Abstract
Purpose
Teprotumumab, a blocking antibody to the insulin like growth factor 1 receptor (IGF-1R) has been shown to significantly reduce proptosis in recent phase 2 and 3 trials in patients with inflammatory thyroid eye disease (TED). Herein, we investigate the impact of teprotumumab on patients with non-inflammatory TED. We also investigate the expression of the IGF-1R on orbital tissues from patients with inflammatory and non-inflammatory TED compared to controls.
Methods
Consecutive patients with non-inflammatory TED (clinical activity score, CAS ≤ 1, for at least 4 months, were treated with teprotumumab. They received a complete course (total eight infusions) of teprotumumab (10 mg/kg for the first infusion and 20 mg/kg for subsequent infusions every 3 weeks). The primary outcome was a proptosis response at week 24. Further, IGF-1R α and β expression was evaluated on orbital tissue from patients with inflammatory and non-inflammatory TED, as well as healthy controls. Non-biased histological analysis of IGF-1R expression was performed using ImageJ.
Results
Four patients met eligibility criteria for the clinical study, with a mean (SD) CAS of 0 (0). Following teprotumumab treatment, there was a mean (SD) reduction in proptosis of 2.6 mm (1.2). Five patients were included for each group of the histological study; inflammatory TED, non-inflammatory TED and controls. IGF-1Rα and IGF-1Rβ expression was significantly greater in the orbital tissues of patients with inflammatory TED and non-inflammatory TED, when compared to controls.
Conclusion
Our findings demonstrate for the first time, that teprotumumab, a blocking antibody to the IGF-1R reduces proptosis in a series of patients with non-inflammatory TED. Overexpression of the IGF-1R in orbital tissue from patients with non-inflammatory disease compared to controls may be an important consideration for effect.
Subject terms: Autoimmunity, Mechanisms of disease
Introduction
The natural history of thyroid eye disease (TED) may be divided into two phases, an inflammatory phase and non-inflammatory phase. Typically, patients present with an initial inflammatory phase which may last between 6 months to 3 years and is characterised by an increase in inflammatory scores such as the clinical activity score (CAS) [1]. Non-inflammatory TED is characterised by fibrosis and or soft tissue expansion [2]. A CAS ≥ 3 may be used to define inflammatory TED, while a CAS < 3 may define non-inflammatory TED [3].
The relationship between inflammation and severity of outcome is not well established [4]. In a previous report, we reported on a group of patients who presented with non-inflammatory TED. This group is presented with progressive strabismus, proptosis or optic neuropathy without having had any signs of inflammation at any point during their condition. They did not have an inflammatory phase, but instead showed signs of a chronic insidious process dominated by fibrosis [5]. This group of patients may represent a therapeutic blind spot, since medical therapy for TED focuses on the resolution of inflammatory signs and therefore, trials evaluating potential treatments have only included patients with inflammatory signs (CAS ≥ 3) and excluded those with non-inflammatory TED [6–9].
Over the past two decades, progress on the understanding of the pathophysiology of TED has focused on the fundamental observation that the insulin like growth factor 1 receptor (IGF-1R) is overexpressed on orbital fibroblasts (OFs) derived from patients with Graves’ disease and TED [10]. Previous work has demonstrated that overexpression of IGF-1R on these tissues and its activation may drive the disease through deposition of hyaluronan by OFs [11]. These fundamental observations led to the clinical development of a blocking molecule which may interfere with this activation. Teprotumumab, an IGF-1R antibody, was recently approved by the FDA for the treatment of TED. Subsequent phase 2 and phase 3 trials demonstrated a significant clinical reduction in proptosis and the CAS in patients with active TED [12, 13]. These trials only included patients with inflammatory TED (CAS ≥ 4) and excluded those with non-inflammatory TED. Therefore, questions remain regarding the efficacy of teprotumumab in patients with non-inflammatory disease.
We hypothesise that teprotumumab may have a clinical effect in patients with non-inflammatory TED. In this study, we report for the first time on (i) the improvement of proptosis of patients who have received teprotumumab, and (ii), demonstrate a sustained expression of IGF-1R in orbital tissues from patients with non-inflammatory TED compared to normal controls.
Methods
This study adhered to the tenets of the Declaration of Helsinki, was performed in accordance with the Health Insurance Portability and Accountability Act and was approved by our institutional review board.
Patients
Consecutive patients with non-inflammatory TED (CAS ≤ 1), for at least 4 months, prior to initiation of teprotumumab therapy were screened for study eligibility. The CAS was evaluated by two separate observers. Patients received a complete course (total eight infusions) of teprotumumab (10 mg/kg for the first infusion and 20 mg/kg for subsequent infusions) every 3 weeks. Patients who had previous orbital surgery or radiation were excluded.
Measurement of clinical outcomes
The primary outcome was measurement of proptosis. This was assessed using the same Hertel exophthalmometer by the same observer at each clinical site.
Immunohistochemistry
Tissue collection
Orbital fat from consecutive patients with inflammatory and non-inflammatory TED was collected during orbital decompression surgery for exophthalmos or compressive optic neuropathy. Patients were excluded if they had previous radiation therapy. Control tissues were obtained during enucleation surgery. Patients in this group were excluded if they had any medical or surgical pathology that might have an impact on orbital fat or muscle. Only patients with intraocular pathology and no extension of this pathology beyond the sclera were included in this group.
Tissue samples were collected from five patients with inflammatory TED, five with non-inflammatory TED and five controls. All TED patients had received systemic steroids. For the controls, one patient underwent enucleation for a choroid solitary fibroma, three patients underwent enucleation for a choroid melanoma, and one had an enucleation due to a retinoblastoma. There were no signs of orbital extension on imaging for both patients.
Sample processing
Following collection, tissues were immediately stored in a universal tube with 10% formalin solution before being processed for embedding in paraffin. Paraffin section thickness was 3 µm and sections were cut every 0.6 mm, to generate six sections per sample. Prior to tissue staining, paraffin was removed, and each section was rehydrated with graded concentrations of alcohol. The primary antibodies used were anti IGF-1R alpha (Santa Cruz Biotechnology, Dallas, TX, USA, serial no. sc-271606 and sc-461) and anti-IGF-1R beta (Cell Signalling Technology, Beverly, MA, USA, serial no. 3027S and from Santa Cruz Biotechnology, Dallas, TX, USA, serial no. sc-81167). The antigen–antibody reaction was visualised with the streptavidin-biotin HRP/DAB (Horseradish Peroxide/3,3′-Diaminobenzidine) method. Cell nuclei were stained with haematoxylin. Histological image analysis was performed using Image Pro Plus, version 6.2 software (Media Cybernetics, Bethesda, MD, USA) installed on a PC, which received its signal through a KY-F550E colour video camera (JVC) mounted on a Zeiss Axioscope microscope (Carl Zeiss, Welwyn Garden City, UK). Images were analysed at a magnification of ×100.
Analysis of immunohistochemistry
A tiled image of each section was acquired and stored on a PC as a JPEG file. Each image was then analysed using ImageJ (NIH, Bethesda, MD, USA) with the immunohistochemistry plugin. For analysis of stained particles, each image was first converted to 8-bit grayscale. The threshold function was then used to manually delineate stained regions, to suppress low intensity signals. The ‘analyse particles’ function was then used to automatically count the number of stained regions in each image. This is a non-biased feature that automatically counts stained regions based on the colour (stain) within each pixel.
Statistical analysis
Statistical analysis was performed using SPSS version 22.0 (SPSS, Inc, Chicago, Illinois, USA). The difference between pre and post treatment exophthalmometry measurements was calculated using a dependent t test. Statistical significance was defined as p < 0.05. Differences in staining between inflammatory, non-inflammatory and controls were analysed using an independent t test.
Results
Clinical study
Four consecutive patients treated with teprotumumab with CAS ≤ 1 met eligibility criteria. Clinical and demographic details are provided in Table 1. Following treatment with teprotumumab, there was a significant reduction in proptosis (mean, 2.6 mm, SD, 1.2 mm), p < 0.01 (Fig. 1). The CAS and diplopia scores remained at 0.
Table 1.
Clinical and demographic details of patients.
| Case | Gender | Age | Duration of TED (months) | Smoker? | Exophthalmometry (mm) at Baseline | Exophthalmometry (mm) post treatment | CAS | Gorman Diplopia Score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| OD | OS | OD | OS | |||||||
| 1 | F | 22 | 12 | No | 24 | 25 | 21 | 21 | 0 | 0 |
| 2 | F | 32 | 7 | No | 20 | 21 | 19 | 19 | 0 | 0 |
| 3 | F | 47 | 4 | No | 18 | 16 | 15 | 14 | 0 | 0 |
| 4 | M | 21 | 5 | No | 21 | 19 | 17 | 16 | 0 | 0 |
Fig. 1. Change in proptosis.
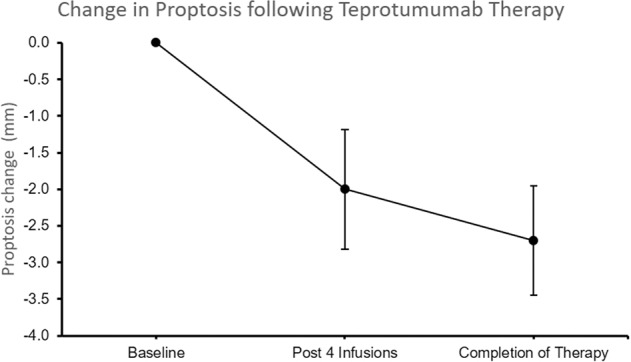
Change in proptosis following Teprotumumab therapy.
IGFR expression is increased on tissue samples of inflammatory and non-inflammatory TED compared to controls
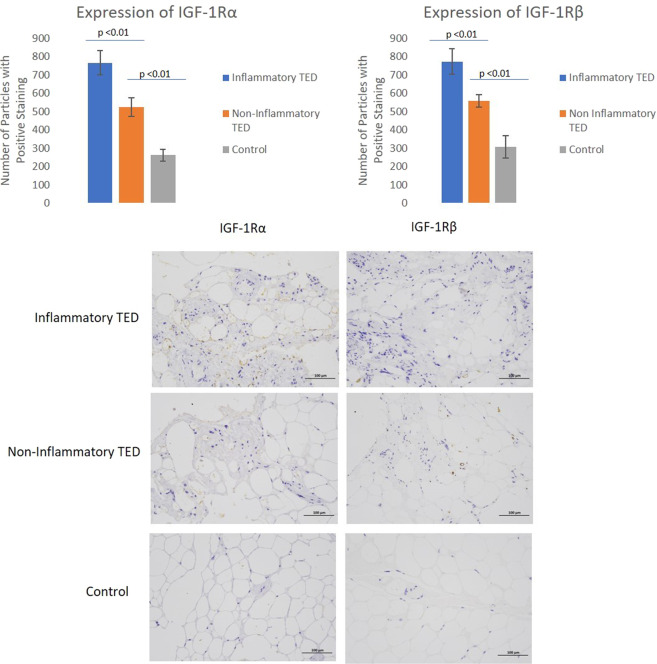
Orbital tissue was obtained from five patients with inflammatory TED, five with non-inflammatory TED and five controls. Clinical and demographic information is provided in Table 2. For each patient, there were 12 sections. The mean (SD) discrete regions staining positively with IGF-1Rα antibody for inflammatory patients was 767 (439), while a mean (SD) of 525 (333) was found in non-inflammatory TED patients and 262 (208) for controls. The difference between inflammatory and non-inflammatory patients was significant (p < 0.01). Further, the difference between non-inflammatory TED patients and controls was also significant (p < 0.01) (Fig. 2).
Table 2.
Clinical and demographic details of patients and controls who contributed samples for IGF-1R staining.
| Case | Gender | Age | Smoker? | Duration of TED (months) | CAS |
|---|---|---|---|---|---|
| Non-Inflammatory TED 1 | M | 43 | Yes (20 years) | 9 | 1 |
| Non-Inflammatory TED 2 | M | 70 | No | 18 | 1 |
| Non-Inflammatory TED 3 | F | 47 | No | 12 | 1 |
| Non-Inflammatory TED 4 | F | 33 | No | 9 | 1 |
| Non-Inflammatory TED 5 | F | 57 | No | 108 | 1 |
| Inflammatory TED 1 | M | 29 | Yes (8 years) | 6 | 5 |
| Inflammatory TED 2 | M | 35 | Yes (10 years) | 14 | 4 |
| Inflammatory TED 3 | F | 50 | No | 88 | 3 |
| Inflammatory TED 4 | F | 49 | No | 15 | 3 |
| Inflammatory TED 5 | M | 53 | Yes (30 years) | 12 | 3 |
| Control 1 | M | 48 | No | – | – |
| Control 2 | F | 63 | No | – | – |
| Control 3 | M | 47 | No | – | – |
| Control 4 | F | 42 | No | – | – |
| Control 5 | M | 53 | No | – | – |
Fig. 2. IGF1α and IGF1β staining.
Differences in IGF-1R α and β staining in inflammatory, non-inflammatory TED and controls.
When staining with IGF-1Rβ, an average (SD) of 773 (441) discrete regions stained positively in the samples collected from inflammatory TED patients, whereas an average (SD) of 557 (401) regions stained positively in samples from non-inflammatory TED patients and an average (SD) 307 (218) of discrete regions stained positively in control samples. The difference between inflammatory TED samples and stable samples was significant (<0.01). The difference between non-inflammatory TED samples and controls was also significant (p < 0.01) (Fig. 2).
Discussion
Teprotumumab was shown to significantly reduce proptosis in patients with inflammatory TED in phase 2 [12] and three trials [13] defined as having a CAS > 3. However, these trials were restricted to patients with inflammatory TED and specifically excluded patients who lacked inflammatory signs of TED but exhibited proptosis. We demonstrate for the first time in a clinical series that teprotumumab effectively reduced proptosis of patients with non-inflammatory TED. The current study also demonstrates an increased expression of IGF-1Rα and IGF-1Rβ in the orbital tissue of patients with both inflammatory and non-inflammatory TED when compared to controls.
Given the overexpression of IGF-1R on fibroblasts in patients with inflammatory TED, our data suggest that persistent increased expression of the IGF-1R is also likely in the non-inflammatory phase. In previous studies, stimulation of TED fibroblasts with IGF-1 led to an increased production of hyaluronan [14]. Orbital tissue from non-inflammatory TED, also has increased expression of IGF-1R which may explain the reduction in proptosis by teprotumumab. Given that overexpression of IGF-1R persists in non-inflammatory TED and the clinical response in this study, there is role for the use of teprotumumab therapy in patients who have had TED for long periods with no inflammation.
The limitations of the clinical study relate to the modest number of patients. However, this is circumvented by the large magnitude of impact of teprotumumab on proptosis and the longitudinal nature of the clinical study. Further, despite a modest number of patients being used for the histopathological study, the difference in expression of both IGF-1Rs (α and β) in inflammatory, non-inflammatory and control patients was marked.
At present, there are no medical treatments for patients with non-inflammatory TED. This study for the first time, demonstrates an effective medical therapy in patients with non-inflammatory TED.
Summary
What was known before
Teprotumumab, an IGF-1R inhibitor reduces proptosis and inflammation in active thyroid eye disease.
The expression of the IGF-1R is increased in active thyroid eye disease.
What this study adds
We have found that Teprotumumab, also reduces proptosis and inflammation in inactive thyroid eye disease.
The expression of the IGF-1R is also increased in inactive thyroid eye disease.
Compliance with ethical standards
Conflict of interest
RD—Consultant Horizon Therapeutics, Immunovant Corporation. The other authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–38. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ugradar S, Rootman DB. Case Series. Ophthal Plast Reconstr Surg 2018:1. http://insights.ovid.com/crossref?an=00002341-900000000-98532. Accessed 27 Mar 2018.
- 3.Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–44. doi: 10.1136/bjo.73.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JW, Woo YJ, Yoon JS. Is modified clinical activity score an accurate indicator of diplopia progression in Graves’ orbitopathy patients? Endocr J. 2016;63:1133–40. doi: 10.1507/endocrj.EJ16-0165. [DOI] [PubMed] [Google Scholar]
- 5.Ugradar S, Rootman DB Case Series. Ophthal Plast Reconstr Surg 2018:1. http://www.ncbi.nlm.nih.gov/pubmed/29465483. Accessed 28 Feb 2018.
- 6.Bartalena L, Krassas GE, Wiersinga W, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97:4454–63. doi: 10.1210/jc.2012-2389. [DOI] [PubMed] [Google Scholar]
- 7.DPF L, S R, M B, et al. Low dose of rituximab for corticosteroid-resistant graves’ orbitopathy. Eur Thyroid J. 2014;3:110. [Google Scholar]
- 8.Kahaly GJ, Riedl M, König J, et al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol. 2018;6:287–98. doi: 10.1016/S2213-8587(18)30020-2. [DOI] [PubMed] [Google Scholar]
- 9.Rajendram R, Taylor PN, Wilson VJ, et al. Combined immunosuppression and radiotherapy in thyroid eye disease (CIRTED): a multicentre, 2 × 2 factorial, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6:299–309. doi: 10.1016/S2213-8587(18)30021-4. [DOI] [PubMed] [Google Scholar]
- 10.Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181:4397–405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168:942–50. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 12.Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376:1748–61. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382:341–52. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 14.Smith TJ, Hoa N. Immunoglobulins from patients with graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. J Clin Endocrinol Metab. 2004;89:5076–80. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]