Abstract
Introduction
Bladder-sparing chemoradiation therapy is a definitive first-line treatment option for muscle-invasive bladder cancer. Randomized trials have demonstrated that the addition of neoadjuvant chemotherapy to radical cystectomy or radiation monotherapy results in a survival benefit. Whether neoadjuvant chemotherapy improves outcomes when used with definitive chemoradiation is unknown.
Patients and Methods
We identified 2,566 patients in the National Cancer Data Base with cT2–4N0M0 urothelial cell carcinoma of the bladder treated with definitive intent concurrent chemoradiation from 2004–2015. The exposure of interest was receipt of neoadjuvant chemotherapy (versus those without neoadjuvant chemotherapy). The primary outcome was overall survival defined from the time of diagnosis. Kaplan Meier and multivariable Cox proportional hazards were used to compare survival between groups. Sensitivity analyses tested 1) an interaction term for clinical T stage and 2) defining survival from start of radiation (opposed to time of diagnosis) to address potential leading time bias.
Results
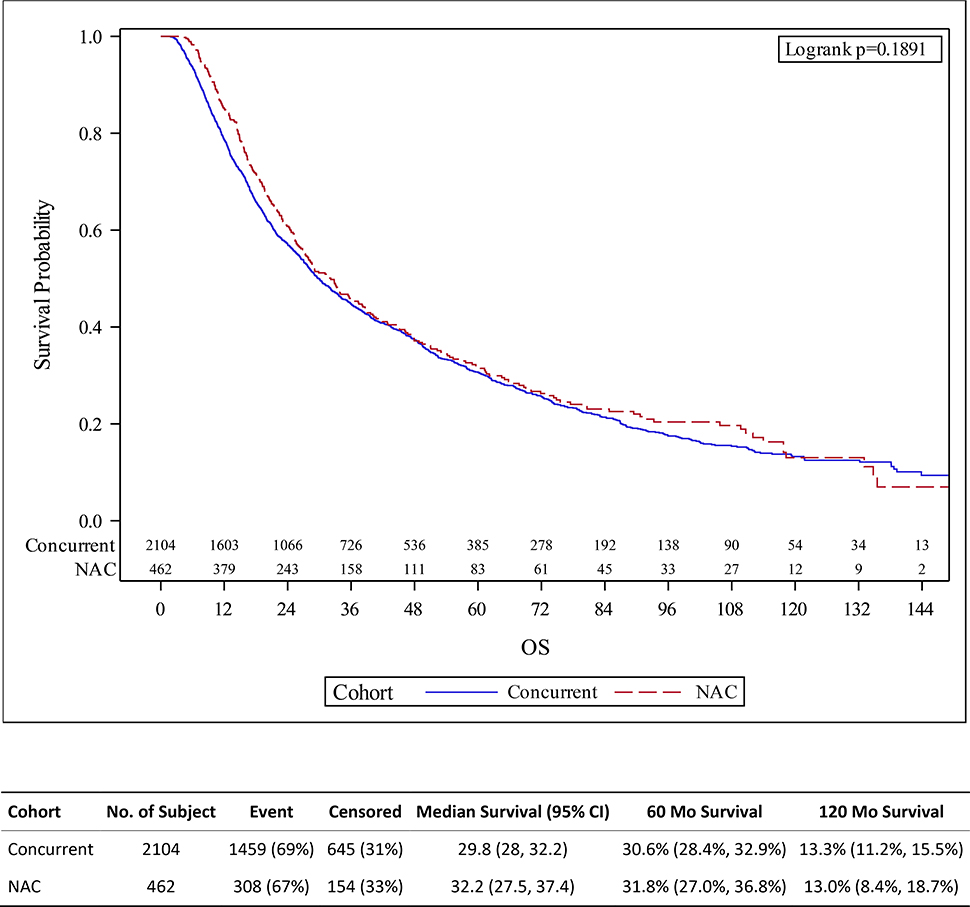
We identified 462 patients treated with neoadjuvant chemotherapy followed by chemoradiation and 2104 patients treated with chemoradiation alone. With a median follow up of 6.2 years, we found no difference in survival between groups (5-year or 10-year overall survival of 30.6% [95%CI: 28.4–32.9%] in the neoadjuvant group versus 31.8% [27.0–36.8%] in the standard chemoradiation therapy group and 13.3% [11.2–15.5%] versus 13.0% [8.4–18.7%], respectively; log-rank p-value 0.19). On multivariable analysis we found no association between receipt of neoadjuvant chemotherpay and overall survival (HR 1.01, 95%CI: 0.88–1.15; p=0.921). On sensitivity analyses we found no differential effect by clinical T stage nor by defining survival from start of radiation.
Conclusion
These results do not support the routine addition of neoadjuvant chemotherapy to definitive chemoradiation for bladder cancer, and optimizing the chemotherapy sequencing and regimens for bladder-preserving approaches to muscle invasive bladder cancer should continue to be studied under prospective clinical trials.
Keywords: muscle invasive bladder cancer, radiation, chemotherapy, radical cystectomy, trimodality therapy
Microabstract:
The benefit of adding neoadjuvant chemotherapy to bladder-sparing chemoradiation for muscle invasive bladder cancer remains unclear. This retrospective large database study of 2,566 patients found no survival benefit with the addition of neoadjuvant chemotherapy to definitive chemoradiation. These results do not support the routine addition of neoadjuvant chemotherapy to definitive chemoradiation for bladder cancer, which should be investigated under trial.
Introduction
Bladder cancer is the sixth most common cancer in the United States and accounts for nearly 81,000 new cases and 18,000 deaths per year.1 Nearly 25% of new cases are muscle-invasive bladder cancer (MIBC) (T2-T4 disease), which has a 5-year survival of <15% without treatment.2 Both bladder-removing radical cystectomy (RC) and bladder-sparing tri-modality therapy (TMT) are definitive, first-line treatment options for MIBC recommended by professional society guidelines.3–5 TMT is a multidisciplinary, organ-preserving approach involving transurethral resection of the bladder tumor followed by concurrent chemoradiation. Chemotherapy is an important component of TMT, with the randomized BC2001 trial showing that chemoradiation provides superior locoregional disease free survival compared to radiation alone.6
Randomized controlled trials and meta-analyses have shown the addition of neoadjuvant chemotherapy (NAC) to RC improves outcomes with an estimated 5% overall survival benefit.7–9 Further, the addition of NAC to definitive radiation-alone provides a 6% overall survival benefit.10 However, the impact of NAC to bladder-sparing TMT (i.e. definitive chemoradiation) is unknown, but there is increasing interest that the addition of NAC could further improve outcomes for these patients.11,12 The RTOG 88–02 trial was a single-arm phase II study that demonstrated the feasibility of TMT with a NAC component.13,14 The RTOG 89–03 trial further attempted to answer this question by randomizing patients to TMT with or without NAC, but it closed prematurely due to unexpected high rates of neutropenia.15 Therefore, the purpose of this study is to evaluate whether the addition of NAC to TMT improves outcomes in a contemporary cohort of patients with MIBC.
Patients and Methods
Data Source
The National Cancer Data Base (NCDB) is a national hospital-based registry jointly sponsored by the American College of Surgeons and the American Cancer Society. It captures the first course of cancer treatment16 and collects data from more than 1,500 Commission on Cancer-accredited facilities and captures approximately 70% of incident cancers in the United States, annually. The data accuracy and quality is continually validated via data quality reviews, site surveys, and internal monitoring.17 Methods regarding data coding have been described elsewhere.18 This study received institutional review board exemption.
Study population
We identified patients aged ≥18 with a diagnosis of cT2–4N0M0 urothelial cell carcinoma of the bladder treated with definitive intent concurrent chemoradiation from 2004–2015. Patients had to receive a transurethral resection of bladder tumor (TURBT) prior to chemoradiation. Patient were included if they had a total radiation dose ≥40 Gy. We excluded those who were post-cystectomy, node positive (N1+), metastatic (M1), had unknown stage, non-urothelial cell carcinoma (i.e. non-transitional cell carcinoma, or variant histologies19), a history of prior malignancy, or received palliative intent therapy. These criteria left 2,566 patients for analysis. Detailed patient selection schema is shown in the Appendix (eTable 1).
Measurements
The exposure of interest was receipt of NAC (versus those without NAC). The NAC group was defined as chemotherapy that was started 31–120 days prior to concurrent chemoradiation; concurrent chemoradiation was defined as chemotherapy and radiation starting within 30 days of each other.20 The primary outcome of interest was overall survival from date of diagnosis, censoring at last follow up for patients still alive.
Statistical analyses
Descriptive statistics presented the baseline characteristics between groups: TMT versus NAC+TMT. Categorical variables were evaluated via Chi-square tests and continuous variables by ANOVA. Patients were stratified by variables of interest including age, T-stage, diagnosis year, race/ethnicity, hospital setting, Charlson-Deyo comorbidity index, insurance status, US region, residence type, education level, household income, and distance to treatment facility. Kaplan-Meier and multivariable Cox proportional hazards were used to assess the association with overall survival (OS). For the multivariable model, backward selection with an alpha level of removal of 0.2 was used, eliminating the following variables from the model: travel distance, facility, residence, and time to radiation.
Two sensitivity analyses were performed. The first, by running the multivariable Cox proportional hazards model and adding an interaction term with clinical T stage subgroup (cT2 versus cT3–4) to test the subgroup interaction by T-stage, hypothesizing that perhaps there may be a benefit to the addition of NAC in more advanced cancers (i.e. clinical T3-T4). The second, to account for potential lead-time bias, we evaluated overall survival defined as start of radiation until death (opposed to from date of diagnosis until death). Analyses used SAS 9.4 (SAS Institute Inc., Cary, NC) and SAS macros.21 Tests were 2-sided with a 0.05 level of significance.
Results
We identified 462 patients in the NAC+TMT group and 2104 patients in the TMT group. The NAC+TMT cohort was younger with 33.8% aged 80+ years versus 42.4% in the TMT group (p=0.001). There were no other differences identified between baseline characteristics of groups including sociodemographic variables (Table 1). Mean time from diagnosis to TURBT was not found to be different between groups (1.8–1.9 weeks; p=0.704). Mean time from diagnosis to radiation start for NAC+TMT versus TMT was 14.7 versus 9.4 weeks, respectively (p<0.001).
Table 1.
Cohort characteristics among patients who received trimodality therapy (TMT) for muscle invasive bladder cancer, stratified by receipt of neoadjuvant chemotherapy (NAC).
| TMT N=2104 |
NAC+TMT N=462 |
|||||
|---|---|---|---|---|---|---|
| Variable | Level | N | % | N | % | P-value* |
|
| ||||||
| Age at Diagnosis | <60 | 147 | 7.0 | 48 | 10.4 | 0.001 |
| 60–69 | 362 | 17.2 | 97 | 21.0 | ||
| 70–79 | 703 | 33.4 | 160 | 34.6 | ||
| 80+ | 892 | 42.4 | 157 | 34.0 | ||
| Race | White | 1876 | 89.2 | 413 | 89.4 | 0.700 |
| Black | 128 | 6.1 | 29 | 6.3 | ||
| Asian-Indian-Pacific | 30 | 1.4 | 9 | 1.9 | ||
| Hispanic | 43 | 2.0 | 8 | 1.7 | ||
| Other/unknown | 27 | 1.3 | 3 | 0.6 | ||
| Year | 2004–2006 | 447 | 21.2 | 84 | 18.2 | 0.315 |
| 2007–2009 | 514 | 24.4 | 107 | 23.2 | ||
| 2010–2012 | 457 | 21.7 | 113 | 24.5 | ||
| 2013–2015 | 686 | 32.6 | 158 | 34.2 | ||
| Charlson-Deyo Score | 0 | 1340 | 63.7 | 300 | 64.9 | 0.613 |
| 1+ | 764 | 36.3 | 162 | 35.1 | ||
| Clinical T stage | T2 | 1728 | 82.1 | 389 | 84.2 | 0.289 |
| T3–4 | 376 | 17.9 | 73 | 15.8 | ||
| Residence | Metro | 1698 | 80.7 | 360 | 77.9 | 0.591 |
| Urban | 310 | 14.7 | 79 | 17.1 | ||
| Rural | 41 | 1.9 | 10 | 2.2 | ||
| Unknown | 55 | 2.6 | 13 | 2.8 | ||
| Insurance | Other | 151 | 7.2 | 28 | 6.1 | 0.099 |
| Private | 344 | 16.3 | 94 | 20.3 | ||
| Medicare | 1609 | 76.5 | 340 | 73.6 | ||
| Median income | < $38,000 | 335 | 16.0 | 67 | 14.6 | 0.325 |
| $38,000-$47,999 | 510 | 24.4 | 124 | 27.0 | ||
| $48,000-$62,999 | 597 | 28.6 | 141 | 30.7 | ||
| >=$63,000 | 647 | 31.0 | 127 | 27.7 | ||
| No high school degree (%) | ≥21.0% | 304 | 14.5 | 62 | 13.4 | 0.318 |
| 13.0–20.9% | 503 | 24.1 | 127 | 27.5 | ||
| 7.0–12.9% | 769 | 36.8 | 172 | 37.3 | ||
| <7.0% | 514 | 24.6 | 100 | 21.7 | ||
| Facility | Non-academic | 1536 | 73.1 | 328 | 71.1 | 0.401 |
| Academic | 566 | 26.9 | 133 | 28.9 | ||
| Region | Northeast | 531 | 25.3 | 99 | 21.5 | 0.190 |
| South | 662 | 31.5 | 142 | 30.8 | ||
| Midwest | 571 | 27.2 | 145 | 31.5 | ||
| West | 338 | 16.1 | 75 | 16.3 | ||
| Travel distance (miles) | <25 | 1740 | 83.3 | 378 | 82.0 | 0.650 |
| 25–50 | 210 | 10.0 | 53 | 11.5 | ||
| >50 | 140 | 6.7 | 30 | 6.5 | ||
|
| ||||||
| TURBT (weeks from dx) | Median (IQR) | 0.0 | 0.0–2.0 | 0.0 | 0.0–2.0 | 0.593 |
| Chemo start (weeks from dx) | Median (IQR) | 8.1 | 5.7–12 | 4.0 | 0.0–7.4 | <0.001 |
| Radiation start (weeks from dx) | Median (IQR) | 8.1 | 5.9–12 | 14.6 | 9.7–18.9 | <0.001 |
| Total radiation dose | Median (IQR) | 64.5 | 59.4–64.8 | 64.8 | 59.4–64.8 | 0.796 |
P-value by ANOVA for numerical covariates and chi-square test for categorical covariates
Abbreviations: TURBT, transurethral resection of bladder tumor; DX, diagnosis; IQR, intraquartile range
Median follow up time was 6.2 years. There was no difference between those who received TMT versus NAC+TMT in estimated 5-year or 10-year OS (30.6% [95%CI: 28.4–32.9%] versus 31.8% [27.0–36.8%] and 13.3% [11.2–15.5%] versus 13.0% [8.4–18.7%], respectively; log-rank p-value 0.19; Figure 1). Further, on multivariable analysis we found no association between overall survival and receipt of NAC (HR 1.01, 95%CI: 0.88–1.15; p=0.921). Age at diagnosis, year of diagnosis, comorbidity, T2 stage, insurance status, education, were all independent predictors of survival (Table 2).
Figure 1.
Kaplan-Meier survival estimates for patients treated with trimodality therapy (TMT) with or without neoadjuvant chemotherapy (NAC) for muscle invasive bladder cancer, with overall survival defined from date of diagnosis.
Table 2.
Multivariate overall survival (OS) analysis among patients who received trimodality therapy (TMT) for muscle invasive bladder.
| Variable | Level | N | Hazard Ratio (95% CI) | P-value* |
|---|---|---|---|---|
|
| ||||
| Cohort | TMT | 2108 | - | |
| NAC+TMT | 463 | 1.01 (0.88–1.15) | 0.921 | |
| Age at Diagnosis | <60 | 192 | - | - |
| 60–69 | 456 | 1.30 (1.02–1.66) | 0.035 | |
| 70–79 | 857 | 1.75 (1.38–2.23) | <0.001 | |
| 80+ | 1040 | 2.13 (1.67–2.70) | <0.001 | |
| Race | White | 2270 | - | - |
| Black | 155 | 1.17 (0.95–1.43) | 0.132 | |
| Asian-Indian-Pacific | 39 | 0.66 (0.42–1.04) | 0.075 | |
| Hispanic | 51 | 0.76 (0.51–1.12) | 0.165 | |
| Other/unknown | 30 | 1.12 (0.80–1.83) | 0.372 | |
| Year | 2004–2006 | 522 | - | - |
| 2007–2009 | 615 | 1.13 (0.99–1.29) | 0.074 | |
| 2010–2012 | 567 | 1.06 (0.92–1.22) | 0.395 | |
| 2013–2015 | 841 | 1.16 (1.01–1.34) | 0.037 | |
| Charlson-Deyo Score | 0 | 1624 | - | - |
| 1+ | 921 | 1.27 (1.15–1.40) | <0.001 | |
| Clinical T stage | T2 | 2099 | - | - |
| T3–4 | 446 | 1.43 (1.27–1.61) | <0.001 | |
| Insurance | Private | 435 | - | - |
| Medicare | 1933 | 1.14 (0.98–1.32) | 0.079 | |
| Other | 177 | 1.34 (1.07–1.68) | 0.010 | |
| Median income | >=$63,000 | 782 | - | - |
| < $38,000 | 402 | 0.92 (0.76–1.12) | 0.423 | |
| $38,000–$47,999 | 632 | 1.10 (0.94–1.28) | 0.248 | |
| $48,000–$62,999 | 738 | 0.96 (0.83–1.10) | 0.523 | |
| No high school degree (%) | <7.0% | 621 | - | - |
| 7.0–12.9% | 939 | 1.06 (0.92–1.22) | 0.441 | |
| 13.0–20.9% | 630 | 1.12 (1.02–1.44) | 0.025 | |
| ≥21.0% | 365 | 1.12 (0.91–1.37) | 0.293 | |
| Region | West | 409 | - | - |
| Northeast | 623 | 0.90 (0.77–1.06) | 0.206 | |
| South | 799 | 0.97 (0.83–1.14) | 0.732 | |
| Midwest | 714 | 1.14 (0.98–1.33) | 0.087 | |
Abbreviation: CI, confidence interval
On the first sensitivity analysis we found no association between overall survival and receipt of NAC using an interaction term for clinical T stage subgroup with a HR 0.96 (95%CI: 0.83–1.10; p=0.50) for cT2 and 1.19 (0.88–1.61; p=0.30) cT3–4 patients (Table 3).
Table 3.
Multivariate overall survival (OS) analysis among patients who received trimodality therapy (TMT) with or without neoadjuvant chemo (NAC) for muscle invasive bladder sensitivity analysis using an interaction term with clinical T stage subgroup.
| Variable | Level | N | Hazard Ratio (95% CI) | P-value* |
|---|---|---|---|---|
|
| ||||
| T2 | TMT | 1713 | - | - |
| NAC+TMT | 386 | 1.05 (0.91–1.21) | 0.536 | |
| T3–4 | TMT | 374 | - | - |
| NAC+TMT | 72 | 0.84 (0.62–1.13) | 0.253 | |
The estimated stratified treatment effect was controlled by: age, comorbidity, facility, income, education, insurance, race, and year.
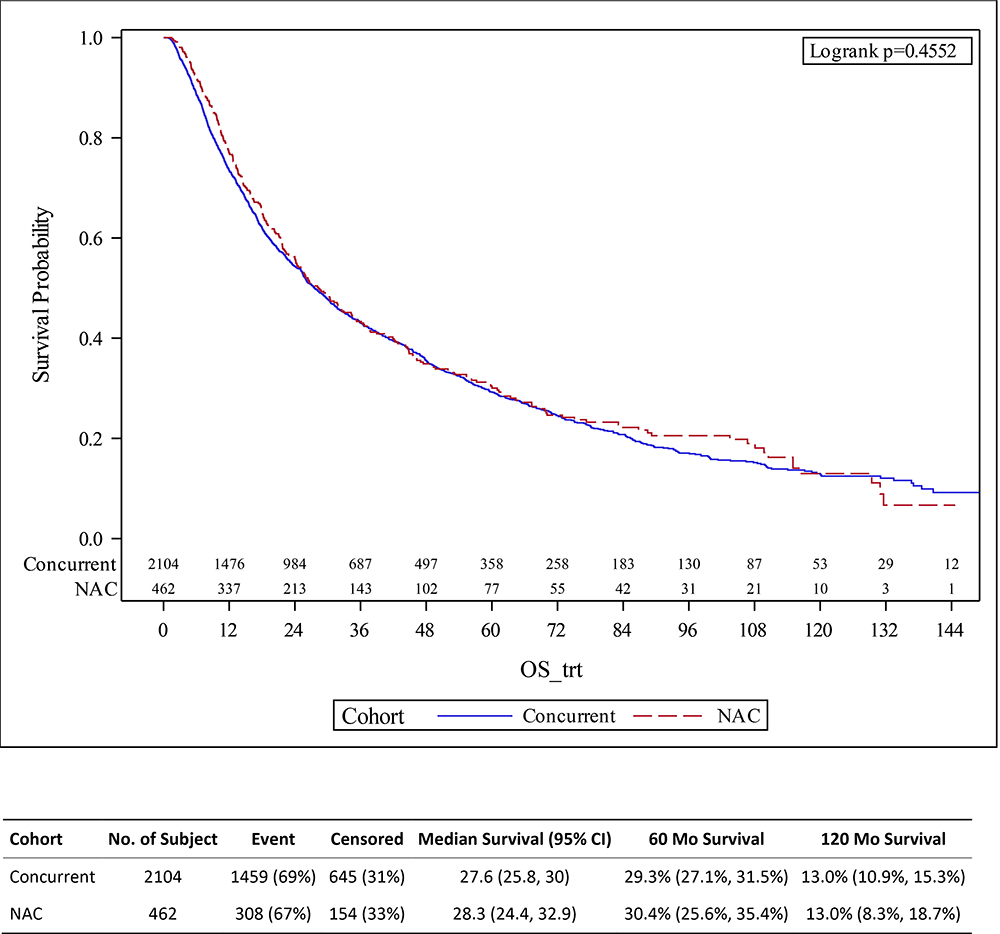
On the second sensitivity analysis, we again found no association between overall survival and receipt of NAC when, to account for potential lead-time bias, we evaluated overall survival defined as start of radiation until death (opposed to from date of diagnosis until death) (Table 4, Figure 2).
Table 4.
Multivariate overall survival (OS) analysis among patients who received trimodality therapy (TMT) with or without neoadjuvant chemo (NAC) for muscle invasive bladder sensitivity analysis using overall survival defined from start date of radiation.
| Variable | Level | N | Hazard Ratio (95% CI) | P-value* |
|---|---|---|---|---|
|
| ||||
| Cohort | TMT | 2087 | - | |
| NAC+TMT | 458 | 0.97 (0.86–1.10) | 0.618 | |
The estimated stratified treatment effect was controlled by: age, comorbidity, facility, income, education, insurance, race, region, T stage, and year.
Figure 2.
Kaplan-Meier survival estimates for patients treated with trimodality therapy (TMT) with or without neoadjuvant chemotherapy (NAC) for muscle invasive bladder cancer, with overall survival defined from start date of radiation.
Discussion
In this study, we consistently found no survival benefit with the addition of NAC to TMT. Our analysis included 2,566 patients with a median follow up of 6.2 years, and these findings held true when looking at both 5-year and 10-year survival rates, and also on multivariable analysis. Further, on sensitivity analysis, when we included an interaction term for the clinic T stage subgroup, hypothesizing that perhaps there may be a benefit to the addition of NAC in more advanced cancers (i.e. clinical T3-T4), we again found no difference. Finally, to account for the potential of lead time bias, a sensitivity result measuring survival from start of radiation (opposed to from diagnosis) we again found no benefit with the addition of NAC.
The clinical significance of these findings are that they do not support the hypothesis that adding NAC to TMT would improve outcomes.12 This finding stands in contrast to the known benefit, observed via prospective randomized trials, of adding NAC to RC7–9 or to radiation alone.10
These results are important in that they fill an existing gap in the literature regarding the question of whether there is an added benefit with NAC to chemoradiation. While the optimal approach to evaluate this question is in the form of a prospective randomized trial, these have been conducted with mixed success. The RTOG 89–03 randomized trial compared a course of neoadjuvant methotrexate, cisplatin, and vinblastine chemotherapy followed by radiation with concurrent cisplatin to a course of radiation with concurrent cisplatin alone. Although the study closed prematurely due to unexpected high rate of neutropenia, they found no impact on 5-year overall survival with the addition of NAC to chemoradiation.15 Additionally, the BC2001 randomized trial of 360 patients was designed to evaluate the impact of the addition of concurrent chemotherapy to radiation therapy, and a recent post hoc analysis revealed a small subset of 56 patients who received NAC followed by chemoradiation;11 this analysis was also underpowered to adequately evaluate a benefit of NAC in the chemoradiation group. Finally, the results of our study are supported by two smaller retrospective or post hoc single-institutional series that have demonstrated no clear benefit with the addition of NAC to chemoradiation.22,23
A limitation of the study is the possibility that some of the NAC+TMT patients did not receive chemotherapy concurrently with radiation (i.e. received NAC followed by radiation alone), but this is likely uncommon given the known advantage of concurrent chemo in addition to radiation as shown in the BC2001 trial.6 Additional limitations include its retrospective nature and the lack of other oncologic endpoints available in the registry database, such as progression-free survival or salvage cystectomy rates, which would be clinically relevant outcomes. The dataset also lacks important data regarding adverse events (e.g. neutropenia) or patient reported outcomes. Finally, our dataset lacks granularity regarding the extent of TURBT or regarding specific types of chemotherapy regimens (e.g. cisplatin versus non-cisplatin-based regimens). For example, it is plausible an inability to discriminate between cisplatin-based versus carboplatin-based chemotherapy could reduce the power of the survival analysis, given the lack of known survival benefit from carboplatin-based chemotherapy. Further, lack of granular lab data limits the ability to control for certain confounders, for example using kidney function a surrogate for receipt of carboplatin. An additional open question is what role, if any, adjuvant chemotherapy may have in MIBC; these results do not speak to that.
Conclusions
Our study found no survival benefit with the addition NAC to a definitive course of TMT for MIBC. These results do not support the routine addition of neoadjuvant chemotherapy to definitive chemoradiation for bladder cancer, and optimizing the chemotherapy sequencing and regimens for bladder-preserving approaches to muscle invasive bladder cancer should continue to be studied under prospective clinical trials.
Supplementary Material
Clinical Practice Points:
Definitive chemoradiation therapy is a first-line treatment option for muscle invasive bladder cancer. Neoadjuvant chemotherapy improves survival when muscle invasive bladder cancer is treated with surgery or radiation. However, how neoadjuvant chemotherapy improves outcomes for those treated with definitive chemoradiation therapy is unknown. This retrospective large database study of 2,566 patients found no survival benefit with the addition of neoadjuvant chemotherapy to definitive chemoradiation. These results do not support the routine addition of neoadjuvant chemotherapy to definitive chemoradiation for bladder cancer, and optimizing the chemotherapy sequencing and regimens for bladder-preserving approaches for muscle invasive bladder cancer should continue to be studied under prospective clinical trials.
Acknowledgment:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Funding:
Dr Royce is supported by grant K12CA120780 from the National Institutes of Health via the UNC Oncology Clinical Translational Research Training Program. The research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292.
Abbreviations:
- TMT
trimodality therapy
- RC
radical cystectomy
- NAC
neoadjuvant chemotherapy
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Lobo N, Mount C, Omar K, Nair R, Thurairaja R, Khan MS. Landmarks in the treatment of muscle-invasive bladder cancer. Nat Rev Urol. 2017. doi: 10.1038/nrurol.2017.82 [DOI] [PubMed] [Google Scholar]
- 3.Royce TJ, Feldman AS, Mossanen M, et al. Comparative Effectiveness of Bladder-preserving Trimodality Therapy Versus Radical Cystectomy for Muscle-invasive Bladder Cancer. Clin Genitourin Cancer. 2019;17(1):23–31.e3. doi: 10.1016/j.clgc.2018.09.023 [DOI] [PubMed] [Google Scholar]
- 4.Leow JJ, Bedke J, Chamie K, et al. SIU–ICUD consultation on bladder cancer: treatment of muscle-invasive bladder cancer. World J Urol. 2019;37(1):61–83. doi: 10.1007/s00345-018-2606-y [DOI] [PubMed] [Google Scholar]
- 5.NCCN. Bladder Cancer (NCCN Guidelines Version 6.2020). NCCN Guidelines. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.Published2020. Accessed November 18, 2020. [Google Scholar]
- 6.James ND, Hussain SA, Hall E, et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N Engl J Med. 2012;366(16):1477–1488. doi: 10.1056/nejmoa1106106 [DOI] [PubMed] [Google Scholar]
- 7.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer — NEJM. 2003. 10.1056/NEJMoa022148. [DOI] [PubMed]
- 8.Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant Cisplatinum Based Combination Chemotherapy in Patients with Invasive Bladder Cancer: A Combined Analysis of Two Nordic Studies. Eur Urol. 2004;45(3):297–303. doi: 10.1016/j.eururo.2003.09.019 [DOI] [PubMed] [Google Scholar]
- 9.Vale C Neoadjuvant chemotherapy in invasive bladder cancer: A systematic review and meta-analysis. Lancet. 2003;361(9373):1927–1934. doi: 10.1016/S0140-6736(03)13580-5 [DOI] [PubMed] [Google Scholar]
- 10.Griffiths G International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171–2177. doi: 10.1200/JCO.2010.32.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain SA, Porta N, Hall E, et al. Outcomes in Patients with Muscle-invasive Bladder Cancer Treated with Neoadjuvant Chemotherapy Followed by (Chemo)radiotherapy in the BC2001 Trial. Eur Urol. December2020. doi: 10.1016/j.eururo.2020.11.036 [DOI] [PubMed] [Google Scholar]
- 12.Winquist E, Booth CM. Trimodality therapy for muscle-invasive bladder cancer: Concurrent chemotherapy is not enough. J Clin Oncol. 2020;38(24):2709–2711. doi: 10.1200/JCO.19.02959 [DOI] [PubMed] [Google Scholar]
- 13.Tester W, Caplan R, Heaney J, et al. Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer: results of Radiation Therapy Oncology Group phase II trial 8802. J Clin Oncol. 1996;14(1):119–126. doi: 10.1200/JCO.1996.14.1.119 [DOI] [PubMed] [Google Scholar]
- 14.Jani AB, Efstathiou JA, Shipley WU. Bladder Preservation Strategies. Hematol Oncol Clin North Am. 2015;29(2):289–300. doi: 10.1016/j.hoc.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89–03. J Clin Oncol. 1998;16(11):3576–3583. doi: 10.1200/JCO.1998.16.11.3576 [DOI] [PubMed] [Google Scholar]
- 16.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research A Review. JAMA Oncol. 2017;3(12):1722–1728. doi: 10.1001/jamaoncol.2016.6905 [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of Commission on Cancer-approved and -nonapproved hospitals in the United states: Implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27(25):4177–4181. doi: 10.1200/JCO.2008.21.7018 [DOI] [PubMed] [Google Scholar]
- 18.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. doi: 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royce TJ, Lin CC, Gray PJ, Shipley WU, Jemal A, Efstathiou JA. Clinical characteristics and outcomes of nonurothelial cell carcinoma of the bladder: Results from the National Cancer Data Base. Urol Oncol Semin Orig Investig. 2018;36(2). doi: 10.1016/j.urolonc.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 20.Torgeson A, Lloyd S, Boothe D, et al. Multiagent induction chemotherapy followed by chemoradiation is associated with improved survival in locally advanced pancreatic cancer. Cancer. 2017;123(19):3816–3824. doi: 10.1002/cncr.30780 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Nickleach DC, Zhang C, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS ®macros. F1000Research. 2019;7. doi: 10.12688/f1000research.16866.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giacalone NJ, Shipley WU, Clayman RH, et al. Long-term Outcomes After Bladder-preserving Trimodality Therapy for Patients with Muscle-invasive Bladder Cancer: An Updated Analysis of the Massachusetts General Hospital Experience. Eur Urol. 2017:1–9. doi: 10.1016/j.eururo.2016.12.020 [DOI] [PubMed] [Google Scholar]
- 23.Jiang D (Maria), Jiang H, Chung PWM, et al. Neoadjuvant Chemotherapy Before Bladder-Sparing Chemoradiotherapy in Patients With Nonmetastatic Muscle-Invasive Bladder Cancer. Clin Genitourin Cancer. 2019;17(1):38–45. doi: 10.1016/j.clgc.2018.09.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.