effect of two or more conditions—on CVD risk in people with HIV (PWH). We used data from 5621 CVD-free participants (51% PWH) in the Veteran’s Aging Cohort Study-8, a prospective, observational study of veterans followed from 2002 to 2014 to assess the association between this syndemic and incident CVD by HIV status. Diagnostic codes identified cases of CVD (acute myocardial infarction, stroke, heart failure, peripheral artery disease, and coronary revascularization). Validated measures of alcohol use, smoking, and depressive symptoms were used. Baseline number of syndemic conditions was categorized (0, 1, ≥ 2 conditions). Multivariable Cox Proportional Hazards regressions estimated risk of the syndemic (≥ 2 conditions) on incident CVD by HIV-status. There were 1149 cases of incident CVD (52% PWH) during the follow-up (median 10.1 years). Of the total sample, 64% met our syndemic definition. The syndemic was associated with greater risk for incident CVD among PWH (Hazard Ratio [HR] 1.87 [1.47–2.38], p < 0.001) and HIV-negative veterans (HR 1.70 [1.35–2.13], p < 0.001), compared to HIV-negative with zero conditions. Among those with the syndemic, CVD risk was not statistically significantly higher among PWH vs. HIV-negative (HR 1.10 [0.89, 1.37], p = .38). Given the high prevalence of this syndemic combined with excess risk of CVD, these findings support linked-screening and treatment efforts.
Keywords: Alcohol, Smoking, Depression, Cardiovascular, HIV
Introduction
While combination antiretroviral therapy has extended the survival of people with HIV (PWH) by decades, this success has been offset by increasing morbidity of non-AIDS diseases [1, 2]. Cardiovascular disease (CVD) is a common comorbidity and cause of death among PWH [3, 4]. After adjustment for possible confounders, PWH experience an increased risk of acute myocardial infarction [5, 6], heart failure [7], ischemic stroke [8, 9], peripheral artery disease [10], and total coronary heart disease [11], compared to HIV-negative people. This risk persists even with sustained HIV viral suppression [5]. While some of this excess risk is likely due to the virus itself [12, 13], behavioral and mental health factors [14], including unhealthy alcohol use, cigarette use, and depression [15], likely also play an important role.
Smoking prevalence among PWH is two-fold higher than in HIV-negative populations [16–18] and is associated with 40–100% increase risk for CVD [5, 11, 19, 20], compared to non-smokers. Unhealthy alcohol use is reported in 25–45% [21–23] of PWH and is associated with nearly 50% higher prevalence of CVD [24], compared to low risk use. Depression, the most common psychological condition among PWH, affects 20–30% [25–27] and is associated with 68% higher risk of CVD [20], compared to those without depression. Further, a longitudinal analysis from the Veteran’s Aging Cohort Study (VACS) showed that unhealthy alcohol use, smoking, and depressive symptoms among PWH were temporally concordant and that removal of any one factor was associated with the cessation of the other two, suggesting that the co-occurrence of these conditions constitutes a unique syndemic [15].
A syndemic refers to two or more adverse conditions—in this case behavioral and mental health factors – that cluster and interact to exacerbate poor health outcomes [28]. While there are unique challenges to assessing the effect of syndemics, more importantly are the unique opportunities to screen and address them in clinical practice. Unhealthy alcohol use, smoking, and depression are behaviorally and biologically linked [29], offering clinicians the opportunity to perform linked-screening and treatment with combined behavioral therapy and pharmacotherapy. Specifically, there is emerging evidence for parsimonious treatment for alcohol and tobacco use with selective nicotinic receptor partial agonists such as varenicline [30], that could mitigate polypharmacy [31]. In a recent study of veterans, 50% of the participants had two and 15% had all three of these conditions [32]. Among those with the syndemic, PWH had a 36% increased risk of death compared to HIV-negative counterparts [32]. Whether this syndemic contributes to the excess risk of CVD among PWH is unknown. Therefore, our objective was to (1) assess the association between this syndemic and incident CVD among PWH and HIV-negative veterans from the VACS and to (2) determine if the association between the syndemic and incident CVD differed by HIV status.
Methods
We analyzed data from the Veteran’s Aging Cohort Study (VACS), an observational, longitudinal cohort of veterans living with HIV and HIV-negative veterans matched 1:1 on age, sex, race/ethnicity, and clinical site [33] to conduct a retrospective longitudinal analysis of prospectively collected cohort data. The VACS undergoes continuous enrollment, beginning June of 2002. Clinical and demographic data are extracted from the VA Corporate Data Warehouse and the VA electronic medical record Health Factor data set. Vital status is determined using the VA vital status file, the Social Security Administration death master file, the Beneficiary Identification and Records Locator Subsystem, and the Veterans Health Administration Medical Statistical Analysis Systems inpatient data sets. Approval of the VACS was obtained from the institutional review board of the Yale School of Medicine. The following analyses were approved by the institutional review board of Vanderbilt University Medical Center.
Participants were included in this analysis if they were CVD-free at baseline and followed from baseline date until date of known cardiovascular disease, death, the last follow-up date, or censored on December 31, 2014 (n = 5995). Based on our previous work [5, 9, 10], prevalent CVD at baseline was defined by validated International Classification of Diseases, Ninth Revision (ICD-9) codes and Current Procedures Terminology (CPT) codes for acute myocardial infarction [34], ischemic stroke [35], heart failure [36], unstable angina [37], peripheral artery disease [38], and coronary revascularization (i.e., stent placement, coronary artery bypass grafting, and percutaneous coronary intervention) [39]. Those with prevalent CVD at baseline were excluded from the analysis.
Exclusion criteria included those with no follow-up after a baseline visit (n = 22), more than one missing component of the Patient Health Questionnaire (PHQ-9, n = 96), and those who self-reported never drinking (n = 256) as the risk profile between never and former drinkers (which may include “sick-quitters”- previous heavy drinkers who stopped drinking due to declining health) is quite disparate [40, 41]. Further, missing data for never-drinkers was 50% or greater at each follow-up visit.
Independent Variable
Unhealthy alcohol use, cigarette smoking, and depressive symptoms were assessed at survey baseline. The Alcohol Use Disorders Identification Test (AUDIT-C) [42] identified unhealthy drinking (> 14 drinks per week for men and > 7 drinks per week for women) and heavy episodic drinking (> 4 drinks for men and > 3 drinks for women on one occasion). The AUDIT-C has been validated across multiple clinical and research settings, with area under the receiver operator curve > 0.80 [43–48]. The AUDIT-C is widely used in HIV research to quantify alcohol use [49, 50]. Cigarette smoking was measured through self-report. The Patient Health Questionnaire-9 items (PHQ-9) identified clinically significant depressive symptoms and has strong reliability and validity for diagnosing major depressive disorders at a cut-off of ≥ 10 [51]. Those with a PHQ-9 score ≥ 10 were considered positive for depressive symptoms. The PHQ-9 is widely validated across many clinical populations, including among PWH [52]. We created a syndemic variable, categorized as having zero, one, and ≥ two conditions (syndemic). We chose to categorize those with 2 and 3 conditions together for the following reasons: (1) to be consistent with the definition of a syndemic (2 or more conditions that increase risk for poor outcomes); (2) the survival curves of those with 2 and 3 conditions are nearly identical overtime, with the 95% confidence intervals overlapping completely; (3) although 15% have all 3 conditions, the number of CVD cases in this group becomes sparse over follow-up creating an issue with stability of the effects. The syndemic score was included as a time-varying predictor of incident CVD.
Dependent Variable
Incident CVD was defined using the same criteria as prevalent CVD, using relevant ICD-9 and CPT codes.
Covariates
Defined in prior work [5, 11], we adjusted for several baseline demographic and CVD-related variables. Briefly, diabetes was defined as having any of the following indicators: abnormal glucose measurement (> 200 mg/dL), use of medications for diabetes treatment, or at least one inpatient or two outpatient ICD-9 codes for diabetes [53]. Hypertension was categorized using American Heart Association cut-offs [54] and integrated use of blood pressure (BP) lowering medication [normal BP (< 140/90 mm Hg with no antihypertensive medication use), controlled (BP < 140/90 mm Hg with antihypertensive medication use), and uncontrolled (BP ≥ 140/90 mm Hg)], and was calculated with the average BP measurement of three routine outpatient clinical assessments closest to baseline date. Low density lipoprotein and high density lipoprotein cholesterol and triglycerides were measured during routine clinical practice and were considered as continuous variables. Obesity was defined as a body mass index ≥ 30 kg/m2 [55]. Anemia and renal disease were ascertained using laboratory values of hemoglobin (< 13 g/dL men; < 12 g/dL women) [56] and estimated glomerular filtration rate levels (mL/min/1.73m2 < 30, 30–59, ≥ 60) [57], respectively. Past year illicit drug use was self-reported using standardized and validated measures [33] which included the use of cocaine/crack, stimulants (amphetamines), and opioids (heroin, morphine, codeine, opium). Opiate use disorder was defined by at least one inpatient or two outpatient ICD-9 codes. Hepatitis C infection was defined as a positive antibody test or at least one inpatient or two outpatient ICD-9 codes [58]. Prevalent chronic obstructive pulmonary disease [59] and posttraumatic stress disorder were defined using ICD-9 codes for diagnoses at baseline. We collected data on CD4 + T-cell counts and HIV-1 RNA values from baseline through the last follow-up date.
Statistical Analysis
Descriptive statistics for all variables by syndemic categories and HIV status were assessed using t tests for continuous variables and × 2 tests for categorical variables. We calculated age-adjusted incident CVD rates per 1000 person-years and age-adjusted survival curves by syndemic categories for the total sample and stratified by HIV status. Cox Proportional Hazards models estimated the hazard ratio (HR) and 95% confidence intervals (CI) to assess the association between the time-updated syndemic and incident CVD. Our primary analysis included the interaction of HIV status and the syndemic categories as a 6-level HIV/syndemic variable on incident CVD, with HIV-negative people with zero conditions as the common referent group. Pairwise comparisons of the syndemic effect by HIV status are presented. Lastly, Cox Proportional Hazards models were conducted among the total sample and by HIV status, controlling for the afore-mentioned covariates. In HIV-stratified models, we included baseline and time-updated HIV viral load and CD4 count as additional covariates to test attenuation of effect.
Missing covariate data were included in the analyses through multiple imputations using chained equations with five separate imputed datasets, generated based on predictive mean matching methods using the Hmisc library of R programming language. Cox survival models were fit in each imputed dataset and finally combined to obtain pooled HRs and standard errors. All analyses were performed using R software (version 3.3.3; www.rproject.org).
Power-analysis
With the observed CVD incident of 14.7% among those without any syndemic conditions (n = 524) and 21.8% among those with ≥ 2 conditions (n = 3617), we anticipated a minimum detectable hazard ratio of 1.35 at 90% power with a Type I error rate of 5%.
Results
The total sample included 5621 participants (2864 PWH and 2757 HIV-negative; Table 1) with a median age of 49.1 years (interquartile range [IQR] 43.6, 54.6). Most of the sample were male (94%) and non-White (65% Black). Of the total sample, 9% had no conditions, 26% had one condition, and 65% had the syndemic (≥ 2 conditions; 51% in PWH, 49% in HIV-negative, Table 1). Nearly 15% of the total sample had all three conditions. During the median follow-up period of 10.1 years, there were 1149 incident cases of CVD (21% in PWH, 20% in HIV-negative). Of the total sample, age-adjusted CVD rates increased with each increasing syndemic category (Fig. 1, Log-Rank Tests P < 0.001). Incidence rates were not statistically significantly higher among PWH compared to HIV-negative people at each syndemic category (Table 2, zero: 18.3 vs. 13.8 (p = 0.22); one: 23.2 vs. 19.8 (p = 0.19); two or more 27.7 vs. 25.7 (p = 0.29), respectively).
Table 1.
Baseline characteristics of study population by number of syndemic behavioral conditions
| Characteristics | Number of syndemic conditions | |||
|---|---|---|---|---|
| Total N = 5621 |
0 524 (9.3%) |
1 1480 (26.3%) |
≥ 2 3617 (64.3%) |
|
| Column frequency (%) | ||||
| Incident CVD* | 1149 (20.4) | 77 (14.7) | 285 (19.3) | 787 (21.8) |
| Age, years, Median [Interquartile] | 49.1 [43.6, 54.6] | 45.2 [38.3, 53.6] | 48.7 [42.1, 55.2] | 49.6 [44.8, 54.4] |
| Sex | ||||
| Women | 317 (5.6) | 56 (10.7) | 115 (7.8) | 146 (4.0) |
| Men | 5304 (94.4) | 468 (89.3) | 1365 (92.2) | 3471 (96.0) |
| Race/Ethnicity | ||||
| White, non-Hispanic | 1196 (21.3) | 127 (24.2) | 371 (25.1) | 698 (19.3) |
| Black, non-Hispanic | 3651 (65.0) | 322 (61.5) | 894 (60.4) | 2435 (67.3) |
| Hispanic | 567 (10.1) | 60 (11.5) | 143 (9.7) | 364 (10.1) |
| Other | 207 (3.7) | 15 (2.9) | 72 (4.9) | 120 (3.3) |
| Education | ||||
| < High school | 352 (6.3) | 11 (2.1) | 59 (4.0) | 282 (7.8) |
| High school or equivalent | 1836 (32.7) | 83 (15.8) | 408 (27.6) | 1345 (37.2) |
| > High school | 3384 (60.2) | 426 (81.3) | 998 (67.4) | 1960 (54.2) |
| Diabetes (glucose measurement, antidiabetic agent use, and/or at least 1 inpatient/2 outpatient ICD-9 codes for diabetes) | 828 (14.7) | 79 (15.1) | 203 (13.7) | 546 (15.1) |
| Hypertension | ||||
| No (BP < 140/90 mm Hg, no antihypertensive medication) | 2250 (40.0) | 246 (46.9) | 596 (40.3) | 1408 (38.9) |
| Controlled (BP < 140/90 mm Hg, on antihypertensive medication) | 1946 (34.6) | 162 (30.9) | 514 (34.7) | 1270 (35.1) |
| Uncontrolled (BP ≥ 140/90 mm Hg) | 1421 (25.3) | 115 (21.9) | 369 (24.9) | 937 (25.9) |
| Low-density lipoprotein cholesterol ≥ 160 mg/dL, blood | 457 (8.1) | 46 (8.8) | 140 (9.5) | 271 (7.5) |
| High-density lipoprotein cholesterol < 40 mg/dL, blood | 2499 (44.5) | 260 (49.6) | 720 (48.6) | 1519 (42.0) |
| Triglycerides ≥ 200 mg/dL, blood | 1237 (22.0) | 98 (18.7) | 351 (23.7) | 788 (21.8) |
| Obesity (body mass index ≥ 30) | 1496 (26.6) | 164 (31.3) | 430 (29.1) | 902 (24.9) |
| Chronic obstructive pulmonary disease | 515 (9.2) | 21 (4.0) | 99 (6.7) | 395 (10.9) |
| Hepatitis C infection | 1934 (34.4) | 53 (10.1) | 310 (20.9) | 1571 (43.4) |
| Anemia (hemoglobin < 13 g/dL men; < 12 g/dL women) | 1068 (19.0) | 84 (16.0) | 266 (18.0) | 718 (19.9) |
| Renal disease (estimated glomerular filtration rate, mL/min/1·73 m2) | ||||
| < 30 | 40 (0.7) | 4 (0.8) | 7 (0.5) | 29 (0.8) |
| 30–59 | 216 (3.8) | 27 (5.2) | 62 (4.2) | 127 (3.5) |
| ≥ 60 | 5281 (94.0) | 481 (91.8) | 1389 (93.9) | 3411 (94.3) |
| Posttraumatic stress disorder (ICD-9 code) | 792 (14.1) | 22 (4.2) | 108 (7.3) | 662 (18.3) |
| Illicit drug use | 2174 (38.7) | 16 (3.1) | 259 (17.5) | 1899 (52.5) |
| Opioid use disorder | 938 (16.7) | 4 (0.8) | 88 (5.9) | 846 (23.4) |
| Cigarette Use | ||||
| Current | 2836 (50.5) | 0 (0.0) | 417 (28.2) | 2419 (66.9) |
| Former | 1369 (24.4) | 0 (0.0) | 325 (22.0) | 1044 (28.9) |
| Never | 1416 (25.2) | 524 (100.0) | 738 (49.9) | 154 (4.3) |
| Alcohol consumption | ||||
| Not current drinker | 1791 (31.9) | 0 (0.0) | 320 (21.6) | 1471 (40.7) |
| Not hazardous | 1495 (26.6) | 524 (100.0) | 825 (55.7) | 146 (4.0) |
| Unhealthy or alcohol use disorder | 2335 (41.5) | 0 (0.0) | 335 (22.6) | 2000 (55.3) |
| Depressive symptoms (PHQ-9 ≥ 10) | 1191 (21.2) | 0 (0.0) | 83 (5.6) | 1108 (30.6) |
| HIV Status | ||||
| HIV-negative (HIV-) | 2757 (49.0) | 276 (52.7) | 719 (48.6) | 1762 (48.7) |
| HIV-positive (HIV +) | 2864 (51.0) | 248 (47.3) | 761 (51.4) | 1855 (51.3) |
| HIV-1 RNA viral load ≥ 400 copies/milliliter (among HIV +) | 1522 (53.1) | 120 (48.4) | 406 (53.4) | 996 (53.7) |
| CD4 + T-cell count < 500 cells/microliter3 (among HIV +) | 1954 (68.2) | 161 (64.9) | 520 (68.3) | 1273 (68.6) |
| On antiretroviral therapy (% of HIV +) | 2282 (79.7) | 197 (79.4) | 628 (82.5) | 1457 (78.5) |
AUDIT-C Alcohol Use Disorders Identification Test, BP blood pressure, CVD cardiovascular disease, ICD-9 International Classification of Diseases, Ninth Revision, PHQ-9 Patient Health Questionnaire, % percentage
CVD: acute myocardial infarction, unstable angina, revascularization, ischemic stroke, heart failure, and peripheral artery disease
Diabetes was identified using a previously validated metric that incorporates glucose measurements, antidiabetic agent use, and/or at least 1 inpatient or 2 outpatient ICD-9 codes for diabetes; Hepatitis C virus sero-positivity was defined as a positive hepatitis C virus antibody test result or at least 1 inpatient or 2 outpatient ICD-9 codes for this diagnosis
Fig. 1.
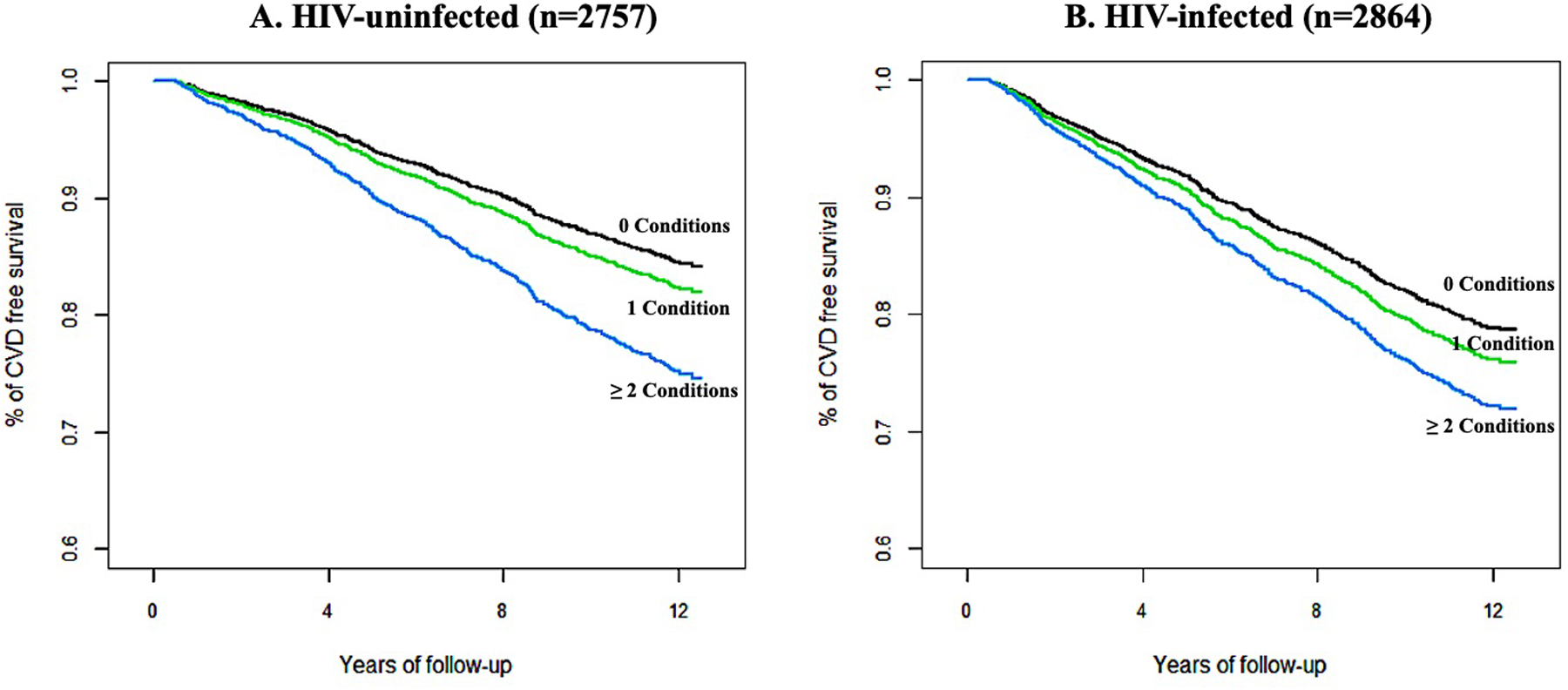
Age-adjusted curves of CVD-free survival by baseline number of syndemic conditions by HIV status. p-value of trend test: < 0.001 (HIV−); < 0.012 (HIV +). Age-adjusted (49.1 years) CVD-free survival among HIV-uninfected. (B) Age-adjusted (49.1 years) CVD-free survival among HIV-infected
Table 2.
Unadjusted cardiovascular disease incidence rates per 1000 person years (95% CI) by number of syndemic behavioral conditions
| Number of conditions | ||||
|---|---|---|---|---|
| Total N = 5621 |
0 524 |
1 1480 |
≥2 3617 |
|
| CVD (column %) | 1149 (20.4) | 77 (14.7) | 285 (19.3) | 787 (21.8) |
| Incidence rates (95% CI) | 24.1 (22.8–25.5) | 15.9 (12.6–19.7) | 21.5 (19.1–24.1) | 26.6 (24.8–28.6) |
| HIV-negative | 22.8 (20.9–24.7) | 13.8 (9.8–18.8) | 19.8 (16.6–23.4) | 25.7 (23.2–28.3) |
| HIV-positive | 25.5 (23.5–27.6) | 18.3 (13.3–24.5) | 23.2 (19.7–27) | 27.7 (25.1–30.4) |
| p-value of test of incidence rate difference | 0.054 | 0.216 | 0.188 | 0.291 |
Conditions refers to unhealthy alcohol use, cigarette smoking, and depressive symptoms
In fully adjusted models (Table 3), compared to HIV-negative people with zero conditions, PWH with the syndemic had 87% higher CVD risk (HR 1.87, 95% CI 1.47, 2.38, p < 0.001), whereas HIV-negative people with the syndemic had 70% greater risk (HR 1.70, 95% CI 1.35, 2.13, p < 0.001). Among those with the syndemic, there was no statistically significant difference in CVD incidence by HIV status (HR 1.10, 95% CI 1.89, 1.37, p = 0.38). In HIV-status stratified models (Table 4), the association between the syndemic and incident CVD among PWH remained after adjustment for baseline HIV viral load and CD4 count (HR 1.37, 95% CI 1.09, 1.72, p = 0.007. After further adjustment for time-updated HIV viral load and CD4 counts, the point estimate remained stable (HR = 1.34 95% CI 1.07, 1.68, p = 0.012).
Table 3.
Hazard ratios (95% CI) for interactive effect of HIV status and syndemic conditions on incident cardiovascular disease
| HIV-uninfected | HIV-infected | Pairwise comparison | |||||
|---|---|---|---|---|---|---|---|
| Number of conditions | HR (95% CI) | p-value | Number of conditions | HR (95% CI) | p-value | HR (95% CI) | p-value |
| HIV-/0 conditions | 1·0 (ref) | – | HIV +/0 conditions | 1.31 (1.05, 1.63) | 0.015 | ||
| HIV-/1 condition | 1.44 (1.17, 1.78) | 0.001 | HIV +/1 condition | 1.66 (1.34, 2.06) | < 0.001 | 1.15 (0.95, 1.39) | 0.160 |
| HIV-/≥ 2 conditions | 1.70 (1.35, 2.13) | < 0.001 | HIV + / ≥ 2 conditions | 1.87 (1.47, 2.38) | < 0.001 | 1.10 (0.89–1.37) | 0.380 |
HIV− HIV-uninfected, HIV + HIV-infected, HR hazard ratio, CI confidence interval, Conditions refer to unhealthy alcohol use, cigarette smoking, and depressive symptoms
Adjusted for age, race/ethnicity, education, cardiovascular disease risk factors (hypertension, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, and obesity), Hepatitis C infection, hemoglobin, eGFR, PTSD, illicit drug use and OUD
Table 4.
Adjusted hazard ratios (95% CI) for association between syndemic conditions and incident cardiovascular disease by HIV status
| Total sample | |||
|---|---|---|---|
| Number of conditions | Age-adjusted incidence rate | HR (95% CI) | p |
| 0 Conditions | 18.2 (16.2, 20.2) | 1·0 (ref) | |
| 1 Condition | 23.6 (21.4, 25.8) | 1.34 (1.16, 1.55) | < 0.001 |
| ≥ 2 Conditions | 25.2 (22.5, 27.8) | 1.55 (1.32, 1.83) | < 0.001 |
| HIV-uninfected | |||
| Number of conditions | Age-adjusted incidence rate | HR (95% CI) | p |
| 0 Conditions | 14.8 (12.3, 17.3) | 1·0 (ref) | |
| 1 Condition | 21.1 (18.1, 24) | 1.48 (1.2, 1.83) | < 0.001 |
| ≥ 2 Conditions | 24.1 (20.6, 27.7) | 1.82 (1.43, 2.33) | < 0.001 |
| HIV-infected | |||||
|---|---|---|---|---|---|
| Number of conditions | Age-adjusted incidence rate | HR (95% CI) | p | HR (95% CI) | p |
| With baseline viral load and CD4 count | With time-updated viral load | ||||
| 0 Conditions | 21.7 (18.5, 24.8) | 1·0 (ref) | 1·0 (ref) | ||
| 1 Condition | 26.0 (22.6, 29.3) | 1.22 (1.00, 1.48) | 0.048 | 1.21 (1.00, 1.48) | 0.054 |
| ≥ 2 Conditions | 26.2 (22.2, 30.1) | 1.37 (1.09, 1.72) | 0.007 | 1.34 (1.07, 1.68) | 0.012 |
CI confidence interval, % percentage, HR hazards ratio, ref reference, Conditions refers to unhealthy alcohol use, cigarette smoking, and depressive symptoms
Incidence rates per 1000 person-years
All models adjusted for baseline age, race/ethnicity, education, cardiovascular disease risk factors (hypertension, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, and obesity), Hepatitis C infection, hemoglobin, eGFR, PTSD, illicit drug use, and OUD. In models with the full sample, HIV status was included for adjustment. In models with only HIV-infected patients, HIV-1 RNA viral load and CD4 + T-cell count were included for adjustment
Discussion
Having at least two of the syndemic conditions (i.e., unhealthy alcohol use, smoking, depressive symptoms) was common (64%) among PWH and HIV-negative veterans. The observed prevalence of having all three conditions was 15%, which exceeded the expected prevalence of 6.9% (6.4% for HIV-negative and 7.5% for PWH), based on individual prevalence of each condition. Having two or more of these conditions was associated with increased risk for incident CVD, regardless of HIV-status, even after adjusting for traditional CVD risk factors and comorbidities.
Prior studies have reported that unhealthy alcohol use [24], cigarette smoking [5, 11, 19, 20, 60], and depression [20] are individually associated with higher CVD risk. The current study reports that 2/3 of VACS participants have at least two of these conditions. These results have important implications for the linked-screening and management of unhealthy alcohol use, smoking, and depression among PWH. In the current era, over 50% of PWH are estimated to be over age 50, and non-AIDS related comorbidities are now the leading causes of mortality among PWH [1, 2]. Even among PWH on treatment with suppressed viremia, there is a 50% excess risk of CVD [5]. While some of this excess risk is attributed to direct effects of the virus on vascular and other tissues [61], it is also likely that the behavioral health factors that comprise this syndemic are under-identified [62–64], under-treated [63, 65–67], and are driving excess risk for CVD. Therefore, a 2-pronged approach to the management of this syndemic should include linked-screening and treatment.
Linked-screening
The US Preventive Services Task Force [68] recommends that clinicians assess all adults aged 18 years and older for unhealthy alcohol use, tobacco use, and depression and provide support to reduce alcohol consumption and tobacco use, and ensure effective diagnosis, treatment, and follow-up to those with depression. Regardless of these recommendations, unhealthy alcohol use, cigarette use, and depression remain under-screened and under-treated among PWH compared to HIV-negative populations [64, 67]. Further, guidelines do not address the simultaneous assessment or management of these conditions. If a patient screens positive for one condition, they will likely screen positive for at least one or both other conditions. Therefore, if screening is not commonplace in practice, then providers may not only be missing one, but potentially all three conditions that have clinical impact on cardiovascular health. Leveraging existing, validated brief screening tools for smoking (one question) [69], unhealthy alcohol use (AUDIT-C, three questions) [44], and depression (PHQ-2, two questions) [70] could make detection of these conditions feasible across general primary care, cardiology, and infectious disease care settings. Further, this process can take place as part of Patient-Reported Outcome Measures [71, 72] programs that many medical centers are adopting.
Treatment
While clinical practice guidelines for addressing unhealthy alcohol use, smoking, and depression [73–75] are available, these conditions are perceived as difficult to effectively treat. Therefore, treatment of these individual conditions is generally low: only 1/3 of smokers making a quit-attempt report using cessation treatments [76]; 24% of those with an alcohol use disorder are ever treated [77]; 37% of those with major depression received related-care within 1-year, with an average delay of eight years from the time of diagnosis [78, 79].
Research supports the combination of behavioral therapy and pharmacotherapy as the most effective treatment for smoking cessation, alcohol use disorder, and depression [80]. Shared biological mechanisms [29, 81, 82] of these conditions support the possibility of parsimonious behavioral and pharmacologic treatment, through cognitive behavior therapy and medications such as varenicline, a partial agonist of the α4β2 nicotinic acetylcholine receptor, which has demonstrated effectiveness for smoking cessation and potential efficacy for treatment of alcohol use in humans [30, 83]. The fact that these conditions are syndemic, are associated with increased risk of CVD, and may be treated parsimoniously is particularly important for PWH and their providers because this clinical population is at great risk for polypharmacy [31]. Models of effective integrated (Screening, Brief Intervention and Referral to Treatment) [84] and/or collaborative care [85–88] exist that have linked cardiology, infectious disease, and general medicine.
The American College of Cardiology has stressed the importance of recognizing tobacco use and implementing evidence-based therapies into cardiovascular medicine [89]. Further, cardiovascular medicine has been recognized as an important setting to screen and treat depression as a modifiable risk factor for CVD [90]. Our findings bolster these recommendations by adding another reason for doing so – smoking, depressive symptoms, and unhealthy alcohol use are often concurrent, and this combination of conditions is associated with increased risk for CVD. Therefore, cardiovascular medicine provides a unique opportunity to implement, at a minimum, linked-screening and ideally treatment in either an integrative or collaborative fashion.
Limitations
The current study has several limitations. First, CVD was based on ICD-9 or CDT codes and was not adjudicated. Therefore, some misclassification may have occurred, likely biasing our results toward the null. Second, as the sample consisted of mostly men, our findings may not be generalizable to women. Related, while the prevalence of smoking and depressive symptoms among PWH is similar to estimates found in other HIV-cohorts, unhealthy alcohol use was markedly higher in this veteran cohort which may not be representative of non-veteran PWH. Third, this study was not powered to model differential magnitude of effect of each specific condition, which was beyond the scope of this analysis. However, this method is similar to scores used in clinical practice that give equal weight to conditions that cluster, including the AHA Life’s Simple 7 [91] and the metabolic syndrome score [92, 93]. Fourth, while illicit drug use (particularly opioid use) is epidemic, the literature linking opioid use and CVD is sparse, with inconsistent findings. We, therefore, chose to focus specifically on the syndemic of unhealthy alcohol use, smoking, and depressive symptoms, each of which is associated with future CVD events. Related, while diagnosis of PTSD is understandably prevalent in this veteran population and relevant to syndemic conditions of interest, the time-varying nature and severity of posttraumatic stress symptoms [94] is not captured in this data. Therefore, we do not include PTSD within the framework of the time-varying syndemic of interest. Since PTSD is associated with unhealthy drinking [95] as well as increased risk for CVD [96], we include ever being diagnosed with PTSD as a potential covariate.
Conclusions
The syndemic of unhealthy alcohol use, cigarette use, and depressive symptoms is common and having two or more of these conditions (syndemic) increased incident CVD risk. This association did not differ significantly by HIV status. Given the high prevalence of this syndemic and the excess risk of CVD, these findings support linked-screening and treatment efforts. Because of the high concurrence of unhealthy alcohol use, smoking, and depression, further research focusing on potential genetic correlates among these conditions is warranted and may provide insight on opportunities for precision medicine. Lastly, this research should be extended to understand disparities in receipt of care among those with syndemic conditions and how disparities may differ by HIV status.
Acknowledgements
We would like to thank the participants of the Veterans Aging Cohort Study.
Funding
This work is supported by The National Heart, Lung, and Blood Institute (K12HL143956 to M.F., N.C.); The National Institute on Alcohol Abuse and Alcoholism (K01AA029042 to N.C.); ViTAL: The Vanderbilt Center for Tobacco, Addiction and Lifestyle (to H.T., M.F., N.C.); V-CREATE: Vanderbilt Clinical Cardiovascular Outcomes Research and Trials Evaluation (to M.F., H.T., N.C.); The Veterans Aging Cohort Study was funded by the National Institute on Alcohol Abuse and Alcoholism U24-AA020794, U01-AA020790, U01-AA02201, and U10-AA013566. The Funders had no involvement in the conduct, collection, analysis, or interpretation of the data, nor the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of Interest The authors have no conflicts of interest to report.
Availability of Data and Material VACS codebooks are available online at https://medicine.yale.edu/intmed/vacs/. Data and code used for the current manuscript is available upon request and approval from the VACS Executive Committee.
Code Availability SAS code pertaining to the current manuscript is available upon request.
Ethical Approval Approval of the Veterans Aging Cohort Study (VACS) was obtained from the institutional review board of the Yale School of Medicine. The following analyses were approved by the institutional review board of Vanderbilt University Medical Center.
Consent to Participate All participants gave written informed consent to participate in the VACS-8 survey cohort.
References
- 1.Eyawo O, Franco-Villalobos C, Hull MW, et al. Changes in mortality rates and causes of death in a population-based cohort of persons living with and without HIV from 1996 to 2012. BMC Infect Dis. 2017;17(1):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farahani M, Mulinder H, Farahani A, Marlink R. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS. 2017;28(7):636–50. [DOI] [PubMed] [Google Scholar]
- 3.Hanna DB, Ramaswamy C, Kaplan RC, et al. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clin Infect Dis. 2016;63(8):1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith CJ, Ryom L, Weber R, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384(9939):241–8. [DOI] [PubMed] [Google Scholar]
- 5.Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverberg MJ, Leyden WA, Xu L, et al. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65(2):160–6. [DOI] [PubMed] [Google Scholar]
- 7.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171(8):737–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus JL, Leyden WA, Chao CR, et al. HIV infection and incidence of ischemic stroke. AIDS. 2014;28(13):1911–9. [DOI] [PubMed] [Google Scholar]
- 9.Sico JJ, Chang CC, So-Armah K, et al. HIV status and the risk of ischemic stroke among men. Neurology. 2015;84(19):1933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckman JA, Duncan MS, Alcorn CW, et al. Association of human immunodeficiency virus infection and risk of peripheral artery disease. Circulation. 2018;138(3):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freiberg MS, Chang CC, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4(4):425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerrato E, D’Ascenzo F, Biondi-Zoccai G, et al. Cardiac dysfunction in pauci symptomatic human immunodeficiency virus patients: a meta-analysis in the highly active antiretroviral therapy era. Eur Heart J. 2013;34(19):1432–6. [DOI] [PubMed] [Google Scholar]
- 13.Longenecker CT, Funderburg NT, Jiang Y, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14(6):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chichetto NE, Polanka BM, So-Armah KA, et al. Contribution of behavioral health factors to non-AIDS-related comorbidities: an updated review. Curr HIV/AIDS Rep. 2020;17(4):354–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braithwaite RS, Fang Y, Tate J, et al. Do alcohol misuse, smoking, and depression vary concordantly or sequentially? A longitudinal study of HIV-infected and matched uninfected veterans in care. AIDS Behav. 2016;20(3):566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med. 2017;376(4):342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regan S, Meigs JB, Grinspoon SK, Triant VA. Determinants of smoking and quitting in HIV-infected individuals. PLoS ONE. 2016;11(4):e0153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Center for Disease Control and Prevention. Current cigarette smoking among adults in the United States. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/.Accessed2 June 2020.
- 19.Khambaty T, Stewart JC, Gupta SK, et al. Association between depressive disorders and incident acute myocardial infarction in human immunodeficiency virus-infected adults: Veterans aging cohort study. JAMA Cardiol. 2016;1(8):929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White JR, Chang CC, So-Armah KA, et al. Depression and human immunodeficiency virus infection are risk factors for incident heart failure among veterans: Veterans Aging Cohort Study. Circulation. 2015;132(17):1630–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deiss RG, Mesner O, Agan BK, et al. Characterizing the association between alcohol and HIV virologic failure in a military cohort on antiretroviral therapy. Alcohol Clin Exp Res. 2016;40(3):529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kader R, Seedat S, Govender R, Koch JR, Parry CD. Hazardous and harmful use of alcohol and/or other drugs and health status among South African patients attending HIV clinics. AIDS Behav. 2014;18(3):525–34. [DOI] [PubMed] [Google Scholar]
- 23.Monroe AK, Lau B, Mugavero MJ, et al. Heavy alcohol use is associated with worse retention in HIV care. J Acquir Immune Defic Syndr. 2016;73(4):419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freiberg MS, McGinnis KA, Kraemer K, et al. The association between alcohol consumption and prevalent cardiovascular diseases among HIV-infected and HIV-uninfected men. J Acquir Immune Defic Syndr. 2010;53(2):247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–30. [DOI] [PubMed] [Google Scholar]
- 26.Tsai AC. Reliability and validity of depression assessment among persons with HIV in sub-Saharan Africa: systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2014;66(5):503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–8. [DOI] [PubMed] [Google Scholar]
- 28.Lancet T Syndemics: health in context. Lancet. 2017;389(10072):881. [DOI] [PubMed] [Google Scholar]
- 29.Bickel WK, Moody L, Quisenberry AJ, Ramey CT, Sheffer CE. A Competing Neurobehavioral Decision Systems model of SES-related health and behavioral disparities. Prev Med. 2014;68:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litten RZ, Ryan ML, Fertig JB, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7(4):277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Justice AC, Gordon KS, Skanderson M, et al. Nonantiretroviral polypharmacy and adverse health outcomes among HIV-infected and uninfected individuals. AIDS. 2018;32(6):739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chichetto NE, Kundu S, Freiberg MS, et al. Association of syndemic unhealthy alcohol use, cigarette use, and depression with all-cause mortality among adults living with and without hiv infection: veterans aging cohort study. Open Forum Infect Dis. 2019;6(6):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Justice AC, Dombrowski E, Conigliaro J, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care. 2006;44(8 Suppl 2):S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: a systematic review. PLoS ONE. 2014;9(3):e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of Diagnostic Codes for Acute Stroke in Administrative Databases: A Systematic Review. PLoS ONE. 2015;10(8):e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PLoS ONE. 2014;9(8):e104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varas-Lorenzo C, Castellsague J, Stang MR, Tomas L, Aguado J, Perez-Gutthann S. Positive predictive value of ICD-9 codes 410 and 411 in the identification of cases of acute coronary syndromes in the Saskatchewan Hospital automated database. Pharmacoepidemiol Drug Saf. 2008;17(8):842–52. [DOI] [PubMed] [Google Scholar]
- 38.Bali V, Yermilov I, Coutts K, Legorreta AP. Novel screening metric for the identification of at-risk peripheral artery disease patients using administrative claims data. Vasc Med. 2016;21(1):33–40. [DOI] [PubMed] [Google Scholar]
- 39.Davis LA, Mann A, Cannon GW, Mikuls TR, Reimold AM, Caplan L. Validation of diagnostic and procedural codes for identification of acute cardiovascular events in US veterans with rheumatoid arthritis. EGEMS (Wash DC). 2013;1(3):1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;2(8623):1267–73. [DOI] [PubMed] [Google Scholar]
- 41.Mukamal KJ, Rimm EB. Alcohol’s effects on the risk for coronary heart disease. Alcohol Res Health. 2001;25(4):255–61. [PMC free article] [PubMed] [Google Scholar]
- 42.Saunders JB, Aasland OG, Amundsen A, Grant M. Alcohol consumption and related problems among primary health care patients: WHO collaborative project on early detection of persons with harmful alcohol consumption–I. Addiction. 1993;88(3):349–62. [DOI] [PubMed] [Google Scholar]
- 43.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–95. [DOI] [PubMed] [Google Scholar]
- 44.Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–17. [DOI] [PubMed] [Google Scholar]
- 45.Aertgeerts B, Buntinx F, Ansoms S, Fevery J. Screening properties of questionnaires and laboratory tests for the detection of alcohol abuse or dependence in a general practice population. Br J Gen Pract. 2001;51(464):206–17. [PMC free article] [PubMed] [Google Scholar]
- 46.McGinnis KA, Tate JP, Williams EC, et al. Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend. 2016;168:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Connor EA, Perdue LA, Senger CA, et al. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: updated evidence report and systemeatic review for the US Preventive Services Task Force. JAMA. 2018;320(18):1910–28. [DOI] [PubMed] [Google Scholar]
- 48.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Test. Guidelines for Use in Primary Care. Second ed.World Health Organization, Department of Mental Health and Substance Dependence. [Google Scholar]
- 49.McGinnis KA, Justice AC, Kraemer KL, Saitz R, Bryant KJ, Fiellin DA. Comparing alcohol screening measures among HIV-infected and -uninfected men. Alcohol Clin Exp Res. 2013;37(3):435–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marshall BD, Operario D, Bryant KJ, et al. Drinking trajectories among HIV-infected men who have sex with men: a cohort study of United States veterans. Drug Alcohol Depend. 2015;148:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monahan PO, Shacham E, Reece M, et al. Validity/reliability of PHQ-9 and PHQ-2 depression scales among adults living with HIV/AIDS in western Kenya. J Gen Intern Med. 2009;24(2):189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Butt AA, Fultz SL, Kwoh CK, Kelley D, Skanderson M, Justice AC. Risk of diabetes in HIV infected veterans pre- and post-HAART and the role of HCV coinfection. Hepatology. 2004;40(1):115–9. [DOI] [PubMed] [Google Scholar]
- 54.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2018;138(17):e426–83. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. Overweight & Obesity: Defining Adult Obesity 2020; https://www.cdc.gov/obesity/adult/defining.html.Accessed8 Jan 2021. [Google Scholar]
- 56.Blanc B, Finch CA, Hallberg L, et al. Nutritional anaemias. Report of a WHO Scientific Group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 57.National Kidney Foundation. Estimated Glomerular Filtration Rate (eGFR). 2020; https://www.kidney.org/atoz/content/gfr.Accessed8 Jan 2021.
- 58.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–82. [DOI] [PubMed] [Google Scholar]
- 59.Cooke CR, Joo MJ, Anderson SM, et al. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raposeiras-Roubin S, Abu-Assi E, Iniguez-Romo A. Tobacco, illicit drugs use and risk of cardiovascular disease in patients living with HIV. Curr Opin HIV AIDS. 2017;12(6):523–7. [DOI] [PubMed] [Google Scholar]
- 61.Tawakol A, Ishai A, Li D, et al. Association of arterial and lymph node inflammation with distinct inflammatory pathways in human immunodeficiency virus infection. JAMA Cardiol. 2017;2(2):163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asch SM, Kilbourne AM, Gifford AL, et al. Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med. 2003;18(6):450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chander G, Monroe AK, Crane HM, et al. HIV primary care providers–Screening, knowledge, attitudes and behaviors related to alcohol interventions. Drug Alcohol Depend. 2016;161:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. Decreased awareness of current smoking among health care providers of HIV-positive compared to HIV-negative veterans. J Gen Intern Med. 2007;22(6):749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shuter J, Salmo LN, Shuter AD, Nivasch EC, Fazzari M, Moadel AB. Provider beliefs and practices relating to tobacco use in patients living with HIV/AIDS: a national survey. AIDS Behav. 2012;16(2):288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weaver MR, Conover CJ, Proescholdbell RJ, et al. Utilization of mental health and substance abuse care for people living with HIV/AIDS, chronic mental illness, and substance abuse disorders. J Acquir Immune Defic Syndr. 2008;47(4):449–58. [DOI] [PubMed] [Google Scholar]
- 67.Williams EC, Lapham GT, Shortreed SM, et al. Among patients with unhealthy alcohol use, those with HIV are less likely than those without to receive evidence-based alcohol-related care: A national VA study. Drug Alcohol Depend. 2017;174:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.U.S. Preventive Services Task Force. https://www.uspreventiveservicestaskforce.org/Page/Name/home.AccessedJune 1, 2020.
- 69.Center for Disease Control and Prevention. Tobacco questions for surveys: A subset of key questions from the Global Adult Tobacco Survey (GATS). Atlanta, GA: Center for Disease Control and Prevention; 2011. [Google Scholar]
- 70.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–92. [DOI] [PubMed] [Google Scholar]
- 71.Engler K, Lessard D, Lebouche B. A review of HIV-specific patient-reported outcome measures. Patient. 2017;10(2):187–202. [DOI] [PubMed] [Google Scholar]
- 72.Blumenthal DM, Strom JB, Valsdottir LR, et al. Patient-reported outcomes in cardiology. Circ Cardiovasc Qual Outcomes. 2018;11(11):e004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice Guidelines for the Treatment of Patients with Major Depressive Disorder. Third ed: American Psychiatric Association; 2010. [Google Scholar]
- 74.Reus VI, Fochtmann LJ, Bukstein O, et al. The American psychiatric association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Focus (Am Psychiatr Publ). 2019;17(2):158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fiore MC. US public health service clinical practice guideline: treating tobacco use and dependence. Respir Care. 2000;45(10):1200–62. [PubMed] [Google Scholar]
- 76.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102–11. [DOI] [PubMed] [Google Scholar]
- 77.Algur Y, Elliott JC, Aharonovich E, Hasin DS. A cross-sectional study of depressive symptoms and risky alcohol use behaviors among HIV primary care patients in New York city. AIDS Behav. 2018;22(5):1423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pence BW, O’Donnell JK, Gaynes BN. The depression treatment cascade in primary care: a public health perspective. Curr Psychiatry Rep. 2012;14(4):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang PS, Berglund P, Olfson M, Pincus HA, Wells KB, Kessler RC. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):603–13. [DOI] [PubMed] [Google Scholar]
- 80.Ait-Daoud N, Lynch WJ, Penberthy JK, Breland AB, Marzani-Nissen GR, Johnson BA. Treating smoking dependence in depressed alcoholics. Alcohol Res Health. 2006;29(3):213–20. [PMC free article] [PubMed] [Google Scholar]
- 81.Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koob GF. Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry. 2013;4:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Malley SS, Zweben A, Fucito LM, et al. Effect of varenicline combined with medical management on alcohol use disorder with comorbid cigarette smoking: A randomized clinical trial. JAMA Psychiat. 2018;75(2):129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hargraves D, White C, Frederick R, et al. Implementing SBIRT (Screening, Brief Intervention and Referral to Treatment) in primary care: lessons learned from a multi-practice evaluation portfolio. Public Health Rev. 2017;38:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huffman JC, Mastromauro CA, Beach SR, et al. Collaborative care for depression and anxiety disorders in patients with recent cardiac events: the Management of Sadness and Anxiety in Cardiology (MOSAIC) randomized clinical trial. JAMA Intern Med. 2014;174(6):927–35. [DOI] [PubMed] [Google Scholar]
- 86.Rollman BL, Belnap BH, LeMenager MS, et al. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA. 2009;302(19):2095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chuah FLH, Haldane VE, Cervero-Liceras F, et al. Interventions and approaches to integrating HIV and mental health services: a systematic review. Health Policy Plan. 2017;32:iv27–iv47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rollman BL, Herbeck Belnap B, Abebe KZ, et al. Effectiveness of online collaborative care for treating mood and anxiety disorders in primary care: A randomized clinical trial. JAMA Psychiat. 2018;75(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: A report of the american college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol. 2018;72(25):3332–65. [DOI] [PubMed] [Google Scholar]
- 90.Borlaug BA. Moving beyond cardio-centricity in heart failure risk stratification. Circulation. 2015;132(17):1602–3. [DOI] [PubMed] [Google Scholar]
- 91.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. [DOI] [PubMed] [Google Scholar]
- 92.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–31. [DOI] [PubMed] [Google Scholar]
- 93.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28(2):385–90. [DOI] [PubMed] [Google Scholar]
- 94.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). 5 ed.Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 95.Debell F, Fear NT, Head M, et al. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol. 2014;49(9):1401–25. [DOI] [PubMed] [Google Scholar]
- 96.Edmondson D, von Kanel R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry. 2017;4(4):320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


