Abstract
Purpose
The purpose of this study is to examine the seasonal patterns of incidence, demographic factors and microbiological profiles of infectious keratitis (IK) in Nottingham, UK.
Methods
A retrospective study of all patients who were diagnosed with IK and underwent corneal scraping during 2008–2019 at a UK tertiary referral centre. Seasonal patterns of incidence (in per 100,000 population-year), demographic factors, culture positivity rate and microbiological profiles of IK were analysed.
Results
A total of 1272 IK cases were included. The overall incidence of IK was highest during summer (37.7, 95% confidence interval (CI): 31.3–44.1), followed by autumn (36.7, 95% CI: 31.0–42.4), winter (36.4, 95% CI: 32.1–40.8) and spring (30.6, 95% CI: 26.8–34.3), though not statistically significant (p = 0.14). The incidence of IK during summer increased significantly over the 12 years of study (r = 0.58, p = 0.049), but the incidence of IK in other seasons remained relatively stable throughout the study period. Significant seasonal variations were observed in patients’ age (younger age in summer) and causative organisms, including Pseudomonas aeruginosa (32.9% in summer vs. 14.8% in winter; p < 0.001) and gram-positive bacilli (16.1% in summer vs. 4.7% in winter; p = 0.014).
Conclusion
The incidence of IK in Nottingham was similar among four seasons. No temporal trend in the annual incidence of IK was observed, as reported previously, but there was a significant yearly increase in the incidence of IK during summer in Nottingham over the past decade. The association of younger age, P. aeruginosa and gram-positive bacilli infection with summer was likely attributed to contact lens wear, increased outdoor/water activity and warmer temperature conducive for microbial growth.
Subject terms: Epidemiology, Corneal diseases
Introduction
Infectious keratitis (IK) is a common ophthalmic emergency characterised by a variety of manifestations, including corneal ulceration, stromal infiltrates and varying degree of anterior chamber reaction. It is responsible for ~2 million monocular blindness per year, with higher rates reported in developing countries [1]. A wide range of microorganisms, including bacteria, fungi, viruses and parasites, particularly Acanthamoeba, have been implicated in IK [2, 3]. In view of the diverse causative microorganisms, broad-spectrum topical antimicrobial treatment is often commenced initially and supplemented by adjuvant therapies when required [4–6].
IK is primarily diagnosed on clinical grounds with the support of microbiological investigations, commonly in the form of corneal scraping for microscopy, culture and sensitivity testing. However, this current diagnostic approach is challenged by several issues, including the variably low culture yield, the slow turnaround time for positive results (usually 24–48 h from the corneal samples being taken), contamination and the possibility of polymicrobial infection [1, 7, 8]. As the specific cause of IK is often indistinguishable from the clinical features, gaining knowledge about the patterns of microbiological profiles of IK in a particular region may provide additional guidance to the clinicians on the antimicrobial therapy.
Geographical and temporal variations of IK have been well reported in the literature, with bacteria and fungi being shown as the most common microorganisms responsible for IK in developed and developing countries, respectively [2, 3, 9]. However, examination of the seasonal trends in the incidence and causative microorganisms of IK remains limited (Table 1) [10–16]. So far, there are only three studies in the literature that examined the seasonal variations in the rate of IK in the UK [11, 13, 16]. Otri et al. [13] previously reported a higher proportion of IK during the summer season in Nottingham between 2007 and 2010; however, only 129 cases of sight-threatening IK were included in the study. In addition, only one UK study, conducted in Manchester, examined the seasonal variations in the causative microorganisms of IK [16].
Table 1.
Summary of the seasonal trends in the rate and microbiological profiles of infectious keratitis in the literature, in the order of chronology.
| Year | Authors | Study period | Sample sizea | Location | Overall seasonal rate | Microbiological profilesb |
|---|---|---|---|---|---|---|
| 2008 | Green et al. [10] | 1999–2004 | 253 | Brisbane, Australia | Not examined |
P. aeruginosa (in summer); S. pneumonia (in winter) |
| 2009 | Ibrahim et al. [11] | 1997–2003 | 1786 | Portsmouth, UK | Summer > winter > autumn > spring | Not examined |
| 2012 | Lin et al. [12] | 2006–2009 | 6967 | Southeast India | Summer > winter > spring/autumn |
Fungi (in summer); P. aeruginosa (in July–December) |
| 2013 | Otri et al. [13] | 2007–2010 | 129 | Nottingham, UK | Summer > spring > winter > autumn | Not examined |
| 2015 | Ni et al. [14] | 2009–2012 | 313 | Philadelphia, US | Spring > autumn > summer > winter | Bacteria (in spring) |
| 2016 | Gorski et al. [15] | 2008–2013 | 155 | New York, US | Summer > winter > spring > autumn | P. aeruginosa (in summer) |
| 2018c | Walkden et al. [16] | 2004–2015 | 4229 | Manchester, UK | Winter > autumn > spring > summer |
P. aeruginosa (in summer); CoNS (in autumn); Candida (in summer) |
| 2020 |
Ting et al. (current study) |
2008–2019 | 1272 | Nottingham, UK | Summer > autumn > winter > spring |
P. aeruginosa (in summer); Gram-positive bacilli (in summer) |
aNumber of cases of infectious keratitis.
bCausative microorganisms that demonstrated significant seasonal predilection.
cThe reported seasonal rate refers to the culture positivity rate of infectious keratitis but not the overall rate of infectious keratitis.
We recently reported the incidence, causative microorganisms and in vitro antibiotic susceptibility of IK In Nottingham, UK, over the past decade [17]. We observed a relatively stable trend of incidence (estimated at 34.7 per 100,000 population-year) and Pseudomonas aeruginosa was found to be the most common organism for IK. However, the seasonal patterns of these aspects have not been elucidated. In view of the paucity of literature, this study aimed to provide an up-to-date and comprehensive examination of the seasonal variations in the incidence, demographic factors, culture positivity rate, microbiological profiles and antibiotic susceptibility of IK in Nottingham.
Materials and methods
This was a retrospective study of all patients who were diagnosed with IK and underwent corneal scraping between January 2008 and December 2019 (a 12-year period) at the Queen’s Medical Centre (QMC), Nottingham, UK. The study method used was similar to the previous study but with a different objective and a slightly different study period [17]. Cases were identified through the local microbiology electronic database. QMC was the only tertiary ophthalmic referral centre in the city of Nottingham with an embedded eye casualty that was open 24 h a day throughout the year to manage patients with emergency and urgent ophthalmic conditions, including IK. There were two other nearby hospitals in the East Midlands region, including Kings Mill Hospital and Derby Royal Hospital, which covered a different subset of the population outside Nottingham and were not included in our local database.
Based on the departmental guideline for IK, all patients presenting with sight-threatening corneal ulcers—defined as size > 1 mm diameter, central location, associated melting or hypopyon or atypical presentation—were subjected to microbiological investigation such as corneal scraping for microscopy (with gram staining), microbial culture and sensitivity testing [17]. Corneal scrapes were inoculated on chocolate agar (for fastidious organisms), blood agar (for bacteria) and Sabouraud dextrose agar (for fungi). For suspected cases of Acanthamoeba keratitis, non-nutrient Escherichia coli-enriched agar plate was used for inoculation. All cultures were incubated for at least 1 week (and up to 3 weeks for suspected Acanthamoeba keratitis cases). The identity of the microorganisms was confirmed through standard culture and bacteriology tests. Corneal scraping was repeated in the same eye when the patient was unresponsive to treatment regardless of positive or negative outcome of the first culture. These cases were only counted as one clinical episode.
For descriptive and analytic purposes, the causative microorganisms were categorised into gram-positive and gram-negative bacteria, fungi and Acanthamoeba. Seasons were divided into winter (22 December to 21 March), spring (22 March to 21 June), summer (22 June to 21 September) and autumn (22 September to 21 December), as defined by the internationally recognised astronomical seasons and previous studies [14, 15, 18]. The population in Nottingham was estimated at the range between 300,000 and 328,000 people during the 12-year study period (https://www.ukpopulation.org/nottingham-population/). The number of populations used to estimate the yearly incidence of IK in Nottingham, UK, is provided in Supplementary Table 1.
The study was conducted in accordance with the tenets of Declaration of Helsinki and was approved by the Nottingham University Hospitals NHS Trust as a service evaluation study (reference number: 19–265C).
Statistical analysis
Statistical analysis was performed using SPSS version 26.0 (IBM SPSS Statistics for Windows, Armonk, NY, USA). Chi-square test or one-way analysis of variance (ANOVA) was performed, where appropriate, to analyse the seasonal patterns of incidence, demographic factors and microbiological profiles of IK among the four seasons. All continuous data were presented as mean ± standard deviation and/or 95% confidence interval (CI). Pearson’s correlation coefficient (r) analysis was performed to examine the incidence of IK in each season over time and was interpreted as weak (r = 0.00–0.40), moderate (r = 0.41–0.69) or strong (r = 0.70–1.00), with negative values being interpreted in the same way [19]. p value of ≤0.05 was considered statistically significant. When multiple subgroups were analysed in chi-square test, crude Bonferroni-type adjustment was used to keep the overall false positive rate or alpha level at 0.05 (e.g., if comparison of five subgroups was performed, the adjusted p value of ≤0.01 (based on 0.05/5) was considered significant) [20].
Results
Overall description
During the 12-year study period, a total of 1272 cases of IK were included. The mean patient’s age was 50.0 ± 22.2 years and 50.2% were male. Of all cases, 468 (36.8%) cases were culture positive with 549 microorganisms being identified (Table 2).
Table 2.
Summary of the seasonal patterns in demographic factors, culture positivity rate and microbiological profiles of infectious keratitis in Nottingham, UK, between January 2008 and December 2019.
| Winter n (%) | Spring n (%) | Summer n (%) | Autumn n (%) | p value* | |
|---|---|---|---|---|---|
| Age, years | 52.4 ± 22.6 | 51.1 ± 22.5 | 48.3 ± 22.2 | 48.4 ± 21.5 | 0.044 |
| Gender | 0.88 | ||||
| Female | 163 (49.7) | 138 (50.2) | 174 (51.3) | 159 (48.2) | |
| Male | 165 (50.3) | 137 (49.8) | 165 (48.7) | 171 (51.8) | |
| Culture result | 0.69 | ||||
| Positive | 114 (34.8) | 101 (36.7) | 133 (39.2) | 120 (36.4) | |
| Negative | 214 (65.2) | 174 (63.3) | 206 (60.8) | 210 (63.6) | |
| Organismsa | |||||
| Gram-positive | 70 (54.7) | 73 (60.3) | 77 (49.7) | 78 (53.8) | 0.37 |
| Staphylococci | 46 (35.9) | 38 (31.4) | 35 (22.5) | 40 (27.6) | 0.055 |
| Streptococcic | 18 (14.1) | 17 (14.0) | 17 (11.0) | 24 (16.6) | 0.50 |
| Bacilli | 6 (4.7) | 18 (14.9) | 25 (16.1) | 14 (9.7) | 0.014 |
| Gram-negative | 48 (37.5) | 38 (31.4) | 70 (45.2) | 55 (37.9) | 0.14 |
| P. aeruginosa (PA) | 19 (14.8) | 17 (14.0) | 51 (32.9) | 38 (26.2) | <0.001 |
| Non-PA | 29 (22.7) | 21 (17.4) | 19 (12.2) | 17 (11.7) | 0.036 |
| Fungi | 4 (3.1) | 4 (3.3) | 4 (2.6) | 5 (3.4) | 0.98 |
| Acanthamoeba | 6 (4.7) | 6 (5.0) | 4 (2.6) | 7 (4.8) | 0.70 |
| Antibiotics, % (Y/N)b | |||||
| Cephalosporin | 81.8 (27/6) | 90.9 (20/2) | 85.2 (23/4) | 90.0 (18/2) | 0.75 |
| Aminoglycoside | 97.5 (79/2) | 92.6 (63/5) | 97.2 (104/3) | 97.7 (86/2) | 0.28 |
| Fluoroquinolone | 92.4 (97/8) | 93.2 (82/6) | 96.6 (115/4) | 97.2 (104/3) | 0.27 |
Significant p-values are underlined.
Continuous values are presented in mean ± standard deviation.
*Comparison was made among the four seasons using chi-square test or ANOVA test, where appropriate. p value of ≤0.05 was considered statistically significant. Adjusted p value, using crude Bonferroni-type adjustment, was used when multiple pair-wise comparisons were performed. This adjustment was performed for analysis of organisms (at first and second levels separately) and antibiotics.
aIncluded all culture positive cases only and some cases cultured more than one organism. Comparison of organisms among four seasons was performed; (1) first level examining the four main groups, namely, gram-positive and gram-negative bacteria, fungi and Acanthamoeba; and (2) second level examining only the difference in the five bacterial subgroups.
bRefers to antibiotic susceptibility, presented in % of susceptibility (Y = susceptible/N = resistant). The total number may vary as not all organisms were tested against all three classes of antibiotics.
cIncluded two cases of Enterococcus faecalis (one in spring and one in summer).
Seasonal pattern in incidence of IK
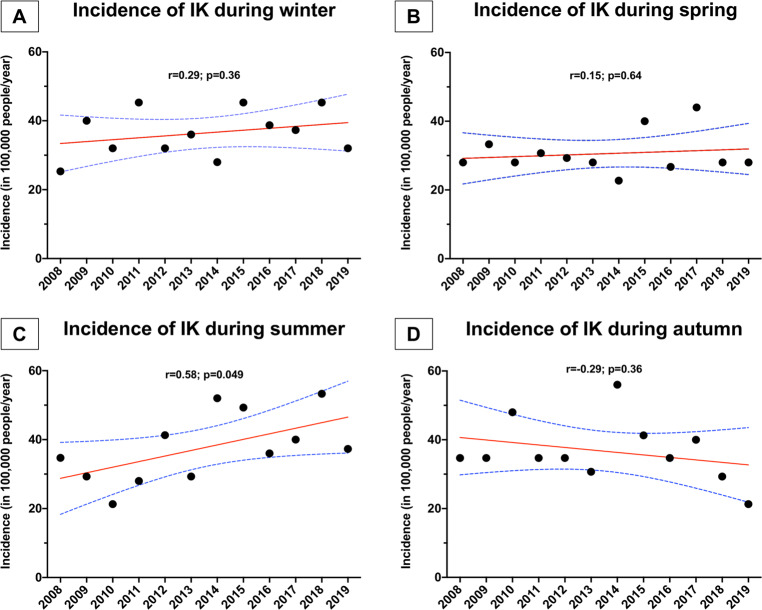
The overall incidence of IK (in per 100,000 population-year) was highest during summer (37.7, 95% CI: 31.3–44.1), followed by autumn (36.7, 95% CI: 31.0–42.4), winter (36.4, 95% CI: 32.1–40.8) and spring (30.6, 95% CI: 26.8–34.3), though the overall difference was not statistically significant (p = 0.14; Fig. 1). Over the 12-year study period, there was a significant yearly increase in the incidence of IK during summer (r = 0.58, p = 0.049), but the incidence of IK in other seasons remained stable over time (Fig. 2).
Fig. 1. Seasonal patterns in the incidence of infectious keratitis in Nottingham, UK, between January 2008 and December 2019.
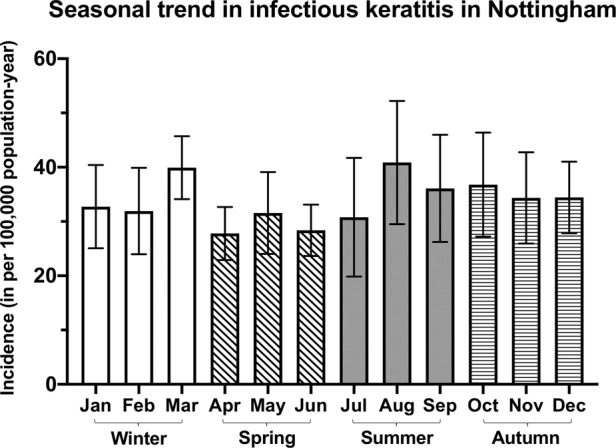
The monthly incidence is presented as mean with 95% confidence interval (depicted by the error bars). For better graphical presentation purpose, “22 Dec–21 Jan” was referred to as month “January”, “22 Jan–21 Feb” was referred to as month “February”, and so on.
Fig. 2. A summary of the temporal changes of the annual incidence of infectious keratitis of each season.
Temporal changes of the incidence of infectious keratitis in Nottingham, UK, during: A winter; B spring; C summer; and D autumn.
Seasonal patterns of demographic factors and microbiological profiles of IK
A total of 549 causative microorganisms were identified during the study period. There was a small but significant difference in the patient’s age among the four seasons (p = 0.044), with a younger group of patients (48.3 ± 22.2 years) presenting during the summer and older group of patients (52.4 ± 22.6 years) presenting during the winter (Table 2). In addition, seasonal predilection was observed in some causative organisms such as P. aeruginosa (32.9% in summer vs. 14.8% in winter; p < 0.001) and gram-positive bacilli (16.1% in summer vs. 4.7% in winter; p = 0.014), which included Propionibacterium spp., Corynebacterium spp. and Bacillus spp. (Table 2). There were no seasonal variations in gender, culture positivity rate and antibiotic susceptibility of IK demonstrated among the four seasons.
Discussion
Seasonal cyclicity is a common feature of infectious diseases in general [21]. Depending on the causative pathogens, geographical and temporal factors and host susceptibility, certain diseases are more common in particular seasons [21, 22]. For instance, influenza and rotavirus-related gastroenteritis were shown to be more common during the winter season (in temperate zones) [21, 23], whereas tuberculosis peaked during summer in some countries such as the UK [23, 24]. Understanding of the seasonal patterns of infectious diseases, including IK, could have important implications on the public health, disease control and biology [21].
To the best of our knowledge, this represents the most up-to-date and largest study examining the seasonal variations in incidence, demographic factors and microbiological profiles of IK in Nottingham, UK. We observed that IK was most prevalent during summer (37.7 per 100,000 population-year), accompanied by a significant increase over the past decade. This was similar to other studies conducted in the UK [11, 13] and in other parts of the world such as India [12] and the US [15], which also reported and a higher rate of IK during summer. Gaining knowledge on the seasonal rate or incidence of IK helps increase the vigilance for IK among clinicians, including ophthalmologists and non-ophthalmologists who work at the front-line service such as accident and emergency department and primary care setting, during the prevalent season.
Plausible explanations for this seasonal phenomenon include raised temperature, which may help the microorganisms to flourish, increased outdoor activities, contact with water and use of contact lenses during the summer period, which could increase the risk of corneal injury and infection [15]. However, further studies examining the seasonal variations of the risk factors are required to elucidate the findings observed in our study. Interestingly, Walkden et al. [16] reported that the culture positivity rate of IK was highest during winter and lowest during summer but it is uncertain whether the overall seasonal incidence of IK in their region could be inferred from these findings.
In addition, we observed significant seasonal variations in P. aeruginosa and gram-positive bacilli during the past decade. P. aeruginosa infection was most commonly observed during summer and was responsible for 33% of all IK. Similarly, a higher rate of P. aeruginosa infection in summer has been reported in other studies [10, 12, 15, 16], which was attributed to warmer temperature and use of contact lens. We also observed a significantly higher proportion of gram-positive bacilli infection during summer when compared to winter. Gram-positive bacilli, including Propionibacterium spp. and Corynebacterium spp., are common ocular surface commensals [25, 26] and the growth has been shown to be most active or optimal at the temperature between 30 and 37 °C [27], which may account for the higher rate of these infections during summer. Furthermore, Lin et al. [12] have also demonstrated a significantly higher rate of fungal infection during summer in Southeast India. The number of fungal or Acanthamoeba infection was very low (<5%) in our study and any seasonal variation was observed.
Interestingly, studies have also shown that postoperative infection may be higher during summer. For instance, Anthony et al. [28] demonstrated that surgical site infections following knee and hip arthroplasty were most common in summer, with increased re-admission for treatment of post-surgical infection during the same season. It would be interesting to examine whether this observation can be generalised to IK following ocular surface and/or refractive surgeries, particularly our study found that there was a significant higher rate of infection related to ocular surface commensals (i.e., Propionibacterium spp. and Corynebacterium spp.) during the summer season.
One of the limitations of our study is that we only included IK cases that had undergone corneal scraping; therefore, the overall incidence of IK in our region is likely to be underestimated. Nevertheless, there was no seasonal disparity in the practice pattern (e.g., culture method or threshold for performing corneal scraping) in our unit, suggesting that the findings related to the seasonal variations of IK observed in our study should not be affected. Cases referred from elsewhere usually have scrapes performed and antibiotic treatment initiated at the referring hospital. Culture results from these patients would not be captured in our microbiology database. Another limitation is that the full representation of the causative microorganisms in this study was hindered by the relatively low positive culture rate, which is a common issue in many IK studies [1]. Emerging investigative techniques such as in vivo confocal microscopy [29, 30], MALDI-TOF mass spectrometry [31, 32], polymerase chain reaction and/or next generation sequencing [33] and artificial intelligence-assisted systems [34] could potentially enhance the diagnostic yield of IK in the future.
In conclusion, there has been a significant increase in IK during summer in Nottingham, UK, over the past decade. Increased awareness of IK during this season should be raised among the general public and the healthcare service. Gram-positive bacilli and P. aeruginosa infections are significantly more common in summer and these observations may provide additional guidance on the antimicrobial therapy used in our region. Further studies investigating the correlations between these observations and the predisposing factors of IK will be beneficial.
Summary
What was known before
Infectious keratitis is a common yet potentially sight-threatening ocular condition.
Seasonal variation in the rate/incidence of infectious keratitis has been previously demonstrated; however, influence on the causative microorganisms is not well known.
What this study adds
This represents the most up-to-date and comprehensive study examining the seasonal pattern of infectious keratitis in Nottingham, UK.
There has been a significant increase in the incidence of infectious keratitis during summer in Nottingham.
Infections caused by Pseudomonas aeruginosa and gram-positive bacilli are more commonly observed during the summer season.
Supplementary information
Acknowledgements
Funding
DSJT acknowledges support from the Medical Research Council/Fight for Sight Clinical Research Fellowship (MR/T001674/1) and the Fight for Sight/John Lee, Royal College of Ophthalmologists Primer Fellowship (24CO4).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41433-020-01272-5) contains supplementary material, which is available to authorised users.
References
- 1.Ung L, Bispo PJM, Shanbhag SS, Gilmore MS, Chodosh J. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64:255–71. doi: 10.1016/j.survophthal.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khor WB, Prajna VN, Garg P, Mehta JS, Xie L, Liu Z, et al. The Asia Cornea Society Infectious Keratitis Study: a prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol. 2018;195:161–70. doi: 10.1016/j.ajo.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Ting DSJ, Settle C, Morgan SJ, Baylis O, Ghosh S. A 10-year analysis of microbiological profiles of microbial keratitis: the North East England Study. Eye. 2018;32:1416–7. doi: 10.1038/s41433-018-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124:1678–89. doi: 10.1016/j.ophtha.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, Glidden DV, et al. Corticosteroids for bacterial keratitis: the Steroids for Corneal Ulcers Trial (SCUT) Arch Ophthalmol. 2012;130:143–50. doi: 10.1001/archophthalmol.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ting DSJ, Henein C, Said DG, Dua HS. Photoactivated chromophore for infectious keratitis—Corneal cross-linking (PACK-CXL): a systematic review and meta-analysis. Ocul Surf. 2019;17:624–34. doi: 10.1016/j.jtos.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes M, Vira D, Dey M, Tanzin T, Kumar N, Sharma S. Comparison between polymicrobial and fungal keratitis: clinical features, risk factors, and outcome. Am J Ophthalmol. 2015;160:873–81.e2. doi: 10.1016/j.ajo.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Ting DSJ, Bignardi G, Koerner R, Irion LD, Johnson E, Morgan SJ, et al. Polymicrobial keratitis with Cryptococcus curvatus, Candida parapsilosis, and Stenotrophomonas maltophilia after penetrating keratoplasty: a rare case report with literature review. Eye Contact Lens. 2019;45:e5–10. doi: 10.1097/ICL.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 9.Shah A, Sachdev A, Coggon D, Hossain P. Geographic variations in microbial keratitis: an analysis of the peer-reviewed literature. Br J Ophthalmol. 2011;95:762–7. doi: 10.1136/bjo.2009.169607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green M, Apel A, Stapleton F. A longitudinal study of trends in keratitis in Australia. Cornea. 2008;27:33–9. doi: 10.1097/ICO.0b013e318156cb1f. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93:1319–24. doi: 10.1136/bjo.2008.151167. [DOI] [PubMed] [Google Scholar]
- 12.Lin CC, Lalitha P, Srinivasan M, Prajna NV, McLeod SD, Acharya NR, et al. Seasonal trends of microbial keratitis in South India. Cornea. 2012;31:1123–7. doi: 10.1097/ICO.0b013e31825694d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otri AM, Fares U, Al-Aqaba MA, Miri A, Faraj LA, Said DG, et al. Profile of sight-threatening infectious keratitis: a prospective study. Acta Ophthalmol. 2013;91:643–51. doi: 10.1111/j.1755-3768.2012.02489.x. [DOI] [PubMed] [Google Scholar]
- 14.Ni N, Nam EM, Hammersmith KM, Nagra PK, Azari AA, Leiby BE, et al. Seasonal, geographic, and antimicrobial resistance patterns in microbial keratitis: 4-year experience in eastern Pennsylvania. Cornea. 2015;34:296–302. doi: 10.1097/ICO.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 15.Gorski M, Genis A, Yushvayev S, Awwad A, Lazzaro DR. Seasonal variation in the presentation of infectious keratitis. Eye Contact Lens. 2016;42:295–7. doi: 10.1097/ICL.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 16.Walkden A, Fullwood C, Tan SZ, Au L, Armstrong M, Brahma AK, et al. Association between season, temperature and causative organism in microbial keratitis in the UK. Cornea. 2018;37:1555–60. doi: 10.1097/ICO.0000000000001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ting DSJ, Ho CS, Cairns J, Elsahn A, Al-Aqaba MA, Boswell T, et al. 12-year analysis of incidence, microbiological profiles, and in vitro antimicrobial susceptibility of infectious keratitis: the Nottingham Infectious Keratitis Study. Br J Ophthalmol. 2020. 10.1136/bjophthalmol-2020-316128. [DOI] [PMC free article] [PubMed]
- 18.Chew HF, Yildiz EH, Hammersmith KM, Eagle RC, Jr., Rapuano CJ, Laibson PR, et al. Clinical outcomes and prognostic factors associated with Acanthamoeba keratitis. Cornea. 2011;30:435–41. doi: 10.1097/ICO.0b013e3181ec905f. [DOI] [PubMed] [Google Scholar]
- 19.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–8. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong RA. When to use the Bonferroni correction. Ophthalmic Physiol Opt. 2014;34:502–8. doi: 10.1111/opo.12131. [DOI] [PubMed] [Google Scholar]
- 21.Martinez ME. The calendar of epidemics: seasonal cycles of infectious diseases. PLoS Pathog. 2018;14:e1007327. doi: 10.1371/journal.ppat.1007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowell SF. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg Infect Dis. 2001;7:369–74. doi: 10.3201/eid0703.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook SM, Glass RI, LeBaron CW, Ho MS. Global seasonality of rotavirus infections. Bull World Health Organ. 1990;68:171–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Koh GC, Hawthorne G, Turner AM, Kunst H, Dedicoat M. Tuberculosis incidence correlates with sunshine: an ecological 28-year time series study. PLoS ONE. 2013;8:e57752. doi: 10.1371/journal.pone.0057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki T, Sutani T, Nakai H, Shirahige K, Kinoshita S. The microbiome of the meibum and ocular surface in healthy subjects. Invest Ophthalmol Vis Sci. 2020;61:18. doi: 10.1167/iovs.61.2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang SD, He JN, Niu TT, Chan CY, Ren CY, Liu SS, et al. Bacteriological profile of ocular surface flora in meibomian gland dysfunction. Ocul Surf. 2017;15:242–7. doi: 10.1016/j.jtos.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Achermann Y, Goldstein EJ, Coenye T, Shirtliff ME. Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin Microbiol Rev. 2014;27:419–40. doi: 10.1128/CMR.00092-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthony CA, Peterson RA, Sewell DK, Polgreen LA, Simmering JE, Callaghan JJ, et al. The seasonal variability of surgical site infections in knee and hip arthroplasty. J Arthroplast. 2018;33:510–4.e1. doi: 10.1016/j.arth.2017.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chidambaram JD, Prajna NV, Palepu S, Lanjewar S, Shah M, Elakkiya S, et al. In vivo confocal microscopy cellular features of host and organism in bacterial, fungal, and Acanthamoeba keratitis. Am J Ophthalmol. 2018;190:24–33. doi: 10.1016/j.ajo.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ting DSJ, Said DG, Dua HS. Interface haze after Descemet stripping automated endothelial keratoplasty. JAMA Ophthalmol. 2019;137:1201–2. doi: 10.1001/jamaophthalmol.2019.2745. [DOI] [PubMed] [Google Scholar]
- 31.Singhal N, Kumar M, Kanaujia PK, Virdi JS. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ting DSJ, McKenna M, Saidq SN, Martin J, Mudhar HS, Meeney A, et al. Arthrographis kalrae keratitis complicated by endophthalmitis: a case report with literature review. Eye Contact Lens. 2020. 10.1097/ICL.0000000000000713. [DOI] [PubMed]
- 33.Ung L, Bispo PJM, Doan T, Van Gelder RN, Gilmore MS, Lietman T, et al. Clinical metagenomics for infectious corneal ulcers: rags to riches? Ocul Surf. 2020;18:1–12. doi: 10.1016/j.jtos.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ting DSJ, Foo VH, Yang LWY, Sia JT, Ang M, Lin H, et al. Artificial intelligence for anterior segment diseases: emerging applications in ophthalmology. Br J Ophthalmol. 2020. 10.1136/bjophthalmol-2019-315651. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.