Abstract
Purpose:
Very preterm (VPT) infants are at high risk for motor and behavioral deficits. We investigated microstructural differences using diffusion tensor imaging (DTI) among VPT infants with different grades of intraventricular hemorrhage (IVH), their association with early motor function and temperament ratings, and the potential moderating effect of IVH severity on the above structure-function relations.
Methods:
Fifty-seven VPT (≤32 weeks gestational age) infants with IVH [Low Grade (Papile grading I/II): 42; High Grade (III/IV): 15] were studied. DTI was acquired between 39-44 weeks postmenstrual age and was analyzed using the tract-based spatial statistics approach. Early motor function and temperament were assessed at 3-month corrected age based on the Hammersmith Infant Neurological Examination (HINE) and Infant Behavioral Questionnaire – Revised, Short Version (IBQ-R-S), respectively.
Results:
Significantly lower fractional anisotropy and higher mean, axial, and/or radial diffusivity were found in VPT infants with High Grade IVH compared to Low Grade IVH (p<0.05). Significant associations were found between DTI metrics and motor function in both IVH groups and between DTI and Fear temperament ratings in the High Grade IVH Group (all p<0.05). IVH severity had a significant moderating effect on the relation between DTI and motor and Fear ratings (p<0.05).
Conclusion:
DTI is a sensitive neuroimaging biomarker providing a refined understanding of the impact and location of differing severities of IVH on the developing white matter of VPT infants. Early motor and behavioral outcomes are associated with microstructural changes that are influenced by severity of IVH.
Introduction
Infants born very preterm (VPT; ≤32 weeks gestational age) are at higher risk for motor, cognitive, social, and behavioral impairments as compared with infants born at term [1–4]. Currently, diagnosis of such impairments is delayed on average until 2 to 3 years of age. There is wide consensus that earlier diagnosis, soon after birth, is urgently needed in order to address identified deficits during the critical windows of early brain development [5,6]. Unfortunately, we still lack a validated early, accurate prognostic models of neurodevelopmental impairments.
Intraventricular hemorrhage (IVH) is a common neuropathological diagnosis in infants born VPT. Research has shown that infants born prematurely with a perinatal diagnosis of IVH exhibited abnormal white matter diffusion properties on diffusion tensor imaging (DTI) [e.g., lower fractional anisotropy (FA) or higher mean diffusivity (MD), etc., compared to controls] accompanied by delayed neurodevelopment [7–9]. DTI is an advanced MRI technique that uses diffusion properties of water molecules as probes to examine tissue structure, revealing characteristics of microscopic organization. Diffusion properties are strongly influenced by the micro-structural components of white matter and provide an indication of the integrity of these structures [10,11]. DTI has been used as a sensitive and non-invasive imaging biomarker to quantify white matter microstructural changes in preterm infants. Diffusion MRI microstructural measures have shown promise as early biomarkers of motor and cognitive impairments in very preterm infants [12–15].
Currently, there is a knowledge gap regarding the predictive value of DTI to identify early biomarkers of neurodevelopmental impairments in preterm infants with different levels of IVH severity. Several studies included preterm infants with low IVH severity (Grade I&II) and showed significant DTI abnormalities [lower FA and/or higher MD and Radial Diffusivity (RD)] when compared to controls [7,9] or a significant correlation between DTI and structural abnormalities [16] in the infants. Other studies included both low grade and high grade IVH patients and showed similar results based on combined data [8,17]. Tam et al. (2009) was the only study identified that reported the results derived from DTI by separate IVH severity groups [18]. They reported a significant association with abnormal DTI in severe IVH with small sample size (n=4) but not in the mild IVH group (n=11). No studies have investigated whether abnormal DTI metrics in infants born VPT with different IVH severities are associated with motor function and/or early temperament ratings (i.e., individual differences in reactivity and self-regulation that serve as a precursor to cognitive and social development) [19], or whether the association between neuroimaging and these neurodevelopmental outcomes is affected by IVH severity.
In the present study, we aimed to investigate microstructural differences in the white matter based on DTI in VPT infants with different IVH severities, their associations with early motor function and temperament ratings, and the potential moderating effect of IVH severity on these association. We hypothesized that (1) diffusion microstructural parameters derived from DTI at term-equivalent age in infants born VPT with mild IVH (low-grade IVH, including Grade I&II) will differ significantly from those with severe IVH (high-grade IVH, including Grade III&IV); (2) DTI indices assessed at term-equivalent age will be associated with motor function and temperament ratings at 3-months corrected age in both low-grade and high-grade IVH patients; (3) there will be a significant interaction between IVH severity and the association between the DTI metrics and motor scores and temperament ratings.
Methods
Participants
All structural and diffusion MR imaging data were prospectively acquired as part of a large multi-center longitudinal neuroimaging study of infants born VPT (hereinafter referred to as the parent study). A total of 395 infants born at or before 32 weeks gestational age (GA) were enrolled between September 2016 and November 2019 in the parent study. Infants were excluded if they met any of the following criteria: 1) known chromosomal or congenital anomalies affecting the central nervous system; 2) cyanotic heart disease; or 3) hospitalization and mechanical ventilation with greater than 50% supplemental oxygen at 45 weeks postmenstrual age (PMA). Five level III/IV academic or community NICUs involved in the study include four NICUs in Cincinnati, Ohio [Cincinnati Children’s Hospital Medical Center (CCHMC), University of Cincinnati Medical Center, Good Samaritan Hospital, and St. Elizabeth’s Healthcare] and one NICU in Dayton, Ohio (Kettering Medical Center). The CCHMC Institutional Review Board approved the study, and the review boards of the other hospitals approved the study based on a reliance agreement. The caregivers of all infants provided written informed consent. Additional information about the parent cohort have been reported elsewhere [20,21].
Of the 395 infants born prematurely that enrolled in the parent study, a total of 72 patients with IVH were identified using a hybrid classification system based on the Papile et al. criteria [22] and Volpe’s system. Consistent with the American Academy of Pediatrics, all of our NICUs used a guideline that recommends a cranial ultrasound at 7 to 10 days after birth or before if needed, in VPT. Radiologists readings were coded as Grade I IVH when there was germinal matrix and/or subependymal hemorrhage, as Grade II when there was IVH without ventricular dilation, as Grade III when there was IVH with acute ventricular dilation, and as Grade IV when there was IVH with parenchymal hemorrhage/infarct. Fifteen of the 72 patients were excluded from the present study (2 for missing DTI scans, 5 for using an alternate DTI protocol, and 8 for poor image quality and/or having severe ventriculomegaly causing misregistration). The remaining 57 patients were categorized to the Low Grade IVH group (34 with Grade I IVH; 8 with Grade II IVH) or High Grade IVH group (7 with Grade III IVH; 8 with Grade IV IVH).
Procedures
In the larger prospective cohort study from which the data in the current study were derived, caregivers were approached in the NICU. If the infant met inclusion criteria and caregiver consented, an MRI was scheduled at 41±1 weeks PMA. All cranial ultrasound scan readings for infants were acquired from the NICU records of the five participating institutions. Based on these readings of cranial ultrasound images, patients with IVH were identified as described above. At 3-months corrected age, the Hammersmith Infant Neurological Examination (HINE) and Prechtl’s General Movements Assessment (GMA) were administered by a single trained occupational therapist who was blinded to clinical history and neuroimaging findings. Caregivers also completed the Infant Behavior Questionnaire-Revised, Short Version (IBQ-R-S).
Measures
The quantitative measures reported in the present study include motor data based on the HINE, and temperament ratings on the IBQ-R-S.
Hammersmith Infant Neurological Examination (HINE) [23] –
The HINE is an infant neurological exam that assesses various neurological functions and domains including cranial nerve function, posture, movements, muscle tone, reflexes and reactions. The HINE score ranges from 0-78 with cut off scores <56 at three months of age considered abnormal [23]. Asymmetries are noted between the sides of the body to further describe unilateral motor abnormalities [24,25]. In the present study, the HINE was administered at 3-months corrected age to evaluate early motor function. One patient in the Low IVH Grade group (Grade I) and one patient in the High IVH Grade group (Grade IV) did not return for HINE data testing. Therefore, analyses with this measure were based on data from 55 patients (41 in the Low IVH Grade group, 14 in the High IVH Grade group).
Infant Behavioral Questionnaire – Revised, Short Version (IBQ-R-S) –
The IBQ-R-S is a parent-report measure used to assess temperamental characteristics in infants between 3 and 12 months of age [26]. Caregivers respond to 91 questions about their children’s typical behavior on a 7-point Likert-type scale (1 = never, 4 = about half the time, and 7 = always). The IBQ-R-S yields 14 subscales: Activity, Smiling and Laughing), High Intensity Pleasure, Perceptual Sensitivity, Approach , Vocal Reactivity, Distress, Fear, Falling Reactivity, Sadness, Soothability, Duration of Orienting, Low Intensity Pleasure, and Cuddliness. Reliability and validity of the IBQ-R-S subscales have been supported for samples from different cultures, with Cronbach’s alphas ranging from 0.77 to 0.96 [27,28]. Inter-rater reliability has been demonstrated for mother and father-report [27,29], with validity of this instrument supported by studies incorporating the IBQ-R and laboratory indicators of temperament [30–32,29]. IBQ-R-S data were acquired from caregivers of 50 patients (37 in the Low Grade IVH group, 13 in the High Grade IVH group) at the 3-month corrected age visit; 7 parents did not complete the IBQ-R-S).
MRI/DTI Data Acquisition, Processing, and Analysis
All MRI data were acquired on a 3T Phillips Ingenia scanner with 32-channel phased array head coil. DTI data were acquired using a 36-direction spin echo EPI sequence with the following specifications: TR/TE = 6972/88 msec; field of view = 160x160 mm; in-plane resolution = 2 mm x 2 mm; slice thickness = 2 mm; number of slices = 54; number of average = 1; diffusion weighting factor b-value = 800 s/mm2. Four frames of images without diffusion weighting (b0) were acquired. A high-resolution 3D T2-weighted anatomical data set (voxel size = 1 x 1 x 1 mm) was acquired in the axial direction for image registration and review.
All MR imaging data preprocessing and analysis were performed using the FMRIB Software Library (FSL, www.fmrib.ox.ac.uk/fsl). Skull stripping was performed using the brain extraction tool (BET) function. Eddy current and head motion artifact were corrected in FSL by aligning diffusion weighed images to the first b0 image with an affine DTI transformation with 12 degrees of freedom. The four commonly used DTI measures, including FA, MD, Axial Diffusivity (AD), and RD, were calculated using standard methods [33].
The tract-based spatial statistics (TBSS) approach was used for the group level analyses [34]. TBSS is a method developed to ameliorate the registration error at the boundary of narrow white matter fiber bundles, a common source of error in voxel-based style analyses. We followed standard TBSS analysis steps and applied a skeleton threshold of 0.15. Threshold-free cluster enhancements (TFCE) [35] with 5000 permutations were used to correct for multiple comparisons. Anatomical locations of white matter structures with significant findings from the statistical analysis were localized using the John Hopkins University ICBM-DTI-81 white matter labels atlas [36]. In all the imaging analysis, PMA at MRI and infant sex were included as covariates.
The TBSS approach was used to analyze the between-group DTI differences and assess the association between DTI metrics measured at term-equivalent age and HINE scores and IBQ-R-S ratings at 3 months corrected age. The approach was also used to explore the potential moderating effect of IVH severity on the association between DTI metrics and HINE and IBQ-R-S scores. To assess the moderating effect, DTI data were used as continuous predictor variables in a general linear model with IVH group included as an independent variable (Low Grade vs. High Grade) to evaluate their potentially unique interactive effect with each of the outcome measures (HINE and IBQ-R-S). For IBQ-R-S, due to the large number of subscales, the analysis of the within group correlations and the interaction effects were only performed for those subscales with significant (p<0.05) or marginally significant (p<0.10) group differences (i.e., High vs Low IVH).
The descriptive statistics and the subsequent statistical analysis of group differences and regression analyses were all performed using SPSS software. The group difference of GA, PMA at MRI, and fronto-occipital horn ratio (FOHR) were tested using the Mann-Whitney U test. The group difference of sex was assessed using the Fisher’s exact test.
Results
Participants
Among the 57 patients with IVH, 30 were females. These patients were born between 24.00 to 32.86 weeks (Median = 28.29 weeks; Mean ± SD = 28.29 ± 2.53 weeks) GA. MRI data were acquired between 39.57 and 44.71 weeks of PMA (Median = 43.14 weeks; Mean ± SD = 42.88 ± 2.53 weeks). The ventricle size based on FOHR ranged from 0.30 to 0.44 (Median = 0.37; Mean ± SD = 0.37 ± 0.03).
Forty two of the 57 patients were in the Low Grade IVH group (Grade I or II) and 15 of the 57 patients were in the High Grade IVH group (Grade III or IV). The demographic characteristics of the patients in the two groups are presented in Table 1. No significant group differences in GA, age at MRI, or sex ratio was found between the two groups (Table 1). The FOHR in the High Grade IVH group was significantly higher than that in the Low Grade IVH group (p<0.05, Table 1). The information about the number of patients (and percentage in each group) with sepsis, necrotizing enterocolitis, Bronchopulmonary dysplasia, retinopathy of prematurity, and white matter injury are also included in Table 1.
Table 1.
Demographics by group
| Low IVH Grade (n=42) | High IVH Grade (n=15) | Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Median | Mean | SD | Range | Median | Mean | SD | Range | U/χ2 | p | ||
| GA (weeks) | 27.71 | 28.40 | 2.56 | 24.00-32.71 | 27.71 | 27.99 | 2.51 | 24.43-32.86 | 346 | 0.580 | |
| PMA at MRI (weeks) | 43.29 | 42.83 | 1.32 | 39.57-44.57 | 43.29 | 43.05 | 1.26 | 41.00-44.71 | 288 | 0.624 | |
| Sex | F/M=19/23 | F/M=11/4 | 2.46 | 0.117 | |||||||
| FOHR | 0.39 | 0.36 | 0.03 | 0.30-0.44 | 0.39 | 0.39 | 0.03 | 0.34-0.44 | 183 | 0.017 | |
| Sepsis, n(%) | 3 (7.1%) | 4 (26.7%) | NA | 0.070 | |||||||
| Necrotizing enterocolitis, n(%) | 6 (14.3%) | 3 (20.0%) | NA | 0.685 | |||||||
| Bronchopulmonary dysplasia, n(%) | 26 (61.9%) | 12 (80.0%) | NA | 0.339 | |||||||
| Retinopathy of prematurity, n(%) | 18 (42.9%) | 9 (60.0%) | 1.31 | 0.254 | |||||||
| White matter injury, n(%) | 2 (4.8%) | 2 (13.3%) | NA | 0.281 | |||||||
Note: GA = Gestational Age; PMA = Post-menstrual age; FOHR = Fronto-occipital horn ratio; IVH = intraventricular hemorrhage; NA = not applicable because calculated using Fisher’s exact test
Of the 57 infants with IVH, seven had cystic periventricular leukomalacia (all independent of IVH), one had cerebellar injury/atrophy, one had punctate white matter lesions. We also derived a global brain score using the Kidokoro et al’s scoring system [37]. The median (IQR) score for these 57 infants was 3.5 (0-11). Detailed information about the scan reading have been reported elsewhere [21]. Ten of the 15 infants with severe IVH had PHVD; none of these infants required neurosurgical intervention.
Motor Function and Temperament Ratings
HINE:
As shown in Table 2, the mean HINE score in the High Grade IVH group was lower than the score in the Low Grade IVH group but the group difference did not reach statistical significance (Mann-Whitney U test, U=197, p=0.112).
Table 2.
Motor function and temperament ratings
| Low IVH Grade | High IVH Grade | Statistics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| n | Median | Mean | SD | Range | n | Median | Mean | SD | Range | U | p | ||
| HINE | 41 | 58.50 | 58.88 | 4.90 | 47.00-67.00 | 14 | 57.00 | 54.14 | 8.91 | 36.00-67.00 | 197 | 0.112 | |
| IBQ-R-S | |||||||||||||
| Activity | 37 | 4.14 | 4.15 | 1.08 | 1.57-6.60 | 13 | 3.83 | 3.75 | 1.17 | 2.14-5.14 | 202 | 0.388 | |
| Distress | 37 | 4.00 | 4.10 | 1.02 | 2.14-6.00 | 13 | 3.50 | 3.63 | 1.13 | 1.29-5.57 | 180 | 0.180 | |
| Fear | 36 | 3.00 | 3.32 | 1.37 | 1.33-6.50 | 13 | 2.33 | 2.32 | 0.85 | 1.00-4.00 | 135 | 0.024 | |
| DUR | 37 | 4.00 | 4.05 | 1.34 | 1.33-7.00 | 13 | 3.83 | 3.67 | 1.74 | 1.00-6.60 | 205 | 0.425 | |
| SMIL | 37 | 4.60 | 4.48 | 1.25 | 1.86-7.00 | 13 | 3.50 | 3.56 | 1.64 | 1.00-6.00 | 158 | 0.066 | |
| HIP | 37 | 4.86 | 4.98 | 1.23 | 2.33-7.00 | 13 | 4.60 | 4.53 | 1.38 | 1.00-6.29 | 200 | 0.364 | |
| LIP | 37 | 6.00 | 5.67 | 1.04 | 2.50-7.00 | 13 | 5.29 | 4.91 | 1.29 | 2.80-6.57 | 156 | 0.060 | |
| Soothability | 37 | 5.43 | 5.37 | 0.90 | 3.43-7.00 | 13 | 5.29 | 5.23 | 0.76 | 3.43-6.57 | 209 | 0.477 | |
| FALL | 37 | 5.40 | 5.19 | 0.89 | 3.50-6.75 | 13 | 5.00 | 5.00 | 0.87 | 3.67-6.00 | 212 | 0.527 | |
| Cuddliness | 37 | 6.00 | 5.92 | 0.78 | 4.00-7.00 | 13 | 6.60 | 6.34 | 0.59 | 5.33-7.00 | 162 | 0.080 | |
| PERC | 34 | 4.00 | 4.24 | 1.35 | 1.17-7.00 | 13 | 1.50 | 2.59 | 1.78 | 1.00-6.00 | 101 | 0.004 | |
| Sadness | 37 | 3.50 | 3.57 | 1.08 | 1.00-5.33 | 13 | 2.83 | 2.88 | 1.04 | 1.00-4.33 | 157 | 0.064 | |
| Approach | 36 | 4.55 | 4.37 | 1.38 | 1.00-7.00 | 13 | 3.50 | 3.51 | 1.56 | 1.00-6.00 | 157 | 0.081 | |
| VOC | 37 | 5.00 | 4.90 | 1.15 | 2.17-7.00 | 13 | 3.83 | 3.87 | 1.53 | 1.00-6.00 | 142 | 0.028 | |
Note: HINE = Hammersmith Infant Neurological Examination; IBQ-R-S = Infant Behavior Questionnaire – Revised, Short Version; DUR = Duration of Orienting; SMIL = Smiling and Laughing; HIP = High Intensity Pleasure; LIP = Low Intensity Pleasure; FALL = Falling Reactivity; PERC = Perceptual Sensitivity; VOC = Vocal Reactivity
IBQ-R-S:
The High Grade IVH Group scored significantly lower than the Low Grade Group on 3 out of the 14 subscales (Table 2; Fear, p=0.024; Perceptual Sensitivity, p=0.004; Vocal Reactivity, p=0.028). Findings were marginally significant with the High Grade IVH group scoring lower than the Low Grade group for five subscales (Smiling and Laughter, Low Intensity Pleasure, Cuddliness, Sadness, and Approach; all p<0.1, Table 2). No trend of difference (at p<0.1 level) was found for the other 8 subscales.
White Matter Regions with Significant DTI Differences between IVH Severity Levels
As shown in Figure 1, after controlling for age at MRI and sex, participants in the High Grade IVH group were found to have significantly lower FA (Figure 1A), higher MD (Figure 1B), AD (Figure 1C) and/or RD (Figure 1D) than participants in the Low Grade IVH group in multiple white matter areas (all p<0.05, TFCE corrected). The white matter regions with significant DTI group differences were located in the genu, body, and splenium of corpus callosum, anterior, superior, and posterior corona radiata, thalamic radiation, and cingulum (Figure 1, A–D).
Figure 1.
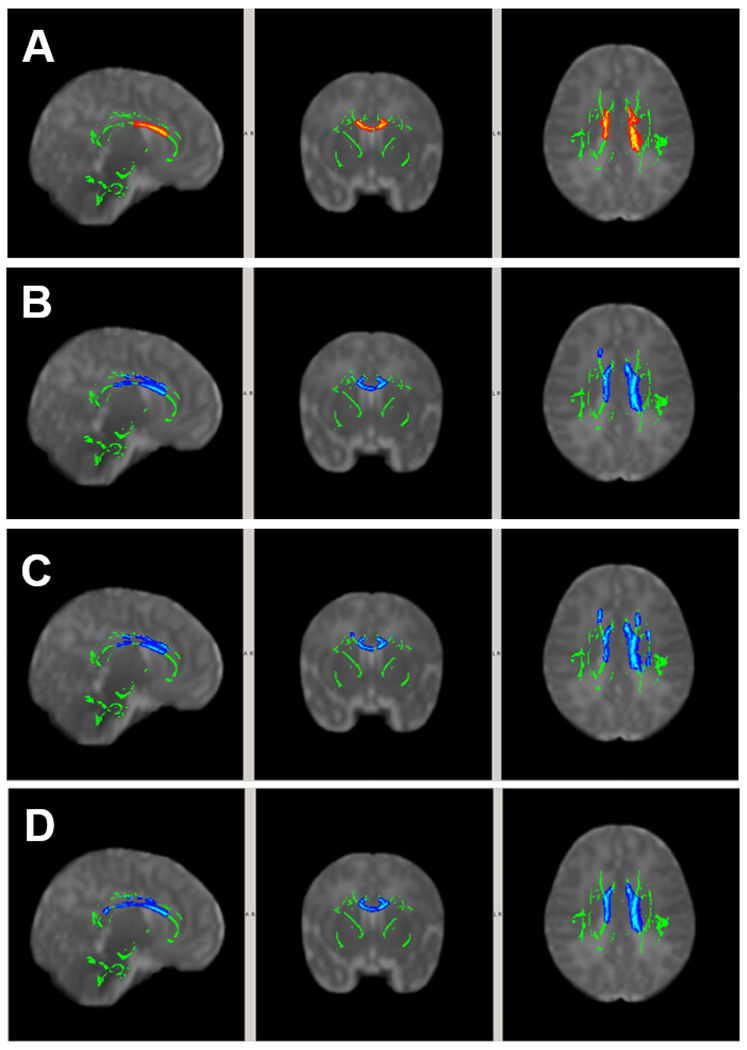
White matter areas with significant group difference in DTI (p<0.05, TFCE corrected). Covariates included postmenstrual age at MRI and sex. (A) FALow Grade > FAHigh Grade; (B) MDLow Grade < MDHigh Grade; (C) ADLow Grade < ADHigh Grade; and (D) RDLow Grade < RDHigh Grade.
The size of the areas with significant group DTI difference for individual anatomical regions can be found in the supplemental materials (Table S1).
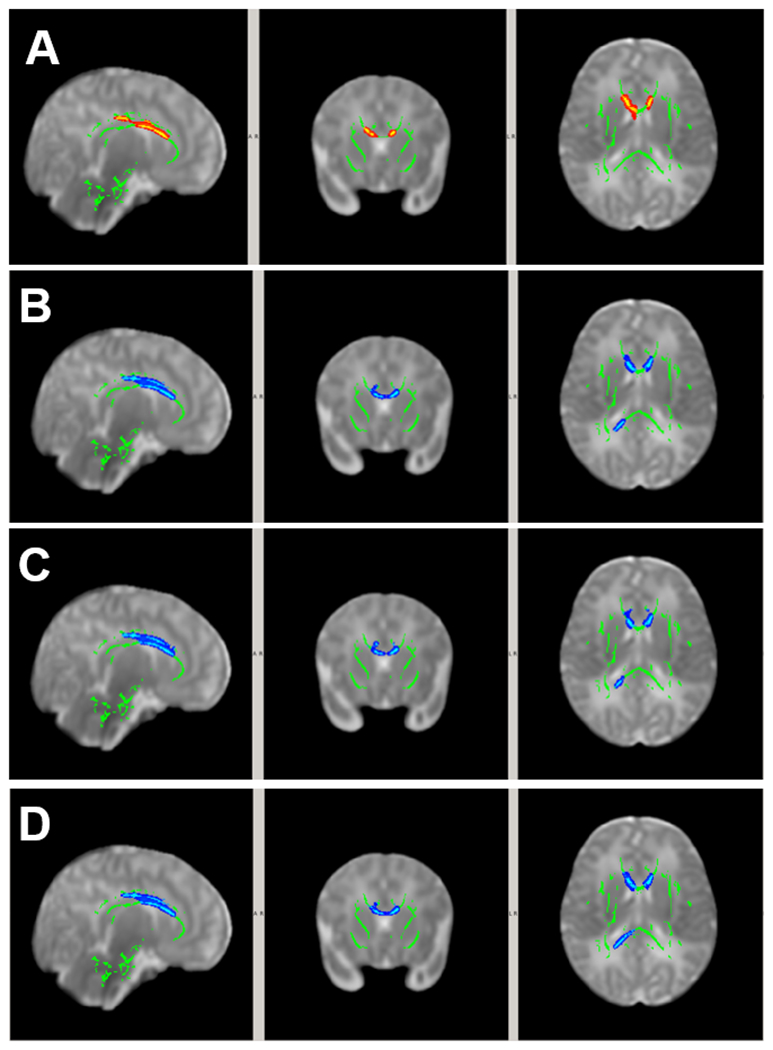
Within Group Correlations between DTI and HINE Motor Function
In participants in the Low Grade IVH group, significant correlations between DTI metrics and early motor development (HINE score) were found in the white matter in which higher FA (Figure 2A) and/or lower RD (Figure 2B) at term age was significantly correlated with higher HINE score at 3 months corrected age. The white matter regions with significant positive correlation between FA and HINE (Figure 2A) included primarily the genu, body, and splenium of corpus callosum, anterior, superior, and posterior corona radiata (all bilateral), and external capsule (right). The white matter regions with significant inverse correlation between RD and HINE were located in much smaller regions involving superior corona radiata, posterior corona radiata, and posterior thalamic radiations (all left, Figure 2B).
Figure 2.
White matter areas in patients with significant correlation between DTI at term and HINE score at 3 months corrected age (p<0.05, TFCE corrected). Covariates included postmenstrual age at MRI and sex. (A) Positive correlation between FA and HINE in the Low Grade IVH Group; (B) Negative correlation between RD and HINE in the Low Grade IVH Group; (C) Positive correlation between FA and HINE in the High Grade IVH Group.
In participants in the High Grade IVH group, significant positive correlations between FA and HINE were found in white matter regions involving the body and splenium of corpus callosum, and the superior and posterior corona radiata (both left, Figure 2C). No significant correlations between MD, AD or RD and HINE were found in any white matter region in the High Grade IVH group.
The size of the areas with significant within-group correlation between DTI and HINE for individual anatomical regions can be found in the supplemental materials (Table S2).
Within Group Correlation between DTI and IBQ-R-S Temperament Ratings
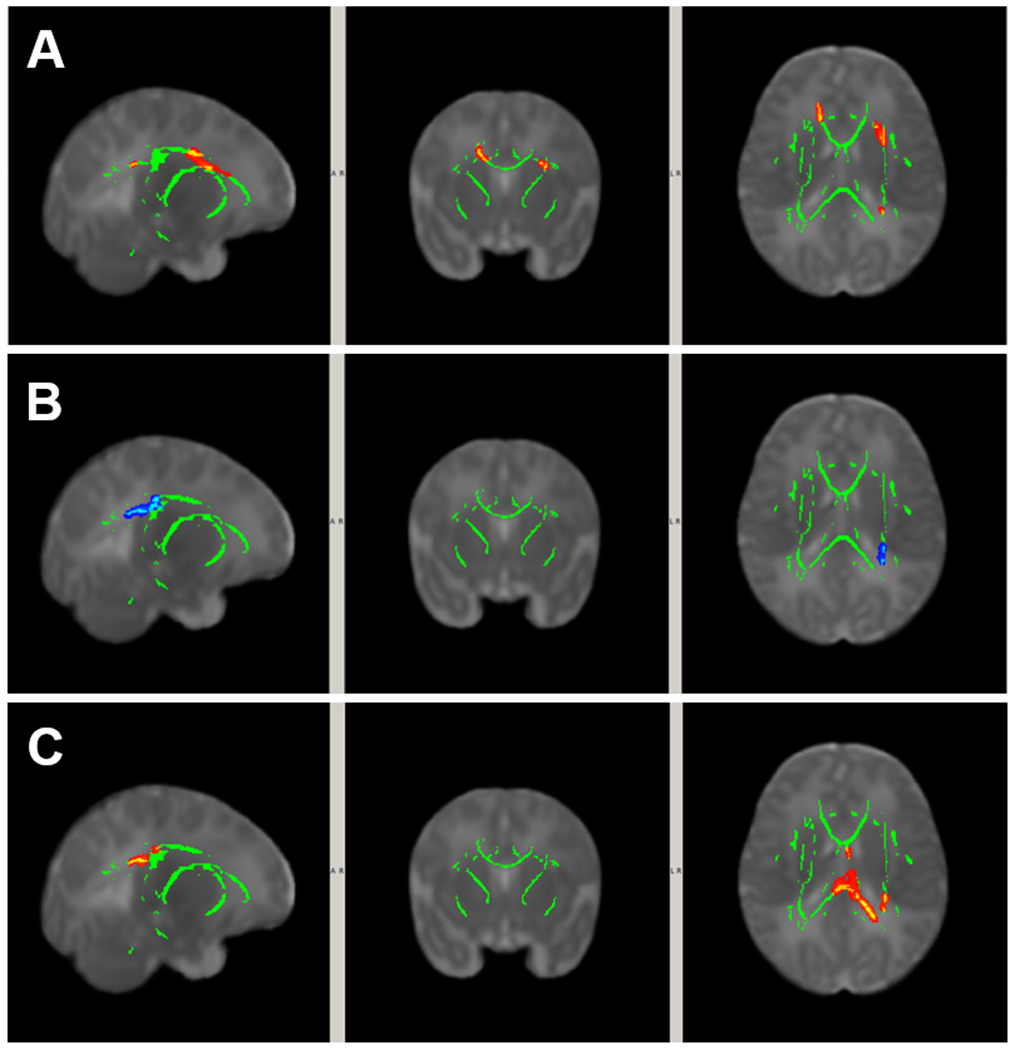
Among the three IBQ-R-S subscales for which the Low Grade and High Grade IVH groups differed significantly (i.e., Fear, Perceptual Sensitivity, and Vocal Reactivity), a statistically significant correlation was found between the four DTI metrics (i.e., FA, MD, AD, RD) and Fear (all p<0.05, TFCE corrected) for the High Grade IVH Group (Figure 3 A–D) in white matter regions involving primarily the genu and body of corpus callosum, superior corona radiata (left), and cingulum (bilateral). No statistically significant correlations were found between DTI metrics and Fear in the Low Grade IVH Group. No DTI metrics correlated significantly with Perceptual Sensitivity or Vocal Reactivity in either the Low Grade or High Grade IVH groups.
Figure 3.
White matter areas with significant correlation between DTI at term age and temperament ratings of Fear at 3 months corrected age (p<0.05, TFCE corrected) within High Grade IVH group. Covariates included postmenstrual age at MRI and sex. (A) Positive correlation between FA and Fear; (B) Negative correlation between MD and Fear; (C) Negative correlation between AD and Fear; and (D) Negative correlation between RD and Fear.
The size of the areas with significant within-group correlation between DTI and IBQ-R-S for individual anatomical regions can be found in the supplemental materials (Table S2).
Similar correlation analyses were also performed between the DTI metrics and the five IBQ-R-S subscales that showed marginally significant group differences. The results are included in the Supplemental Materials (Table S3).
Moderating effect of IVH severity on the association between DTI and HINE scores
In the model that associated HINE (at 3-month corrected age) with MD (at term age), after controlling for age at MRI and sex, a significant interaction between IVH severity and MD was found. The white matter areas associated with the significant interaction were located in the right anterior corona radiata and right external capsule (Figure 4A). Post-hoc analysis of the association between MD within the area with significant interaction and HINE are demonstrated in Figure 4B. No significant interactions were observed in any white matter area for the association between FA, AD, or RD and HINE score.
Figure 4.
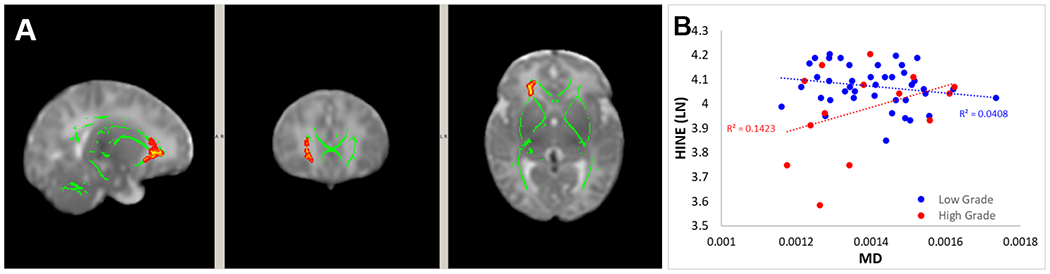
(A) White matter regions with significant interaction effect of IVH severity on the association between MD and HINE at 3 months corrected age. (B) Post-hoc comparison of the association between DTI and outcomes in the two study groups within the area with significant interaction of IVH severity on the association between MD and HINE
The size of the areas with significant interaction effect (in number of voxels) for individual anatomical regions can be found in the supplemental materials (Table S4).
Moderating effect of IVH Severity on the association between DTI and IBQ-R-S Ratings
A significant interaction effect was found for IVH severity on the association between Fear and FA in white matter regions (Figure 5A) including primarily the genu, body and splenium of corpus callosum, the anterior, superior, and posterior corona radiata (bilateral), and right sagittal stratum (bilateral). A significant interaction effect was also found for IVH severity in the association between Fear and MD (Figure 5C) and between Fear and AD (Figure 5E). Post-hoc analysis of the association between DTI within the areas with significant interaction and Fear are demonstrated in Figure 5B, 5D, and 5F (for FA, MD, AD, respectively).
Figure 5.
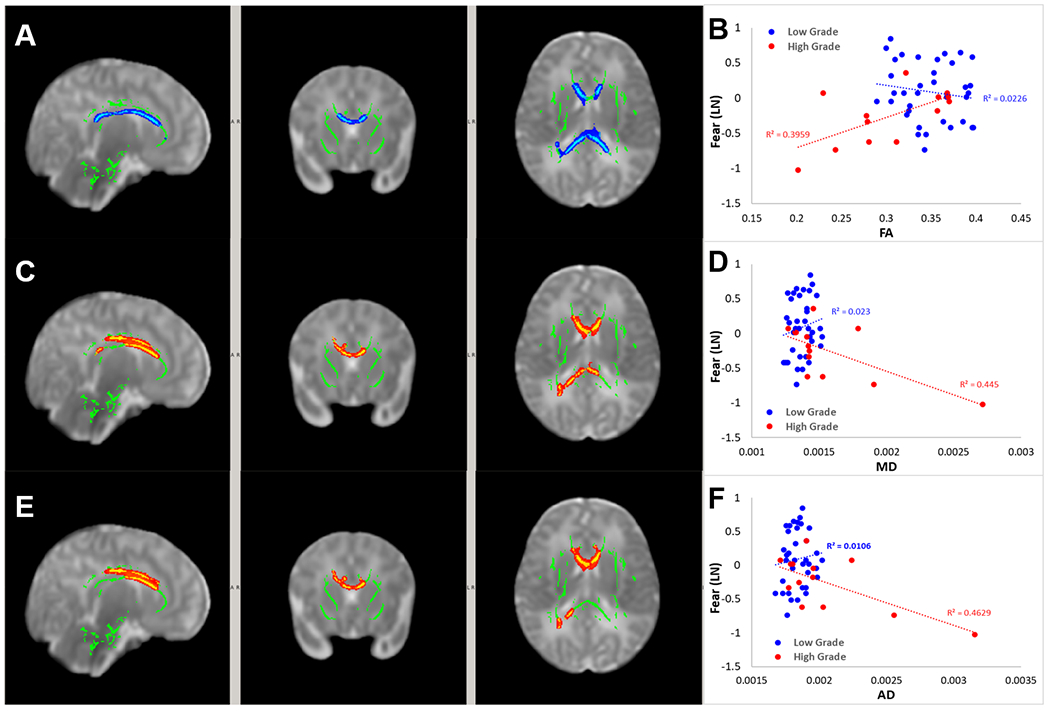
White matter regions with significant interaction effect of IVH severity on the association between FA and Fear score (A), between MD and Fear score (C) and between AD and Fear score (E). Post-hoc comparison of the association between DTI and outcomes in the two study groups within the area with significant interaction of IVH severity on the association between FA and Fear (B), between MD and Fear (D) and between AD and Fear (F).
No significant interaction effects were found in any white matter area for IVH severity on the association between DTI metrics and other IBQ-R-S ratings.
The size of the areas with a significant interaction for IVH severity in the associations between DTI metrics and IBQ-R-S Fear ratings can be found in Table S4.
Discussion
Summary:
In the present study, our data showed that there were significant differences in DTI metrics between infants born VPT at term age in Low Grade IVH Group and those in High Grade IVH Group. Abnormalities in DTI metrics were associated with the HINE score and IBQ-R-S Fear ratings in both Low Grade and High Grade IVH groups. In addition, our data also suggested that IVH severity had significant moderating effect on the relation between DTI and HINE score and between DTI and IBQ-R-S fear ratings.
The observed IVH group differences in DTI metrics found in the present study are in line with our expectations. The High Grade IVH group had significantly lower FA and higher MD, AD, and/or RD when compared to the Low Grade IVH group. The direction of the group differences is generally consistent with findings in other DTI studies of infants born preterm. For example, Morita et al. (2015) reported lower FA in a pre-determined region of interest, superior cerebellar peduncle, in infants born preterm with low grade IVH (Grade I or Grade II) when compared to infants without IVH [7]. Another study of pre-term infants with low Grade IVH reported abnormally low FA, high MD and RD in the corpus callosum limbic pathway, and cerebellar white matter tracts [9]. More recently, Young et al. (2018) investigated the white matter abnormalities based on diffusion MRI data in a cohort of infants born VPT including both low grade and high grade IVH (7 with Grade I/II IVH; 6 with Grade III/IV IVH) [8]. While the direction of DTI alterations (i.e., lower FA higher RD in infants born VPT compared to the controls) in this study were similar to that from the studies with only low grade IVH participants, and the abnormalities appeared involve more extensive white matter regions, no further analyses were conducted to explore potential differences based on IVH severity due possibly to the small sample size of the patient group. The only study that explored the potential effects of IVH severity on white matter integrity based on DTI was conducted by Tam et al. (2009) in which severe IVH was found to be associated with lower FA and higher MD in the middle cerebellar peduncle, deep cerebellar nuclear hila, and or cerebellar cortex [18]. However, the sample size was very small, especially in the severe IVH group (11 with mild IVH, 4 with severe IVH), and the investigation was limited to three regions of interest in the cerebellum, both of which significantly limit the generalizability of their findings. To our knowledge, the present study is the first to investigate the contrast of DTI metrics between infants born VPT with different IVH severity levels and to explore whether IVH severity affect the relationship between DTI metrics and motor function and temperament ratings in a large cohort of subjects. Of note, all the analyses were performed using a voxel-wise approach in the entire brain, including white matter regions in both the cerebrum and cerebellum, adding novel information.
Analysis of within-group correlations between DTI and outcome showed that DTI metrics obtained at term equivalent age may be predictive of future HINE and IBQ-R-S ratings of Smiling and Laughing in both Low Grade and High Grade IVH severity groups. DTI was also found to be predictive of IBQ-R-S ratings of Fear and Low Intensity Pleasure in High Grade IVH; however, no significant correlation was found for these relations in the Low Grade IVH group. Of note, the HINE correlations are in the direction expected assuming lower FA and higher MD/AD/RD in preterm patients with IVH when compared with normal controls or in low grade IVH patients compared with high grade IVH. Specifically, our data showed that higher HINE at 3 months corrected age was predicted by higher FA in both Low Grade and High Grade IVH groups and also by lower RD in the Low Grade group tested at term age. It is more difficult to interpret the directionality of the association of DTI metrics and IBQ-R-S ratings given that temperament dimensions can be potentially problematic if extreme in either direction (e.g., extreme fearfulness or lack thereof could potentially negatively impact a child’s functioning). Nonetheless these findings add to a growing literature suggesting individual differences in temperament may be influenced by brain abnormalities. Higher IBQ-R-S Fear ratings were predicted by higher FA, lower MD, AD, and/or RD in the High Grade IVH group, and higher Smiling and Laughing scores were predicted by higher FA in the Low Grade IVH group. Higher IBQ-R-S Smiling and Laughing scores were positively correlated with MD, AD, and/or RD in the High Grade IVH group; and the Low Intensity Pleasure score was inversely correlated with FA and positively correlated with MD and/or RD in the High Grade IVH group. The variability in directionality of IBQ-R-S findings may reflect the complex nature of the disease status, how the IVH of different severity may have affected the progression of the microstructural impairment in white matter, and how these differences may have impacted neurodevelopment in this young and vulnerable patient population. Interestingly, fear, defined on the IBQ-R-S as anticipated pain, distress, or threat to novel stimuli, ratings do seem to be specifically affected by brain abnormalities. For example, fear ratings on various versions of the Infant Behavior Questionnaire have been linked with functional connectivity in full term infants [38,39] and with cerebellar abnormalities in infants born VPT [20]. Further, reduced amygdala volume in infants born preterm was related to fear-processing abilities on the Laboratory Temperament Assessment Battery [40].
It is unclear as to how the DTI abnormalities, which are most commonly interpreted as a reflection of underlying microstructural changes, e.g, axonal membrane injury and/or delayed myelination/demyelination in the white matter, are affected by IVH in general, and whether there are different mechanisms when IVH of different severities affect the underling brain network. For the latter question, the moderating effect of IVH severity on the association between DTI and outcomes found in this study add further urgency for the need to delineate the association among the characteristics of the insults (IVH with different severity), the mechanisms of brain microstructural injury, and the subsequent influence on neurodevelopment. In IVH, typically hemosiderin deposition is followed by the generation of free radicals. The free iron and subsequent chain reaction can lead to cell toxicity, lipids, proteins, and DNA damage, leading to cell membrane damage and cell death. For patients with low grade IVH, hemosiderin is located in subependymal region or ventricle while for patients with high grade IVH there is a leakage of hemosiderin into ventricles causing ventriculomegaly/hydrocephalus (Grade III) or even into brain parenchyma (intraparenchymal hemorrhage, Grade IV) after the break down of blood brain barrier. As reported by Ley and colleagues using a preterm rabbit pup model of IVH, extracellular hemoglobin was found in periventricular areas, including extensive WM regions even in normal appearing brain regions based on conventional MRI [41]. Specifically, it was found that there was a concentration gradient along which hemoglobin diffuse “toward the less cellular dense white matter axonal tracts” in the ependyme [41]. A more recent study based on the magnetic susceptibility imaging, a MR technique known to be sensitive to myelin integrity and myelination progression during development in white matter and iron concentration (hemosiderin and/or ferritin) in the deep gray matter nuclei [42,43], showed evidences for the existence of changes in magnetic susceptibility affecting both white matter surrounding IVH and white matter distant from the IVH [44]. Taken these findings together, it is believed that the difference in the distribution of blood cells and the release and migration of extracellular hemoglobin in IVH with different severities may initiate different cascading effects, leading to differential damage and affect neurodevelopment through different underlying mechanisms.
This study had its limitations. While the patients included in the present study represented the largest cohort reported so far for infants born VPT with IVH, our study was still limited by sample size which did not allow for correction for potential multiple comparison error in the statistical analysis related to the 14 IBQ-R-S subscales. The study was also not powered to include all potential confounding factors, e.g., post-menstral age, ventricle size, periventricular venous infarct, laterality of the infarct, and other disorders, that allow for comprehensive and robust statistical analyses. Second, several subjects with severe ventriculomegaly were excluded to allow for appropriate registration in the TBSS data processing and analysis. This practice not only lowered the total number of subjects but also brough in bias with more subjects with high-Grade IVH excluded, which may have introduced Type I error in the between group comparisons and make the analysis less sensitive to the group differences. Third, we cannot rule out the susceptibility effect due to the extracellular hemoglobin in different forms, especially in periventricular white matter, on the DTI data acquisition and the findings. The present study is also limited by the low diffusion weighting factor (b= 800 s/mm2) and the tensor model used to quantify diffusion properties in the white matter. While this b-value is common for studying neonate and infant brains, the single tensor model does not allow for reconstructing diffusion features in areas with crossing fiber or in even more complex configurations. The constrained spherical deconvolution, a technique that allows for generating more precise representation for crossing or fanning fibers in both adult and pediatric populations [45,46], may help to improve the findings in the current study. DTI is also limited in differentiating diffusion properties in different microstructural compartments. A new diffusion model called neurite orientation and density imaging (NODDI) [47] has emerged as a potential alternative. Using multiple shell high angular resolution diffusion imaging protocol, NODDI allows for modeling for three brain tissue compartments, providing diffusion metrics to assess the dispersion of neurites and quantify intra-neurite space (extracellular space between axons), quantify the axonal density in the intra-cellular compartment, and characterize the diffusion feature in the CSF [48–50]. Moreover, the outcomes in the present study were assessed at 3 months corrected age. Future studies with longer follow-up, e.g., at 2-year corrected age or older would benefit the field and fill the knowledge gap regarding the temporal progression of the underlying changes in the white matter microstructure and how these alterations are affected by the IVH, potentially differently at different severity levels.
Conclusions
Our data suggest that DTI is a sensitive neuroimaging biomarker for detecting differences in white matter diffusion properties in VPT infants with different IVH severities. The results from within group correlation analysis showed that association between DTI and outcomes may exist in High Grade IVH group only, or in both Low Grade and High Grade IVH group, depending on the domain of outcomes studied. Combined with the moderating effect, the findings from the present study warrant further large-scale prospective study to investigate the differential effects of IVH with increasing severity on the underlying white matter microstructural alteration and their association with their short and/or long term neurodevelopmental outcomes.
Supplementary Material
Acknowledgements
This research was supported by grants R01-NS094200 and R01-NS096037 from the National Institute of Neurological Disorders and Stroke (NINDS). We sincerely thank the Cincinnati Infant Neurodevelopment Early Prediction Study (CINEPS) Investigators (names in in alphabetical order; coauthors not included): Anita Arnsperger, RRT, Traci Beiersdorfer, RN BSN, Kaley Bridgewater, RT(MR) CNMT, Tanya Cahill, MD, Kim Cecil, PhD, Kent Dietrich, RT, Christen Distler, BSN RNC-NIC, Juanita Dudley, RN BSN, Brianne Georg, BS, Cathy Grisby, RN BSN CCRC, Lacey Haas, RT(MR) CNMT, Lili He, PhD, Scott K. Holland, PhD, V.S. Priyanka Illapani, MS, Kristin Kirker, CRC, Julia E. Kline, PhD, Beth M. Kline-Fath, MD, Hailong Li, PhD, Matt Lanier, RT(MR) RT(R), Stephanie L. Merhar, MD MS, Greg Muthig, BS, Brenda B. Poindexter, MD MS, David Russell, JD, Kari Tepe, BSN RNC-NIC, Julia Thompson, RN BSN, Jean A. Tkach, PhD, Hui Wang, PhD, Jinghua Wang, PhD, Brynne Williams, RT(MR) CNMT, Kelsey Wineland, RT(MR) CNMT, Sandra Wuertz, RN BSN CCRP, Donna Wuest, AS. We are also grateful to Jennifer Notestine, RN and Valerie Marburger, NNP for serving as our Nationwide Children’s study coordinators and Mark Smith, MS, for serving as the study MR technologist. We are most thankful for the families that made this research possible.
Funding -
This research was supported by grants R01-NS094200 and R01-NS096037 from the National Institute of Neurological Disorders and Stroke (NINDS). -
Footnotes
Conflict of interest - The authors declare that they have no conflict of interest.
Ethical approval - All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent - Informed consent was obtained from the caregivers of all infants who participated the study.
Competing Financial Interests
The authors declare no competing interests.
Reference
- 1.Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P (2008) Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol 50 (5):334–340. doi: 10.1111/j.1469-8749.2008.02047.x [DOI] [PubMed] [Google Scholar]
- 2.Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O (2017) Cerebral Palsy-Trends in Epidemiology and Recent Development in Prenatal Mechanisms of Disease, Treatment, and Prevention. Front Pediatr 5:21. doi: 10.3389/fped.2017.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J (2009) Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124 (2):717–728. doi: 10.1542/peds.2008-2816 [DOI] [PubMed] [Google Scholar]
- 4.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ (2002) Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 288 (6):728–737. doi: 10.1001/jama.288.6.728 [DOI] [PubMed] [Google Scholar]
- 5.Johnston MV (2004) Clinical disorders of brain plasticity. Brain Dev 26 (2):73–80. doi: 10.1016/S0387-7604(03)00102-5 [DOI] [PubMed] [Google Scholar]
- 6.Johnston MV (2009) Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev 15 (2):94–101. doi: 10.1002/ddrr.64 [DOI] [PubMed] [Google Scholar]
- 7.Morita T, Morimoto M, Yamada K, Hasegawa T, Morioka S, Kidowaki S, Moroto M, Yamashita S, Maeda H, Chiyonobu T, Tokuda S, Hosoi H (2015) Low-grade intraventricular hemorrhage disrupts cerebellar white matter in preterm infants: evidence from diffusion tensor imaging. Neuroradiology 57 (5):507–514. doi: 10.1007/s00234-015-1487-7 [DOI] [PubMed] [Google Scholar]
- 8.Young JM, Vandewouw MM, Morgan BR, Smith ML, Sled JG, Taylor MJ (2018) Altered white matter development in children born very preterm. Brain Struct Funct 223 (5):2129–2141. doi: 10.1007/s00429-018-1614-4 [DOI] [PubMed] [Google Scholar]
- 9.Tortora D, Martinetti C, Severino M, Uccella S, Malova M, Parodi A, Brera F, Morana G, Ramenghi LA, Rossi A (2018) The effects of mild germinal matrix-intraventricular haemorrhage on the developmental white matter microstructure of preterm neonates: a DTI study. Eur Radiol 28 (3):1157–1166. doi: 10.1007/s00330-017-5060-0 [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15 (7-8):435–455. doi: 10.1002/nbm.782 [DOI] [PubMed] [Google Scholar]
- 11.Ducreux D, Huynh I, Fillard P, Renoux J, Petit-Lacour MC, Marsot-Dupuch K, Lasjaunias P (2005) Brain MR diffusion tensor imaging and fibre tracking to differentiate between two diffuse axonal injuries. Neuroradiology 47 (8):604–608. doi: 10.1007/s00234-005-1389-1 [DOI] [PubMed] [Google Scholar]
- 12.Hollund IMH, Olsen A, Skranes J, Brubakk AM, Haberg AK, Eikenes L, Evensen KAI (2018) White matter alterations and their associations with motor function in young adults born preterm with very low birth weight. Neuroimage Clin 17:241–250. doi: 10.1016/j.nicl.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DY, Park HK, Kim NS, Hwang SJ, Lee HJ (2016) Neonatal diffusion tensor brain imaging predicts later motor outcome in preterm neonates with white matter abnormalities. Ital J Pediatr 42 (1):104. doi: 10.1186/s13052-016-0309-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pogribna U, Burson K, Lasky RE, Narayana PA, Evans PW, Parikh NA (2014) Role of diffusion tensor imaging as an independent predictor of cognitive and language development in extremely low-birth-weight infants. AJNR Am J Neuroradiol 35 (4):790–796. doi: 10.3174/ajnr.A3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh NA, Hershey A, Altaye M (2019) Early Detection of Cerebral Palsy Using Sensorimotor Tract Biomarkers in Very Preterm Infants. Pediatr Neurol 98:53–60. doi: 10.1016/j.pediatrneurol.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeong HJ, Shim SY, Cho HJ, Cho SJ, Son DW, Park EA (2016) Cerebellar Development in Preterm Infants at Term-Equivalent Age Is Impaired after Low-Grade Intraventricular Hemorrhage. J Pediatr 175:86–92 e82. doi: 10.1016/j.jpeds.2016.05.010 [DOI] [PubMed] [Google Scholar]
- 17.Young JM, Vandewouw MM, Mossad SI, Morgan BR, Lee W, Smith ML, Sled JG, Taylor MJ (2019) White matter microstructural differences identified using multi-shell diffusion imaging in six-year-old children born very preterm. Neuroimage Clin 23:101855. doi: 10.1016/j.nicl.2019.101855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tam EW, Ferriero DM, Xu D, Berman JI, Vigneron DB, Barkovich AJ, Miller SP (2009) Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr Res 66 (1):102–106. doi: 10.1203/PDR.0b013e3181a1fb3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothbart MK (1981) Measurement of temperament in infancy. Child Dev (52):569–578 [Google Scholar]
- 20.Tamm L, Patel M, Peugh J, Kline-Fath BM, Parikh NA, Cincinnati Infant Neurodevelopment Early Prediction Study G (2020) Early brain abnormalities in infants born very preterm predict under-reactive temperament. Early Hum Dev 144:104985. doi: 10.1016/j.earlhumdev.2020.104985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harpster K, Merhar S, Priyanka Illapani VS, Peyton C, Kline-Fath B, Parikh NA (2021) Associations Between Early Structural MRI, Hammersmith Infant Neurological Exam, and General Movements Assessment in Very Preterm Infants. J Pediatr. doi: 10.1016/j.jpeds.2020.12.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92 (4):529–534. doi: 10.1016/s0022-3476(78)80282-0 [DOI] [PubMed] [Google Scholar]
- 23.Romeo DM, Cioni M, Scoto M, Mazzone L, Palermo F, Romeo MG (2008) Neuromotor development in infants with cerebral palsy investigated by the Hammersmith Infant Neurological Examination during the first year of age. Eur J Paediatr Neurol 12 (1):24–31. doi: 10.1016/j.ejpn.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 24.Einspieler C (2008) Early markers for unilateral spastic cerebral palsy in premature infants. Nat Clin Pract Neurol 4 (4):186–187. doi: 10.1038/ncpneuro0745 [DOI] [PubMed] [Google Scholar]
- 25.Roze E, Harris PA, Ball G, Elorza LZ, Braga RM, Allsop JM, Merchant N, Porter E, Arichi T, Edwards AD, Rutherford MA, Cowan FM, Counsell SJ (2012) Tractography of the corticospinal tracts in infants with focal perinatal injury: comparison with normal controls and to motor development. Neuroradiology 54 (5):507–516. doi: 10.1007/s00234-011-0969-5 [DOI] [PubMed] [Google Scholar]
- 26.Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E (2014) Development and assessment of short and very short forms of the infant behavior questionnaire-revised. J Pers Assess 96 (4):445–458. doi: 10.1080/00223891.2013.841171 [DOI] [PubMed] [Google Scholar]
- 27.Gartstein MA, Rothbart MK (2003) Studying infant temperament via the Revised Infant Behavior Questionnaire. Journal of Infant Behavior and Development 26:64–86 [Google Scholar]
- 28.Gartstein MA, Slobodskaya HR, Kinsht IA (2003) Cross-cultural differences in temperament in the first year of life: United States of America (US) and Russia. International Journal of Behavioral Development 27:316–328 [Google Scholar]
- 29.Parade SH, Leerkes EM (2008) The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behav Dev 31 (4):637–646. doi: 10.1016/j.infbeh.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aureli T, Coppola G, Picconi L, Grazia A, Ponzetti S (2015) Relationships between regulatory temperament dimensions and self-regulatory behaviors at 4 and 6 months of age. Infant Behav Dev 38:162–166. doi: 10.1016/j.infbeh.2014.12.013 [DOI] [PubMed] [Google Scholar]
- 31.Gartstein MA, Bridgett DJ, Rothbart MK, Robertson C, Iddins E, Ramsay K, Schlect S (2010) A latent growth examination of fear development in infancy: Contributions of maternal depression and the risk for toddler anxiety. Dev Psychol 46 (3):651–668. doi: 10.1037/a0018898 [DOI] [PubMed] [Google Scholar]
- 32.Gartstein MA, Marmion J (2008) Fear and positive affectivity in infancy: Convergence/discrepancy between parent-report and laboratory-based indicators. Infant Behav Dev 31 (2):227–238. doi: 10.1016/j.infbeh.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basser PJ, Pierpaoli C (1998) A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med 39 (6):928–934 [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31 (4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 35.Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44 (1):83–98. doi: 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- 36.Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40 (2):570–582. doi: 10.1016/j.neuroimage.2007.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kidokoro H, Neil JJ, Inder TE (2013) New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 34 (11):2208–2214. doi: 10.3174/ajnr.A3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, Styner M, Entringer S, Wadhwa PD, Fair DA (2016) Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev Cogn Neurosci 18:12–25. doi: 10.1016/j.dcn.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas E, Buss C, Rasmussen JM, Entringer S, Ramirez JSB, Marr M, Rudolph MD, Gilmore JH, Styner M, Wadhwa PD, Fair DA, Graham AM (2019) Newborn amygdala connectivity and early emerging fear. Dev Cogn Neurosci 37:100604. doi: 10.1016/j.dcn.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cismaru AL, Gui L, Vasung L, Lejeune F, Barisnikov K, Truttmann A, Borradori Tolsa C, Huppi PS (2016) Altered Amygdala Development and Fear Processing in Prematurely Born Infants. Front Neuroanat 10:55. doi: 10.3389/fnana.2016.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ley D, Romantsik O, Vallius S, Sveinsdottir K, Sveinsdottir S, Agyemang AA, Baumgarten M, Morgelin M, Lutay N, Bruschettini M, Holmqvist B, Gram M (2016) High Presence of Extracellular Hemoglobin in the Periventricular White Matter Following Preterm Intraventricular Hemorrhage. Front Physiol 7:330. doi: 10.3389/fphys.2016.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Argyridis I, Li W, Johnson GA, Liu C (2014) Quantitative magnetic susceptibility of the developing mouse brain reveals microstructural changes in the white matter. Neuroimage 88:134–142. doi: 10.1016/j.neuroimage.2013.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W, Wu B, Batrachenko A, Bancroft-Wu V, Morey RA, Shashi V, Langkammer C, De Bellis MD, Ropele S, Song AW, Liu C (2014) Differential developmental trajectories of magnetic susceptibility in human brain gray and white matter over the lifespan. Hum Brain Mapp 35 (6):2698–2713. doi: 10.1002/hbm.22360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tortora D, Severino M, Sedlacik J, Toselli B, Malova M, Parodi A, Morana G, Fato MM, Ramenghi LA, Rossi A (2018) Quantitative susceptibility map analysis in preterm neonates with germinal matrix-intraventricular hemorrhage. J Magn Reson Imaging 48 (5):1199–1207. doi: 10.1002/jmri.26163 [DOI] [PubMed] [Google Scholar]
- 45.Tournier JD, Calamante F, Connelly A (2007) Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35 (4):1459–1472. doi: 10.1016/j.neuroimage.2007.02.016 [DOI] [PubMed] [Google Scholar]
- 46.Toselli B, Tortora D, Severino M, Arnulfo G, Canessa A, Morana G, Rossi A, Fato MM (2017) Improvement in White Matter Tract Reconstruction with Constrained Spherical Deconvolution and Track Density Mapping in Low Angular Resolution Data: A Pediatric Study and Literature Review. Front Pediatr 5:182. doi: 10.3389/fped.2017.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC (2012) NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61 (4):1000–1016. doi: 10.1016/j.neuroimage.2012.03.072 [DOI] [PubMed] [Google Scholar]
- 48.Genc S, Malpas CB, Holland SK, Beare R, Silk TJ (2017) Neurite density index is sensitive to age related differences in the developing brain. Neuroimage 148:373–380. doi: 10.1016/j.neuroimage.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 49.Churchill NW, Caverzasi E, Graham SJ, Hutchison MG, Schweizer TA (2017) White matter microstructure in athletes with a history of concussion: Comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Hum Brain Mapp 38 (8):4201–4211. doi: 10.1002/hbm.23658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palacios EM, Owen JP, Yuh EL, Wang MB, Vassar MJ, Ferguson AR, Diaz-Arrastia R, Giacino JT, Okonkwo DO, Robertson CS, Stein MB, Temkin N, Jain S, McCrea M, MacDonald CL, Levin HS, Manley GT, Mukherjee P, Investigators T-T (2020) The evolution of white matter microstructural changes after mild traumatic brain injury: A longitudinal DTI and NODDI study. Sci Adv 6 (32):eaaz6892. doi: 10.1126/sciadv.aaz6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


